Abstract
STUDY QUESTION
What are the systemic molecular profiles of endometriosis diagnosed in adolescents and young adults?
SUMMARY ANSWER
Significant enrichment and increased activation of proteins related to angiogenesis and cell migration pathways were observed in endometriosis cases compared to controls (P-value < 2.4 × 10−8).
WHAT IS KNOWN ALREADY
Little is known about the pathophysiology of adolescent endometriosis despite the fact that over 50% of adults with endometriosis report onset of severe pelvic pain during adolescence.
STUDY DESIGN, SIZE, DURATION
A cross-sectional analysis using data on 142 laparoscopically confirmed endometriosis cases and 74 controls from the observational longitudinal cohort of Women’s Health Study: From Adolescence to Adulthood (A2A).
PARTICIPANTS/MATERIALS, SETTING, METHODS
We measured 1305 plasma protein levels using the validated, multiplex aptamer-based proteomics discovery platform, SOMAscan. We calculated odds ratios and 95% CIs using logistic regression adjusting for age, BMI, fasting status and hormone use at blood draw for differentially expressed proteins (P < 0.05). Ingenuity Pathway Analysis and STRING analysis were performed to identify biological pathways and protein interactions. We also examined proteins and pathways associated with superficial peritoneal lesion colors (i.e. red, vascularized, white, blue/black, brown).
MAIN RESULTS AND THE ROLE OF CHANCE
Average age at blood draw was 18 years for endometriosis cases and 22 years for controls. We identified 63 proteins associated with endometriosis with type-I error set at 0.05, and absolute fold change >1.2, revealing significant enrichment of dysregulated proteins in biological pathways associated with endometriosis. Increased activation of pathways related to angiogenesis and cell migration was observed in plasma from endometriosis cases compared to controls (P-value < 2.4 × 10−8). Furthermore, when we examined proteins and pathways associated with lesion colors, vascularized lesions were associated with upregulation of pathways related to immune cell migration/activation and inflammation, whereas white, blue/black and brown lesions were associated with downregulation of these pathways.
LIMITATIONS, REASONS FOR CAUTION
Validation of our results in independent datasets and mechanistic studies are warranted to further our understanding of the pathophysiological characteristics of this common but understudied patient population.
WIDER IMPLICATIONS OF THE FINDINGS
To our knowledge, this was the first study to comprehensively examine circulating proteins in predominantly adolescents and young adult women with and without endometriosis. Results from this study provide novel biological insight that will build toward further research to elucidate endometriosis pathophysiology during the earlier course of the disease trajectory.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the Department of Defense (W81XWH1910318) and the 2017 Boston Center for Endometriosis Trainee Award. Financial support for establishment of and data collection within the A2A cohort were provided by the J. Willard and Alice S. Marriott Foundation. N.S., A.F.V., S.A.M., K.L.T. have received funding from Marriott Family Foundation. S.A.M. and K.L.T. are supported by NICHD (R01 HD94842). S.A.M. serves as an advisory board member for AbbVie and Roche; neither are related to this study. The authors report no conflict of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: endometriosis, proteomics, adolescents, lesion color, angiogenesis
Introduction
Endometriosis is a chronic inflammatory disorder associated with severe pelvic pain and infertility, estimated to affect about 10% of reproductive-aged women, corresponding to an estimated 200 million women and girls worldwide (Adamson et al., 2010; Zondervan et al., 2018, 2020). It is characterized by the presence of endometrial-like tissue outside the uterus that responds to hormonal cues (Zondervan et al., 2020). Endometriosis has been reported to be associated with a proinflammatory response (Drosdzol-Cop and Skrzypulec-Plinta, 2012; Kianpour et al., 2012; Jiang et al., 2016; Mu et al., 2018). Inflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α and C-reactive protein, are elevated in the peritoneal fluid and peripheral blood of women with endometriosis compared to controls (Drosdzol-Cop and Skrzypulec-Plinta, 2012; Kianpour et al., 2012). However, these studies predominantly include adults, resulting in a lack of knowledge about molecular profiles associated with endometriosis diagnosed during adolescence and young adulthood.
It is possible that endometriosis diagnosed in adolescence may have a different molecular phenotype than endometriosis diagnosed in adulthood, given the differences in clinical presentation. Endometriosis diagnosed in adolescents and young adults typically presents with severe pelvic pain and superficial peritoneal lesions, while endometriosis diagnosed in adults is most often also characterized by pain and superficial lesions, but also presents with infertility and deep fibrotic lesions (Shah and Missmer, 2011; DiVasta et al., 2018; Shafrir et al., 2018). Furthermore, we recently reported that plasma CA125 levels, which are elevated in endometriosis diagnosed in adults, were not elevated in adolescent endometriosis (Sasamoto et al., 2020a). Elucidating molecular profiles associated with endometriosis diagnosed in adolescents and young adults will provide new information about the pathophysiology during the earlier course of the disease trajectory. Therefore, the objective of this study was to examine plasma protein markers in adolescents and young adults with and without endometriosis and investigate biological markers and pathways associated with endometriosis diagnosed in this young population.
Materials and methods
Study population
The Women’s Health Study: From Adolescence to Adulthood (A2A) is a US-based longitudinal cohort that enrolled participants from 2012 to 2018. Details of the study have been described previously (Sasamoto et al., 2020,b). Briefly, laparoscopically confirmed endometriosis cases were identified from two academic hospitals in Boston, MA, USA. Controls were recruited from clinics at these two academic hospitals as well as from the local community they served through local advertisement, online postings and word of mouth. Of the 1839 eligible people approached, 1569 (85%) consented to join the study. Participants completed a baseline questionnaire at enrollment that was expanded from the World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project (WERF EPHect) standard clinical questionnaire (Vitonis et al., 2014), which consists of demographic, behavioral and reproductive characteristics, as well as details on pelvic pain, treatment regimen and medication use. An EPHect compliant surgical form was used to collect operative data for each endometriosis case. Information collected on the surgical form included hormone use at time of surgery, revised American Society for Reproductive Medicine (rASRM) score and superficial peritoneal lesion colors (Becker et al., 2014).
Blood samples were collected at baseline, and plasma aliquots were stored at ≤−80°C following the standard protocols of WERF EPHect fluid biospecimen collection (Rahmioglu et al., 2014) with one exception—bloods were centrifuged at 1790×g for 10 min. At time of blood collection, a biospecimen questionnaire was completed by the participants assessing information including date of last menstrual period, timing of last food/beverages consumed and recent medication use including hormones.
Of the 785 endometriosis cases and 764 controls enrolled in the A2A study, we excluded those who did not complete the baseline questionnaire (156 endometriosis cases and 81 controls), did not have a surgery at baseline (172 endometriosis cases), those who had an endometrioma(s) or deep lesion(s) (9 endometriosis cases), did not provide blood at baseline (180 endometriosis cases and 177 controls) or blood not collected within 90 days prior to the surgery (13 endometriosis cases), those whose blood was drawn more than 6 months from questionnaire completion (19 endometriosis cases and 9 controls) and those missing data on dysmenorrhea or acyclic pelvic pain (29 endometriosis cases and 41 controls). Due to other future analyses utilizing these samples, we further excluded those who did not complete the Year 1 questionnaire (54 cases and 176 controls). Among the remaining 153 cases, we included those with post-surgical blood samples (89 endometriosis cases) and randomly selected 53 additional cases. Among the remaining 280 controls, we selected 74 controls, frequency matching them to the cases on age, race, BMI and hormone use. As a result, we measured plasma proteins from the blood sample collected at baseline in 142 endometriosis cases and 74 controls.
Ethical approval
This study was approved by the Boston Children’s Hospital (BCH) Institutional Review Board on behalf of both BCH and Brigham and Women’s Hospital. All participants provided written consent for participation in the study, with parental consent plus participant assent for participants age <18 years old.
Covariates
Demographic and behavioral characteristics were obtained from the baseline questionnaire. BMI (kg/m2) was calculated based on self-reported height and weight and categorized as follows: for participants aged ≥20 years: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥30 kg/m2) according to World Health Organization criteria (Eveleth, 1996); for participants younger than 20 years, the age- and gender-specific BMI Z-score was calculated, and participants were categorized as underweight (Z-score ≤ −2), normal weight (Z-score >−2 to <1), overweight (Z-score 1–2) or obese (Z-score >2) (Barlow, 2007). Hormone use and fasting status were abstracted from the biospecimen questionnaire and reflect the status at time of blood draw. Dysmenorrhea severity was assessed at baseline using the 11-point numeric rating scale (NRS). Superficial peritoneal lesion colors were documented by the surgeon and were categorized as having any if the patient had at least one endometriotic lesion of the color of interest (e.g. any red, white, blue/black, brown lesion) or if vascularized. For example, if the endometriosis case had at least one red lesion, the case was categorized as having ‘any red lesion’. Therefore, individuals were included in more than one category if they had superficial peritoneal lesions with different colors. Clear lesions were extremely common (94%), and given this lack of variation among the cases, was not explored as a lesion color category.
SOMAscan proteomics assay
In plasma samples (50 µl), we measured relative protein levels in plasma using the SOMAscan Assay Kit for human plasma 1.3k, which allows quantification of 1305 proteins simultaneously using highly selective single-stranded Slow Off-rate Modified DNA Aptamers (SOMAmer), following the manufacturer’s standard protocol (SomaLogic; Boulder, CO, USA). Five pooled human plasma controls and one no-protein buffer control were run in parallel with the plasma test samples. Sample to sample variability was further controlled by several hybridization spikes-in controls. This assay has been previously reported to have high reproducibility and within-person stability over time (Kim et al., 2018). Quality control, calibration and normalization of the data were conducted according to the manufacturer’s protocol as previously described (Hathout et al., 2015). We included 30 blinded quality control (QC) samples that were randomly distributed among the participants’ samples. Overall, in the blinded QC samples, 98% of proteins had intra-batch coefficient of variation (CVs) <25%, 99% had inter-batch CV <25%; there were no missing protein values.
Statistical analysis
We used logistic regression adjusting for age at blood draw (years), BMI (underweight or normal, overweight or obese), hormone use at blood draw (no, yes) and fasting at blood draw (no, yes) to calculate odds ratios (ORs) and 95% CIs per 1 SD increase in protein levels. We conducted sensitivity analyses restricting to White race and additionally adjusting for regular analgesic use. We also conducted sensitivity analyses stratifying by age at blood sample collection [i.e. adolescents (age <20 years) versus young adults (age ≥20 years)] and pain severity of dysmenorrhea (i.e. NRS <7 versus 7+). False discovery rate was calculated to account for multiple testing. Fold change of protein levels was calculated by dividing the mean protein level from the cases to that of the controls. If this ratio was <1, we inverted the ratio and multiplied by −1.
To acquire new insights into potential pathophysiological pathways underlying endometriosis-specific plasma protein signatures, we performed functional category analysis of all dysregulated proteins with a P-value <0.05 and absolute fold change >1.2 from the logistic regression analysis using Ingenuity Pathway Analysis (IPA) (QIAGEN, Redwood City, CA, USA) (Krämer et al., 2013). Additional network and functional enrichment analysis were performed using the STRING database version 11.0 for protein–protein functional and physical interactions, the results of which were displayed as a functional network (Szklarczyk et al., 2019). Interactions were considered with a STRING confidence score of ≥0.4 (out of 1.0) garnered from the ‘experimental’ and ‘databases’ categories. Proteins without associations to other proteins in the network were removed. A k-means clustering algorithm was performed to select connected proteins (k-means/number of clusters = 6). Functional description of clusters was assigned based on a manually curated evaluation of enriched KEGG pathway, Gene Ontology (GO), Reactome, STRING local network clusters terms and PubMed literature search. All statistical analyses were performed using SAS/STAT version 9.4 (SAS Institute Inc., Cary, NC, USA); systems biology analyses were performed using the Ingenuity Pathways Knowledge Base (Qiagen, Redwood City, CA, USA).
Results
We measured 1305 proteins in plasma samples from 142 laparoscopically confirmed endometriosis cases and 74 controls. Average age at blood draw was 18 years for endometriosis cases and 22 years for controls (Table I). Majority were normal weight (>60%), White (>84%) and were on hormones at time of blood draw (>70%). More than half of the endometriosis cases were fasting at blood draw (68%), whereas only six controls were fasting at blood draw (8%). Endometriosis cases had superficial peritoneal lesions only with 97% being rASRM stage I/II at diagnosis, which is a typical clinical presentation of endometriosis diagnosed in adolescents. Having any clear lesion was most common (>90%), red lesion was observed in 84%, while white lesions, blue/black lesions, brown lesions or vascularized lesions each were observed in about 20–30% of endometriosis cases.
Table I.
Baseline characteristics of endometriosis cases and controls in the Women’s Health Study: From Adolescence to Adulthood (A2A) cohort (n = 216).
| Endometriosis cases | Controls | |
|---|---|---|
| (n = 142) | (n = 74) | |
| Age at blood draw, years, mean (SD) | 17.9 (4.0) | 22.0 (3.0) |
| BMIa, kg/m2 | ||
| Underweight | 0 (0) | 1 (1.4) |
| Normal | 92 (64.8) | 49 (66.2) |
| Overweight | 37 (26.1) | 14 (18.9) |
| Obese | 13 (9.2) | 10 (13.5) |
| Race | ||
| White | 128 (90.1) | 62 (83.8) |
| Black | 2 (1.4) | 2 (2.7) |
| Asian | 0 (0) | 7 (9.5) |
| Other | 12 (8.4) | 2 (2.8) |
| Unknown | 0 | 1 (1.4) |
| On hormones at time of blood drawb | 126 (88.7) | 54 (73.0) |
| Fasting at time of blood draw | 97 (68.3) | 6 (8.2) |
| Regular analgesic usec | 76 (53.5) | 16 (21.6) |
| ASRM stage | ||
| Stage I/II | 137 (97) | NA |
| Stage III/IV | 4 (3) | NA |
| Any clear lesions | 133 (93.7) | NA |
| Any red lesions | 119 (83.8) | NA |
| Any white lesions | 43 (30.3) | NA |
| Any blue/black lesions | 34 (23.9) | NA |
| Any brown lesions | 35 (24.6) | NA |
| Any vascularized lesions | 37 (26.1) | NA |
Unless stated otherwise values are n (%).
For women aged ≥20 years: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) according to World Health Organization criteria; for those <20 years, the age- and gender-specific BMI Z-score was calculated, and participants were categorized as underweight (Z-score ≤ −2), normal weight (Z-score >−2 to <1), overweight (Z-score 1–2) or obese (Z-score > 2).
Among hormone users, type of hormones used were for cases: combined birth control pills (n = 83), progestin only birth control pills (n = 17), Aygestin (n = 17), depo provera (n = 1), ring (n = 3), hormonal intrauterine device (n = 1), implant (n = 2), more than one type (n = 1), unknown (n = 1); for controls: combined birth control pills (n = 54).
Regular use of analgesic medications defined as use at least once a week for a period of 3 months or longer.
When we compared levels of 1305 individual proteins between endometriosis cases and controls, we identified 63 proteins associated with endometriosis (Table II: top 10 up- and downregulated proteins; Supplementary Table SI: all). Compared to controls, 36 proteins were increased, while 27 were decreased in endometriosis. Interestingly, the top upregulated proteins included proteins related to systemic iron homeostasis such as ferritin (OR = 2.45, CI = 1.44–4.17), hepcidin (OR = 1.99, CI = 1.29–3.07) and proteins related to angiogenesis such as angiopoietin-related protein 3 (OR = 14.97, CI = 2.37–94.75), gremlin-1 (OR = 3.74, CI = 1.48–9.45) and hepatocyte growth factor (OR = 2.58, CI = 1.31–5.06). Similar results were observed when we restricted to White race or additionally adjusted for regular analgesic use. When we stratified by pain severity of dysmenorrhea (NRS <7 versus 7+), we observed similar associations in the top proteins.
Table II.
Top 10 positively and negatively associated plasma proteins between endometriosis cases and controls with absolute fold-change >1.2 in the A2A cohort (n = 216).
| Protein name | Entrez gene symbol | Fold change | OR (95% CI)a | Unadjusted P-value | FDR |
|---|---|---|---|---|---|
| Proteins positively associated with endometriosis | |||||
|
| |||||
| Protein kinase C zeta type | PRKCZ | 1.20 | 3.87 (1.80–8.32) | 0.0005 | 0.07 |
| Ferritin | FTH1 FTL | 1.22 | 2.45 (1.44–4.17) | 0.0009 | 0.08 |
| Hepcidin | HAMP | 1.25 | 1.99 (1.29–3.07) | 0.002 | 0.08 |
| Peroxiredoxin-6 | PRDX6 | 1.38 | 2.53 (1.40–4.57) | 0.002 | 0.09 |
| Ephrin-B3 | EFNB3 | 1.25 | 4.47 (1.62–12.32) | 0.004 | 0.12 |
| Angiopoietin-related protein 3 | ANGPTL3 | 1.30 | 14.97 (2.37–94.75) | 0.004 | 0.12 |
| RNA-binding protein 39 | RBM39 | 1.20 | 2.52 (1.32–4.81) | 0.005 | 0.14 |
| Gremlin-1 | GREM1 | 1.23 | 3.74 (1.48–9.45) | 0.005 | 0.14 |
| Hepatocyte growth factorb | HGF | 1.50 | 2.58 (1.31–5.06) | 0.006 | 0.15 |
| Cathepsin G | CTSG | 1.20 | 1.98 (1.21–3.23) | 0.006 | 0.16 |
|
| |||||
| Proteins negatively associated with endometriosis | |||||
|
| |||||
| Glucagon | GCG | −1.92 | 0.31 (0.17–0.56) | 0.0001 | 0.07 |
| Endoplasmic reticulum aminopeptidase 1 | ERAP1 | −1.26 | 0.44 (0.29–0.68) | 0.0002 | 0.07 |
| dCTP pyrophosphatase 1 | DCTPP1 | −1.29 | 0.46 (0.30–0.69) | 0.0002 | 0.07 |
| Glypican-3 | GPC3 | −1.29 | 0.47 (0.31–0.71) | 0.0003 | 0.07 |
| cAMP-dependent protein kinase catalytic subunit alpha | PRKACA | −1.41 | 0.47 (0.31–0.71) | 0.0004 | 0.07 |
| T-lymphocyte surface antigen Ly-9 | LY9 | −1.21 | 0.47 (0.31–0.72) | 0.0004 | 0.07 |
| CD109 antigen | CD109 | −1.20 | 0.49 (0.32–0.75) | 0.0009 | 0.08 |
| Endoglin | ENG | −1.22 | 0.43 (0.26–0.72) | 0.001 | 0.08 |
| Semaphorin-6B | SEMA6B | −1.20 | 0.51 (0.33–0.77) | 0.002 | 0.08 |
| Gamma-enolase | ENO2 | −1.23 | 0.53 (0.35–0.79) | 0.002 | 0.09 |
OR, odds ratio; FDR, false discovery rate.
Adjusted for characteristics at blood draw—age (years), BMI (underweight, normal, overweight, obese), fasting (yes, no), hormone use (yes, no); per 1 SD increase in protein levels.
Removed one outlier measured in a sample from a control.
We further stratified by age at blood sample collection to examine differences in proteomic profiles associated with endometriosis between adolescents and young adults. Interestingly, while many of the proteins showed similar direction of associations in both adolescents and young adults, there were several proteins that showed difference in direction of associations, including proteins related to inflammation such as CTSG (OR = 0.78, 95% CI = 0.13–4.75 in adolescents and OR = 2.17, 95% CI = 1.26–4.05 in young adults) and MMP9 (OR = 0.66, 95% CI = 0.29–1.54 in adolescents and OR = 2.23, 95% CI = 0.14–3.88 in young adults) (Supplementary Table SII).
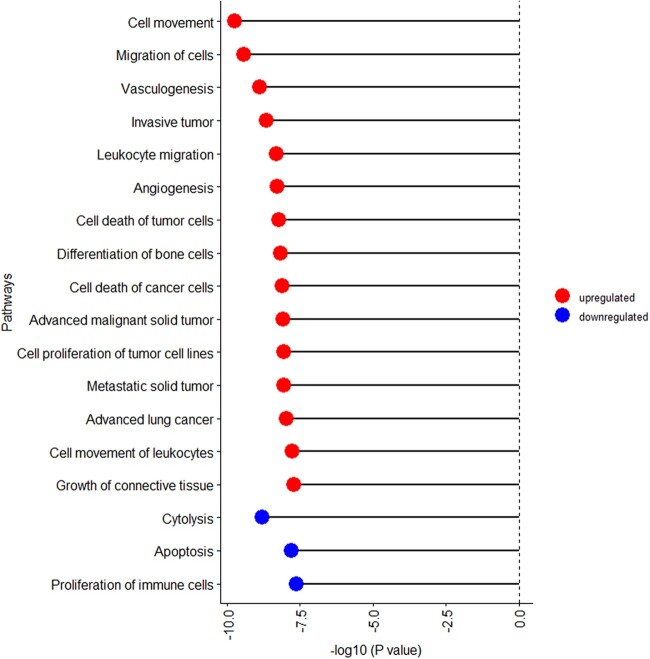
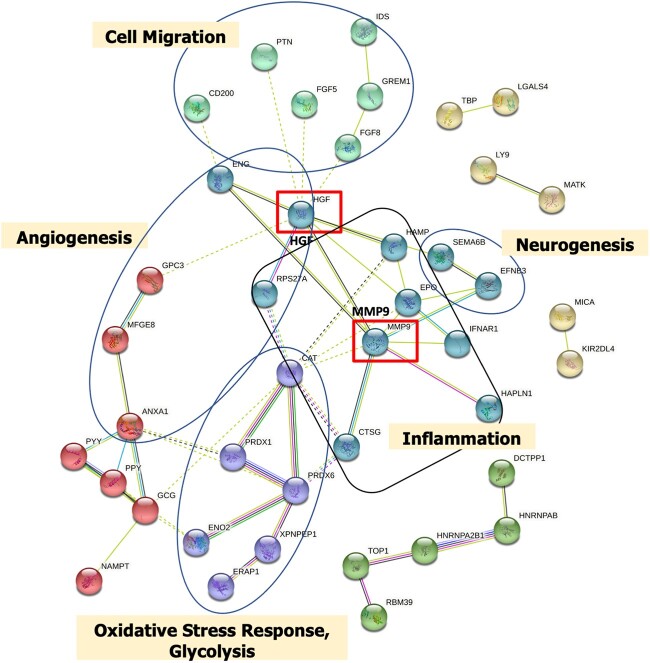
IPA was performed using the 63 proteins associated with endometriosis. Of the top 20 pathways (P-value < 2.4 × 10−8), pathways related to cell movement, leukocyte migration and angiogenesis were upregulated in endometriosis cases compared to controls (Fig. 1, Supplementary Table SIII). In contrast, pathways related to cytolysis, apoptosis and proliferation of immune cells were downregulated in endometriosis cases compared to controls. We performed network and cluster analysis using the STRING database of functional and physical protein associations curated across major data repositories. Forty-one of the 63 proteins formed distinct interacting protein clusters that were enriched in pathways associated with angiogenesis (e.g. HGF, ENG, GPC3), cell migration (CD200, PTN, FGF5), oxidative stress (CAT, PRDX1, PRDX6) and inflammation (MMP9, IFNAR1, CTSG) and created a complex network, demonstrating that these pathways are interrelated (Fig. 2).
Figure 1.
Pathways associated with endometriosis based on 63 proteins with P-value <0.05 and absolute fold-change >1.2 in the A2A cohort (n = 216). Biological pathways associated with endometriosis are grouped by the direction of association (i.e. activation z-score) and presented within group ordered by P-value. Upregulated pathways are denoted by red bubbles and downregulated pathways are denoted by blue bubbles. Of the top 20 statistically significant pathways, two pathways (morphology of bone and uveitis pathways) were removed due to activation z-score of 0.0.
Figure 2.
STRING analysis and visualization of protein–protein interaction clusters (k-means = 6 clusters indicated by node color) and relevant pathways associated with endometriosis in the A2A cohort (n = 216). Protein–protein interaction network was created based on the 63 proteins that were significantly associated with endometriosis with unadjusted P-value <0.05 and absolute fold-change >1.2. Related functional categories are labeled based on proteins with their reported functional involvement in the pathways of angiogenesis (red node), cell migration (light green node), inflammation (dark green node), oxidative stress response (purple node) and neurogenesis (yellow node). Solid line represents within-cluster, dashed gray line represents between-cluster interactions. Line thickness indicates strength of data support. Red box indicates major hub nodes.
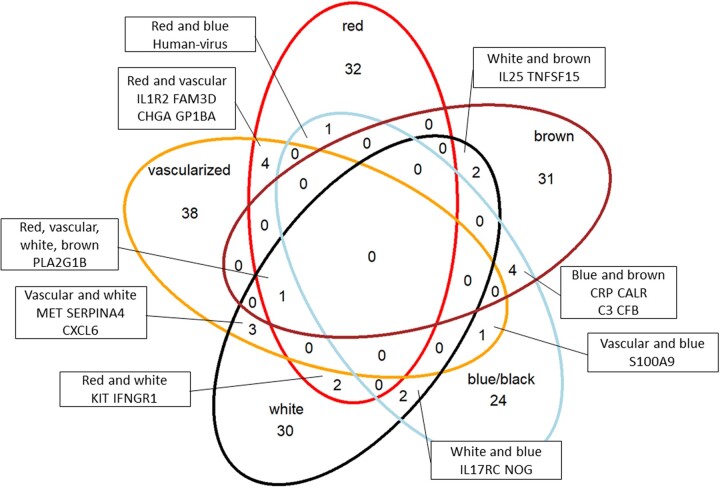
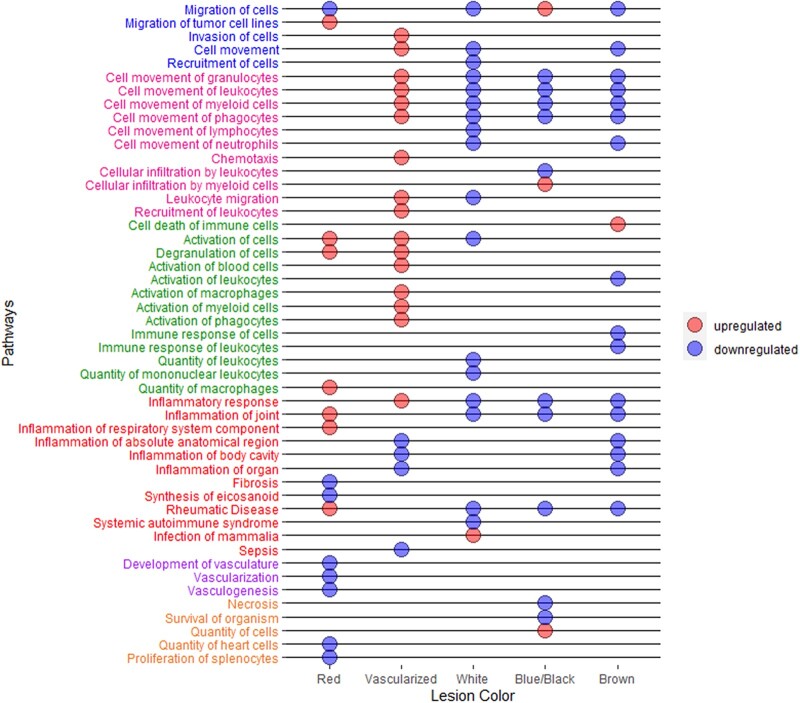
We further investigated circulating proteins and biological pathways associated with different superficial peritoneal endometriotic lesion colors (i.e. red, vascularized, white, blue/black, brown). There were 40 proteins associated with having any red lesions compared to having no red lesions with unadjusted P-value <0.05 (Supplementary Table SIV). Similarly, there were 40 proteins associated with having any white lesions, 32 proteins associated with having any blue/black lesions, 39 proteins associated with having any brown lesions and 47 proteins associated with having any vascularized lesions. When we examined the overlap of proteins associated with each lesion color, there was very little overlap among lesion colors (Fig. 3). Furthermore, when we examined the top pathways associated for each lesion color, having any vascularized lesions was associated with upregulation of multiple pathways related to immune cell migration/activation and inflammation whereas having any white, blue/black or brown lesions were associated with downregulation of these pathways (Fig. 4, Supplementary Table SV). Having any red lesions was associated with upregulation of pathways related to immune cell activation and inflammation and downregulation of vascular functions.
Figure 3.
Venn diagram showing unique and overlapping proteins by lesion colors among endometriosis cases in the A2A cohort (n = 142). There were 40 proteins associated with having any red lesions (red circle), 40 proteins associated with having any white lesions (black circle), 32 proteins associated with having any blue/black lesions (light blue circle), 39 proteins associated with having any brown lesions (brown circle) and 47 proteins associated with having any vascularized lesions (yellow circle), with unadjusted P-value <0.05. Entrez gene symbols of proteins that overlapped between two or more lesion colors are presented.
Figure 4.
Top pathways associated with lesion colors in the A2A cohort (n = 142). Biologic pathways associated with each lesion color are presented in the dot chart. Upregulated pathways are denoted by red dots and downregulated pathways are denoted by blue dots. Of the top 20 statistically significant pathways, the following pathways were removed due to activation z-score being equivalent to 0.0: red lesion (uterine serous papillary cancer, fasciculation of axons); white lesion (chronic inflammatory disorder); blue lesion (chronic inflammatory disorder). The pathway names are colored in the following groups: pathways related to cell migration (blue font), pathways related to immune cell movement (pink font), pathways related to immune cell activation (green font), pathways related to inflammation (red font), pathways related to vascularization (purple font) and others (orange font).
Discussion
We conducted a large-scale analysis of the human plasma proteome and identified proteomic profiles associated with endometriosis in a population of adolescents and young adults in the A2A study. Several biological pathways related to angiogenesis and cell migration were upregulated in endometriosis cases compared to controls. Furthermore, our analysis examining proteomic profiles by lesion colors revealed key differences in biological pathways of immune cell migration/activation and inflammation among lesion colors.
Upregulation of angiogenesis pathways were observed in endometriosis cases compared to controls in our young population. Angiogenesis, which refers to the growth of new blood vessels, has been reported to play a key role in endometriosis development. Increased levels of biomarkers related to angiogenesis have been observed in peritoneal fluid of adult endometriosis patients (McLaren et al., 1996; Taylor et al., 2002). Dense vascularization is often observed in endometriotic lesions and therefore angiogenesis is thought to play an important role in the development of endometriosis (Laschke et al., 2011). Furthermore, endometriotic lesions secrete cytokines such as IL-1β, IL-6 and growth factors such as hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), which are known to enhance angiogenesis (Cohen et al., 1996; Lebovic et al., 2000a,b). Indeed, we observed a significant increase of the important angiogenesis factor HGF in adolescent endometriosis cases, which has been reported to be induced by pro-inflammatory cytokines and prostaglandins in the pelvic cavity of women with endometriosis (Khan et al., 2004; Yoshida et al., 2004). HGF enhances degradation of the extracellular matrix at least partially via increasing the expression of metalloproteinases such as MMP9. In our analyses, we observed that MMP9 was increased in adolescent endometriosis cases, and it has been previously demonstrated to be increased in serum and ascites of women with endometriosis (Liu et al., 2015, 2016). HGF and MMP9 were identified as key focus hubs in our STRING analysis of interactions between the 63 endometriosis-associated proteins that link to various other protein clusters of the endometriosis protein signature.
Interestingly, pathways related to inflammation, which have been associated with endometriosis among adults ( Harada et al., 2001; Lebovic et al., 2001; Jiang et al., 2016; Mu et al., 2018) did not emerge as associated protein pathways in our analysis. Further, this lack of association was not due to confounding by analgesic use, as our main results were similar after additionally adjusting for regular analgesic use. Therefore, difference in analgesic use between cases and controls does not explain this null inflammatory pathway observation. One reason for this observation could be that angiogenesis may be more important earlier in endometriosis natural history. A small study among adults reported higher serum VEGF levels in rASRM stage I/II endometriosis patients compared to stage III/IV patients as well as controls (Kopuz et al., 2014). Studies using mouse models reported neutrophils and macrophages as well as secretion of interleukin-10 promoted angiogenesis early in the experimental model endometriosis establishment (Lin et al., 2006; Suen et al., 2019). Since these adolescent endometriosis patients presented with severe pelvic pain, our results of angiogenic pathways being one of the significant pathways could also be reflecting the upregulation of neuroangiogenesis, which can simultaneously occur with angiogenesis (Teng et al., 2008) and is thought to contribute to the development of endometriosis-associated pain (Asante and Taylor, 2011; Machairiotis et al., 2021).
We also observed pathways related to cell migration and invasion being upregulated in endometriosis. While the etiology of endometriosis is still largely unknown, the Sampson theory argues that endometriosis develops through implantation of endometrial cells from retrograde menstruation (Zondervan et al., 2020). This implantation theory suggests that cell migration and invasion play an important role in endometriosis development (Giudice and Kao, 2004; Hull et al., 2008). Thus, our results are in line with the implantation theory and with prior mechanistic studies (Chang et al., 2013; Li et al., 2018; Fu et al., 2021). We also observed upregulation of pathways related to leukocyte migration in endometriosis cases, which is in line with prior studies reporting enhanced recruitment of immune cells to ectopic endometriosis lesions (Jones et al., 1998, 2005). Further, abnormal cell survival in ectopic sites is necessary for endometriosis to develop. Downregulation of apoptosis-related gene expression and proteins has been observed in endometriotic tissue (Gebel et al., 1998; Dufournet et al., 2006), which is consistent with our results showing downregulation of apoptosis pathways in endometriosis patients.
While we could not conduct direct comparison between adolescents versus adults with endometriosis, when we stratified by age at blood sample collection, we observed some differences in individual protein associations among our top hits, including proteins related to inflammation (Khokha et al., 2013; Gao et al., 2018). However, our study population is oversampled to include a large proportion of adolescents, and thus the age-defined stratum-specific samples sizes are small and thus yielded less precise estimates (i.e. wide confidence intervals). Future analyses building on these findings to directly compare these adolescent/young adult endometriosis plasma proteomic profiles to samples collected from adults are needed to further inform similarities and differences by age at sample collection, time since endometriosis diagnosis, and also by time since endometriosis-associated symptom onset. Like the A2A cohort, larger and more diverse longitudinal studies designed to maximize retention of participants over many years regardless of their clinical interaction and pelvic pain-related treatment journeys are needed. Serial biological sample collection measures are essential to adequately quantify the proteomic milieu as it changes across the life course.
Few studies have examined differences in molecular features by endometriotic lesion colors (Nisolle et al., 1993; Donnez et al., 1998; Strehl et al., 2014). One reported strongest vascularization and highest level of proliferative activity in red lesions and lowest level of proliferative activity in white lesions (Nisolle et al., 1993). Another study reported that red lesions have high concentration of VEGF compared to blue/black lesions (Donnez et al., 1998). We examined systemic plasma proteomic profiles associated with lesion colors and observed upregulation of inflammation pathways in red and vascularized lesions and downregulation of inflammation in blue/black, brown and white lesions, which is consistent with prior findings. Interestingly, having any vascularized lesions was associated with upregulation of multiple pathways related to immune cell migration/activation and having any white, blue/black or brown lesions were associated with downregulation of immune cell migration pathways. Unexpectedly, our result showed having any red lesions were associated with downregulation of vascularization pathways which was different from prior studies (Nisolle et al., 1993; Donnez et al., 1998). This difference could be due to prior studies examining protein expression in the ectopic lesions, whereas our study compared the systemic proteomic profiles.
Little is known about the pathophysiology of adolescent endometriosis despite the fact that over 50% of adults with endometriosis report onset of severe pelvic pain during adolescence (Nnoaham et al., 2011; Zondervan et al., 2020). Thus, furthering our understanding on the molecular profiles of endometriosis diagnosed in adolescents and young adults is likely to inform pathophysiology of endometriosis during the earlier course of the disease trajectory and may indicate potential new treatment strategies for these young patients. Moreover, unique blood biomarker profiles of endometriosis patients diagnosed in this young population may be promising candidates for non-invasive earlier diagnostic markers for endometriosis. While validation of our results in independent datasets and mechanistic studies are warranted to further our understanding of the biological mechanism, our current study is one of the first large studies reporting comprehensive proteomics profiles of endometriosis diagnosed in adolescents and young adults, shedding light into the molecular profile of this common but understudied patient population. This study also supports the promising application of proteomics technology to discover novel proteomic biomarkers for endometriosis.
Our study has several strengths. This was the first study to comprehensively examine circulating proteins in adolescents and young adult women with and without endometriosis. Although there have been several studies that conducted untargeted proteomic analyses to identify circulating proteins that differ between endometriosis cases and controls (Ferrero et al., 2008; Wölfler et al., 2009; Zheng et al., 2011; Long et al., 2013; Manousopoulou et al., 2019), we used a validated proteomic platform showing high reproducibility and reliability assuring validity of the measurements (Kim et al., 2018). We used blood samples that were collected and processed using a standardized protocol, minimizing measurement error due to pre-analytic blood sample collection factors (Tworoger and Hankinson, 2006). Another strength of our study is that while many prior studies compared endometriosis cases to surgical controls (which would yield null results if the non-endometriosis pathology that indicated the surgery is also associated with the measured protein levels or pathways), we included controls who were sampled from the underlying population that gave rise to the endometriosis cases (Missmer, 2019). However, our sample size limited the ability to identify statistically significant individual proteins associated with endometriosis after multiple testing correction. We were also only able to explore any presence of a lesion color and could not evaluate presence or absence of multiple lesion color combinations.
In conclusion, we identified circulating proteins and biological pathways associated with laparoscopically confirmed endometriosis in adolescents and young adults, revealing molecular characteristics of this unique patient population. Results from this study suggest angiogenesis and cell migration pathways may be important biological pathways in endometriosis diagnosed in adolescents and young adults. Future studies directly comparing the molecular characteristics of adolescent/young adult versus adult endometriosis are warranted to reveal disease pathophysiology during the earlier course of the disease trajectory, implicitly differentiating between endometriosis diagnosed in adolescence versus adulthood.
Supplementary Material
Acknowledgements
The authors would like to thank all the participants of the Women’s Health Study: From Adolescence to Adulthood for their valuable contributions and staff of the Boston Center for Endometriosis.
Authors’ roles
N.S. was responsible for formulation of the study hypotheses and study design, results interpretation, manuscript writing, revision and finalization. L.N. was responsible for statistical analyses, results interpretation, manuscript revision and finalization. A.F.V. was responsible for data collection development and implementation, study analysis methods, results interpretation, manuscript revision and finalization. S.T.D. was responsible for proteomics data generation, results interpretation, manuscript revision and finalization. S.A.M. was responsible for data collection development and implementation, study design and analysis methods, results interpretation, manuscript revision and finalization. T.A.L. was responsible for formulation of the study hypotheses and study design, statistical analyses, results interpretation, manuscript revision and finalization. K.L.T. was responsible for formulation of the study hypotheses and study design, results interpretation, manuscript revision and finalization.
Funding
This study was supported by the Department of Defense (W81XWH1910318) and the 2017 Boston Center for Endometriosis Trainee Award. Financial support for establishment of and data collection within the A2A cohort were provided by the J. Willard and Alice S. Marriott Foundation. N.S., A.F.V., S.A.M. and K.L.T. have received funding from Marriott Family Foundation. S.A.M. and K.L.T. are supported by NICHD (R01 HD94842).
Conflict of interest
S.A.M. serves as an advisory board member for AbbVie and Roche; neither are related to this study. The authors report no conflict of interest.
Contributor Information
Naoko Sasamoto, Department of Obstetrics and Gynecology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Boston Center for Endometriosis, Boston Children’s Hospital and Brigham and Women’s Hospital, Boston, MA, USA.
Long Ngo, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA; Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Allison F Vitonis, Department of Obstetrics and Gynecology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Boston Center for Endometriosis, Boston Children’s Hospital and Brigham and Women’s Hospital, Boston, MA, USA.
Simon T Dillon, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA; Genomics, Proteomics, Bioinformatics and Systems Biology Center, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Stacey A Missmer, Boston Center for Endometriosis, Boston Children’s Hospital and Brigham and Women’s Hospital, Boston, MA, USA; Department of Obstetrics, Gynecology, and Reproductive Biology, Michigan State University College of Human Medicine, Grand Rapids, MI, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Towia A Libermann, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA; Genomics, Proteomics, Bioinformatics and Systems Biology Center, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Kathryn L Terry, Department of Obstetrics and Gynecology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Boston Center for Endometriosis, Boston Children’s Hospital and Brigham and Women’s Hospital, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Data Availability
Data are not publicly available due to information that could compromise research participants’ privacy and consent. However, experienced scientists who would like to inquire regarding use of data from this study to address specific hypotheses may submit an application and research proposal. Data requests must be reviewed and approved by the BWH Institutional Review Board (https://www.brighamandwomens.org/research/research-administration). All inquiries should be directed to the Boston Center for Endometriosis leadership committee (womenshealthstudy@bwh.harvard.edu). Data sharing will require a fully executed Data Usage Agreement.
References
- Adamson GD, Kennedy S, Hummelshoj L.. Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. J Endometr 2010;2:3–6. [Google Scholar]
- Asante A, Taylor RN.. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol 2011;73:163–182. [DOI] [PubMed] [Google Scholar]
- Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120(Suppl 4):S164–S192. [DOI] [PubMed] [Google Scholar]
- Becker CM, Laufer MR, Stratton P, Hummelshoj L, Missmer SA, Zondervan KT, Adamson GD; WERF EPHect Working Group. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil Steril 2014;102:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Au HK, Lee WC, Chi CC, Ling TY, Wang LM, Kao SH, Huang YH, Tzeng CR.. Expression of the pluripotent transcription factor OCT4 promotes cell migration in endometriosis. Fertil Steril 2013;99:1332–1339.e5. [DOI] [PubMed] [Google Scholar]
- Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ.. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 1996;271:736–741. [DOI] [PubMed] [Google Scholar]
- DiVasta AD, Vitonis AF, Laufer MR, Missmer SA.. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am J Obstet Gynecol 2018;218:324.e1–324.e11. [DOI] [PubMed] [Google Scholar]
- Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M.. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod 1998;13:1686–1690. [DOI] [PubMed] [Google Scholar]
- Drosdzol-Cop A, Skrzypulec-Plinta V.. Selected cytokines and glycodelin A levels in serum and peritoneal fluid in girls with endometriosis. J Obstet Gynaecol Res 2012;38:1245–1253. [DOI] [PubMed] [Google Scholar]
- Dufournet C, Uzan C, Fauvet R, Cortez A, Siffroi JP, Daraï E.. Expression of apoptosis-related proteins in peritoneal, ovarian and colorectal endometriosis. J Reprod Immunol 2006;70:151–162. [DOI] [PubMed] [Google Scholar]
- Eveleth PB. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. Am J Hum Biol 1996;8:786–787. [Google Scholar]
- Ferrero S, Gillott DJ, Remorgida V, Ragni N, Venturini PL, Grudzinskas JG.. Proteomics technologies in endometriosis. Expert Rev Proteomics 2008;5:705–714. [DOI] [PubMed] [Google Scholar]
- Fu X, Yao M, Ye C, Fang T, Wu R.. Osteopontin regulates endometrial stromal cell migration in endometriosis through the PI3K pathway: osteopontin regulates endometrial cell migration in endometriosis. Reprod Sci 2021;28:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Zhu H, Zuo X, Luo H.. Cathepsin G and its role in inflammation and autoimmune diseases. Arch Rheumatol 2018;33:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP.. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril 1998;69:1042–1047. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC.. Endometriosis. Lancet 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- Harada T, Iwabe T, Terakawa N.. Role of cytokines in endometriosis. Fertil Steril 2001;76:1–10. [DOI] [PubMed] [Google Scholar]
- Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, Furlong P, Gordish-Dressman H, Hache L, Henricson E, Hoffman EP. et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 2015;112:7153–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull ML, Escareno CR, Godsland JM, Doig JR, Johnson CM, Phillips SC, Smith SK, Tavaré S, Print CG, Charnock-Jones DS.. Endometrial-peritoneal interactions during endometriotic lesion establishment. Am J Pathol 2008;173:700–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yan Y, Liu Z, Wang Y.. Inflammation and endometriosis. Front Biosci (Landmark Ed) 2016;21:941–948. [DOI] [PubMed] [Google Scholar]
- Jones RK, Bulmer JN, Searle RF.. Phenotypic and functional studies of leukocytes in human endometrium and endometriosis. Hum Reprod Update 1998;4:702–709. [DOI] [PubMed] [Google Scholar]
- Jones RL, Morison NB, Hannan NJ, Critchley HO, Salamonsen LA.. Chemokine expression is dysregulated in the endometrium of women using progestin-only contraceptives and correlates to elevated recruitment of distinct leukocyte populations. Hum Reprod 2005;20:2724–2735. [DOI] [PubMed] [Google Scholar]
- Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T.. Higher activity by opaque endometriotic lesions than nonopaque lesions. Acta Obstet Gynecol Scand 2004;83:375–382. [DOI] [PubMed] [Google Scholar]
- Khokha R, Murthy A, Weiss A.. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 2013;13:649–665. [DOI] [PubMed] [Google Scholar]
- Kianpour M, Nematbakhsh M, Ahmadi SM.. C-reactive protein of serum and peritoneal fluid in endometriosis. Iran J Nurs Midwifery Res 2012;17:S115–S119. [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Tworoger SS, Stampfer MJ, Dillon ST, Gu X, Sawyer SJ, Chan AT, Libermann TA, Eliassen AH.. Stability and reproducibility of proteomic profiles measured with an aptamer-based platform. Sci Rep 2018;8:8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopuz A, Kurt S, Demirtas O, Toz E, Tasyurt A.. Relation of peritoneal fluid and serum vascular endothelial growth factor levels to endometriosis stage. Clin Exp Obstet Gynecol 2014;41:547–550. [PubMed] [Google Scholar]
- Krämer A, Green J, Pollard J Jr, Tugendreich S.. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2013;30:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laschke MW, Giebels C, Menger MD.. Vasculogenesis: a new piece of the endometriosis puzzle. Hum Reprod Update 2011;17:628–636. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Bentzien F, Chao VA, Garrett EN, Meng YG, Taylor RN.. Induction of an angiogenic phenotype in endometriotic stromal cell cultures by interleukin-1beta. Mol Hum Reprod 2000a;6:269–275. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN.. Immunobiology of endometriosis. Fertil Steril 2001;75:1–10. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Shifren JL, Ryan IP, Mueller MD, Korn AP, Darney PD, Taylor RN.. Ovarian steroid and cytokine modulation of human endometrial angiogenesis. Hum Reprod 2000b;15(Suppl 3):67–77. [DOI] [PubMed] [Google Scholar]
- Li N, Zhang L, Li Q, Du Y, Liu H, Liu Y, Xiong W.. Notch activity mediates oestrogen-induced stromal cell invasion in endometriosis. Reproduction 2018;157:371–381. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Lai MD, Lei HY, Wing LY.. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology 2006;147:1278–1286. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang J, Wang H, Tang N, Li Y, Zhang Y, Hao T.. Correlation between matrix metalloproteinase-9 and endometriosis. Int J Clin Exp Pathol 2015;8:13399–13404. [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang J, Wang H, Tang N, Li Y, Zhang Y, Hao T.. The plasma and peritoneal fluid concentrations of matrix metalloproteinase-9 are elevated in patients with endometriosis. Ann Clin Biochem 2016;53:599–605. [DOI] [PubMed] [Google Scholar]
- Long X, Jiang P, Zhou L, Zhang W.. Evaluation of novel serum biomarkers and the proteomic differences of endometriosis and adenomyosis using MALDI-TOF-MS. Arch Gynecol Obstet 2013;288:201–205. [DOI] [PubMed] [Google Scholar]
- Machairiotis N, Vasilakaki S, Thomakos N.. Inflammatory mediators and pain in endometriosis: a systematic review. Biomedicines 2021;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manousopoulou A, Hamdan M, Fotopoulos M, Garay-Baquero DJ, Teng J, Garbis SD, Cheong Y.. Integrated eutopic endometrium and non-depleted serum quantitative proteomic analysis identifies candidate serological markers of endometriosis. Proteomics Clin Appl 2019;13:e1800153. [DOI] [PubMed] [Google Scholar]
- McLaren J, Prentice A, Charnock-Jones DS, Smith SK.. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod 1996;11:220–223. [DOI] [PubMed] [Google Scholar]
- Missmer SA. Why so null? Methodologic necessities to advance endometriosis discovery. Paediatr Perinat Epidemiol 2019;33:26–27. [DOI] [PubMed] [Google Scholar]
- Mu F, Harris HR, Rich-Edwards JW, Hankinson SE, Rimm EB, Spiegelman D, Missmer SA.. A prospective study of inflammatory markers and risk of endometriosis. Am J Epidemiol 2018;187:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisolle M, Casanas-Roux F, Anaf V, Mine JM, Donnez J.. Morphometric study of the stromal vascularization in peritoneal endometriosis. Fertil Steril 1993;59:681–684. [PubMed] [Google Scholar]
- Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT; World Endometriosis Research Foundation Global Study of Women's Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366–373.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmioglu N, Fassbender A, Vitonis AF, Tworoger SS, Hummelshoj L, D'Hooghe TM, Adamson GD, Giudice LC, Becker CM, Zondervan KT. et al. ; WERF EPHect Working Group. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil Steril 2014;102:1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamoto N, DePari M, Vitonis AF, Laufer MR, Missmer SA, Shafrir AL, Terry KL.. Evaluation of CA125 in relation to pain symptoms among adolescents and young adult women with and without surgically-confirmed endometriosis. PLoS One 2020a;15:e0238043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamoto N, Farland LV, Vitonis AF, Harris HR, DiVasta AD, Laufer MR, Terry KL, Missmer SA.. In utero and early life exposures in relation to endometriosis in adolescents and young adults. Eur J Obstet Gynecol Reprod Biol 2020b;252:393–398. [DOI] [PubMed] [Google Scholar]
- Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA.. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol 2018;51:1–15. [DOI] [PubMed] [Google Scholar]
- Shah DK, Missmer SA.. Scientific investigation of endometriosis among adolescents. J Pediatr Adolesc Gynecol 2011;24:S18–S19. [DOI] [PubMed] [Google Scholar]
- Strehl JD, Hackl J, Wachter DL, Klingsiek P, Burghaus S, Renner SP, Fasching PA, Hartmann A, Beckmann MW.. Correlation of histological and macroscopic findings in peritoneal endometriosis. Int J Clin Exp Pathol 2014;7:152–162. [PMC free article] [PubMed] [Google Scholar]
- Suen JL, Chang Y, Shiu YS, Hsu CY, Sharma P, Chiu CC, Chen YJ, Hour TC, Tsai EM.. IL-10 from plasmacytoid dendritic cells promotes angiogenesis in the early stage of endometriosis. J Pathol 2019;249:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019;47:D607–D613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RN, Lebovic DI, Mueller MD.. Angiogenic factors in endometriosis. Ann N Y Acad Sci 2002;955:89–100; discussion 118, 396–406. [DOI] [PubMed] [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M. et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab 2008;28:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tworoger SS, Hankinson SE.. Use of biomarkers in epidemiologic studies: minimizing the influence of measurement error in the study design and analysis. Cancer Causes Control 2006;17:889–899. [DOI] [PubMed] [Google Scholar]
- Vitonis AF, Vincent K, Rahmioglu N, Fassbender A, Buck Louis GM, Hummelshoj L, Giudice LC, Stratton P, Adamson GD, Becker CM. et al. ; WERF EPHect Working Group. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil Steril 2014;102:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfler MM, Schwamborn K, Otten D, Hornung D, Liu H, Rath W.. Mass spectrometry and serum pattern profiling for analyzing the individual risk for endometriosis: promising insights? Fertil Steril 2009;91:2331–2337. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Harada T, Mitsunari M, Iwabe T, Sakamoto Y, Tsukihara S, Iba Y, Horie S, Terakawa N.. Hepatocyte growth factor/Met system promotes endometrial and endometriotic stromal cell invasion via autocrine and paracrine pathways. J Clin Endocrinol Metab 2004;89:823–832. [DOI] [PubMed] [Google Scholar]
- Zheng N, Pan C, Liu W.. New serum biomarkers for detection of endometriosis using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Int Med Res 2011;39:1184–1192. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P.. Endometriosis. Nat Rev Dis Primers 2018;4:9. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Missmer SA.. Endometriosis. N Engl J Med 2020;382:1244–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not publicly available due to information that could compromise research participants’ privacy and consent. However, experienced scientists who would like to inquire regarding use of data from this study to address specific hypotheses may submit an application and research proposal. Data requests must be reviewed and approved by the BWH Institutional Review Board (https://www.brighamandwomens.org/research/research-administration). All inquiries should be directed to the Boston Center for Endometriosis leadership committee (womenshealthstudy@bwh.harvard.edu). Data sharing will require a fully executed Data Usage Agreement.