Abstract
Objective
Youth and adolescents with type 1 diabetes (T1D) are at risk for poor health outcomes. Understanding if psychological factors shortly following diagnosis, such as diabetes distress and resilience, predict glycated hemoglobin (A1C) trajectories may help inform both optimal timing and content of psychosocial interventions for youth with T1D.
Methods
Youth and adolescents (N = 34) with newly diagnosed T1D completed distress and resilience measures at baseline and 3 months following diagnosis. Using multilevel modeling, we predicted A1C trajectories up to 3 years following diagnosis.
Results
We found that in separate models, higher 3-month diabetes distress and lower 3-month resilience predicted larger increases in A1C years 1–3 following diagnosis.
Conclusions
Our findings suggest that targeting resilience and diabetes distress within 3 months following diagnosis has implications for the yearly rate of A1C increase up to 3 years later.
Keywords: distress, resilience, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is among the most common chronic diseases experienced by children and adolescents, affecting approximately one in every 400–600 youth and adolescents (Dabelea et al., 2021). Youth and adolescents with newly diagnosed T1D are at risk for especially poor physical health outcomes (Jones et al., 2019; Varni et al., 2018). Indeed, suboptimal glycated hemoglobin (A1C) is prevalent in older youth starting at the age of 10 years and persisting until the 16–17 year range (Clements et al., 2016), before plateauing and then improving in adulthood. Even though disease management begins to improve when patients enter adulthood, suboptimal A1C during childhood and adolescence still predicts increased risk for long-term microvascular (e.g., retinopathy) and macrovascular (e.g., cardiovascular) complications (Maahs et al., 2010; Samuelsson et al., 2020, 2021).
Researchers have increasingly identified socioemotional and psychosocial factors as relevant in predicting glycemic variability in youth with T1D (Helgeson et al., 2010; Hilliard et al., 2013; Yi-Frazier et al., 2015). The Diabetes Resilience Model posits that positive factors are equally as important in conceptualizing positive outcomes in this high-risk group as negative factors (Hilliard et al., 2012). As such, self-perceived resilience and diabetes distress are two relevant psychosocial factors that may be important in conceptualizing youth both at risk for and those who may be protected against suboptimal long-term glycemic variability. Associations between diabetes distress, which refers to specific distress around the disease experience of diabetes, and A1C in youth and adolescents are well established (Hilliard et al., 2016). Around one-third of adolescents with T1D will experience elevated diabetes distress, which, in addition to suboptimal A1C levels, is also associated with low self-efficacy and reduced self-care (Hagger et al., 2016). Resilience, which we conceptualize as a process of harnessing resources to sustain wellbeing during times of adversity, protects against higher A1C in youth and adults (Yi-Frazier et al., 2010, 2015). Those with higher levels of resilience experience less diabetes distress and higher quality of life (Yi-Frazier et al., 2015). Finally, both diabetes distress and resilience are malleable targets for intervention in youth with serious illness (Rosenberg et al., 2015). However, it is less clear if resilience and diabetes distress shortly following diagnosis matter for longer term glycemic variability, as challenges with psychological adjustment following diagnosis is considered normative (DeCosta et al., 2020).
Understanding when psychosocial factors matter for less optimal A1C trajectories beginning at the 1-year mark may help researchers identify high-risk groups and inform optimal timing of psychosocial intervention. We argue that 3 months following diagnosis is an important time point for determining whether resilience and diabetes distress matter for long-term A1C trajectories, particularly given that higher A1C levels shortly after diagnosis is a major risk factor for suboptimal glycemic control during the early years after diagnosis in children (Paes et al., 2020). The 3-month mark following diagnosis represents a crucial time point regarding the development of diabetes self-care habits, the establishment of diabetes care, and the heightened risk for the development of clinically significant mental health disorders. While many individuals with diabetes are experiencing a partial remission following diagnosis (i.e., honeymoon period) (Sokołowska et al., 2016), by 3 months, all youth need to fully engage and commit to reliable glucose monitoring and insulin administration. At 3 months following diagnosis, American Diabetes Association (ADA) guidelines state that most patients typically initiate insulin therapy, have received diabetes management education, and have established a plan for quarterly visits with their diabetes care team (American Diabetes Association, 2021). Finally, up to one-third of children and adolescents with T1D will develop clinically significant psychological symptoms in the early months following diagnosis (Butwicka et al., 2015; Kovacs et al., 1985; McGill et al., 2018; Rechenberg et al., 2017). In sum, 3 months following diagnosis is an important time point for evaluating and troubleshooting barriers to disease management, and is likely when resilience and diabetes distress may become impactful predictors beyond clinical and demographic variables already associated with A1C trajectories, such as Diabetes Ketoacidosis (DKA) at diagnosis (Duca et al., 2019), income (Clements et al., 2016), patient sex (Moore & Snell-Bergeon, 2019), and baseline psychosocial presentations (i.e., resilience and stress at baseline) (Yi-Frazier et al., 2018). Examining whether distress and resilience 3 months following diagnosis predicts A1C trajectories may help researchers and clinicians identify ideal intervention targets and timing.
Both the ADA and the International Society for Pediatric and Adolescent Diabetes recommend incorporating psychosocial care as part of a multidisciplinary care team, with an early focus on psychological adjustment to the first few months of diagnosis (American Diabetes Association, 2021; Pihoker et al., 2018). However, treatment recommendations shortly following diagnosis are less clear regarding what to screen for, when to intervene, and what interventions should target to reduce distress and bolster resources. Researchers have identified this gap as crucial and essential for informing interventions to support disease control (Clements et al., 2016), especially because the year following diagnosis is also associated with the highest risk of developing a comorbid mental health disorder (Butwicka et al., 2015).
To address these gaps in the literature, this study aimed to investigate if diabetes distress and resilience 3 months following diagnosis predicted yearly increases in A1C in youth with T1D starting at 1 year and up to 3 years later. Understanding A1C trajectories relative to distress and resilience may help researchers and clinicians better understand when to target relevant psychosocial factors preventatively.
Methods
Participants
This was a follow-up analysis of a new onset diabetes in youth study (see Yi-Frazier et al., 2018). Newly diagnosed youth and adolescents with T1D (aged 10–18 years) and their parents were approached in the ambulatory clinic or inpatient hospital unit within 6 weeks of diagnosis over the course of 18 months at a children’s hospital located in a metropolitan city. Parents provided informed consent for themselves and their child, and patients provided informed consent or assent. Demographic and clinical variables were extracted from each patient’s medical record, including age and sex (see Table I). For the purposes of this analysis, we utilized psychosocial measures collected at baseline and 3 months post-diagnosis. Patients completed surveys by paper or online via REDCap, a HIPAA-compliant Web-based survey collection tool, at home or in clinic.
Table I.
Sample Characteristics
| M | SD | Range | |
|---|---|---|---|
| Age at diagnosis | 13.21 | 2.08 | 10.07–17.32 |
| Stress at baseline | 4.11 | 2.46 | 1.00–10.00 |
| Diabetes distress at 3 months | 58.86 | 26.28 | 26.00–124.00 |
| Resilience at baseline | 29.22 | 4.74 | 14.00–38.00 |
| Resilience at 3 months | 27.89 | 6.17 | 15.00–40.00 |
| Average A1C (N = 401 data points) | 8.32 | 1.92 | 3.10–14.00 |
| Time from diagnosis to approach for enrollment (days) | 11.54 | 12.47 | 0.00–37.00 |
| Patient sex (% male) | 43.2% | ||
| Patient race (% White) | 92.0% | ||
| Patient diagnosed with DKA (% yes) | 21.6% | ||
| Caregiver education (% high school or less) | 46.0% | ||
| Family income (% greater than $100K) | 51.0% |
Measures
Diabetes-Specific Distress
The Problem Areas in Diabetes-Teen (PAID-T) consists of 26 items that assess diabetes-specific distress in adolescents (Weissberg-Benchell & Antisdel-Lomaglio, 2011) on a 6-point Likert scale (1 = Not a problem to 6 = Serious problem), as reported per adolescents. Scores in our sample ranged from 26 to 124 points, and reliability was high (α = .96). Diabetes-related distress as measured by the PAID-T refers to questions around diabetes management, treatment plans, reactions from peers in social situations, and how occupied with diabetes adolescents are throughout the day. Diabetes distress as measured by the PAID-T is responsive to intervention (Welch et al., 2003), with most interventions improving PAID-T scores by 6–9 points. A 10-point higher score on the PAID-T is associated with a 2.6 increase in A1C (Weissberg-Benchell & Antisdel-Lomaglio, 2011). The PAID-T has excellent psychometric properties (α = .96) and has been validated in adolescents with diabetes (Shapiro et al., 2018; Weissberg-Benchell & Antisdel-Lomaglio, 2011). Diabetes distress was only measured at 3 months. The PAID-T is not typically administered at diagnosis because the questions rely on having had diabetes for a period of time. Higher scores indicate higher levels of diabetes-specific distress.
Resilience
The Connor-Davidson Resilience Scale (CDRISC-10) consists of 10 items that assess patient perceptions of their own resilience (Connor & Davidson, 2003) on a 5-point Likert scale (1 = not true at all to 5 = true nearly all of the time; α =.85). This measure has been used in both adolescent and young adult populations (Campbell-Sills et al., 2006) and patients with diabetes (Steinhardt et al., 2009). In our sample, the range was 12–40 points, accounting for the measurement at both time points. Higher scores indicate higher perceptions of resilience. In a sample of young adults with T1D, a 10-point higher resilience score predicted a lower A1C by 5.4 points (Huber et al., 2018). The CDRISC-10 was collected at baseline and at 3 months following diagnosis.
Acute Diabetes-Specific Stress
Adolescents were asked, “What is your overall stress level about your diabetes right now?” on a Likert scale ranging from “1” I’m not at all stressed to “5” I’m moderately stressed, to “10” I’m extremely stressed. Stress-o-meters have been validated in populations experiencing chronic illness (Keegan et al., 2015; Linehan et al., 2017; Snowden et al., 2011). Acute diabetes-specific stress was assessed at baseline.
Demographic and Clinical Variables
Glycemic variability was assessed by A1C levels. All available A1C values were extracted from each patient’s electronic medical record starting at year 1 following diagnosis through year 3. In total, there were N = 401 data points of A1C for this sample, ranging from 2 to 11 data points per person. Whether or not the patient had been diagnosed with DKA and age at diagnosis were also recorded from the medical record. Patients self-reported their race/ethnicity. Parents self-reported on their family’s income and their own level of education.
Statistical Analysis Plan
We tested the hypothesis that diabetes distress and resilience at 3 months following diagnosis predicted a yearly, linear increase in A1C over years 1–3 following diagnosis. To examine changes in A1C over time, we estimated a series of growth curve models with a Multilevel Modeling (MLM) approach using Hierarchical Linear and Nonlinear Modeling software (Version 7.01, Raudenbush et al., 2019). Multilevel models can be utilized to model both within person trajectories (Level 1) and between person differences in trajectories (Level 2). Additionally, MLM can account for missing data and can still estimate trajectories based on varying amounts of missing data per patient. We included cases with at least two values of A1C given this strength of MLM methods.
First, we estimated an unconditional linear growth term model. The intercept parameter indicated A1C at 1 year following diagnosis. The linear parameter represented the rate (and directionality) of change over time in A1C. Therefore, we modeled A1C values at Level 1 by time since diagnosis and a residual term to examine the variance component of each term in the unrestricted model. The time term had significant variance, χ2 = 300.36, p<.001. All models were estimated using robust standard errors.
Diabetes Distress Model
Next, we constructed a model to test whether 3-month diabetes distress predicted the linear term, which represented the yearly increase in A1C. A1C values were modeled by the time since diagnosis, indicating the within person repeated measures portion of the model. At Level 2, we modeled the intercept and slope terms by 3-month diabetes distress, covarying for age at diagnosis, DKA, sex, income, and baseline stress.
Resilience Model
Finally, we constructed a model to test whether 3-month self-reported resilience predicted a yearly increase in A1C. A1C values were modeled by the time since diagnosis, indicating the within person repeated measures portion of the model. At Level 2, we modeled the intercept and linear term by 3-month resilience, covarying for age at diagnosis, DKA, sex, income, and baseline resilience.
Results
Participants
We approached 108 patients with newly diagnosed T1D within 6 weeks of diagnosis. A total of 60 patients enrolled on study (56% of those approached). One participant was later diagnosed with type 2 diabetes and was withdrawn. A prior study examining this data set found that those who enrolled versus those who did not differ regarding sex, age, or presence of DKA. However, race and ethnicity did differ between groups; patients who enrolled were more likely to identify as White and non-Hispanic (92%) compared to patients who declined (76%) (Yi-Frazier et al., 2018).
A total of N = 34 patients had complete data for this analysis; meaning they completed psychosocial measures at baseline and 3 months post-diagnosis and had at least two measures of A1C recorded in their medical record. For this subsample included in our analyses, the majority of the sample was female (56.8%), White (92%), and on average, M = 13.21 years old (SD = 2.08). Approximately 51% (as reported by parents) had a family income above $100K. On average, patients enrolled on study 11.5 days following diagnosis, on average and approximately 60% completed baseline surveys while inpatient at T1D onset. If we conceptualize moderate to high distress as having a score of 70 points or above on this version of the PAID-T, then 12 patients (32%) had moderate to high distress at 3 months (Hagger et al., 2017). See Table I for sample characteristics.
Those with full data compared to those without were not statistically different in regard to sex, age, race, or DKA (p’s > .05). However, those with full data (M = 4.12) compared to those without (M = 3.05) were approximately one quintile higher in regard to their income, t(51) = 2.65, p = .012.
Change in A1C over Time
First, we estimated a simple Level 1 model to describe A1C change over time. Here, the sample evinced a significant increase in A1C per year (B10 = .50, SE = 0.15, p < .001). At baseline, participants had an average A1C at year 1 of 7.33 (intercept, B00 = 7.33, SE = 0.27, p < .001).
Diabetes Distress and A1C Increase per Year
Next, we estimated trajectories of A1C as predicted by diabetes distress. Our analyses indicated that—when controlling for age at diagnosis, DKA, sex, income, and baseline stress—higher distress at 3 months uniquely predicted a larger linear slope coefficient, indicating that higher diabetes distress at 3 months predicted a larger yearly increase in A1C over years 1–3 post-diagnosis (B= .02, SE = 0.01, p= .036). In other words, a 10-point increase in diabetes distress 3 months following diagnosis was associated with a .40 increase in A1C between years 1 and 3 following diagnosis (Table II;Figure 1).
Table II.
Level 2 Predictors of A1C
| Predictor | Unstandardized coefficient | SE | p |
|---|---|---|---|
| A1C modeled by 3-month diabetes distress (robust SEs) | |||
| One year (intercept) | |||
| Constant | 7.47 | 0.41 | <.001 |
| Age | –0.24 | 0.14 | .083 |
| DKA | 1.60 | 0.65 | .021 |
| Sex | 1.63 | 0.83 | .062 |
| Income | –0.38 | 0.33 | .255 |
| Baseline stress | 0.33 | 0.20 | .116 |
| Three-month diabetes distress | –0.05 | 0.02 | .036 |
| Linear trajectory over years 1–3 (linear term) | |||
| Constant | 0.38 | 0.26 | .150 |
| Age | 0.05 | 0.07 | .488 |
| DKA | –0.63 | 0.40 | .126 |
| Sex | –0.53 | 0.41 | .206 |
| Income | –0.06 | 0.19 | .735 |
| Baseline stress | –0.11 | 0.10 | .271 |
| Three-month diabetes distress | 0.02 | 0.01 | .036 |
| A1C modeled by 3-month resilience (robust SEs) | |||
| One year (intercept) | |||
| Constant | 6.25 | 0.68 | <.001 |
| Age | –0.30 | 0.12 | .015 |
| DKA | 1.24 | 0.54 | .029 |
| Sex | 0.74 | 0.70 | .302 |
| Income | –0.27 | 0.30 | .375 |
| Baseline resilience | 0.01 | 0.10 | .906 |
| Three-month resilience | 0.07 | 0.06 | .288 |
| Linear trajectory over years 1–3 (linear term) | |||
| Constant | 1.48 | 0.46 | .003 |
| Age | 0.11 | 0.06 | .099 |
| DKA | –0.60 | 0.37 | .114 |
| Sex | –0.11 | 0.38 | .773 |
| Income | –0.07 | 0.15 | .654 |
| Baseline resilience | 0.03 | 0.05 | .570 |
| Three-month resilience | –0.08 | 0.04 | .035 |
Note. In Level 1 models, the outcomes were predicted from time (coded in years from diagnosis). Sex was coded as 1 (Male) or 0 (Female). DKA was coded as 1 (Yes) or 0 (No). All other variables were grand-mean centered.
Figure 1.
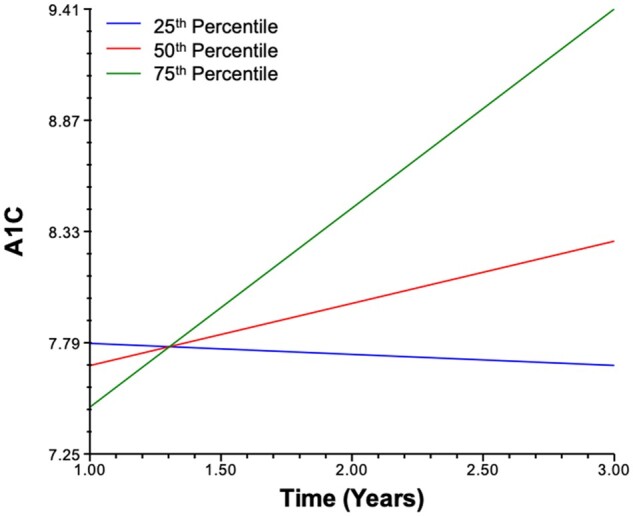
A1C modeled by time for 25th, 50th, and 75th percentile 3-month diabetes distress scores.
Self-Reported Resilience and A1C Increase per Year
Similarly, we estimated a model exploring whether self-reported resilience as measured at 3 months following diagnosis predicted a smaller or larger yearly increase in A1C. We tested the hypothesis that higher resilience at 3 months following diagnosis predicted a smaller A1C increase over time. Our analyses indicated that—when controlling for age at diagnosis, DKA, sex, income, and baseline resilience—higher resilience at 3 months uniquely predicted a smaller linear slope coefficient, indicating that higher resilience predicted a smaller yearly increase in A1C over years 1–3 post-diagnosis (B= –.08, SE = 0.04, p= .035). In other words, a 10-point lower resilience score 3 months following diagnosis was associated with a 1.60-point increase in A1C between years 1–3 following diagnosis (Table II;Figure 2).
Figure 2.
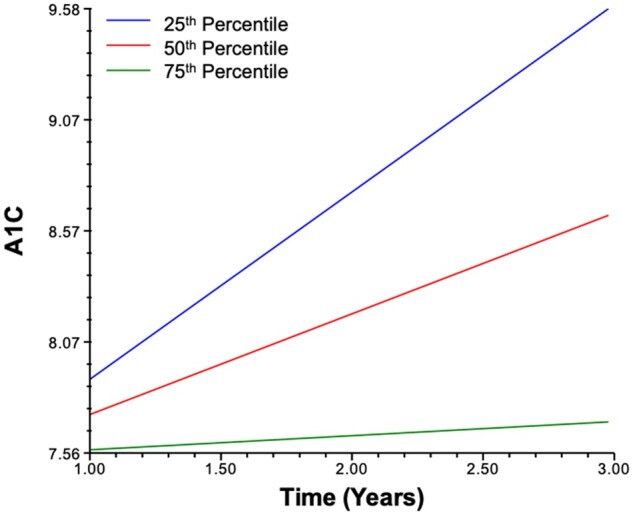
A1C modeled by time for 25th, 50th, and 75th percentile 3-month resilience scores.
Discussion
This study aimed to explore whether resilience and diabetes distress scores at 3 months post-diagnosis affected A1C trajectories over time. In separate models, we found that higher 3-month distress and lower 3-month resilience uniquely predicted yearly increases in A1C, controlling for resilience and acute stress at diagnosis. Our findings suggest that targeting resilience and distress within 3 months following diagnosis has implications for the yearly rate of A1C increase up to 3 years following diagnosis.
While research has identified strong associations between mental health symptoms and A1C in youth with T1D (Hilliard et al., 2016), less is known about when the psychosocial symptoms begin mattering for A1C outcomes. Three months following diagnosis is an important time point for determining whether resilience and diabetes distress matter for long-term A1C trajectories, particularly given that higher A1C levels shortly after diagnosis is a major risk factor for suboptimal glycemic control during the early years after diagnosis in children (Paes et al., 2020). The 3-month mark following diagnosis also represents a crucial time point regarding the development of diabetes self-care habits, the establishment of diabetes care, and the heightened risk for the development of clinically significant mental health disorders (American Diabetes Association, 2021; Butwicka et al., 2015; Kovacs et al., 1985; McGill et al., 2018; Rechenberg et al., 2017). Here, we found that resilience and distress as early as 3 months post-diagnosis, even when controlling for resilience and stress at baseline, following diagnosis predicted yearly increases in A1C. Certainly, there is variability in each patient’s experience. Some will develop clinically significant mental health disorders past this window (Bernstein et al., 2013), and diabetes care per ADA guidelines may take longer to establish or be of varying quality due to health care disparities (Valenzuela et al., 2014). However, given the established literature framing the early months following diagnosis as important for these reasons, we focused on the 3-month time point.
Many psychosocial intervention studies in youth with severe chronic illness often list inclusion criteria as having had the diagnosis for several months prior to enrollment (Duncan et al., 2013; Nansel et al., 2012). However, our findings suggest that boosting resilience and decreasing distress early following diagnosis (i.e., 3 months) should be considered. The psychological adjustment period following diagnosis has been characterized as normative, with symptoms subsiding by the 1-year mark following diagnosis (DeCosta et al., 2020). Our results suggest that while perhaps normative, early resilience and diabetes distress do have long-term implications for A1C. As such, researchers and clinicians should re-think this adjustment period as crucial for early intervention to prevent suboptimal A1C.
Youth who can cultivate resilience resources and reduce distress early in their illness experience may be better prepared for adhering to regimented health behaviors necessary for maintaining more optimal A1C levels (Hilliard et al., 2015). Those who take longer to build resilience resources and reduce distress may be more at risk for suboptimal diabetes self-care. It is also possible that better resilience and lower distress are also protective for biological processes associated with disease management and/or progression early in disease course. For instance, inflammatory processes inherent to some types of mental health symptoms (Slavich et al., 2020) may contribute to more rapid pancreatic beta cell dysfunction shortly following diagnosis (Weaver et al., 2012).
If replicated, our findings suggest that both diabetes distress and resilience as reported by patients are promising candidates for intervention to improve A1C up to 3 years following diagnosis, especially if targeted shortly after diagnosis. We did not directly compare the resilience and distress models to determine if there was a more “robust” predictor of A1C growth. However, our results suggest that a 10-point higher resilience score was associated with a more optimal trajectory than a 10-point lower distress score. Future research should better parse out the independent variance from resilience and distress on A1C trajectories. However, should future research also identify resilience as a stronger predictor of more optimal A1C trajectories, this could suggest that interventions should also focus on boosting resources instead of just reducing distress. Interventions should focus on buffering factors, such as improving skills regarding emotion regulation, problem solving, and diabetes management (Kichler & Kaugars, 2015). Future research will also need to examine if the timing of interventions relative to diagnosis has implications for A1C outcomes. We did not compare models predicting A1C from diabetes distress and resilience at 6 or 9 months following diagnosis as we were underpowered to do so, and it is possible that symptoms at these time points could have similar or stronger predictive power for long-term A1C. Additional important future directions are as follows. We focused on A1C as our main dependent variable indicative of glycemic variability; however, researchers have also suggested that an important research direction is to consider time in range (Beck et al., 2019). Also, we relied on patient report on their perceptions of distress, stress, and resilience to inform youth-specific interventions as self-report of the youth’s own experiences may be more accurate than a parent-proxy (Reyes & Kazdin, 2005). We captured personal perceptions of resilience more broadly using the CD-RISC measure; however, future research may consider using a more disease-specific resilience measure, such as the Diabetes Strengths and Resilience measure to better inform intervention targets (Hilliard et al., 2017). Finally, age did not predict the association between distress or resilience and A1C in this analysis. However, future research should consider how developmental differences at diagnosis may affect what psychological factors are most important for predicting A1C trajectories.
Our analysis, while helpful for identifying an optimal intervention window, is not without limitations. While we employed MLM to utilize all 401 data points of A1C in this sample to optimize statistical power, it is also important to maximize the number of Level 2 units (in this case, patients; Brown, 2015). This study had significant attrition, which is common in pediatric chronic illness research (Karlson & Rapoff, 2009). Additionally, our sample was majorly White; however, those with full data compared to those without were not more likely to be White. Those who enrolled in the study compared to those who did not were more likely to be White. The history of mistreatment of people of color and other marginalized groups in medical research settings understandably contributes to mistrust in the medical system, and lower rates of enrollment (Keegan & Parsons, 2018; Nuriddin et al., 2020). Future research and evidence-based recommendations are needed to reduce disparities in study enrollment. Future research certainly needs to include a more racially diverse sample, especially because there are differences in A1C between White youth and youth of color (Coulon et al., 2017), which is likely due to social determinants of health such as systemic racism. Although the sample was racially homogenous, participants had more socioeconomic variability and 46% of our sample had parents with a high school education or less. Since data collection for this study, revised versions of the PAID-T have been developed. The currently revised PAID-T uses 14 items (Shapiro et al., 2018), and the child-specific version of the PAID uses 11 items (Evans et al., 2019). It is possible that results may differ in future samples using updated versions of the PAID-T. Furthermore, norms for the CD-RISC 10-item measure (measuring resilience in the current analyses) and the PAID-T (measuring diabetes distress in the current analyses) for youth with T1D are not yet established. It is unclear whether or not our sample was more or less resilient or distressed than other youth with newly diagnosed T1D. However, the mean CD-RISC and PAID-T scores in our sample were similar to other youth T1D samples more broadly (Rosenberg et al., 2015; Shapiro et al., 2018). We also did not have reliable data regarding technology use (i.e., insulin pumps) for our sample over the duration of time that we pulled A1C information from patients’ medical records and were unable to control for this. Finally, we utilized dimensional measures of distress and resilience, which encompasses subthreshold symptoms and a wider range of affect but does not capture implications of clinically significant mental health disorders for A1C changes over time.
Despite these limitations, our findings importantly demonstrate that, when examined separately, diabetes distress and resilience proximally following diagnosis may have important implications for glycemic deterioration characteristic of this age group up to 3 years following diagnosis. We demonstrated that both negative and positive psychosocial factors early following diagnosis should be considered in the conceptualization of glycemic variability.
Funding
This project was supported by the Seattle Children’s Research Institute’s Center for Clinical and Translational Research Clinical Research Scholars Program and in part by NIH CTSA Grant UL1 TR000423 of the University of Washington (UW), Institute of Translational Health Science (ITHS). Samantha R. Scott is supported by a Graduate Research Fellowship from the National Science Foundation.
Conflicts of interest: None declared.
Contributor Information
Samantha R Scott, Department of Psychology, University of Denver, USA; Palliative Care and Resilience Lab, Center for Clinical and Translational Research, Seattle Children’s Research Institute, USA.
Maeve O’Donnell, Palliative Care and Resilience Lab, Center for Clinical and Translational Research, Seattle Children’s Research Institute, USA.
Erika M Manczak, Department of Psychology, University of Denver, USA.
Kaitlyn Fladeboe, Palliative Care and Resilience Lab, Center for Clinical and Translational Research, Seattle Children’s Research Institute, USA.
Britney Ellisor, Palliative Care and Resilience Lab, Center for Clinical and Translational Research, Seattle Children’s Research Institute, USA.
Abby R Rosenberg, Palliative Care and Resilience Lab, Center for Clinical and Translational Research, Seattle Children’s Research Institute, USA; Department of Pediatrics, University of Washington School of Medicine, USA; Fred Hutchinson Cancer Research Center, USA.
Faisal S Malik, Department of Pediatrics, University of Washington School of Medicine, USA.
Joyce P Yi-Frazier, Palliative Care and Resilience Lab, Center for Clinical and Translational Research, Seattle Children’s Research Institute, USA.
Data Availability
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
References
- American Diabetes Association (2021). Children and adolescents: Standards of medical care in diabetes—2021. Diabetes Care, 44(Suppl. 1), S180–S199. 10.2337/dc21-S013 [DOI] [PubMed] [Google Scholar]
- Beck R. W., Bergenstal R. M., Riddlesworth T. D., Kollman C., Li Z., Brown A. S., Close K. L. (2019). Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care, 42(3), 400–405. 10.2337/DC18-1444/-/DC1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein C. M., Stockwell M. S., Gallagher M. P., Rosenthal S. L., Soren K. (2013). Mental health issues in adolescents and young adults with type 1 diabetes: Prevalence and impact on glycemic control. Clinical Pediatrics, 52(1), 10–15. 10.1177/0009922812459950 [DOI] [PubMed] [Google Scholar]
- Brown T. M. (2015). Power and sample size in clinical studies. Journal of Nuclear Cardiology, 22(6), 1314–1315. 10.1007/s12350-015-0288-z [DOI] [PubMed] [Google Scholar]
- Butwicka A., Frisén L., Almqvist C., Zethelius B., Lichtenstein P. (2015). Risks of Psychiatric disorders and suicide attempts in children and adolescents with type 1 diabetes: A population-based cohort study. Diabetes Care, 38(3), 453–459. 10.2337/dc14-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L., Cohan S. L., Stein M. B. (2006). Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behaviour Research and Therapy, 44(4), 585–599. 10.1016/j.brat.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Clements M. A., Foster N. C., Maahs D. M., Schatz D. A., Olson B. A., Tsalikian E., Lee J. M., Burt-Solorzano C. M., Tamborlane W. V., Chen V., Miller K. M., Beck R. W;. for the T1D Exchange Clinic Network (2016). Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatric Diabetes, 17(5), 327–336. 10.1111/pedi.12295 [DOI] [PubMed] [Google Scholar]
- Connor K. M., Davidson J. R. T. (2003). Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC). Depression and Anxiety, 18(2), 76–82. 10.1002/da.10113 [DOI] [PubMed] [Google Scholar]
- Coulon S. J., Velasco-Gonzalez C., Scribner R., Park C. L., Gomez R., Vargas A., Stender S., Zabaleta J., Clesi P., Chalew S. A., Hempe J. M. (2017). Racial differences in neighborhood disadvantage, inflammation and metabolic control in black and white pediatric type 1 diabetes patients. Pediatric Diabetes, 18(2), 120–127. 10.1111/pedi.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D., Sauder K. A., Jensen E. T., Mottl A. K., Huang A., Pihoker C., Hamman R. F., Lawrence J., Dolan L. M., Agostino R. D., Wagenknecht L., Mayer‐Davis E. J., Marcovina S. M. (2021). Twenty years of pediatric diabetes surveillance: What do we know and why it matters. Annals of the New York Academy of Sciences, 1495(1), 99–120. 10.1111/nyas.14573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCosta P., Grabowski D., Skinner T. C. (2020). The psychosocial experience and needs of children newly diagnosed with type 1 diabetes from their own perspective: A systematic and narrative review. Diabetic Medicine, 37(10), 1640–1652. 10.1111/dme.14354 [DOI] [PubMed] [Google Scholar]
- Duca L. M., Reboussin B. A., Pihoker C., Imperatore G., Saydah S., Mayer-Davis E., Rewers A., Dabelea D. (2019). Diabetic ketoacidosis at diagnosis of type 1 diabetes and glycemic control over time: The SEARCH for diabetes in youth study. Pediatric Diabetes, 20(2), 172–179. 10.1111/pedi.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Hogan M. B., Tien K. J., Graves M. M., Chorney J. M., Zettler M. D., Koven L., Wilson N. W., Dinakar C., Portnoy J. (2013). Efficacy of a parent-youth teamwork intervention to promote adherence in pediatric asthma. Journal of Pediatric Psychology, 38(6), 617–628. 10.1093/jpepsy/jss123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. A., Weil L. E. G., Shapiro J. B., Anderson L. M., Vesco A. T., Rychlik K., Hilliard M. E., Antisdel J., Weissberg-Benchell J. (2019). Psychometric properties of the parent and child problem areas in diabetes measures. Journal of Pediatric Psychology, 44(6), 703–713. 10.1093/jpepsy/jsz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger V., Hendrieckx C., Cameron F., Pouwer F., Skinner T. C., Speight J. (2017). Cut points for identifying clinically significant diabetes distress in adolescents with type 1 diabetes using the PAID-T: Results from diabetes MILES youth-Australia. Diabetes Care, 40(11), 1462–1468. 10.2337/dc17-0441 [DOI] [PubMed] [Google Scholar]
- Hagger V., Hendrieckx C., Sturt J., Skinner T. C., Speight J. (2016). Diabetes distress among adolescents with type 1 diabetes: A systematic review. Current Diabetes Reports, 16(1), 9–14. 10.1007/s11892-015-0694-2 [DOI] [PubMed] [Google Scholar]
- Helgeson V. S., Escobar O., Siminerio L., Becker D. (2010). Adolescents with diabetes: Five-year longitudinal study. Health Psychology, 29(2), 153–159. 10.1037/a0018163.Relation [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. E., Harris M. A., Weissberg-Benchell J. (2012). Diabetes resilience: A model of risk and protection in type 1 diabetes. Current Diabetes Reports, 12(6), 739–748. 10.1007/s11892-012-0314-3 [DOI] [PubMed] [Google Scholar]
- Hilliard M. E., Iturralde E., Weissberg-Benchell J., Hood K. K. (2017). The Diabetes Strengths and Resilience Measure for Adolescents with Type 1 Diabetes (DSTAR-Teen): Validation of a new, brief self-report measure. Journal of Pediatric Psychology, 42(9), 995–1005. 10.1093/JPEPSY/JSX086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. E., McQuaid E. L., Nabors L., Hood K. K. (2015). Resilience in youth and families living with pediatric health and developmental conditions: Introduction to the special issue on resilience. Journal of Pediatric Psychology, 40(9), 835–839. 10.1093/jpepsy/jsv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. E., Wu Y. P., Rausch J., Dolan L. M., Hood K. K. (2013). Predictors of deteriorations in diabetes management and control in adolescents with type 1 diabetes. The Journal of Adolescent Health, 52(1), 28–34. 10.1016/j.jadohealth.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. E., Yi-Frazier J. P., Hessler D., Butler A. M., Anderson B. J., Jaser S. (2016). Stress and A1c among people with diabetes across the lifespan. Current Diabetes Reports, 16(8), 67. 10.1007/s11892-016-0761-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J., Fox C., Hill A., McDonald T., Shields B., Jones A. (2018). Psychosocial resilience contributes to better glycaemic control in people living with type 1 diabetes. 32nd Annual Conference of the European Health Psychology Society. https://research.brighton.ac.uk/en/publications/psychosocial-resilience-contributes-to-better-glycaemic-control-i
- Jones S., Khanolkar A. R., Gevers E., Stephenson T., Amin R. (2019). Cardiovascular risk factors from diagnosis in children with type 1 diabetes mellitus: A longitudinal cohort study. BMJ Open Diabetes Research & Care, 7(1), e000625. 10.1136/bmjdrc-2018-000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson C. W., Rapoff M. A. (2009). Attrition in randomized controlled trials for pediatric chronic conditions. Journal of Pediatric Psychology, 34(7), 782–793. 10.1093/jpepsy/jsn122 [DOI] [PubMed] [Google Scholar]
- Keegan D., Byrne K., Cullen G., Doherty G. A., Dooley B., Mulcahy H. E. (2015). The stressometer: A simple, valid, and responsive measure of psychological stress in inflammatory bowel disease patients. Journal of Crohn’s and Colitis, 9(10), 881–885. 10.1093/ecco-jcc/jjv120 [DOI] [PubMed] [Google Scholar]
- Keegan T. H. M., Parsons H. M. (2018). Adolescent angst: Enrollment on clinical trials. Hematology. American Society of Hematology. Education Program, 2018(1), 154–160. 10.1182/ASHEDUCATION-2018.1.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichler J. C., Kaugars A. S. (2015). Topical review: Applying positive development principles to group interventions for the promotion of family resilience in pediatric psychology. Journal of Pediatric Psychology, 40(9), 978–980. 10.1093/jpepsy/jsu115 [DOI] [PubMed] [Google Scholar]
- Kovacs M., Feinberg T. L., Paulauskas S., Finkelstein R., Pollock M., Crouse-Novak M. (1985). Initial coping responses and psychosocial characteristics of children with insulin-dependent diabetes mellitus. The Journal of Pediatrics, 106(5), 827–834. 10.1016/S0022-3476(85)80368-1 [DOI] [PubMed] [Google Scholar]
- Linehan K., Fennell K. M., Hughes D. L., Wilson C. J. (2017). Use of the distress thermometer in a cancer helpline context: Can it detect changes in distress, is it acceptable to nurses and callers, and do high scores lead to internal referrals? European Journal of Oncology Nursing, 26, 49–55. 10.1016/J.EJON.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Maahs D. M., West N. A., Lawrence J. M., Mayer-Davis E. J. (2010). Epidemiology of type 1 diabetes. Endocrinology and Metabolism Clinics of North America, 39(3), 481–497. 10.1016/j.ecl.2010.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill D. E., Volkening L. K., Pober D. M., Muir A. B., Young-Hyman D. L., Laffel L. M. (2018). Depressive symptoms at critical times in youth with type 1 diabetes: Following type 1 diabetes diagnosis and insulin pump initiation. Journal of Adolescent Health, 62(2), 219–225. 10.1016/J.JADOHEALTH.2017.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. M., Snell-Bergeon J. K. (2019). Trajectories of hemoglobin A1c and body mass index z-score over four decades among 2 to 18 year olds with type 1 diabetes. Pediatric Diabetes, 20(5), 594–603. 10.1111/pedi.12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansel T. R., Iannotti R. J., Liu A. (2012). Clinic-integrated behavioral intervention for families of youth with type 1 diabetes: Randomized clinical trial. Pediatrics, 129(4), e866–e873. 10.1542/peds.2011-2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriddin A., Mooney G., White A. I. R. (2020). Reckoning with histories of medical racism and violence in the USA. The Lancet, 396(10256), 949–951. 10.1016/S0140-6736(20)32032-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes V. M., Barrett J. K., Dunger D. B., Gevers E. F., Taylor-Robinson D. C., Viner R. M., Stephenson T. J. (2020). Factors predicting poor glycemic control in the first two years of childhood onset type 1 diabetes in a cohort from East London, UK: Analyses using mixed effects fractional polynomial models. Pediatric Diabetes, 21(2), 288–299. 10.1111/pedi.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihoker C., Forsander G., Fantahun B., Virmani A., Corathers S., Benitez-Aguirre P., Fu J., Maahs D. M. (2018). ISPAD Clinical Practice Consensus Guidelines 2018: The delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatric Diabetes, 19, 84–104. 10.1111/pedi.12757 [DOI] [PubMed] [Google Scholar]
- Raudenbush, S. W., Bryk, A. S., Cheong, Y. F., & Congdon, R. (2019). HLM 8 for Windows [Computer software]. Scientific Software International, Inc.
- Rechenberg K., Whittemore R., Grey M. (2017). Anxiety in youth with type 1 diabetes. Journal of Pediatric Nursing, 32, 64–71. 10.1016/J.PEDN.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A. D. L., Kazdin A. E. (2005). Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin, 131(4), 483–509. 10.1037/0033-2909.131.4.483 [DOI] [PubMed] [Google Scholar]
- Rosenberg A. R., Yi-Frazier J. P., Eaton L., Wharton C., Cochrane K., Pihoker C., Baker K. S., McCauley E. (2015). Promoting resilience in stress management: A pilot study of a novel resilience-promoting intervention for Adolescents and Young Adults with serious illness. Journal of Pediatric Psychology, 40(9), 992–999. 10.1093/jpepsy/jsv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson J., Samuelsson U., Hanberger L., Bladh M., Åkesson K. (2020). Poor metabolic control in childhood strongly correlates to diabetes-related premature death in persons’ 30 years of age—A population-based cohort study. Pediatric Diabetes, 21(3), 479–485. 10.1111/pedi.12980 [DOI] [PubMed] [Google Scholar]
- Samuelsson U., Anderzen J., Åkesson K., Hanberger L. (2021). The importance of low HbA1c during childhood on glycaemic control in adulthood and the risk of late complications. Acta Paediatrica, 110(4), 1264–1272. 10.1111/apa.15591 [DOI] [PubMed] [Google Scholar]
- Shapiro J. B., Vesco A. T., Weil L. E. G., Evans M. A., Hood K. K., Weissberg-Benchell J. (2018). Psychometric properties of the problem areas in diabetes: Teen and parent of teen versions. Journal of Pediatric Psychology, 43(5), 561–571. 10.1093/jpepsy/jsx146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G. M., Giletta M., Helms S. W., Hastings P. D., Rudolph K. D., Nock M. K., Prinstein M. J. (2020). Interpersonal life stress, inflammation, and depression in adolescence: Testing Social Signal Transduction Theory of Depression. Depression and Anxiety, 37(2), 179–193. 10.1002/da.22987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden A., White C. A., Christie Z., Murray E., McGowan C., Scott R. (2011). The clinical utility of the distress thermometer: A review. British Journal of Nursing (Mark Allen Publishing), 20(4), 220–227. 10.12968/bjon.2011.20.4.220 [DOI] [PubMed] [Google Scholar]
- Sokołowska M., Chobot A., Jarosz-Chobot P. (2016). The honeymoon phase – What we know today about the factors that can modulate the remission period in type 1 diabetes. Pediatric Endocrinology, Diabetes, and Metabolism, 22(2), 66–70. 10.18544/PEDM-22.02.0053 [DOI] [PubMed] [Google Scholar]
- Steinhardt M. A., Mamerow M. M., Brown S. A., Jolly C. A. (2009). A resilience intervention in African American adults with type 2 diabetes: A pilot study of efficacy. The Diabetes Educator, 35(2), 274–284. 10.1177/0145721708329698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela J. M., Seid M., Waitzfelder B., Anderson A. M., Beavers D. P., Dabelea D. M., Dolan L. M., Imperatore G., Marcovina S., Reynolds K., Yi-Frazier J., Mayer-Davis E. J.;. SEARCH for Diabetes in Youth Study Group (2014). Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. Journal of Pediatrics, 164(6), 1369–1375.e1. 10.1016/j.jpeds.2014.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni J. W., Delamater A. M., Hood K. K., Driscoll K. A., Wong J. C., Adi S., Yi-Frazier J. P., Grishman E. K., Faith M. A., Corathers S. D., Kichler J. C., Miller J. L., Raymond J. K., Doskey E. M., Aguirre V., Heffer R. W., Wilson D. P.;. Pediatric Quality of Life Inventory 3.2 Diabetes Module Testing Study Consortium (2018). Diabetes management mediating effects between diabetes symptoms and health-related quality of life in adolescents and young adults with type 1 diabetes. Pediatric Diabetes, 19(7), 1322–1330. 10.1111/pedi.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J. R., Holman T. R., Imai Y., Jadhav A., Kenyon V., Maloney D. J., Nadler J. L., Rai G., Simeonov A., Taylor-Fishwick D. A. (2012). Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Molecular and Cellular Endocrinology, 358(1), 88–95. 10.1016/j.mce.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J., Antisdel-Lomaglio J. (2011). Diabetes-specific emotional distress among adolescents: Feasibility, reliability, and validity of the Problem Areas in Diabetes-Teen version. Pediatric Diabetes, 12(4 Pt 1), 341–344. 10.1111/j.1399-5448.2010.00720.x [DOI] [PubMed] [Google Scholar]
- Welch G., Weinger K., Anderson B., Polonsky W. H. (2003). Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabetic Medicine, 20(1), 69–72. 10.1046/j.1464-5491.2003.00832.x [DOI] [PubMed] [Google Scholar]
- Yi-Frazier J. P., Cochrane K., Whitlock K., Rosenberg A. R., Pascual M., Beauregard N., Mitrovich C., Panlasigui N., Pihoker C. (2018). Trajectories of acute diabetes-specific stress in adolescents with type 1 diabetes and their caregivers within the first year of diagnosis. Journal of Pediatric Psychology, 43(6), 645–653. 10.1093/jpepsy/jsy003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi-Frazier J. P., Smith R. E., Vitaliano P. P., Yi J. C., Mai S., Hillman M., Weinger K. (2010). A person-focused analysis of resilience resources and coping in patients with diabetes. Stress and Health, 26(1), 51–60. 10.1002/smi.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi-Frazier J. P., Yaptangco M., Semana S., Buscaino E., Thompson V., Cochrane K., Tabile M., Alving E., Rosenberg A. R. (2015). The association of personal resilience with stress, coping, and diabetes outcomes in adolescents with type 1 diabetes: Variable- and person-focused approaches. Journal of Health Psychology, 20(9), 1196–1206. 10.1177/1359105313509846 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.


