Abstract
Objective:
Avoidant/restrictive food intake disorder (ARFID) is characterized by food avoidance or dietary restriction not primarily motivated by body weight/shape concerns. Individuals with ARFID can report early satiation, post-prandial fullness, and high inter-meal satiety, but whether these symptoms are related to differences in the biology underlying appetite regulation is unknown. In male and female children and adolescents, we hypothesized that fasting levels of cholecystokinin (CCK), a satiety hormone, would be elevated in participants with ARFID (full or subthreshold) versus healthy controls (HC). Within the ARFID group, we also explored the relations of CCK with weight status, subjective appetite ratings, and ARFID severity and phenotypes.
Methods:
125 participants (83 full/subthreshold ARFID and 42 HC, ages 10-23 years; 59% female; July 2014—December 2019) underwent fasting blood draws for CCK, completed self-report measures assessing subjective state and trait appetite ratings, and completed a semi-structured interview assessing ARFID severity.
Results:
Fasting CCK was higher in full/subthreshold ARFID versus HC with a large effect (F(1)=25.0, p<.001, ηp2=.17), controlling for age, sex, and BMI percentile. Within the ARFID group, CCK was not significantly related to BMI percentile, subjective appetite ratings, or ARFID characteristic measures.
Conclusion:
CCK may contribute to etiology and/or maintenance of ARFID, as children/adolescents with heterogeneous presentations of avoidant/restrictive eating appear to show elevated fasting levels compared to healthy youth. Further research is needed to understand relations between CCK and appetite, weight, and eating behavior in ARFID.
Keywords: Avoidant/restrictive food intake disorder, feeding and eating disorders, appetite regulation, cholecystokinin, neuroendocrinology
INTRODUCTION
Avoidant/restrictive food intake disorder (ARFID) is a recently classified eating disorder defined by food avoidance or restrictive eating that is not primarily motivated by body shape/weight concerns. In contrast to other restrictive eating disorders (e.g., anorexia nervosa), avoidant/restrictive eating in ARFID is motivated by one of three prototypes that often co-occur— sensory sensitivities to food characteristics, disinterest in food/eating or low appetite, and/or fear of aversive consequences (e.g., choking, vomiting).1 Medical (e.g., weight loss, low-weight status, dependence on supplemental nutrition) and/or psychosocial (e.g., social eating difficulty) impairments are sequelae of limited food variety and/or volume in ARFID.1 While early satiation, post-prandial fullness, and high inter-meal satiety are commonly reported as associated with avoidant/restrictive eating in ARFID, whether biological abnormalities in appetite regulation underlie ARFID is largely unknown.
Satiety hormones may be particularly relevant to ARFID, given their role in other restrictive eating disorder presentations with disrupted appetite signaling.2,3 While the motivations for food restriction are different in ARFID, ARFID shares dysregulated eating patterns (e.g., avoidance of specific foods; low food volume intake; absence of food intake for long intervals) with other eating disorders. Research has shown that other restrictive eating disorders (e.g., anorexia nervosa) are characterized by disturbances in gut-derived satiety hormones.3 As ARFID is often characterized by high inter-meal fullness and disinterest in eating,1 elevations in cholecystokinin (CCK), a satiety-promoting gut-derived satiety hormone, may be particularly relevant. CCK is a gut-brain peptide secreted from I-cells in the small bowel, signaling satiation in response to food intake as well as satiety (i.e., between meals).4 Fasting CCK has been shown in some studies to be elevated in anorexia nervosa, but other studies show no differences from controls.5 However, no studies to date have investigated CCK levels in ARFID.
In the current study, we compared fasting concentrations of plasma CCK between adolescents and young adults with ARFID and health controls (HC). For our primary aim, we hypothesized that, similar to some findings for anorexia nervosa,3 fasting CCK levels would be high in youth with ARFID compared to HC. We then explored relations of CCK with ARFID characteristics including weight status (by BMI percentile), subjective appetite ratings, and avoidant/restrictive eating severity and phenotypes in the ARFID group.
METHODS
Participants
One-hundred twenty-five male and female participants ages 10 to 23 years were consecutively recruited for one or both of two NIMH-funded studies on the neurobiology of ARFID or low-weight eating disorders between July 2014 and December 2019. Participants included 83 with full/subthreshold ARFID [49% female, M(SD) age= 15.4 (3.7)] and 42 HC [83% female, M(SD) age= 18.5 (3.0)]. Participants completed a screening visit to determine eligibility, which included a detailed medical history, physical examination including height and weight, a blood sample to rule out anemia, urine pregnancy test, and the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime (KSADS-PL). We measured height on a wall-mounted stadiometer in triplicate and weight on an electronic scale.
Eligibility.
Participants in the full/subthreshold ARFID group6,7 were eligible if they met full criteria for ARFID on the Eating Disorder Assessment-5 (EDA-5)8 or endorsed ARFID symptoms on the Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children-Present and Lifetime version (KSADS-PL) Eating Disorder and Substance-Related Disorders Supplements.9 History of any other feeding or eating disorders (other than ARFID) were ruled out via a clinically significant score (i.e., global score of 4 or higher) or endorsement of self-induced vomiting, fasting, laxatives, diuretics or compensatory exercise in the last 28 days on the Eating-Disorder Examination-Questionnaire (EDE-Q). Participants met full criteria for ARFID if they restricted their intake by volume and/or variety and met DSM-5 criteria A, B, C, and D on the Pica, ARFID, and Rumination Disorder Interview (PARDI).10 Participants met subthreshold criteria for ARFID if they restricted their intake by volume and/or variety, and met DSM-5 criteria B, C, and D, but did not meet any sub-criteria for criterion A (medical and/or psychosocial impairment) at the level of required on the PARDI (e.g., a score of 3 out of 6 on a psychosocial impairment item, when 4 out of 6 was required for full syndrome diagnosis).
Exclusion criteria for both full/subthreshold ARFID and HC groups included systemic hormone use, current pregnancy, breastfeeding within the 8 weeks prior to the study visit, psychosis history, active substance abuse, hematocrit <30%, gastrointestinal (GI) tract surgery history, or other medical conditions (e.g., diabetes) that could lead to low weight or appetite hormone dysregulation. Inclusion criteria for the HC group were: 15th-85th BMI percentiles for age, regular menses (if ≥two years post-menarcheal), no pubertal delay history (i.e., menarche at >16 years or thelarche at >13 years), <10 hours of exercise or <25 miles of running per week in the three months prior (as greater levels of exercise have been shown to influence appetite-regulating hormones),11 and no lifetime history of any psychiatric disorder by KSADS-PL.
Informed consent.
We obtained written informed consent from participants ≥18 years and from parents of subjects <18 years old; we obtained assent from subjects <18 years old. All study procedures were approved by the Mass General Brigham Institutional Review Board.
Procedures
The study visit occurred within 8 weeks of the screening visit. A research nurse practitioner captured an updated medical history and performed a physical examination, including height (on a wall-mounted stadiometer in triplicate) and weight (in a gown on an electronic scale). Participants were asked to fast (with the exception of water intake) for at least 8 hours prior to fasting blood draw.
CCK
Fasting blood was drawn around 08:45AM by trained nursing staff. Plasma samples were immediately placed on ice following venipuncture and spun in a refrigerated centrifuge (all samples were stored at −80 degrees Celsius until analysis). Enzyme-linked immunosorbent assays were used to assess plasma total CCK (RayBio: Peachtree Corners, GA; intra-assay CV <10% and inter-assay CV <15%; lower limit of detection 0.2 pg/mL).
Measures
Visual Analogue Scales (VAS) for subjective appetite assessment include an electronic scale of 0 to 100 to assess levels of hunger and satiety. Participants in both studies completed VAS ratings immediately prior to the fasting blood draw. Questions and rating scales used in this study are as follows: (a) “How hungry do you feel?” (0=“I am not hungry at all”, 100=“I have never been more hungry”), (b) “How satisfied do you feel?” (0=“I am completely empty”, 100=“I can’t eat another bite”), (c) “How full do you feel?” (0=“not at all full”; 100=“totally full”).
The Adult Eating Behavior Questionnaire (AEBQ) is a self-report measure of appetite and interest in eating with a 5-point Likert scale (1=strongly disagree to 5=strongly agree).12 We used the 5-item hunger subscale, with lower scores indicating lower ratings of trait hunger; and the 4-item satiety responsiveness subscale, with higher scores indicating greater satiety and early satiation. Participants in one study (R01MH108595) completed this measure.
The Pica, ARFID, and Rumination Disorder Interview (PARDI) is a validated semi-structured clinical interview that captures ARFID symptoms.10 We used an overall symptom severity rating, and severity ratings for three prototypic ARFID presentations—sensory sensitivity, lack of interest in food or eating, and fear of aversive consequences. PARDI scores range from 0 (no symptoms) to 6 (extreme severity). Participants in one study (R01MH108595) completed this measure.
Statistical Analysis
We used SPSS Statistics v.24 for statistical analyses. Variables were screened prior to analysis, including outlier removal (by determining if any values fell beyond three standard deviations of the mean). Log-transformation improved non-normal distributions for CCK and AEBQ-hunger, but not BMI percentile, age, PARDI-sensory, PARDI-fear, or PARDI-lack of interest. For descriptive purposes, we compared clinical characteristics between groups using Mann Whitney U tests for age and BMI comparisons, analysis of covariance for appetite variables and ARFID severity scores (with age, sex, and BMI percentile as covariates), and chi-square tests for categorical variables.
For our primary aim, we conducted an analysis of covariance (ANCOVA) to compare fasting CCK levels between groups controlling for age, sex, and BMI percentile because descriptive analyses showed differences in these variables between groups (see below). In an exploratory fashion, within the full/subthreshold ARFID group, we conducted a series of Spearman correlations between non-log-transformed CCK levels and each of the following: BMI percentile, Appetite VAS, AEBQ-hunger, AEBQ-satiety responsiveness, PARDI severity scores (applying a Bonferroni correction for multiple testing setting the alpha at p<.004).
RESULTS
Most of the sample identified as White (85.6%; n=107) and non-Hispanic/Latino (90.4%; n=113). Compared to the HC group, the full/subthreshold ARFID group was younger (p=.001), had a greater proportion of males (p<.001), and had lower BMI percentiles (p=.001) (Table 1). Frequencies of current psychiatric and neurodevelopmental comorbidities and psychiatric medications in the full/subthreshold ARFID group are included in Table 2.
Table 1.
Sample characteristics
| Full/Subthreshold ARFID (n=83) |
HC (n=42) |
U, F, or Chia | p-value | Cohen’s d/ηb | |
|---|---|---|---|---|---|
| Age (years), M(SD), range | 15.3 (3.6) | 18.5 (3.0) | 865.0 | <.001 | 0.732 |
| 10.2-23.7 | 10.9-22.7 | ||||
| Sex, n(%) female | 41 (49%) | 35 (83%) | 13.9 | <.001 | -- |
| Race, n(%) | -- | -- | -- | ||
| American Indian/Alaskan Native | 0 (0%) | 0 (0%) | |||
| Asian/Asian American | 3 (4%) | 8 (19%) | |||
| Native Hawaiian or Other Pacific Islander | 0 (0%) | 0 (0%) | |||
| Black/African American | 2 (2%) | 0 (0%) | |||
| White | 74 (89%) | 33 (79%) | |||
| More than one race | 4 (5%) | 1 (2%) | |||
| Ethnicity, n(%) | -- | -- | -- | ||
| Hispanic/Latino | 7 (8%) | 5 (12%) | |||
| Non-Hispanic/Latino | 76 (92%) | 37 (88%) | |||
| BMI-kg/m2, M(SD) | 19.3 (5.5) | 21.7 (2.2) | 945.5 | <.001 | 0.42 |
| Percentile | 35.4 (34.5) | 54.3 (17.6) | 1060.5 | <.001 | 0.75 |
| BMI categories for < 20 years old (percentiles), n(%) c | -- | -- | -- | ||
| Underweight (<5th) | 17 (20.5%) | 0 (0.0%) | |||
| Normal weight (5th to <85th) | 46 (55.4%) | 28 (66.7%) | |||
| Overweight (85th to <95th) | 2 (2.4%) | 0 (0.0%) | |||
| Obesity (≥95th) | 4 (4.8%) | 0 (0.0%) | |||
| BMI categories for ≥ 20 years old (kg/m2), n(%) | -- | -- | -- | ||
| Underweight (<18.5 kg/m2) | 3 (3.6%) | 0 (0.0%) | |||
| Normal weight (18.5-24.9 kg/m2) | 6 (7.2%) | 12 (28.6%) | |||
| Overweight (25.0-29.9 kg/m2) | 2 (2.4%) | 2 (4.8%) | |||
| Obesity (≥ 30.0 kg/m2) | 3 (3.6%) | 0 (0.0%) | |||
| VAS scales, M(SD) (0-100) | |||||
| “How hungry do you feel?” | 58.0 (25.2) | 60.8 (18.2) | 3.1 | .078 | 0.026 |
| “How satisfied do you feel?” | 31.5 (20.2) | 34.5 (17.9) | 0.5 | .480 | 0.004 |
| “How full do you feel?” | 27.4 (22.6) | 24.2 (19.1) | 0.5 | .480 | 0.004 |
| AEBQ Hunger scale, M(SD) (0-5) d | 2.3 (0.7) | 2.1 (0.5) | 1.1 | .289 | 0.015 |
| AEBQ Satiety Responsiveness scale, M(SD) (0-5)d | 2.4 (0.9) | 2.2 (0.8) | 4.6 | .035 | 0.058 |
| PARDI severity scores, M(SD) (0-6) e | |||||
| Overall Severity | 2.4 (0.9) | 0.2 (0.2) | 144.5 | <.001 | 0.649 |
| Sensory Sensitivity | 1.5 (1.1) | 0.0 (0.0) | 37.5 | <.001 | 0.325 |
| Lack of Interest | 1.9 (1.6) | 0.1 (0.1) | 20.7 | <.001 | 0.210 |
| Fear of Aversive Consequences | 0.3 (0.7) | 0.0 (0.0) | 1.9 | .173 | 0.024 |
Mann Whitney U tests used with for age and BMI comparisons. Analysis of covariance used for VAS appetite scores, AEBQ hunger (log-transformed), AEBQ satiety responsiveness, and ARFID severity scores (log-transformed), with age (log-transformed), sex, and BMI percentile (log-transformed) as covariates. Chi-Square Tests used with categorical variables.
Percentages reported among participants of female sex.
BMI percentile categories based on the Center for Disease Control guidelines. HC participants were included in this study if they were in the 15th-85th percentile range.
Data available for n=68 ARFID and n=12 HC.
Data available for n=71 ARFID and n=12 HC.
Abbreviations: ARFID = avoidant/restrictive food intake disorder; HC = healthy controls; BMI=body mass index; VAS = visual analogue scale; AEBQ = Adult Eating Behavior Questionnaire; PARDI = Pica, ARFID, Rumination Disorder Interview.
Table 2.
Current psychiatric/neurodevelopmental comorbidities and medications in the full/subthreshold ARFID group (n=83).
| n(%) | |
|---|---|
| Diagnosis | |
| Generalized anxiety disorder | 20 (24%) |
| ADHD/other specified ADHD | 16 (19%) |
| Social anxiety disorder | 7 (8%) |
| Specific phobia | 6 (7%) |
| Obsessive compulsive disorder | 3 (4%) |
| Autism spectrum disorder | 2 (2%) |
| Oppositional defiant disorder/other specified disruptive, impulse-control, and conduct disorder | 2 (2%) |
| Agoraphobia | 1 (1%) |
| Tourette’s disorder | 1 (1%) |
| Medication | |
| Antidepressants | 22 (27%) |
| Psychostimulants | 8 (10%) |
| Antipsychotics | 2 (2%) |
Abbreviations: ARFID = avoidant/restrictive food intake disorder.
Consistent with our hypothesis, participants with full/subthreshold ARFID had higher fasting CCK than HC (p<.001), while controlling for age, sex, and BMI percentile (Figure 1). Average (SD; range) CCK levels in the full/subthreshold ARFID group were 458.6 (490.6; 39.8-2,816.6) and in the HC group were 143.6 (87.2; 54.6-392.6) pg/mL with a large effect (ηp2=.17). Within the full/subthreshold ARFID group, CCK was not significantly associated with BMI percentile (rho=.026, p=.814), Appetite VAS scores (rho’s=−.035-.013, p’s=.758-.950), AEBQ-hunger (rho=.062, p=.616), AEBQ-satiety responsiveness (rho=.067, p=.589), or PARDI scores (rho’s=−.123-.054, p’s=.309-.746).
Figure 1.
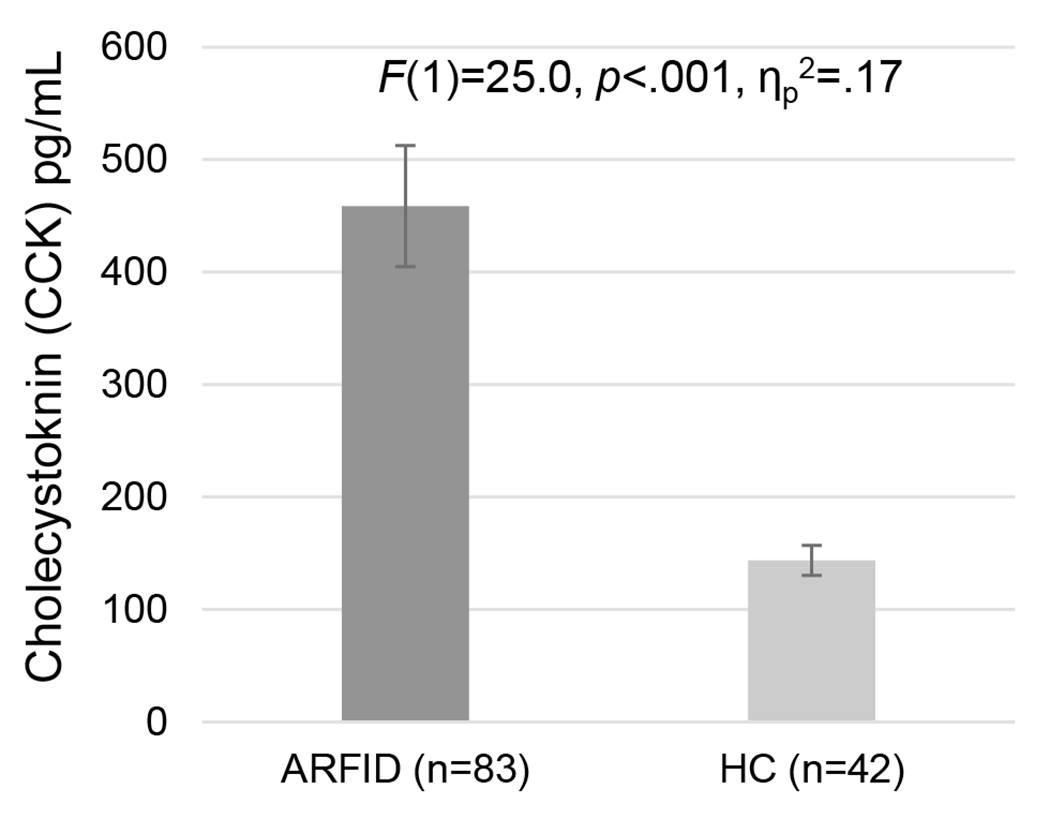
Fasting post-prandial CCK levels in participants with avoidant/restrictive food intake disorder (ARFID) and healthy controls (HC).a
aError bars= standard error of the mean; hormone levels after an 8+ hour overnight fast; between-group comparisons conducted using analysis of covariance (ANCOVA), controlling for age, sex, and BMI percentile.
Abbreviations: ARFID = avoidant/restrictive food intake disorder; HC = healthy controls
Discussion
ARFID is often associated with characteristics of fullness, such as early satiation, post-prandial fullness, and high inter-meal satiety. Given the recent debut of ARFID in the DSM-5, research on biological differences that may underlie dysregulated appetite in ARFID is nascent. The current study investigated the role of satiety-promoting CCK in a fasting state in full/subthreshold ARFID, including exploration of associations with subjective appetite measures. Among male and female children and adolescents, we found that fasting CCK was significantly elevated (over three times higher on average) in those with full/subthreshold ARFID compared to healthy controls. Despite the significant elevation in CCK in the full/subthreshold ARFID group, CCK was not significantly associated with BMI percentile, age, subjective appetite ratings, or ARFID characteristic measures among those with full/subthreshold ARFID. Our study establishes early support for CCK dysregulation in ARFID. However, further longitudinal research is needed, as it is not yet clear whether elevated CCK contributes to development of, is a manifestation of, and/or contributes to maintenance of avoidant/restrictive eating.
Elevated CCK may relate to gut-brain dysregulation in avoidant/restrictive eating in ARFID. Many individuals at all body weights with ARFID describe low appetite and gastrointestinal pain around eating.10 In fact, ARFID has significant overlap with a disorder of gut-brain interaction characterized by dysregulation in satiety called functional dyspepsia, post-prandial distress syndrome (ARFID observed in up to 40%).13,14 The core symptoms of post-prandial distress syndrome include high satiety, early satiation, and post-prandial fullness. Similar to our findings in ARFID, pilot studies have shown elevated fasting CCK in functional dyspepsia compared to controls.15 In addition, although individuals with anorexia nervosa (another primary restrictive eating disorder) do not consistently show higher CCK than healthy controls,2,16 there is evidence that elevated CCK levels at treatment initiation are associated with fullness, pain, and constipation during refeeding.16 CCK antagonists could be a potential therapeutic option for ARFID and have previously been proposed for functional dyspepsia,17 anorexia nervosa,18 and bulimia nervosa.19 However, further research is needed, as we were unable to identify relations between CCK and subjective appetite.
Our exploratory analyses did not show any relationships between CCK and weight status (BMI percentile), self-report measures of appetite, interest in eating, or ARFID symptoms. It is possible that other factors may better explain differences in CCK levels—for example, recent findings suggest that classical conditioning may better explain dysregulation in CCK and other gut-derived appetite hormones.4 It is possible that avoidant/restrictive eating in ARFID may lead to the de-conditioning of CCK as a signal for satiation in response to meal ingestion, leading to dysregulation in secretion patterns and circulating levels in a fasted state. Instead, cues for reduced intake (volume and/or variety) in ARFID may become reliant on other cues related to motivations underlying avoidant/restrictive eating (e.g., sensory characteristics of food, fear of aversive consequences) instead of appetite. Identifying subjective appetite may have also been difficult for many in this sample, as younger adolescents and children may have less insight into their symptoms,10 and research suggests low interoceptive awareness in other eating disorder samples.20 Further, having feeding/eating difficulties for a majority of their lifetime,21 may make it challenging to identify and describe appetite cues without a memory of normal cues. Overall, future longitudinal research is needed to explore if CCK levels influence appetite and eating behavior over time, particularly, following treatment interventions.
To our knowledge, this study is the first to explore levels of CCK in children and adolescents with full/subthreshold ARFID. Strengths include a large sample of individuals with full/subthreshold ARFID diagnosed via structured interview and heterogeneous characteristics in terms of age, sex, weight status, and ARFID presentations. This is a cross-sectional study; therefore, we cannot determine if elevated CCK levels in youth with ARFID represent a consequence of malnutrition that could be remediated with treatment, or a precursor to ARFID symptoms. It is also possible that some of our ARFID assessments may not have adequately captured state and trait appetite in our sample—future research is needed to validate these measures among different populations with ARFID. The sample was also predominantly White, and it is not clear that findings generalize to other populations. Finally, while we controlled for the effects age, sex, and weight status on CCK levels, future research should explore if difference in CCK levels relates to changes in appetite and food intake in ARFID, as well as other factors that could affect risk and maintenance of ARFID such as generalized anxiety.
In sum, our results provide preliminary support for CCK dysregulation in ARFID, but future studies are needed to determine the role of abnormal CCK levels in the etiology and/or maintenance of avoidant/restrictive eating.
CLINICAL CARE POINTS.
Avoidant/restrictive food intake disorder (ARFID) is associated with early satiation, post-prandial fullness, and high inter-meal satiety, but whether these symptoms are related to differences in the biology underlying appetite regulation is unknown.
Cholecystokinin (CCK), a satiety-promoting hormone, may contribute to etiology and/or maintenance of ARFID, as children/adolescents with heterogeneous presentations of avoidant/restrictive eating appear to show elevated fasting levels compared to healthy youth.
Financial support:
Research reported in this publication was supported by the National Institutes of Health: National Institute of Mental Health (KRB, F32MH111127; K23MH125143), (KTE, MM, EAL, R01MH103402), (JJT, EAL, NM, R01MH108595), (LB, T32MH112485), (EAL, K24MH120568); Grant Number 1UL1TR002541-01 (MGH Translational and Clinical Research Centers); and National Institute of Diabetes and Digestive and Kidney Diseases (HBM, K23DK131334).
Role of the funding agency:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest:
NM receives honoraria from the Gertrude von Meissner foundation, for a scientific advisory committee role. MM reports research funding support from Novo-Nordisk and Abbvie, has received honoraria from the American Board of Pediatrics and royalties from UpToDate, has served on the scientific advisory board of Abbvie and Ipsen, and is a consultant for Sanofi and Abbvie. EAL is on the scientific advisory board and has a financial interest in OXT Therapeutics, a company developing an intranasal oxytocin and long-acting analogs of oxytocin to treat obesity and metabolic disease. She also receives royalties from UpToDate. JJT receives royalties from Harvard Health Publications and Hazelden for the sale of her book, Almost Anorexic: Is My (or My Loved One’s) Relationship with Food a Problem? JJT and KTE receive royalties from Cambridge University Press for the sale of their book, Cognitive-Behavioral Therapy for Avoidant/Restrictive Food Intake Disorder: Children, Adolescents, and Adults. JJT, KTE, and KRB receive royalties from Cambridge University Press for their book The Picky Eater’s Recovery Book: Overcoming Avoidant/Restrictive Food Intake Disorder. HBM and JJT receive royalties from Oxford University Press for the sale of their forthcoming book on rumination syndrome. BK has received research support from AstraZeneca, Takeda, Gelesis, Medtronic, Genzyme and has served as a consultant to Shire, Takeda, and Ironwood. HBM, LB, SH, KH, MF, MK, and MJD do not have any disclosures to report.
Footnotes
Previous presentation: A portion of this data will be presented as an oral abstract at the June 2021 International Conference on Eating Disorders.
REFERENCES
- 1.Thomas JJ, Lawson EA, Micali N, Misra M, Deckersbach T, Eddy KT. Avoidant/Restrictive Food Intake Disorder: a Three-Dimensional Model of Neurobiology with Implications for Etiology and Treatment. Current Psychiatry Reports. 2017;19(8):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schorr M, Miller KK. The endocrine manifestations of anorexia nervosa: mechanisms and management. Nat Rev Endocrinol. 2017;13(3):174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailer UF, Kaye WH. A review of neuropeptide and neuroendocrine dysregulation in anorexia and bulimia nervosa. Curr Drug Targets CNS Neurol Disord. 2003;2(1):53–59. [DOI] [PubMed] [Google Scholar]
- 4.Woods SC, May-Zhang AA, Begg DP. How and why do gastrointestinal peptides influence food intake? Physiol Behav. 2018;193(Pt B):218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36(2):110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker KR, Mancuso CJ, Asanza E, et al. Ghrelin and PYY in low-weight females with avoidant/restrictive food intake disorder compared to anorexia nervosa and healthy controls. Psychoneuroendocrinology. 2021;129:105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harshman SG, Jo JH, Kuhnle MC, et al. A moving target: how we define avoidant/restrictive food intake disorder can double its prevalence. . J Clin Psychiatry. 2021;82(5):20m13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sysko R, Glasofer DR, Hildebrandt T, et al. The eating disorder assessment for DSM-5 (EDA-5): Development and validation of a structured interview for feeding and eating disorders. Int J Eat Disord. 2015;48(5):452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children (KSADS-PL). 2013.
- 10.Bryant-Waugh R, Micali N, Cooke L, Lawson EA, Eddy KT, Thomas JJ. The Pica, ARFID, and Rumination Disorder Interview, a multi-informant, semi-structured interview of feeding disorders across the lifespan: A pilot study for ages 10–22. Int J Eat Disord. 2019;52:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackerman KE, Slusarz K, Guereca G, et al. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. American Journal of Physiology-Endocrinology and Metabolism. 2012;302(7):E800–E806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunot C, Fildes A, Croker H, Llewellyn CH, Wardle J, Beeken RJ. Appetitive traits and relationships with BMI in adults: Development of the Adult Eating Behaviour Questionnaire. Appetite. 2016;105:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton Murray H, Bailey AP, Keshishian AC, et al. Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Adult Neurogastroenterology Patients. Clin Gastroenterol Hepatol. 2020;18(9):1995–2002. e1991. [DOI] [PubMed] [Google Scholar]
- 14.Burton Murray H, Jehangir A, Silvernale CJ, Kuo B, Parkman HP. Avoidant/Restrictive Food Intake Disorder Symptoms are Frequent in Patients Presenting for Symptoms of Gastroparesis. Neurogastroenterol Motil. 2020;32(12):e13931. [DOI] [PubMed] [Google Scholar]
- 15.Pilichiewicz AN, Feltrin KL, Horowitz M, et al. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. Am J Gastroenterol. 2008;103(10):2613–2623. [DOI] [PubMed] [Google Scholar]
- 16.Culbert KM, Racine SE, Klump KL. Hormonal Factors and Disturbances in Eating Disorders. Curr Psychiatry Rep. 2016;18(7):65. [DOI] [PubMed] [Google Scholar]
- 17.Van den Houte K, Scarpellini E, Verbeure W, et al. The Role of GI Peptides in Functional Dyspepsia and Gastroparesis: A Systematic Review. Frontiers in Psychiatry. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crow S Investigational drugs for eating disorders. Expert Opin Investig Drugs. 1997;6(4):427–436. [DOI] [PubMed] [Google Scholar]
- 19.Lutter M Emerging Treatments in Eating Disorders. Neurotherapeutics. 2017;14(3):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalsa SS, Adolphs R, Cameron OG, et al. Interoception and Mental Health: A Roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(6):501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas JJ, Eddy K. Cognitive-behavioral therapy for avoidant/restrictive food intake disorder: children, adolescents, and adults. Cambridge: UK: Cambridge University Press; 2019. [Google Scholar]


