Abstract
Enteropathogenic Escherichia coli (EPEC) infection of T84 cells induces a decrease in transepithelial resistance, the formation of attaching and effacing (A/E) lesions, and cytokine production. The purpose of this study was to investigate the ability of EPEC to activate mitogen-activated protein (MAP) kinases in T84 cells and to correlate these signaling pathways with EPEC-induced cell responses. T84 cells were infected with either the wild-type (WT) EPEC strain E2348/69 or two mutants, intimin deletion strain CVD206 (ΔeaeA) and type III secretion apparatus mutant strain CVD452 (ΔescN::aphA). Infection of T84 cells with WT but not mutant EPEC strains induced tyrosine phosphorylation of several proteins in T84 cells, including the p46 and p52 Shc isoforms. Kinetics studies revealed that ERK1/2, p38, and c-Jun N-terminal kinase (JNK) MAP kinases were activated in cells infected with strain E2348/69 but not with the mutant strains. Inhibition of MAP kinases with PD98059 or SB203580 did not affect the EPEC-induced decrease in transepithelial resistance or actin accumulation beneath the WT bacteria, but these two inhibitors significantly decreased interleukin-8 (IL-8) synthesis. We demonstrate that EPEC induces activation of ERK1/2, p38, and JNK cascades, which all depend on bacterial adhesion and expression of the bacterial type III secretion system. ERK1/2 and p38 MAP kinases were equally implicated in IL-8 expression but did not participate in A/E lesion formation or transepithelial resistance modification, indicating that the signaling pathways involved in these events are distinct.
The gram-negative pathogen enteropathogenic Escherichia coli (EPEC) is a major cause of infantile diarrhea in the developing world (29, 33). EPEC infection of epithelial cell progresses in three steps: (i) localized adherence (LA); (ii) signal transduction, and (iii) intimate attachment (39). Associated with the LA phenotype is the induction of attaching and effacing (A/E) lesions, characterized by localized destruction of microvilli and the accumulation of polymerized actin beneath bacteria that promotes the formation of a cuplike pedestal (24, 28). Actin rearrangement is also involved in the internalization of a subpopulation of EPEC by epithelial cells (2, 34). All of the genes needed for A/E lesions are located on the bacterial chromosome in a large (35-kb) region termed the locus enterocyte effacement (LEE) (12). The LEE genes are separated into three functional domains. The first domain includes genes implicated in intimate attachment: the eaeA gene, coding for an adhesin called intimin, and the gene coding for the translocated intimin receptor (Tir) (21, 22). The second domain encodes the type III secretion system that directs the secretion of bacterial proteins. Virulence genes termed EPEC-secreted proteins (Esp) constitute the third LEE domain. At least three secreted proteins, EspA, EspB, and EspD, are involved in the induction of signal transduction pathways within the epithelial cell, including the elevation of intracellular levels of Ca2+ and inositol phosphate (3, 11, 16) and phosphorylation of several cellular proteins (27, 34). Activation of these pathways in the EPEC-infected human intestinal epithelial cell line T84 leads to disruption of the epithelial barrier, host cell cytoskeletal rearrangement, and the secretion of cytokines (31, 38, 40).
Mitogen-activated protein (MAP) kinases are central in many host responses, including the mitogenic response to growth factors for which they are named. MAP kinases have also been implicated in the regulation of cytokine responses, stress responses, and cytoskeletal reorganization (18). MAP kinases form a group of three serine/threonine kinases with isoforms ranging from 40 to 62 kDa. They include extracellular signal-regulated protein kinases 1 and 2 (ERK1 and ERK2) and two stress-activated protein kinases, p38 MAP kinase, also known as the hyperosmolarity glycerol kinase, and c-Jun N-terminal kinase (JNK). Recently, MAP kinases have been implicated in the host cell response to bacterial infection. Activation of ERK, JNK, and p38 MAP kinases has been found in epithelial cells infected by Listeria monocytogenes and Salmonella enterica serovar Typhimurium (19, 41). It has been demonstrated that L. monocytogenes invasion of epithelial cells requires activation of the MEK-1/ERK2 pathway, because a specific inhibitor of this signaling pathway, PD98059 (1), blocks L. monocytogenes invasion of HeLa cells (41). While, activation of p38 MAP kinase is implicated in cytokine synthesis, because cell pretreatment by the specific p38 MAP kinase inhibitor SB203580 (8) prevents Salmonella-induced interleukin-8 (IL-8) synthesis (19). We recently demonstrated that in T84 cells, EPEC can induce the activation of ERK1/2 MAP kinase, which we found is required for EPEC internalization by these cells (9). This study was designed to investigate the ability of EPEC to activate other MAP kinases and to correlate these signaling pathways with cellular responses to EPEC infection. The data presented in this paper show that the wild-type (WT) EPEC strain E2348/69 induced tyrosine phosphorylation of several proteins and concomitant activation of ERK1/2, p38, and JNK MAP kinases pathways in T84 cells. None of the three MAP kinases were stimulated by the intimin-deleted strain CVD206 or the type III secretion system apparatus mutant strain CVD452. Preincubation of T84 cells with PD98059 or SB203580 did not prevent the decrease of WT EPEC-induced transepithelial resistance and pedestal formation, but these inhibitors significantly reduced EPEC-induced IL-8 expression.
MATERIALS AND METHODS
Cell lines, media, and bacterial strains.
The T84 human colonic cell line was obtained from the European Collection of Animal Cell Cultures (Salisbury, England). The T84 culture medium contained a 1:1 mixture of Dulbecco-Vogt modified Eagle medium and Ham's F12 medium (DMEM-F12) supplemented with 50 μg each of penicillin and streptomycin (Sigma, Saint Quentin Fallavier, France) per ml and 5% fetal bovine serum (Dominique Dutcher, Brumath, France). The wild-type (WT) bacterial strain EPEC E2348/69 and mutant strains CVD206 and CVD452 used in this study (Table 1) were kindly provided by J. Kaper (Center for Vaccine Development, University of Maryland, Baltimore). Bacteria were stored in Luria-Bertani medium plus 15% glycerol at −80°C and grown in Luria broth overnight at 37°C without shaking.
TABLE 1.
Bacterial strains used in this study
| Strain | Characteristics (reference) | T84 cell response to infection
|
||
|---|---|---|---|---|
| Barrier function (% change in resistance vs control)a | A/E lesionb | IL-8 (ng/ml)c | ||
| E2348/69 | Parental EPEC, Adh+ Inv+ (26) | −78 ± 0.2 | + | 54 ± 6.8 |
| CVD206 | E2348/69 ΔeaeA, Adh+ intimate Adh− Inv− (10) | −12 ± 0.1 | − | 2.8 ± 0.82 |
| CVD452 | E2348/69 ΔescN::aphA-3, signal transduction affected (20) | +6 ± 0.3 | − | 1.9 ± 0.49 |
TER was monitored on filter-grown T84 cells as described in Materials and Methods. Values presented correspond to resistance measured after 6 h of infection.
Estimated after 3 h of infection by staining with FITC-phalloidin.
Estimated in cell supernatant from 6-h-infected cells by enzyme-linked immunosorbent assay as described in Materials and Methods.
Inhibitors.
The MEK-1 inhibitor PD98059 and the p38 inhibitor SB203580 (Calbiochem, Meudon, France) were stored in dimethyl sulfoxide (DMSO) at −20°C. Prior to infection, the cells were incubated for 90 min with PD98059 (50 μM) and SD203580 (10 μM), alone or together. The inhibitors were maintained during the infection period.
Infection procedure.
Prior to infection, the T84 cells were washed and incubated for at least 2 h in serum- and antibiotic-free DMEM-F12. Infections were carried out in serum- and antibiotic-free DMEM/F12. Bacteria grown overnight in Luria-Bertani medium were pelleted by centrifugation, resuspended in sterile phosphate-buffered saline (PBS), and added to the cells (100 bacteria/cell).
Electrical resistance measurements.
T84 cells were grown on collagen-coated, 0.33-cm2 porous filter membranes (3-μm-diameter pores; Costar; Poly Lab. Paul Block, Strasbourg, France). Transmonolayer electrical resistance (TER) was measured with a Millicell-ERS apparatus (Millipore, Molsheim, France), as described elsewhere (13). Under these conditions, high TER values (>2,000 Ω/cm2) were consistently obtained in 7-day postseeding monolayers.
Immunofluorescence microscopy.
Cells were seeded and grown overnight on glass coverslips. After infection and/or treatment with inhibitors, the cells were washed with PBS and fixed for 30 min with 2% formaldehyde in PBS. The cells were washed five times with PBS, permeabilized for 5 min with 0.1% Triton X-100 in PBS, and washed. Actin filaments were stained for 45 min with fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma).
IL-8 assay.
IL-8 assays were performed at 70 to 90% confluence of T84 monolayers grown in 60-mm-diameter petri dishes. Cells were infected with WT or mutated strains for 6 h in 1.5 ml of serum- and antibiotic-free DMEM/F12. The culture supernatants were then centrifuged for 10 min at 10,000 rpm to pellet residual bacteria. The IL-8 concentration was determined with a Quantikine human IL-8 immunoassay (R&D Systems, Abington, United Kingdom). Where indicated, SB203580 (10 μM) and PD98059 (50 μM) were added 90 min prior to infection and maintained throughout the infection period.
Preparation of cell lysates for Western blotting and immunoprecipitation.
T84 cells were seeded in 100-mm-diameter petri tissue culture dishes. At 70 to 90% confluence, the monolayers were washed twice with serum-free DMEM/F12 and then depleted in fresh culture medium supplemented with 0.1% bovine serum albumin (Sigma) for 12 h. At various times, the infected cells were washed with PBS and solubilized for 30 min at 4°C in lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P 40 (NP-40), 2 mM Na3VO4, 1 mM EDTA, 1 μM aprotinin, 25 μM leupeptin, 1 μM pepstatin, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 10 mM sodium fluoride, 5 mM NaPPi, 10 mM β-glycerophosphate]. The lysate was sonicated and centrifuged at 15,000 rpm for 15 min at 4°C. The protein content of the supernatant was determined using Bio-Rad DC reagents.
Immunoprecipitation of Shc.
One milligram of precleared whole extracts from control and infected cells were incubated with 2 μg of anti-Shc antibody (UBI, Souffelweyersheim, France) for 12 h at 4°C. The immunocomplexes were precipitated by incubation with protein A-Sepharose (Amersham Pharmacia Biotech, Saclay, France) for 1 h at 4°C. Immunopellets were washed four times in PBS with 0.5% NP-40 and once in PBS and then eluted with 40 μl of reduced Laemmli buffer at 100°C for 5 min.
Western blotting.
Equal amounts (50 μg) of whole-cell lysates (WCL) and immunoprecipitates were subjected to electrophoresis on a sodium dodecyl sulfate (SDS)–9% polyacrylamide gel. The proteins were transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore) and incubated overnight at 4°C with biotinylated antiphosphotyrosine (Upstate Biotechnology Incorporated). After three washes with TNN (10 mM Tris-HCl [pH 7.4], 0.15 M NaCl, 1% NP-40), the presence of antibodies was revealed with biotin-streptavidin complex (Amersham) and visualized by the Amersham enhanced chemiluminescence detection system.
Analysis of MAP, JNK, and p38 activation.
Equal amounts (50 μg) of WCL from infected, epidermal growth factor (EGF)-stimulated, and control cells were analyzed by Western blotting, as described above, using anti-phospho-ERK1/2, anti-phospho-JNK, or anti-phospho-p38 rabbit antibodies (New England Biolabs) and horseradish peroxidase-2 conjugated anti-rabbit antibodies (New England Biolabs).
Statistical analysis.
Results are presented as the mean ± standard error of the means. (SE). Statistical significance was determined by the analysis of variance with the StatView program for Macintosh, followed by post hoc comparison with the Bonferroni/Dunn tests.
RESULTS
Induction of tyrosine phosphorylation in T84 cells infected by WT EPEC.
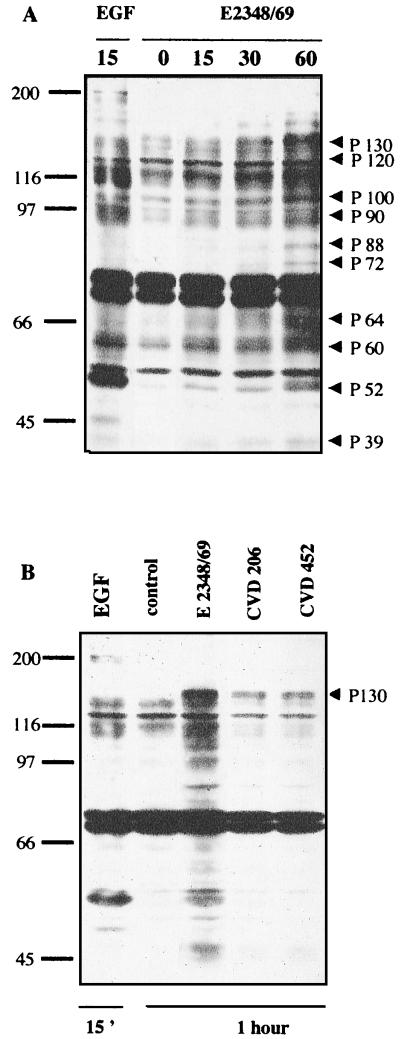
The possibility of tyrosine phosphorylation of cellular proteins in T84 cells infected by the WT EPEC strain E2348/69 was investigated by infecting cells for various times with this strain, followed by antiphosphotyrosine Western blotting analysis of the WCL (Fig. 1A). A few tyrosine-phosphorylated proteins were detected in cells after 15 or 30 min of infection compared to noninfected control cells. However, most of the tyrosine-phosphorylated proteins were visualized only after a 1-h incubation of T84 cells with the WT EPEC strain. Unexpectedly, the antiphosphotyrosine antibodies reacted strongly with a protein migrating at 52 to 46 kDa. This protein presented the same electrophoretic mobility as the major phosphorylated protein in EGF-treated cells.
FIG. 1.
Induction of protein tyrosine phosphorylation in T84 cells infected with EPEC. (A) T84 cells were lysed at various times after infection. Samples were resolved by electrophoresis on an SDS–9% polyacrylamide gel and analyzed by immunoblotting using antiphosphotyrosine antibody. In both panels, the positions of molecular weight standards are shown at the left in kilodaltons. The putative T84 cell-induced tyrosine-phosphorylated proteins are indicated by arrowheads. (B) T84 cells were infected for 1 h with WT strain E2348/69 and mutant strains CVD206 and CVD452. Samples were resolved as described above. The mutant-induced tyrosine phosphorylated protein of 130 kDa is indicated by an arrowhead. The control lane corresponded to uninfected cells. The positive control lane was obtained from cells treated with EGF (10 nM, 15 min). Identical results were obtained at least five times.
To verify whether activation of a cellular tyrosine kinase(s) participates in the pathogenic phenotype of EPEC, we investigated the ability of E2348/69-derived mutants CVD206 and CVD452 to induce tyrosine phosphorylation in T84 cells. The genetic characteristics of these mutants and their cellular responses to infection are described in Table 1. CVD206 was obtained by deleting the eaeA gene from the WT strain E2348/69 (10), and CVD452 was mutated in the type III secretion system (20). As shown in Table 1, mutant CVD452 had no effect on TER and mutant CVD206 induced a small decrease in TER. In contrast to the WT strain E2348/69, the eaeA and escN mutants did not stimulate IL-8 secretion in T84 cells after 6 h of infection and also failed to induce actin accumulation in host cells. Antiphosphotyrosine Western blotting revealed that only a 130-kDa protein was phosphorylated in T84 cells infected for 1 h with strains CVD206 and CVD452 (Fig. 1B). Other phosphorylated proteins with apparent sizes of 120, 100, 90, and 60 kDa present in WT EPEC-infected cells were absent in cells infected by these mutants. These results indicate that both adhesion and signal transduction by the type III secretion system are necessary to induce tyrosine phosphorylation in T84 cells.
WT EPEC induces tyrosine phosphorylation of Shc during infection of T84 cells.
To verify that the phosphorylated proteins of 46 to 52 kDa induced in response to E2348/69 infection corresponded to members of the Shc family, a major target of EGF receptor kinase (35), immunoprecipitation experiments were performed with anti-Shc antibodies followed by Western blotting with antiphosphotyrosine antibody. As shown in Fig. 2, p46 and p52 Shc isoforms were effectively tyrosine phosphorylated in cells exposed for 1 h to the WT strain E2348/69 and 15 min to EGF, used as a positive control. In contrast, Shc was not phosphorylated in cells infected by the EPEC strains carrying a mutation on the intimate adhesion factor (CVD206) or on signal transduction pathways (CVD452). This result strongly supports the hypothesis that a correlation exists between Shc activation and expression of the pathogenic phenotype of EPEC.
FIG. 2.
Effect of WT and various EPEC mutant strains tyrosine activation of p46 and p52 Shc isoforms. Lysates from uninfected T84 cells (control) and cells infected for 1 h with WT strain E2348/69 or mutant strains CVD206 and CVD452 were immunoprecipitated with anti-Shc antibody. The immunocomplex was subjected to electrophoresis on an SDS–9% polyacrylamide gel and analyzed by immunoblotting using antiphosphotyrosine antibodies. The positive control was obtained from cells treated with EGF (10 nM, 15 min). Identical results were obtained at least three times.
Activation of the ERK1/2 pathway is related to E2348/69 pathogenicity.
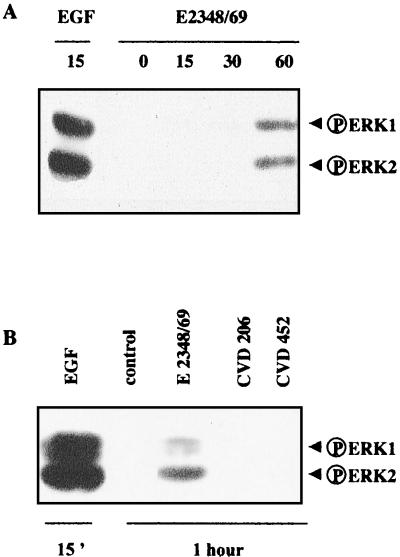
As phosphorylated Shc is an upstream regulatory protein of the ERK1/2 MAP kinase pathway, we investigated the possible activation of the ERK1/2 pathway in E2348/69-infected cells. The time course of ERK1/2 activation was assessed at different times of infection by Western blotting using an antibody that specifically recognizes the dually phosphorylated ERK1/2 forms (Fig. 3A). The active forms of ERK1/2 (p42 and p44) were undetectable in control cells but appeared after a 1-h exposure of T84 cells to E2348/69 bacteria. Western blotting using antibodies recognizing the total amount of ERK1/2 revealed the presence of the same quantity of ERK1 and ERK2 in the analyzed samples (data not shown). Interestingly, activated ERK1/2 forms were not detected in lysates extracted from T84 cells infected for 1 h with strain CVD206 or CVD452 (Fig. 3B). These data suggest that activation of the ERK1/2 pathway is related to the pathogenic phenotype of the WT strain.
FIG. 3.
Activation of ERK1/2 in E2348/69 infected cells. (A) T84 cells were lysed at various times after infection. Samples were resolved by electrophoresis on an SDS–9% polyacrylamide gel and analyzed by immunoblotting using antiphosphorylated ERK1/2 antibodies. (B) T84 cells were infected for 1 h with the WT strain E2348 or with EPEC mutant strain CVD206 or CVD452. Samples were resolved as described above. The control lane corresponds to uninfected T84 cells. The positive control was obtained from cells treated with EGF (10 nM, 15 min). Identical results were obtained at least four times.
WT EPEC strain activates p38 and JNK.
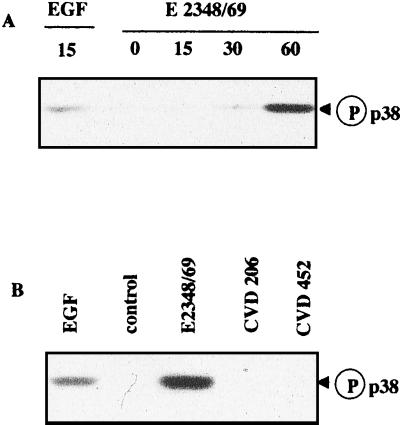
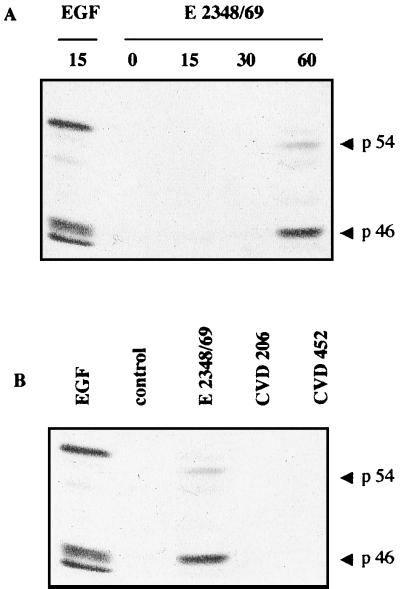
Activation of p38 and JNK MAP kinases has been found in epithelial cells infected by S. enterica serovar Typhimurium and L. monocytogenes (19, 41). We thus investigated the effect of EPEC infection on the activation of these two pathways. The kinetics of p38 and JNK activation after various lengths of infection was assessed by Western blotting using antibodies directed against their phosphorylated forms (Fig. 4A and 5A, respectively). The active forms of p38 and JNK (p46 and p54) appeared after a lag of 1 h following E2348/69 infection, concomitant with activation of the ERK1/2 pathway. Consistent with the above-reported results for ERK1/2, strains CVD206 and CVD452 were ineffective in activating p38 (Fig. 4B) or JNK (Fig. 5B).
FIG. 4.
Activation of p38 MAP kinases in E2348/69-infected cells. (A) T84 cells were lysed at different times after infection. Samples were resolved by electrophoresis on an SDS–9% polyacrylamide gel and analyzed by immunoblotting using anti-phosphorylated p38 antibodies. (B) T84 cells were infected for 1 h with the WT strain E2348/69 and EPEC mutant strains CVD206 and CVD452. Samples were resolved as described above. The control lane corresponds to uninfected T84 cells. The positive control was obtained from cells treated with EGF (10 nM, 15 min). Identical results were obtained at least three times.
FIG. 5.
Activation of JNK in E2348/69 infected cells. (A) T84 cells were lysed at various times after infection. Samples were resolved by electrophoresis on an SDS–9% polyacrylamide gel and analyzed by immunoblotting using anti-phosphorylated JNK antibodies. (B) T84 cells were infected for 1 h with the WT strain E2348/69 and EPEC mutant strains CVD206 and CVD452. Samples were resolved as described above. The control lane corresponds to uninfected T84 cells. The positive control was obtained from cells treated with EGF (10 nM, 15 min). Identical results were obtained at least two times.
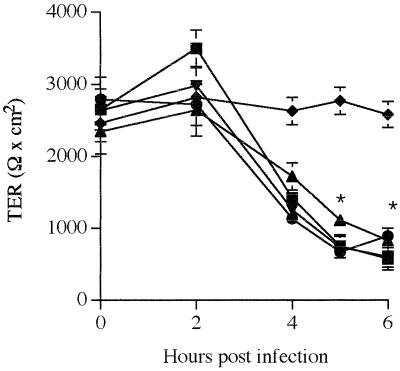
Inhibition of ERK1/2 and p38 does not prevent the EPEC-induced decrease of T84 monolayer resistance.
In contrast to CVD452, which had no effect, and CVD206, which had a small effect, on TER (Table 1), the WT EPEC strain induced a dramatic decrease in TER, reflecting a modification of tight junction-associated proteins (31). Since CVD206 and CVD452 did not activate ERK1/2 and p38, we speculated that MAP kinases might be implicated in the cellular process related to modification of the tight junction structure. For this purpose, T84 monolayers were apically infected with strain E2348/69 alone or in the presence of 10 μM SB203580, a p38 MAP kinase inhibitor (8), and 50 μM PD98059, a highly selective inhibitor of the ERK1/2 pathway (1). We first verified that at these concentrations the two types of inhibitors administered alone or together had no effect on either E2348/69 or T84 viability (data not shown). We also verified that DMSO had no effect on monolayer resistance during the incubation periods tested. As shown in Fig. 6, monolayer resistance in EPEC-infected cells remained unchanged for up to 4 h and then dropped dramatically after 5 h (P < 0.02 versus control monolayers). When infection was performed in the presence of either SB203580 or PD98059, TER was not affected compared to cells infected with EPEC alone, indicating that activation of ERK1/2 and p38 MAP kinases was probably not involved in modification of the barrier function in EPEC-infected T84 cells.
FIG. 6.
MAP kinase inhibitors have no effect on the EPEC-induced decrease of TER in T84 monolayers. Bacteria (100/cell) were added to the apical surface of T84 cells. Resistance decreased in cells infected with the WT strain E2348/69 (■) compared to control monolayers (⧫). When indicated, the inhibitors PD98059 (●) and SB203580 (▴), alone or together (▾), were added to the cell medium 90 min prior to infection, and the inhibitors were present during the infection. In cells treated with inhibitors, TER remained comparable to that in EPEC-infected cells. Each point represents the mean value obtained from at least six individual T84 monolayers. Error bars show the standard deviation. ∗ denotes significantly different from control monolayers (P < 0.02) when compared by the Bonferroni/Dunn tests.
Activation of ERK1/2 and p38 is not required for pedestal formation in T84 cells infected by the WT strain E2348/69.
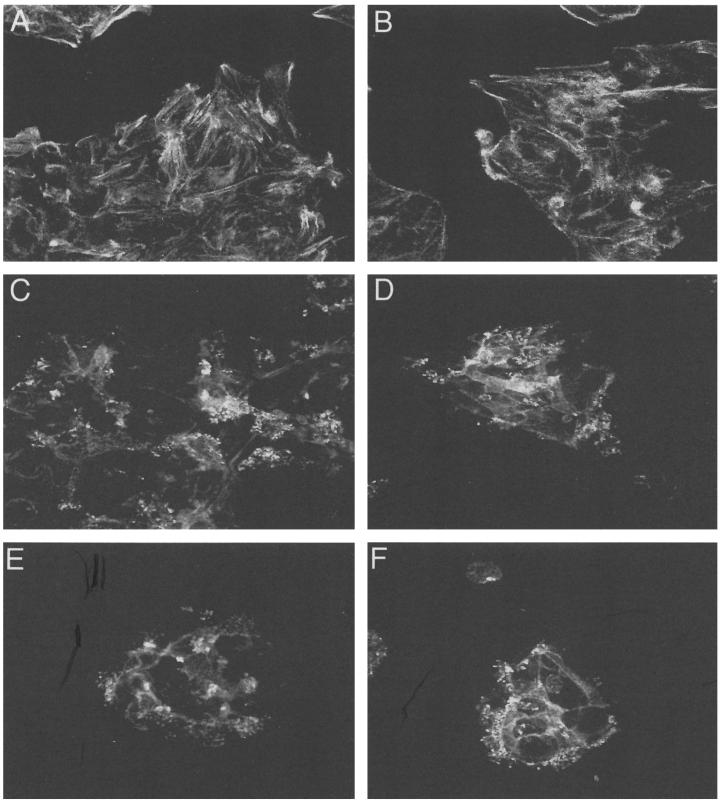
One manifestation of EPEC infection is the modification of cytoskeletal components (actin, ezrin, and talin) situated beneath the adherent bacteria and forming a cuplike pedestal (15, 24). In contrast to mutants CVD206 and CVD452, which failed to form A/E lesions in T84 cells (Table 1), E2348/69 induced actin accumulation in the zone of the bacterial attachment site (Fig. 7C). We thus investigated whether ERK1/2 and p38 MAP kinase activation plays a role in EPEC-induced actin polymerization by cell treatment with the inhibitors PD98059 (50 μM) and SB203580 (10 μM). Incubation of cells with PD98059 (Fig. 7D) or SB203580 (Fig. 7E), alone or together (Fig. 7F), prior to infection and during infection had no effect on the formation of A/E lesions by EPEC. This demonstrates that the ERK1/2 and p38 MAP kinases pathways did not participate in the cytoskeletal reorganization accompanying EPEC infection.
FIG. 7.
Rearrangement of actin filaments in T84 cells upon infection with E2348/69 is not affected by treatment with MAP kinase inhibitors. FITC-phalloidin was used to localize actin filaments in control cells without (A) or with (B) DMSO, EPEC-infected T84 cells (C), and cells treated with PD98059 (D) or SB203580 (E), alone or together (F), before and during infection. Original magnification, ×2,400. Identical results were obtained at least three times.
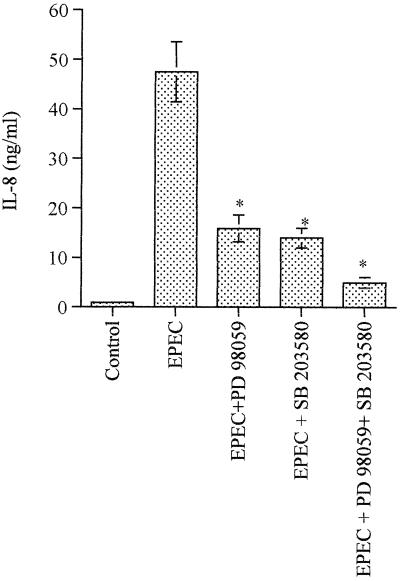
Activation of ERK1/2 and p38 MAP kinases is implicated in EPEC-induced IL-8 expression.
EPEC infection of host intestinal epithelia has been shown to initiate an inflammatory cascade by activating IL-8 transcription (38). Given that CVD206 and CVD452 did not induce IL-8 synthesis (Table 1) and that activation of p38 MAP kinase has been implicated in Salmonella-induced cytokine expression (19), we sought to determine the implication of p38 and ERK1/2 pathways in EPEC-induced expression of IL-8. For this purpose, T84 cells were preincubated for 90 min with PD98059 and SB203580, alone or together. The inhibitors were present during the 6-h infection with WT strain E2348/69. As shown in Fig. 8, SB203580 and PD98059 reduced IL-8 expression by 70%. It is noteworthy that the concomitant presence of the two inhibitors decreased EPEC-induced IL-8 synthesis by 90%, indicating that p38 and ERK1/2 MAP kinase pathways are both required for IL-8 expression.
FIG. 8.
Both ERK1/2 and p38 MAP kinases are involved in EPEC-induced IL-8 synthesis in T84 cells. IL-8 content was estimated in the supernatant of T84 cells after 6 h of infection with WT strain E2348/69. When indicated, the inhibitors PD98059 and SB203580, alone or together, were added to the cell medium 90 min prior to infection, and inhibitors were present during the infection. Error bars show the standard deviation. ∗ denotes significantly different from EPEC-infected monolayers (P < 0.02) when compared by the Bonferroni/Dunn tests.
DISCUSSION
EPEC strains have been shown to induce tyrosine phosphorylation in host epithelial cells (34). Rosenshine et al. (34) reported the tyrosine phosphorylation of three proteins in HeLa cells infected with EPEC, including a major phosphorylated substrate, the 90-kDa protein (Hp90) subsequently identified as Tir, and two minor tyrosine-phosphorylated proteins of 39 kDa (Hp39) and 72 kDa (Hp72). Consistent with these findings, we observed tyrosine-phosphorylated proteins of 90, 72, and 39 kDa in EPEC-infected T84 cells in our study. Interestingly, we found that the WT EPEC strain (E2348/69) could also trigger the tyrosine phosphorylation of several others proteins with apparent sizes of 130, 120, 100, and 40 to 50 kDa; in contrast to HeLa cells (34), Hp90/Tir was not the major phosphorylated substrate in T84 cells. This discrepancy may be related to the different cell model used. Moreover, in HeLa cells, Hp90/Tir was phosphorylated by the mutant CVD206 (34). As shown in this study, the CVD206 and CVD452 mutants failed to induce tyrosine phosphorylation of most proteins in T84 cells, with the exception of the 130-kDa protein, indicating that bacterial adhesion and secretion of bacterial protein through the type III secretion system are crucial for the triggering of tyrosine kinase(s).
We identified one major phosphorylated 46- to 52-kDa substrate in WT EPEC-infected T84 cells as Shc. In a previous study, we reported that EPEC induces ERK1/2 MAP kinase activation in T84 cells (9). Given that Shc, once phosphorylated, is known to act as an upstream regulatory protein of the ERK1/2-MAP kinase pathway (35), we investigated the possible correlation between Shc phosphorylation and the activation of the ERK1/2 MAP pathway induced by EPEC in T84 cells. Time course experiments showed that Shc phosphorylation and ERK1/2 activation occurred concomitantly, 1 h after the beginning of infection. Interestingly, when T84 cells were infected with EPEC mutant CVD206 or CVD452, Shc was not tyrosine phosphorylated and ERK1 and -2 were not activated, supporting a causal relationship between the two events. The activation of the ERK1/2 pathway by the WT EPEC strain prompted us to investigate the activation of other MAP kinase pathways. As demonstrated in this study, EPEC-induced tyrosine phosphorylation and ERK1/2 activation occurred simultaneously with the activation of p38 and JNK, two other MAP kinases congeners.
Activation of the MAP kinases pathways has been investigated previously, mainly in macrophages, in which these signaling pathways are induced by the invasive bacteria S. enterica serovar Typhimurium, L. monocytogenes, and Yersinia pseudotuberculosis (25, 30, 32). MAP kinases appear to play a key role in bacterial internalization, intramacrophage survival, and modulation of cytokine responses. Epithelial cells form the mucosal barrier and are thus the first targets of microbial attacks. Activation of MAP kinases in epithelial cells has been reported only in response to infection by S. enterica serovar Typhimurium and L. monocytogenes (19, 41). Implication of these signaling pathways in EPEC infections, characterized by A/E lesion formation, has not been investigated before. Lipopolysaccharide (LPS), a component of gram-negative bacteria, has been shown to activate MAP kinase pathways in macrophages (42). This activation involves the macrophage receptor CD14, which is not expressed in epithelial cells. Recently, Cario et al. (5) reported that LPS can stimulate the three MAP kinases in intestinal epithelial cells. The Toll-like receptors present at the cell surface of T84 cells appear to mediate LPS stimulation of ERK1/2 and JNK in these cells (5). We thus cannot rule out LPS participation in the activation of ERK1/2 and JNK in response to EPEC infection. However, LPS failed to stimulate the p38 pathway in T84 cells (5), which is activated by the WT EPEC strain. As reported in this study, mutants CVD206 and CVD452, which are not affected in LPS expression, do not activate the ERK1/2, p38, or JNK pathway. Bacterial factors other than LPS are therefore necessary to trigger host cell responses.
In this study, we provide evidence that intimate adhesion of EPEC to T84 cells is necessary for activation of the ERK1/2, p38, or JNK pathway, since mutants CVD206 and CVD452, which attach only in a nonintimate fashion, failed to activate these signaling pathways. Bacterial adhesion has been reported to be essential in MAP kinase activation in gram-positive and gram-negative bacteria. L. monocytogenes presents a surface protein called internalin that is required for invasion into a variety of cultured cell lines. Internalin-deficient mutants, which are unable to adhere efficiently to host cells, do not trigger MAP kinase activation (41). Activation of ERK1/2, p38, and JNK MAP kinases in epithelial cells by S. enterica serovar Typhimurium is strictly dependent on the function of the type III protein secretion system, the activation of which is triggered by adhesion to host cells (17, 19). EPEC also possesses a type III protein secretion system that is activated after bacterial adhesion. The data reported in this study show that only the WT EPEC strain, which expresses localized and intimate adhesion as well as a type III secretion system, allows the concomitant activation of all the three MAP kinase pathways.
The type III secretion system allows the targeting of bacterial protein to host cell membrane or cytoplasm (17). This interaction induces several signaling pathways necessary for the remodeling of the host cell cytoskeletal that is required for pathogen adherence and invasion. For instance, upon adhesion, Salmonella induces membrane ruffling accompanied by profuse macropinocytosis that leads to internalization of the bacteria into host cells (14). The EPEC-induced pedestal is composed of highly organized actin filaments and cytoskeletal components including α-actinin, talin, ezrin, and vinculin (15, 24). Although EPEC and Salmonella use the same signaling components (phospholipase C and Ca2+) to induce cytoskeletal changes, the temporal sequence and spatial colocalization of these signaling events in relation to EPEC-induced pedestal formation are not clearly established (3, 16, 36). The dramatic rearrangements of the actin cytoskeleton observed during bacterial infection suggest the involvement of a small GTP-binding protein family. Chen et al. (6) demonstrated that a Rho subfamily member, Cdc42, is required to mediate Salmonella uptake through membrane ruffling. In contrast, inhibition of Rho, Rac, and Cdc42 by compactin and Clostridium difficile toxin B has no effect on EPEC-induced pedestal formation (4). These results suggest that EPEC use a nontraditional mechanism to rearrange actin. To define the role of MAP kinases in the control of this process, we used PD98059 and SB203580, two specific inhibitors of ERK1/2 and p38, respectively. The absence of effect of these two drugs on pedestal formation leads us to conclude that neither the ERK1/2 nor the p38 pathway is implicated in this process. This observation was confirmed by the use of mutant strains CVD206 and CVD452, which failed to activate these pathways and also failed to induce modification of actin distribution.
Infection of T84 cells with EPEC alters barrier function that results from modification of the tight junction structure (31). Regulation of tight junction permeability occurs at many sites within the epithelial cell, involving the phosphorylation of tight junction-associated protein (7). To date, phosphorylation of myosin light chain in EPEC-infected T84 cells has been directly implicated in this process (44). We thus investigated whether tight junction-associated proteins might be targets for ERK1/2 or p38 MAP kinase. We provide evidence that inhibition of the ERK1/2 and p38 pathways did not affect the drop in TER observed during EPEC infection, ruling out the possible participation of these signaling pathways in the regulation of epithelial permeability. This observation was confirmed by the use of mutant strains: CVD452 did not modify TER, and CVD206 induced only a small decrease in transepithelial resistance that, in contrast to results reported by others authors, did not reach a half-of-wild-type decrease in TER (37). We explain this discrepancy by differences in the infection procedure, i.e., (i) the multiplicity of infection and (ii) the use of bacteria that were not (in this study) or were activated in DMEM/F12. The expression of adherence factors and secretion of virulence factors by EPEC have been reported to be medium dependent (23, 43).
Activation of the p38 MAP kinase pathway has been implicated in the synthesis of IL-8 by epithelial cells in response to S. enterica serovar Typhimurium-infection (19). EPEC infection of T84 cells has been shown to induce IL-8 secretion, which participates in the recruitment and transmigration of polymorphonuclear leukocytes (PMN) (38). We thus investigated a possible implication of the ERK1/2 and p38 MAP kinase pathway in the synthesis of IL-8. Treatment of cells with PD98059 or SB203580 decreased IL-8 synthesis in EPEC-infected T84 cells by 70%. Abrogation of IL-8 synthesis in EPEC-infected cells necessitated cotreatment with both inhibitors. This result indicated that both ERK1/2 and p38 MAP kinases are implicated in IL-8 synthesis. This result was confirmed by the use of mutant strains CVD206 and CVD452. These strains, which failed to activate the ERK1/2 and p38 MAP kinases pathways, also failed to stimulate IL-8 production. Mutant strain CVD206 has been shown to stimulate maximal transmigration of PMN (37), whereas in our study it did not stimulate IL-8. This observation supports the hypothesis that IL-8 probably does not fully account for PMN chemotaxis associated with EPEC infection.
The use of mutant strains to explore the implication of MAP kinase in biological responses to EPEC infection rules out any possible secondary toxic effect of the pharmacological inhibitors of MAP kinases on cell viability.
In conclusion, we demonstrated that EPEC strains induce concomitant activation of ERK1/2, p38, and JNK MAP kinases. The modification of TER and the formation of A/E lesions occur independently of ERK1/2 and p38 MAP kinases, but these signaling pathways are implicated in cytokine responses induced by EPEC, indicating that the signaling pathways involved in these events are distinct.
ACKNOWLEDGMENTS
S.D. and B.M. participated equally in this work.
This study was supported by Laboratoires BIOCODEX, the Région Provence-Alpes Côte d'Azur, the Conseil Général des Alpes Maritimes, the Faculté de Médecine de l'Université de Nice-Sophia Antipolis, and the Centre Hospitalier Régional de Nice.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Andrade J R C, Da Viega V D, De Santa Rosa M R, Suassuna I. An endocytic process in Hep-2 cells induced by enteropathogenic Escherichia coli. J Med Microbiol. 1989;28:49–57. doi: 10.1099/00222615-28-1-49. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin T J, Ward W, Aitken A, Knutton S, Williams P H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Ami G, Ozeri V, Hanski E, Hofmann F, Aktories K, Hahn K M, Bokoch G M, Rosenshine I. Agents that inhibit Rho, Rac, and Cdc42 do not block formation of actin pedestals in HeLa cells infected with enteropathogenic Escherichia coli. Infect Immun. 1998;66:1755–1758. doi: 10.1128/iai.66.4.1755-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cario E, Rosenberg I M, Brandwein S L, Beck P L, Reinecker H-C, Podolsky D. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 6.Chen L M, Hobbie S, Galan J E. Requirement of CDC42 for Salmonella typhimurium-induced cytoskeletal reorganization and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- 7.Collares-Buzato C B, Jepson M A, Simmons N L, Hirst B H. Increased tyrosine phosphorylation causes redistribution of adherence junction and tight junction proteins and perturbs paracellular barrier function in MDCK epithelia. Eur J Cell Biol. 1998;76:85–92. doi: 10.1016/S0171-9335(98)80020-4. [DOI] [PubMed] [Google Scholar]
- 8.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. SB203580 is a specific inhibitor of MAP kinase homologue which is stimulated by cellular stress and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 9.Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Saccharomyces boulardii preserves barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun. 2000;68:5998–6004. doi: 10.1128/iai.68.10.5998-6004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnenberg M S, Donohue-Rolfe A, Keusch G T. Epithelial cell invasion: overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989;160:452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- 11.Dytoc M T, Fedorko L, Sherman P M. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology. 1994;106:1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 12.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y, Lai L-C, Namara B P, Donnenberg M S, Kaper J. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 13.Finlay B B, Falkow S. Salmonella interaction with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 14.Finlay B B, Ruschkovsky S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 15.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC) triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galan J, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 18.Garrington T P, Johnson G L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 19.Hobbie S, Chen L M, Davis R J, Galan J E. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 20.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny B, DeVinney R, Stein M, Reinschied D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 23.Kenny B, Abe A, Stein M, Finlay B. Enteropathogenic Escherichia coli protein secretion in response to conditions similar to those in the gastrointestinal tract. Infect Immun. 1997;65:2606–2612. doi: 10.1128/iai.65.7.2606-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kugler S, Schuller S, Goebel W. Involvement of MAP-kinases and -phosphatases in uptake and intracellular replication of Listeria monocytogenes in J774 macrophage cells. FEMS Lett. 1997;157:131–136. doi: 10.1111/j.1574-6968.1997.tb12763.x. [DOI] [PubMed] [Google Scholar]
- 26.Levine M M, Nalin D, Hornick R, Berquist E, Waterman D, Young C, Sotman S, Rowe B. Escherichia coli strains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are not invasive. Lancet. 1978;i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 27.Manjarrez-Hernandez H A, Baldwin T J, Aitken A, Knutton S, Williams P H. Intestinal epithelial cell protein phosphorylation in enteropathogenic Escherichia coli diarrhoae. Lancet. 1992;339:521–523. doi: 10.1016/0140-6736(92)90340-9. [DOI] [PubMed] [Google Scholar]
- 28.Moon H W, Whipp S C, Argenzio R A, Levine M M, Gianella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nataro J, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-a production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 31.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;33:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 32.Proczyk K J, Kovarik P, von Gabain A, Baccarini M. Salmonella typhimurium and lipopolysaccharide stimulate extracellularly regulated kinase activation in macrophages by a mechanism involving phosphatidylinositol 3-kinase and phospholipase D as novel intermediates. Infect Immun. 1999;67:1011–1017. doi: 10.1128/iai.67.3.1011-1017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosa A C, Mariano A T, Tibana A, Gomes T A, Andrade J R. Enteropathogenicity markers in Escherichia coli isolated from infants with acute diarrhoea and healthy controls in Rio de Janeiro, Brazil. J Med Microbiol. 1998;47:781–790. doi: 10.1099/00222615-47-9-781. [DOI] [PubMed] [Google Scholar]
- 34.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruff-Jamison S, McGlade J, Pawson T, Chen K, Cohen S. Epidermal growth factor stimulates the tyrosine phosphorylation of SHC in the mouse. J Biol Chem. 1993;168:7610–7612. [PubMed] [Google Scholar]
- 36.Ruschkowski S, Rosenshine I, Finlay B B. Salmonella typhimurium induces an inositol phosphate flux in infected epithelial cells. FEMS Microbiol Lett. 1992;74:121–126. doi: 10.1016/0378-1097(92)90416-l. [DOI] [PubMed] [Google Scholar]
- 37.Savkovic S, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savkovic S, Koutsouris A, Hecht G. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 39.Scaletsky I, Silva M L, Trabulski L R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal monolayers diminishes barrier function. Am J Physiol. 1995;31:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 41.Tang P, Sutherland C L, Gold M R, Finlay B B. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect Immun. 1998;66:1106–1112. doi: 10.1128/iai.66.3.1106-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinstein S L, Sanghera J S, Lemke K, DeFranco A L, Pelech S L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992;267:14955–14962. [PubMed] [Google Scholar]
- 43.Vuopio-Varkila J, Scholnik G K. Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J Exp Med. 1991;174:1161–1167. doi: 10.1084/jem.174.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuhan R, Koutsouris A, Savkovic S D, Heicht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gasteroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]