Abstract
The COVID-19 pandemic has substantially impacted the world health systems, causing public health concerns, and the search for new compounds with antiviral activity is of extreme interest. Natural molecules with bioactive potential are a trend, with essential oils (Eos) being the focus of recent studies. Thus, this study evaluates in chemico the d-limonene inhibitory activities in the viral genome of SARS-CoV-2 and analyzes the cytotoxic potential and safety profile of d-limonene and lime and orange EOs with a high content of d-limonene. The EOs were extracted and characterized, and the in chemico computational analysis for the determination as a potential anti-SARS-CoV-2 was performed with d-limonene, the major compound in EOs. The cytotoxicity analysis of EOs and d-limonene was carried out with MRC-5 and HaCaT, and the preliminary safety profile was also evaluated by the HET-CAM assay. d-limonene was suggested as a promising compound for anti-SARS-CoV-2 research, since the molecule does not provide mutagenic and cytotoxic fragments, and does not have irritating potential when diluted, in addition to having favorable pharmacokinetic characteristics, through in chemico analysis. Collectively, the results reveal the antiviral potential of lime and orange EOs, as well as their major compound. In this sense, further studies should be conducted to understand the antiviral mechanisms.
Keywords: Essential oils, d-limonene, anti-Sars-CoV-2, COVID-19
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible and pathogenic coronavirus that emerged in late 2019 and caused an acute respiratory illness pandemic, called “coronavirus disease 2019” (COVID-19). Its unprecedented global social and economic impact marked the third zoonotic introduction of a highly pathogenic coronavirus into the human population (Hu et al., 2020). Following the emergence of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV-2 is the third zoonotic human coronavirus of the century (Rawson et al., 2020). Substantially, the coronavirus disease pandemic impacted healthcare systems around the world, causing infection and mortality (Wang et al., 2020). Despite major vaccination campaigns, many public health measures are still needed to contain the virus transmission, at this time mainly caused by mutations and variants: Omicron, identified for the first time in South Africa, and Delta, identified for the first time in India (CDC, 2021).
Essential oils (EOs) are alternatives for controlling and reducing viral load, with inhibitory activities demonstrated in modeling studies in chemico, in silico, in vitro, and in vivo. Different OEs and its compounds showed antiviral activity against influenza A (H1N1), hepatitis A (HAV), human and simian immunodeficiency virus (HIV and SIV) (Battistini et al., 2019; Brochot et al., 2017; Mediouni et al., 2020). The in chemico studies allow a chemical-based analytical tests in multiple compounds, according to the chemical mechanisms of the molecule when reacting covalently with the viral genome; thus, predicting the decreasing viral load possibility (Taylor, 2019).
Faced with the SARS-CoV-2 pandemic, different modeling studies are analyzing plant-derived natural products, such as OEs and phytotherapeutic agents, effects on the virus genome (Ansari et al., 2022; Khan et al., 2021). Recently, the compounds of garlic, eucalyptus, and tea tree EOs showed antiviral action, opening the possibility of studies to analyze different EOs compounds (My et al., 2020; Sharma & Kaur, 2020; Thuy et al., 2020). Thus, in view of the SARS-CoV-2 pandemic, it is extremely relevant for public health to develop research on compounds that make it possible to inactivate or reduce the viral load. And there is a need for further research to determine which OE components have antiviral characteristics (Silva et al., 2020). Thus, the objective of this study was to evaluate the yet unexplored in chemico the d-limonene inhibitory activities in the viral genome of SARS-CoV-2 and to analyze the cytotoxic potential and safety profile of d-limonene and lime and orange EOs with a high content of d-limonene.
2. Materials and methods
2.1. Chemicals and reagents
Substances used in gas chromatography and cell culture were purchased from Sigma Aldrich Co. (St. Louis, MO), including alkane standard solution, d-limonene standard, C8-C20, culture mediums, fetal bovine serum, antibiotic solution, DMSO (dimethyl sulfoxide), MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide). Medium-chain triglycerides (MCT: capric and caprylic acids) were purchased from Mapric Greentech Company (Diadema, SP, Brazil). Ultrapure water was obtained from a Milli-Q apparatus (Millipore, Merck, Darmstadt, Germany).
2.2. Evaluation of the essential oil chemical composition
Commercial samples of Tahiti lime (Citrus × latifolia) and orange (Citrus sinensis) were purchased from a producer in Canoas, Rio Grande do Sul, Brazil (29° 55′ 12″ S and 51° 10′ 48″ W). Each sample (peels) was placed separately in a 2 L flask and was mixed with ultrapure water and hydrodistilled using a Clevenger apparatus for 90 min at 100°C. A sterilized glass vial was used to collect the EO.
The EO compounds chemical characterization and quantification of lime and orange were carried out through gas chromatographic and mass spectrometry, according to Hussain et al. (2010), with some modifications.
The EO was diluted in ethyl ether and analyzed on a gas chromatograph (GC) (Shimadzu QP2010 Plus), equipped with an AOC-20i auto-sampler and coupled to a QP2010 Plus single quadrupole mass spectrometer (MS) (Shimadzu Corporation, Kyoto, Japan). Compounds were separated using an Rtx-5MS capillary column (Shimadzu, 30 m x 0.25 x 0.25 μm). The injector and detector temperatures were set at 220 and 250°C, respectively. The column temperature was from 80 to 220°C at a rate of 4°C/min. Helium was used as carrier gas with a flow rate of 1.5 mL/min.
An electron ionization mode with 70 eV ionization energy was used for mass spectrum detection. Injector and MS transfer line temperatures were set at 220 and 290°C, respectively. A 1.0 μL aliquot was injected using the split mode (split ratio, 1:40). For the EO components identification, the retention indexes (Eq. (1)) were calculated based on the comparison of their retention indexes with n-alkanes solution (C8-C20). The compounds were identified and authenticated using their mass spectrum data compared to the MS NIST11 library.
| (1) |
Where: RI (retention index); Pz (number of alkane carbons before the peak of interest); Pz+1 (retention time of the alkane after the peak of interest); RT (x) (peak sample retention time to be identified); RT (Pz) (retention time of the alkane before the peak of interest).
2.3. Computational and in chemico analysis
The computational analysis was performed using the OSIRIS program (version 2.15.1, National Center for Biotechnology Information), which allows the identification of possible toxic structural fragments known and previously computed in the RTECS database (Registry of Toxic Effects of Chemical Substances) (Brito, 2011). In addition, the partition coefficient (logP) and molecular weight were determined, aiming to correlate the Lipinski et al. (2001) parameters, where the molecular weight must be <500 Da (Da), and the logP must be <5.
In the in chemico analysis, a structural comparison of limonene (the major compound in the studied EOs) was performed with the SARS-CoV-2 virus complete viral genome obtained from the GenBank database of the National Center for Biotechnology Information (NCBI) (2020). The electronic surface analysis was determined by the MolView web server (Bergwerf, 2015).
2.4. In vitro cytotoxicity assessment
The human keratinocytes (HaCaT) and fibroblasts (MRC-5), were acquired from the Rio de Janeiro Cell Bank, cultivated at 37°C with 5% CO2 in DMEM supplemented with 10% fetal bovine serum and 1% antibiotics solution (penicillin 100 U/mL and 100 μg/mL streptomycin).
To evaluate the cytotoxicity of EOs and d-limonene, the MTT method was described by (Mosmann, 1983) and adapted for 96-well plates. Initially, cells were transferred to 96-well plates at 2x105 cells/mL (HaCaT) and 2x104 cells/mL (MRC-5) and incubated for 24 h under the same atmospheric conditions described above. On the second day of the experiment, solutions of each EO and d-limonene (15.6 to 1000.0 μg/mL) were prepared in DMSO, not exceeding 0.1% v/v DMSO in the wells. Then the solutions were transferred to the plates and the cells were incubated again for 24 h. For positive cytotoxicity control, 6% v/v DMSO solution was applied to the MRC-5 and 1% v/v H2O2 solution to the HaCaT, both prepared in a culture medium. Especially for the positive control of 1% v/v H2O2, an exposure time of 30 min was applied on the last day of the experiment.
After the treatment exposure period, MTT solution (5 mg/mL) was added to all wells. Then, the plates were incubated for 2.5 h and the absorbance was verified at 570 nm and 630 nm, after the formazan crystals solubilization with DMSO. The cell inhibition percentage was calculated based on the negative control absorbance, composed of cells + culture medium according to Equation (2).
| (2) |
Where: AA570 - sample absorbance value at 570 nm; AA630 – sample absorbance value at 630 nm; AC570 - control absorbance value at 570 nm; AC630 – control absorbance value at 630 nm.
2.5. Preliminary safety profile assessment
2.5.1. Obtaining eggs and Ethical aspects
Embryonic chicken eggs were purchased from Aviário de Ensino e Pesquisa da Universidade Federal do Rio Grande do Sul - UFRGS (Porto Alegre, Rio Grande do Sul, Brazil) on the first day of laying and incubated until the tenth day in an automatic incubator (Chocar Chocadeiras, Conceição do Coité, Brazil). Temperature and humidity were maintained at 37.5 ± 0.5°C and 65% ± 5% RH, respectively. After incubation (48 h), the eggs were submitted to automatic turning every 60 min and the development of the embryos was verified by the candling technique, throughout the incubation period. This study was previously approved by the Ethics Committee on the Use of Animals at UFRGS (protocol number: 40087).
2.5.2. Hen's egg chorioallantoic membrane assay (HET-CAM)
The preliminary safety profile of lime and orange EOs, and the d-limonene, were evaluated by the HET-CAM assay (Luepke, 1985). The analyzed groups (n = 4/group) were: 0.1 M NaOH (positive control, hemorrhage and coagulation), 1% w/v sodium lauryl sulfate in water (positive control, vasoconstriction), 0.9% NaCl m/v in water (negative control), MCT (diluent control), d-limonene at 10 mg/mL in MCT, lime EO at 10 mg/mL in MCT, orange EO at 10 mg/mL in MCT. For the assay, 300 μL of each solution was applied to the chorioallantoic membrane of the embryonated eggs (day 10) and the irritating effects (hemorrhage, coagulation, and vasoconstriction) were observed for 5 min. Afterward, the irritation score was calculated for each sample, according to Equation (3). Results were expressed as mean ± standard deviation.
| (3) |
Where: IS (irritation score); H sec (start second of hemorrhage effect); V sec (start second of vasoconstriction effect); C sec (start second of coagulation effect). Classification: IS 0 - 0.9: non-irritant; IS 1.0 – 4.9: slight irritant; IS 5.0 – 8.9 moderate irritant; IS 9.0 – 21.0 severe irritant.
2.6. Statistical analysis
For statistical analysis, the one-way ANOVA test was applied followed by the Tukey post hoc test, with a significance level set at 5% (P<0.05). Analyzes were conducted with GraphPad Prism software, version 6.0.
3. Results and discussion
3.1. Essential oil chemical composition
The lime EO GC-MS analysis identified 18 different constituents representing 96.94% of the total EO mass (Table 1 ). d-limonene (54.71%) was the major constituent followed by γ-terpinene (16.53%), β-pinene (10.29%), α-citral (2.51%), β-bisabolene (2.02%) and β-citral (1.77%). The other constituents identified in the EO ranged from 0.16% to 1.47%. The orange EO GC-MS analysis identified 10 different constituents representing 99.04% of the total EO mass (Table 2 ). d-limonene (90.90%) was the major constituent followed by linalool (2.49%), octanal (1.99%), and β-myrcene (1.34%). The other constituents identified in the EO were in the range of 0.20%–0.88%.
Table 1.
Chemical composition of lime EO (Citrus × latifolia).
| # | Retention time | Compound | % Similarity | Mol Wt | Area | Area % |
|---|---|---|---|---|---|---|
| 1 | 4.128 | β-Phelandrene | 93 | 136 | 438699 | 1.47 |
| 2 | 4.230 | β-pinene | 96 | 136 | 3067369 | 10.29 |
| 3 | 4.377 | β-Myrcene | 96 | 136 | 365882 | 1.23 |
| 4 | 4.992 | (+)-4-Carene | 93 | 136 | 127404 | 0.43 |
| 5 | 5.165 | o-Cymeno | 92 | 134 | 47533 | 0.16 |
| 6 | 5.268 | d-limonene | 95 | 136 | 16302966 | 54.71 |
| 7 | 5.978 | γ-Terpinene | 96 | 136 | 4925308 | 16.53 |
| 8 | 6.760 | Terpinolene | 96 | 136 | 207480 | 0.70 |
| 9 | 7.002 | Linalool | 92 | 154 | 128422 | 0.43 |
| 10 | 9.357 | Terpinen-4-ol | 92 | 154 | 156870 | 0.53 |
| 11 | 9.752 | α-Terpineol | 94 | 154 | 343645 | 1.15 |
| 12 | 10.870 | Nerol | 91 | 154 | 355989 | 1.19 |
| 13 | 11.293 | β-Citral | 95 | 152 | 528429 | 1.77 |
| 14 | 11.685 | Geraniol | 95 | 154 | 429310 | 1.44 |
| 15 | 12.220 | α-Citral | 97 | 152 | 747055 | 2.51 |
| 17 | 16.948 | Caryophyllene | 92 | 204 | 112395 | 0.38 |
| 18 | 19.600 | β-bisabolene | 93 | 204 | 601716 | 2.02 |
Table 2.
Chemical composition of orange EO (Citrus sinensis).
| # | Retention time | Compound | % Similarity | Mol wt | Area | Area % |
|---|---|---|---|---|---|---|
| 1 | 4.134 | Sabinene | 90 | 136 | 81622 | 0.28 |
| 2 | 4.383 | β-Myrcene | 96 | 136 | 392362 | 1.34 |
| 3 | 4.626 | Octanal | 97 | 136 | 583886 | 1.99 |
| 4 | 4.877 | 3-careno | 94 | 128 | 57772 | 0.20 |
| 5 | 5.277 | d-limonene | 95 | 136 | 26664048 | 90.90 |
| 6 | 6.193 | 1-octanol | 96 | 136 | 258528 | 0.88 |
| 7 | 7.005 | Linalool | 92 | 154 | 730035 | 2.49 |
| 8 | 9.759 | α-Terpineol | 93 | 154 | 111897 | 0.38 |
| 9 | 10.149 | Decan | 93 | 154 | 92474 | 0.32 |
| 10 | 11.304 | Citral | 90 | 156 | 75037 | 0.26 |
EOs of various citrus peels such as pomelo (Citrus grandis L.), sweet lime (Citrus limetta), chinotto (Citrus × myrtifolia Raf.), green mandarin (Citrus reticulata) have limonene as their major compound, with the area % of this compound ranging from 76.83% to 97.68% (Caputo et al., 2020; Maurya et al., 2018; Ngan et al., 2020; Ngo et al., 2020). The results presented by Tran et al. (2020) corroborate the lime EO composition obtained in the GC-MS analyses, with the monoterpenes d-limonene (51.64%), γ-terpinene (12.8%), and β-pinene (12.79%) representing the largest volatile compounds area on lime EO. Similar compositions were also reported by Zhang et al. (2019) and Radünz et al. (2021), where d-limonene was the orange EO major constituent with 90.75% and 96% of the area, respectively.
The area (%) of minor compounds such as the sesquiterpene β-bisabolene and the monoterpenes α-phellandrene and β-myrcene also resemble the composition recently described (Ledesma-Escobar et al., 2019). Knowing the minor compounds area is just as important as the major compounds of EOs. Since, with the EOs complex composition, several components may act synergistically. Thus, due to the synergistic action of its compounds, the antiviral activity of an EO may be greater than that of its isolated major compound (Silva et al., 2020).
3.2. d-limonene computational and in chemico analysis
The in chemico analysis allows the evaluation of the compounds chemical similarity with the interest target. It is an important tool to assist in the prediction of pharmacological or toxicological properties, with the advantage of reducing the cost with procedures and mainly, meeting the 3Rs principles (Reduction, Refinement, Replacement), aiming at reducing the animal use in research, as well as optimize the studies conduct (de Astrogildo, 2018).
Based on the volatile profile (Table 1, Table 2), the compound d-limonene was present as the major compound in the orange (90.90%) and lime (54.71%) EOs.
The logP is a parameter used to evaluate the bioactive compounds hydrophilicity. Being an ideal value for drugs in the range of 2 to 5 (Lipinski et al., 2001). Through the OSIRIS, d-limonene presented a logP value equal to 3.36, which makes it a good drug candidate. Value similar to the obtained with the OSIRS was described for d-limonene in PubChem (XLogP3 = 3.4) (NCBI, 2021). In addition, d-limonene had a molecular weight of 136.23 Da, thus suggesting that this substance diffuses across the cell membrane, as it meets the “rule of five” by Lipinski et al. (2001), whose value must be <500 Da.
The OSIRIS computational analysis showed that mutagenic and cytotoxic fragments were not evidenced in the d-limonene molecule. Thus, this predictive analysis through computational resources suggests that d-limonene is a promising compound for antiviral research.
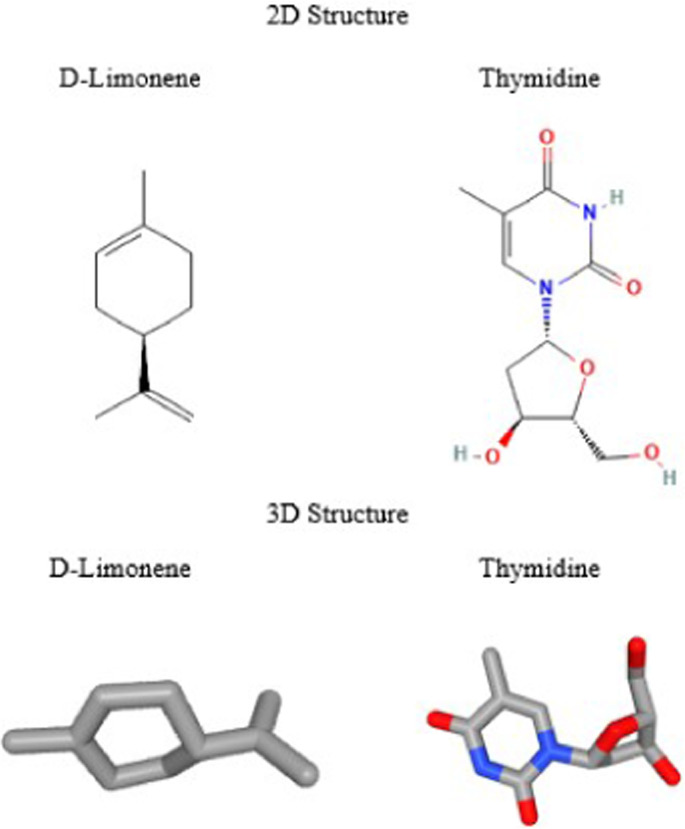
The SARS-CoV-2 genome has numerous thymidine molecules, which have structural similarities to the compound d-limonene in chemico analysis. (Fig. 1 ) (NCBI, 2020). The d-limonene cyclohexene ring resembles the viral thymidine pyrimidine nucleus (NCBI, 2021).
Fig. 1.
Structure of d-Limonene and thymidine compounds (NCBI, 2021, 2022).
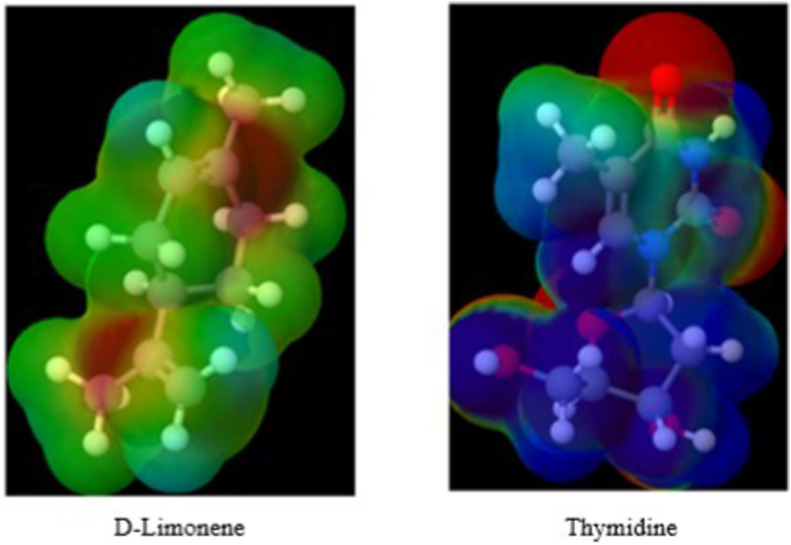
d-limonene has a lipophilic characteristic, due to the absence of oxygen and nitrogen atoms in the molecule, but it has 2 unsaturations that confer greater electron density, as can be seen in red color in Fig. 2. This feature may confer greater nucleophilic affinity of the molecule to the bonds of amino bases, as occurs with alkylating agents that act as cytotoxic agents in clinical chemotherapy. Thereby our observation is corroborated by studies conducted by Chaudhary et al. (2012), who demonstrated that d-limonene can inhibit cancer cells by preventing the incorporation of thymidine, acting as an antimetabolite drug.
Fig. 2.
The electronic surface of d-Limonene and Thymidine molecules. Carbon and Hydrogen: green. Red: unsaturation or oxygen. Blue: nitrogen. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
There is knowledge of drugs available in the pharmaceutical market, which acts as antimetabolites that can disrupt the viral nucleic acid synthesis by substituting for the natural metabolite. An example is zidovudine, nucleoside antiretroviral drugs, due to their similarity to nitrogenous bases (thymidine), is a potent inhibitor of HIV replication (Paton et al., 2021). According to the study by Roberto et al. (2010), there was a reduction in thymidine concentration in cells exposed to d-limonene. In addition, perillic acid, a majoritarian limonene metabolite detected in human plasma, also has an inhibitory effect through thymidine incorporation, which presented a dose-dependent effect (Mukhtar et al., 2018). Thus, there is still a need for further research on how d-limonene acts on the viral genome. However, based on alkylating agents and antimetabolites drugs, by the structural similarities with thymidine, d-limonene may have a biological effect and inhibit the viral protein synthesis (NCBI, 2012; Rao & Shetty, 2022).
3.3. In vitro cytotoxicity
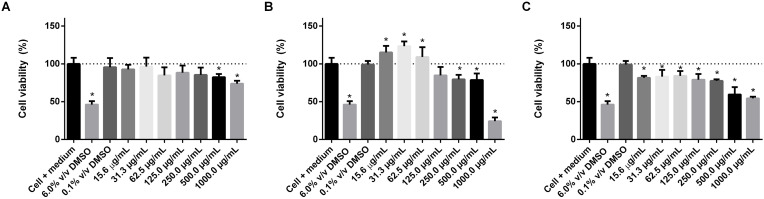
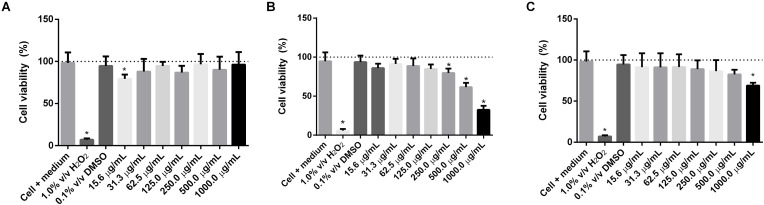
The cytotoxicity assessment of the d-limonene and lime and orange EOs was measured after exposure of MRC-5 and HaCaT cells to solutions from 15.6 to 1000.0 μg/mL. According to the results shown in Fig. 3, Fig. 4 , the DMSO 0.1% v/v in culture medium (negative control), applied as a diluent for lipophilic substances, did not differ from the negative control composed of cells + culture medium.
Fig. 3.
In vitro cytotoxicity assessment of d-limonene (A), lemon EO (B), and Orange EO (C) in MRC-5 cells after 24 h of exposure. Values expressed as mean ± standard deviation. Statistical analysis: One-way ANOVA, post hoc Tukey test, * (P<0.05) compared to the negative control (cell + medium).
Fig. 4.
In vitro cytotoxicity assessment of d-limonene (A), lemon EO (B), and Orange EO (C) in HaCaT cells after 24 h of exposure. Values expressed as mean ± standard deviation. Statistical analysis: One-way ANOVA, post hoc Tukey test, * (P<0.05) compared to the negative control (cell + medium).
Regarding cytotoxicity in MRC-5 cells, d-limonene did not show high cytotoxicity, showing cell viability of 82.43% ± 4.26% and 73.85% ± 3.80% at 500.0 and 1000.0 μg/mL, respectively (Fig. 3-A). The other concentrations evaluated did not differ in cell viability values when compared to the negative control. This cell viability result is in agreement with that obtained in different studies of the cytotoxicity of limonene and other monoterpene derivatives, where limonene did not exhibit significant cytotoxicity (Hajizadeh et al., 2019; Souto et al., 2020).
The lime EO sample showed an increase in cell viability with decreasing concentration, and the concentration of 1000.0 μg/mL showed the lowest cell viability value, 24.15% ± 5.03% (Fig. 3-B), among all groups. According to Nikolić et al. (2017), analyzing the cytotoxicity of Citrus limon L. EO in MRC-5 cells, the high concentration of EO in the cell results in lower cell viability. However, lime EO from 15.6 to 62.5 μg/mL showed superior cell viability than control cells + medium (greater than 100.0%). The orange EO sample showed lower cell viability values than the negative control in the evaluated concentration range. However, concentrations from 15.6 to 250.0 μg/mL showed cell viability values greater than 75% (Fig. 3-C). The lower cytotoxicity of lime EO, when compared to orange EO (at concentrations from 15.6 to 500.0 μg/mL), may be related to the limonene content present in the lime EO (54.71%), when compared to orange EO (90.90%). Since, according to Aumeeruddy-Elalfi et al. (2018), the higher the concentration of limonene in the EO, the lower the cell viability in high amounts of EO in contact with MRC-5. However, consideration should also be given to possible synergistic or antagonistic effects for EO constituents, such as pinenes, γ-terpinene, terpinen-4-ol, cymene, linalool, and others (Silva et al., 2020).
The cell viability profiles of d-limonene and lime and orange EO samples in the HaCaT strain (Fig. 4) were similar to the profiles in MRC-5. When evaluating the cell viability percentages for the concentration range from 15.6 to 1000.0 μg/mL, the lime EO showed slight cytotoxicity, followed by the orange EO, and limonene. Furthermore, at 1000.0 μg/mL the EO of lime presented a lower value of cell viability, 32.40% ± 5.17% (Fig. 4-B), compared to values of 69.03% ± 3.36% (Fig. 4-C) and 96.35% ± 14.88% (Fig. 4-A) obtained for the orange EO and d-limonene samples, respectively. However, no sample showed a difference with the negative control at the mean concentration of 125.0 μg/mL. The cytotoxicity results obtained in this study for d-limonene in the HaCaT are in agreement with Kulig et al. (2022), where the authors suggest that d-limonene has a weak activity for the HaCaT cells. The toxic impact on the viability of HaCaT at high concentrations of lime EO has been previously demonstrated (Lv et al., 2018). However, in the lime and orange EOs at concentrations up to 250.0 μg/mL, the inhibition of growth was less than 30%, which may indicate a low toxic effect for HaCaT (Alexa et al., 2022; Souto et al., 2020).
3.4. Preliminary safety profile assessment
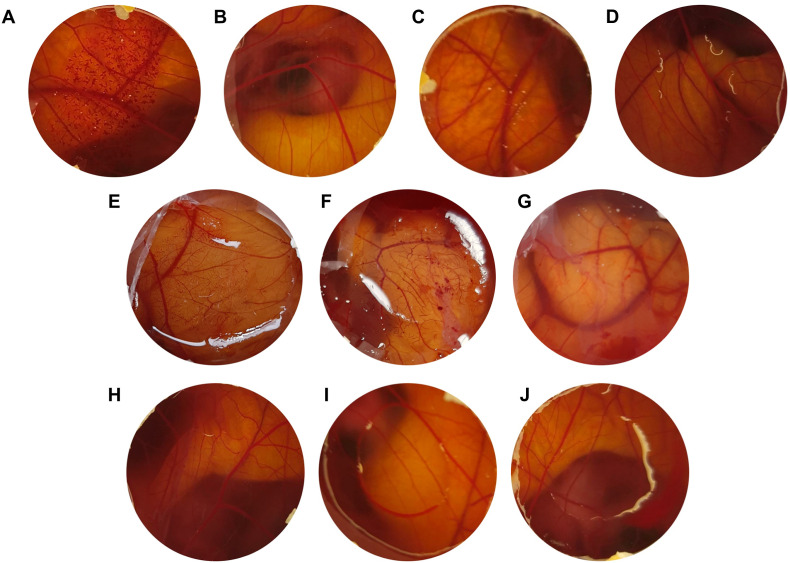
Preliminary safety profile results by the HET-CAM assay are shown in Table 3 and representative images of the chorioallantoic membrane are shown in Fig. 5 . Irritant bleeding and clotting effects were observed in the 0.1 M NaOH positive control (Fig. 5-A) and vasoconstriction effect after application of the positive control sodium lauryl sulfate 1% w/v (Fig. 5-B). The negative control and the diluent (Fig. 5-C and 5-D) had the expected results. Lime EO, orange EO, and d-limonene at 10 mg/mL had no irritating effects on the chorioallantoic membrane (Fig. 5-H, 5-I, and 5-J). Results similar to those presented were obtained by Mehanna et al. (2020), where the HET-CAM test showed no signs of hemorrhage, coagulation, or lysis for the loaded limonene nanoemulgel (levofloxacin-loaded limonene-based nanoemulsion).
Table 3.
Irritation score and classification of damage to the chorioallantoic membrane (n = 4/group).
| Sample | Irritation score (IS) | Classification |
|---|---|---|
| NaOH 0,1 M | 13,31 ± 0,26a | Severe irritant |
| Sodium lauryl sulfate 1% m/v | 10,65 ± 0,23a | Severe irritant |
| NaCl 0,9% m/v | 0,00 ± 0,00b | Non-irritant |
| MCT | 0,00 ± 0,00b | Non-irritant |
| D-limoneno pure | 6,24 ± 0,32a,b | Moderate irritant |
| Pure Lime EO | 8,57 ± 0,24a,b | Moderate irritant |
| Pure Orange EO | 6,95 ± 0,0,29a,b | Moderate irritant |
| d-limonene 10 mg/mL | 0,00 ± 0,00b | Non-irritant |
| Lime EO 10 mg/mL | 0,00 ± 0,00b | Non-irritant |
| Lime EO 10 mg/mL | 0,00 ± 0,00b | Non-irritant |
Statistical analysis: ANOVA, post hoc Tukey test (p<0,05): a - different from negative control (NaCl 0,9% m/v); b – different from positive control (NaOH 0,1 M and sodium lauryl sulfate 1% m/v). MCT: medium-chain triglycerides; EO: essential oil.
Fig. 5.
Assay images of observed effects on the chorioallantoic membrane after 5 min exposure (n = 4/group). (A) 0.1M NaOH; (B) Sodium lauryl sulfate 1% w/v; (C) 0.9% w/v NaCl; (D) Medium chain triglycerides - MCT; (E) pure d-limonene; (F) EO pure lemon; (G) pure orange EO; (H) 10 mg/ml d-limonene in MCT; (I) 10 mg/ml lemon EO in MCT; (J) EO of orange at 10 mg/mL in MCT. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
However, d-limonene and pure lime and orange OEs implied moderate irritation of the chorioallantoic membrane, visualized by the effects of vasoconstriction and hemorrhage (Fig. 5-E, 5-F, and 5-G). Several studies demonstrate that both limonene and citrus EO, when diluted in low concentrations, present no signs of irritation in HET-CAM. However, when the compounds or EOs were analyzed in high concentrations, they showed irritating potential. These results do not prevent the use of these compounds in low concentrations, since several EO are already used diluted in several product formulations (Lanzerstorfer et al., 2021; Mehanna et al., 2020; Weimer et al., 2021).
The results obtained for the pure lime or orange EOs are similar to the values by Lanzerstorfer et al. (2021) testing citrus EO at a 10% (v/v) concentration, where the IS was 6.8 ± 1.1. Furthermore, Weimer et al. (2021) showed IS values of 11.730 ± 0.167 and 10.410 ± 0.147 for EO extracted from leaves and peel of ripe fruits of Citrus aurantiifolia, respectively. These values were classified as severely irritating, unlike the pure lime and orange OEs tested which were classified as a moderate irritant.
4. Conclusions
d-limonene was the major compound in orange and lemon citrus essential oils. Based on in chemico analysis, d-limonene is suggested as a promising compound for anti-SARS-CoV-2 research, as it does not have mutagenic and cytotoxic fragments in the molecule and has good absorption. In addition, d-limonene has structural similarity with the thymidine of the SARS-CoV-2 viral genome since the cyclohexene ring of d-limonene resembles the pyrimidine nucleus of thymidine. d-limonene has a more lipophilic characteristic and higher electron density; it confers greater nucleophilic affinity of the molecule to the bonds of the amino bases. The low cytotoxicity of d-limonene and citrus OEs was confirmed in vitro by analysis in MRC-5 and HaCaT cells, and the dilution of these presented no signs of irritation in HET-CAM analysis. Thus, we determined that citrus lemon and orange OEs are candidates for future studies of their anti-SARS-CoV-2 effects.
CRediT authorship contribution statement
Aldrey Nathália Ribeiro Corrêa: Conceptualization, Validation, Formal analysis, Investigation, and Writing - original draft. Patrícia Weimer: Conceptualization, Validation, Methodology, and Formal analysis. Rochele Cassanta Rossi: Funding acquisition, Project administration, and Resources. Jessica Fernanda Hoffmann: Methodology, Formal analysis. Letícia Scherer Koester: Validation, Methodology, and Formal analysis. Edna Sayuri Suyenaga: Formal analysis, Investigation, and Methodology. Cristiano Dietrich Ferreira: Conceptualization, Funding acquisition, Methodology, Resources, Project administration, Supervision, and Writing - review & editing.
Funding
This study was financed in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) – Finance code 316360/2021–0.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPERGS (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior e Brasil (CAPES).
Data availability
No data was used for the research described in the article.
References
- Alexa V.T., Galuscan A., Soica C.M., Cozma A., Coricovac D., Borcan F., Popescu I., Mioc A., Szuhanek C., Dehelean C.A., Jumanca D. In vitro assessment of the cytotoxic and antiproliferative profile of natural preparations containing bergamot, orange and clove essential oils. Molecules. 2022;27(3) doi: 10.3390/molecules27030990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari W.A., Khan M.A., Rizvi F., Ali K., Hussain M.K., Saquib M., Khan M.F. Computational screening of plant-derived natural products against SARS-CoV-2 variants. Future Pharmacology. 2022;2(4):558–578. doi: 10.3390/futurepharmacol2040034. [DOI] [Google Scholar]
- de Astrogildo T. Considerações sobre o conceito dos 3Rs e o potential conflito com novas compreensões do animal experimental. Revista Brasileira de Zoociencias. 2018;19(2) doi: 10.34019/2596-3325.2018.v19.24741. [DOI] [Google Scholar]
- Aumeeruddy-Elalfi Z., Ismaël I.S., Hosenally M., Zengin G., Mahomoodally M.F. Essential oils from tropical medicinal herbs and food plants inhibit biofilm formation in vitro and are non-cytotoxic to human cells. 3 Biotech. 2018;8(9):1–11. doi: 10.1007/s13205-018-1413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistini R., Rossini I., Ercolini C., Goria M., Callipo M.R., Maurella C., Pavoni E., Serracca L. Antiviral activity of essential oils against hepatitis A virus in soft fruits. Food and Environmental Virology. 2019;11(1):90–95. doi: 10.1007/s12560-019-09367-3. [DOI] [PubMed] [Google Scholar]
- Bergwerf H. MolView. 2015. https://molview.org/ Internet. November 2022.
- Brito M. A. de. Pharmacokinetic study with computational tools in the medicinal chemistry course. Brazilian Journal of Pharmaceutical Sciences. 2011;47(4):797–805. doi: 10.1590/S1984-82502011000400017. [DOI] [Google Scholar]
- Brochot A., Guilbot A., Haddioui L., Roques C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. MicrobiologyOpen. 2017;6(4) doi: 10.1002/mbo3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo L., Cornara L., Bazzicalupo M., De Francesco C., De Feo V., Trombetta D., Smeriglio A. Chemical composition and biological activities of essential oils from peels of three citrus species. Molecules. 2020;25(8):1890. doi: 10.3390/molecules25081890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Variants of the virus. National center for immunization and respiratory diseases (NCIRD), division of viral diseases. 2021. www.cdc.gov/coronavirus/2019-ncov/variants/index.html Internet: November 2022.
- Chaudhary S.C., Siddiqui M.S., Athar M., Alam M.S. D-limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Human & Experimental Toxicology. 2012;31(8):798–811. doi: 10.1177/0960327111434948. [DOI] [PubMed] [Google Scholar]
- Hajizadeh M.R., Maleki H., Barani M., Fahmidehkar M.A., Mahmoodi M., Torkzadeh-Mahani M. In vitro cytotoxicity assay of D-limonene niosomes: An efficient nano-carrier for enhancing solubility of plant-extracted agents. Research in Pharmaceutical Sciences. 2019;14(5):448–458. doi: 10.4103/1735-5362.268206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nature Reviews Microbiology. 2020:1–14. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A.I., Anwar F., Chatha S.A.S., Jabbar A., Mahboob S., Nigam P.S. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Brazilian Journal of Microbiology. 2010;41:1070–1078. doi: 10.1590/S1517-83822010000400027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.F., Khan M.A., Khan Z.A., Ahamad T., Ansari W.A. In-silico study to identify dietary molecules as potential SARS-CoV-2 agents. Letters in Drug Design and Discovery. 2021;18(6):562–573. doi: 10.2174/1570180817999201209204153. [DOI] [Google Scholar]
- Kulig M., Galanty A., Grabowska K., Podolak I. Assessment of safety and health-benefits of Citrus hystrix DC. peel essential oil, with regard to its bioactive constituents in an in vitro model of physiological and pathological skin conditions. Biomedicine & Pharmacotherapy. 2022;151 doi: 10.1016/j.biopha.2022.113151. [DOI] [PubMed] [Google Scholar]
- Lanzerstorfer P., Sandner G., Pitsch J., Mascher B., Aumiller T., Weghuber J. Acute, reproductive, and developmental toxicity of essential oils assessed with alternative in vitro and in vivo systems. Archives of Toxicology. 2021;95(2):673–691. doi: 10.1007/s00204-020-02945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma-Escobar C.A., Priego-Capote F., Robles-Olvera V.J., García-Torres R., Reyes De Corcuera J.I., Luque de Castro M.D. GC-MS study of changes in polar/mid-polar and volatile compounds in Persian lime (Citrus latifolia) during fruit growth. Journal of the Science of Food and Agriculture. 2019;99(3):1020–1028. doi: 10.1002/jsfa.9266. [DOI] [PubMed] [Google Scholar]
- Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Luepke N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association. 1985;23(2):287–291. doi: 10.1016/0278-6915(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Lv X., Cong Z., Liu Z., Ma X., Xu M., Tian Y., Zhang X., Xu B., Zhang J., Tang Z. Improvement of the solubility, photostability, antioxidant activity and UVB photoprotection of trans-resveratrol by essential oil based microemulsions for topical application. Journal of Drug Delivery Science and Technology. 2018;48:346–354. doi: 10.1016/j.jddst.2018.10.017. [DOI] [Google Scholar]
- Maurya A.K., Mohanty S., Pal A., Chanotiya C.S., Bawankule D.U. The essential oil from Citrus limetta Risso peels alleviates skin inflammation: In-vitro and in-vivo study. Journal of Ethnopharmacology. 2018;212:86–94. doi: 10.1016/j.jep.2017.10.018. [DOI] [PubMed] [Google Scholar]
- Mediouni S., Jablonski J.A., Tsuda S., Barsamian A., Kessing C., Richard A., Biswas A., Toledo F., Andrade V.M., Even Y., Stevenson M., Tellinghuisen T., Choe H., Cameron M., Bannister T.D., Valente S.T. Oregano oil and its principal component, carvacrol, inhibit hiv-1 fusion into target cells. Journal of Virology. 2020;94(15) doi: 10.1128/JVI.00147-20. e00147-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna M.M., Mneimneh A.T., Abed El Jalil K. Levofloxacin-loaded naturally occurring monoterpene-based nanoemulgel: A feasible efficient system to circumvent MRSA ocular infections. Drug Development and Industrial Pharmacy. 2020;46(11):1787–1799. doi: 10.1080/03639045.2020.1821048. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mukhtar Y.M., Adu-Frimpong M., Xu X., Yu J. Biochemical significance of limonene and its metabolites: Future prospects for designing and developing highly potent anticancer drugs. Bioscience Reports. 2018;38(6) doi: 10.1042/BSR20181253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- My T.T.A., Loan H.T.P., Hai N.T.T., Hieu L.T., Hoa T.T., Thuy B.T.P., Quang D.T., Triet N.T., Anh T. T. Van, Dieu N.T.X., Trung N.T., Hue N. Van, Tat P. Van, Tung V.T., Nhung N.T.A. Evaluation of the inhibitory activities of COVID-19 of Melaleuca cajuputi oil using docking simulation. ChemistrySelect. 2020;5(21):6312–6320. doi: 10.1002/slct.202000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information (NCBI) LiverTox: Clinical and research information on drug-induced liver injury. Clinical and Research Information on Drug-Induced Liver Injury. 2012 www.ncbi.nlm.nih.gov/books/NBK547849/ Internet: November 2022. [Google Scholar]
- National Center for Biotechnology Information (NCBI) Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome. 2020. https://www.ncbi.nlm.nih.gov/nuccore/1798174254 Internet: November 2022.
- National Center for Biotechnology Information (NCBI) D-limonene. PubChem compound summary for CID 440917. 2021. https://pubchem.ncbi.nlm.nih.gov/compound/D-Limonene Internet. August 2022.
- National Center for Biotechnology Information (NCBI) Thymidine. PubChem compound summary for CID 5789. 2022. https://pubchem.ncbi.nlm.nih.gov/compound/Thymidine Internet: August 2022.
- Ngan T., Muoi N., Quan P., Cang M. Evaluation of physical and chemical properties of pomelo (Citrus grandis L.) essential oil using steam distillation process. Asian Journal of Chemistry. 2020;6:1433–1436. doi: 10.14233/ajchem.2020.22234. [DOI] [Google Scholar]
- Ngo T.C.Q., Tran T.K.N., Nguyen V.M., Mai H.C. Optimization of green Mandarin (Citrus reticulata) essential oil extraction using microwave-assisted hydrodistillation and chemical composition analysis. IOP Conference Series: Materials Science and Engineering. 2020;991(1) doi: 10.1088/1757-899X/991/1/012122. [DOI] [Google Scholar]
- Nikolić M.M., Jovanović K.K., Marković T.L., Marković D.L., Gligorijević N.N., Radulović S.S., Kostić M., Glamočlija J.M., Soković M.D. Antimicrobial synergism and cytotoxic properties of Citrus limon L., Piper nigrum L. and Melaleuca alternifolia (Maiden and Betche) Cheel essential oils. Journal of Pharmacy and Pharmacology. 2017;69(11):1606–1614. doi: 10.1111/jphp.12792. [DOI] [PubMed] [Google Scholar]
- Paton N.I., Musaazi J., Kityo C., Walimbwa S., Hoppe A., Balyegisawa A., Kaimal A., Mirembe G., Tukamushabe P., Ategeka G., Hakim J., Mugerwa H., Siika A., Asienzo J., Castelnuovo B., Kiragga A., Kambugu A. Dolutegravir or darunavir in combination with zidovudine or tenofovir to treat HIV. New England Journal of Medicine. 2021;385(4):330–341. doi: 10.1056/NEJMoa2101609. [DOI] [PubMed] [Google Scholar]
- Radünz M., Mota Camargo T., Santos Hackbart H.C., dos Inchauspe Correa Alves P., Radünz A.L., Avila Gandra E., da Rosa Zavareze E. Chemical composition and in vitro antioxidant and antihyperglycemic activities of clove, thyme, oregano, and sweet orange essential oils. LWT - Food Science and Technology. 2021;138 doi: 10.1016/j.lwt.2020.110632. [DOI] [Google Scholar]
- Rao S.J.A., Shetty N.P. Structure-based screening of natural product libraries in search of potential antiviral drug-leads as first-line treatment to COVID-19 infection. Microbial Pathogenesis. 2022;165 doi: 10.1016/j.micpath.2022.105497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clinical Infectious Diseases. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. An Official Publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto D., Micucci P., Sebastian T., Graciela F., Anesini C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic and Clinical Pharmacology and Toxicology. 2010;106(1):38–44. doi: 10.1111/j.1742-7843.2009.00467.x. j.1742-7843.2009.00467.x. [DOI] [PubMed] [Google Scholar]
- Sharma A.D., Kaur I. Jensenone from eucalyptus essential oil as a potential inhibitor of COVID 19 corona virus infection. Research & Reviews in Biotechnology & Biosciences. 2020;7(1):59–66. doi: 10.5281/zenodo.3748477. [DOI] [Google Scholar]
- Silva J. K. R. da, Figueiredo P.L.B., Byler K.G., Setzer W.N. Essential oils as antiviral agents. Potential of essential oils to treat SARS-CoV-2 infection: An in-silico investigation. International Journal of Molecular Sciences. 2020;21(10) doi: 10.3390/ijms21103426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto E.B., Zielinska A., Souto S.B., Durazzo A., Lucarini M., Santini A., Silva A.M., Atanasov A.G., Marques C., Andrade L.N., Severino P. (+)-Limonene 1,2-epoxide-loaded SLNs: Evaluation of drug release, antioxidant activity, and cytotoxicity in an HaCaT cell line. International Journal of Molecular Sciences. 2020;21(4) doi: 10.3390/ijms21041449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. In: Herrmann K., Jayne K., editors. Vol. 22. Brill; 2019. Recent developments in alternatives to animal testing; pp. 585–609. (Animal experimentation: Working towards a paradigm change). [DOI] [Google Scholar]
- Thuy B.T.P., My T.T.A., Hai N.T.T., Hieu L.T., Hoa T.T., Thi Phuong Loan H., Triet N.T., Anh T. T. Van, Quy P.T., Tat P. Van, Hue N. Van, Quang D.T., Trung N.T., Tung V.T., Huynh L.K., Nhung N.T.A. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5(14):8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T.K.N., Ngo T.C.Q., Tran T.H., Tran T.T., Huynh X.P., Do V.D., Nguyen K.T., Nguyen M.V., Bach L.G. Yields and composition of Persian lime essential oils (Citrus latifolia) from Hau Giang province, Vietnam extracted by three different extraction methods. IOP Conference Series: Materials Science and Engineering. 2020;991(1) doi: 10.1088/1757-899X/991/1/012130. [DOI] [Google Scholar]
- Wang M.-Y., Zhao R., Gao L.-J., Gao X.-F., Wang D.-P., Cao J.-M. SARS-CoV-2: Structure, biology, and structure-based therapeutics development. Frontiers in Cellular and Infection Microbiology. 2020;10 doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P., Lisbôa Moura J.G., Mossmann V., Immig M.L., de Castilhos J., Rossi R.C. Citrus aurantiifolia (Christm) Swingle: Biological potential and safety profile of essential oils from leaves and fruit peels. Food Bioscience. 2021;40 doi: 10.1016/j.fbio.2021.100905. [DOI] [Google Scholar]
- Zhang L.-L., Yang Z.-Y., Fan G., Ren J.-N., Yin K.-J., Pan S.-Y. antidepressant-like effect of Citrus sinensis (L.) Osbeck essential oil and its main component limonene on mice. Journal of Agricultural and Food Chemistry. 2019;67(50):13817–13828. doi: 10.1021/acs.jafc.9b00650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.