Abstract
The ability of the maternally transmitted endosymbiotic bacterium Wolbachia to induce cytoplasmic incompatibility (CI) and virus blocking makes it a promising weapon for combatting mosquito-borne diseases through either suppression or replacement of wild-type populations. Recent field trials show that both approaches significantly reduce the incidence of dengue fever in humans. However, new questions emerge about how Wolbachia-mosquito associations will co-evolve over time and whether Wolbachia-mediated virus blocking will be affected by the genetic diversity of mosquitoes and arboviruses in the real world. Here, we have compared the Wolbachia density and CI expression of two wAlbB-infected Aedes aegypti lines transinfected 15 years apart. We have also assessed wAlbB-mediated virus blocking against dengue (DENV), Zika (ZIKV), and Chikungunya (CHIKV) viruses and examined whether host genetic backgrounds modulate viral blocking effects by comparing ZIKV infection in mosquitoes with a Mexican genetic background to those with a Singaporean background. Our results show that over 15 years, wAlbB maintained the capacity to form a stable association with Ae. aegypti in terms of both density and CI expression. There were variations in wAlbB-induced virus blocking against CHIKV, DENV, and ZIKV, and higher inhibitory effects on ZIKV in mosquitoes on the Singaporean genetic background than on the Mexican background. These results provide important information concerning the robustness and long-term stability of Wolbachia as a biocontrol agent for arbovirus disease control.
Significance Statement.
The successful implementation of Wolbachia for combating mosquito-borne arbovirus diseases relies on a stability of artificial Wolbachia transinfection in target mosquito vectors, with a high virus blocking induced in mosquitoes on various genetic backgrounds across different geographic populations. The present studies showed that the Wolbachia wAlbB strain maintained a stable association with Ae. aegypti over 15 years and virus blocking effects varied among different arboviruses and host genetic backgrounds. Given the ongoing Wolbachia release in multiple countries, these results provide important information to guide the development of optimal Wolbachia release strategies for disease control, highlighting a potential for global deployment of Wolbachia in a region and context specific manner.
Introduction
Both the distribution range of mosquito vectors and the prevalence of mosquito-borne diseases have rapidly increased because of global warming in recent decades (1–3). A highly effective, sustainable, and environmentally-friendly vector control strategy is urgently needed because of the insufficiency of traditional approaches. Recently, significant efforts have been made to develop Wolbachia-based approaches to either reduce the mosquito’s ability to transmit pathogens through population replacement or to suppress the mosquito density below the epidemic risk threshold through population suppression (4–8). Successful field trials have shown that Wolbachia-based population replacement has reduced dengue incidence by 77.1% and hospitalization by 86.2% in Indonesia, among several other countries (4, 7, 9), and population suppression has been shown to produce strong suppression or even elimination of Aedes mosquito vectors in target areas in multiple countries, with dengue incidence being reduced by 71% to 88% in Singapore (5, 8, 10–12).
Estimated to infect more than 65% of all insect species, Wolbachia is a maternally transmitted endosymbiotic bacterium belonging to the order Rickettsiales (13). It is known for its ability to induce cytoplasmic incompatibility (CI), a phenomenon involving early embryonic death that occurs when the Wolbachia-infected male mates with either an uninfected female or a female carrying a different strain of Wolbachia (14). Based on CI, a conditional sterility can be induced in the field by releasing the incompatible males to mate with naturally uninfected wild-type females, resulting in population suppression. CI also provides a reproductive advantage to Wolbachia-infected females as compared to uninfected females, since infected females can produce infected offspring after mating with both infected and uninfected males, whereas uninfected females can reproduce only if they mate with uninfected males. With Wolbachia frequencies surpassing a critical equilibrium determined by fitness costs (15), CI would facilitate the invasion and spread of Wolbachia into uninfected populations and eventually causes the population infected at high frequency, triggering population replacement (16–18).
Multiple Wolbachia strains are able to induce pathogen blocking in mosquitoes (19–22), thus enabling population replacement to reduce pathogen transmission between mosquitoes and humans. Aedes aegypti, the primary dengue vector, does not carry the native Wolbachia infection (23), whereas Ae. albopictus, another important vector of arboviruses, is naturally infected by Wolbachia (24). Both can be transinfected by transfer of Wolbachia strains from other insect hosts via embryonic microinjection (8, 17). A hallmark of successful transinfection in mosquitoes is a stable maternal transmission of Wolbachia at 100% efficiency, a property critical for maintaining a high Wolbachia infection frequency in the field, and for quality production of Wolbachia-infected male mosquitoes for the suppression approach. In addition to maintaining the ability to induce CI, artificial Wolbachia infection in transinfected Ae. aegypti can inhibit a variety of arboviruses, including dengue (DENV), Zika (ZIKV), and Chikungunya (CHIKV) viruses (20). Evidence indicates that the strength of the Wolbachia-mediated viral interference is often correlated with the density of Wolbachia in the somatic tissues, such as the midguts and salivary glands, of transinfected mosquitoes (25). Although not yet fully understood in this context, immune priming and altered metabolism are the two physiological changes that could contribute to the underlying mechanism of Wolbachia-mediated viral blocking (26, 27). Depending on the Wolbachia strain, a transinfected mosquito can show strong, moderate, or no resistance to arboviruses (28–30). While the wMel strain, a complete CI inducer in transinfected mosquito but weak CI inducer in its original Drosophila host (31, 32), has been successfully demonstrated to reduce dengue incidence in field trial, evidence indicates that wAlbB and wMelCS outperform wMel in reducing the viral transmission potential according to viral loads in the mosquito saliva (21). The strength of Wolbachia-mediated viral inhibition can also be affected by both the host and viral genetic backgrounds. Multigenerational artificial selection with regard to pathogen blocking for DENV in wMel-infected Ae. aegypti has resulted in a significant divergence of mosquito populations with either low or high virus blocking, which is associated with single nucleotide polymorphisms of mosquito genes on chromosome 1 (33). Wolbachia-mediated viral blocking has been found to vary among the four DENV serotypes, with inhibition of DENV-1 being consistently less effective than the others (21, 30, 34, 35). Furthermore, evidence indicates global genetic diversity of Ae. aegypti and geographic variation in vector competence (36, 37), likely contributing to the variation of Wolbachia-mediated blocking in these mosquitoes. Accordingly, it is unclear how the efficacy of disease control will be affected by variation in Wolbachia-mediated viral blocking resulting from genetic diversity in mosquitoes and arboviruses in the real world that Wolbachia will encounter when deployed across a global landscape in the future. These emerging pieces of evidence call for an in-depth characterization of the impact of host and virus genomes on viral blocking, knowledge that is essential for widespread use of the replacement strategy.
As the first Wolbachia strain established in both Ae. aegypti and Anopheles mosquitoes, wAlbB induces complete CI, as observed in its original host, Ae. albopictus (38), and strong pathogen blocking in transinfected lines (17, 19, 39, 40). Recent studies have shown that wAlbB is more stable at high temperature than wMel in Ae. aegypti, leading to the proposed release of wAlbB in areas where it is challenging for wMel to establish infection because of its high susceptibility to extreme summer temperatures and wide yearly temperature ranges (41–43). Based on analysis of the wAlbB whole-genome sequence, only four single nucleotide variants have occurred over 15 years of transinfection (43). A benefit of this stable performance is that wAlbB-infected Ae. aegypti have been effectively mass-reared to produce incompatible males for release into the field for population suppression in the United States, Singapore, Australia, and Mexico (5, 6, 11, 12, 44). Release of wAlbB-infected Ae. aegypti for population replacement has also resulted in a significant reduction in dengue incidence in Malaysia (45). After being introduced into various mosquito genetic backgrounds through outcrosses, wAlbB maintains a stable association with Ae. aegypti (43, 45–47). However, one study has shown that the impact of wAlbB on mosquito life traits depends on the host’s genetic background (47), whereas another study has shown that the effects are consistent across two mosquito backgrounds (43). While a recent study provides extensive data showing a decade of stability for wMel in the field after release (48), evidence has also been accumulating over the last few years for wAlbB to explore the long-term stability of the transinfection for disease control (43). Questions remain to fully explore are whether the titers of the artificial Wolbachia infections will attenuate over time and, if so, how quickly that attenuation will occur, resulting in a breakdown of mediated viral blocking or CI expression. One way which has not yet been done to address them is direct comparisons between old lines and ones with a more novel association.
We developed the first wAlbB-infected Ae. aegypti line, WB1, in 2005 (17). Fifteen years later, to test the stability of the wAlbB–Ae. aegypti association, we repeated this transinfection assay to introduce wAlbB from Ae. albopictus into Ae. aegypti and generate the WB2 line. Phenotypic comparisons between WB1 and WB2 have now demonstrated that over the course of those 15 years, wAlbB maintained the capacity to have a stable density and perfect maternal transmission efficiency and induce strong CI in the natural Waco genetic background. We also found that wAlbB induced a significant inhibitory effect against DENV, ZIKV, and CHIKV in Ae. aegypti. In addition, wAlbB-infected Ae. aegypti with a Singaporean genetic background exhibited a stronger viral blocking than did those on a Mexican genetic background, indicating an impact of mosquito host genetic background on Wolbachia-mediated virus blocking. These results demonstrate the stability and robustness of wAlbB transinfection in Ae. aegypti, with the maintenance of properties critical for the suppression of the Ae. aegypti mosquito population and viral blocking ability for reducing arbovirus transmission.
Results
Generation of the Ae. aegypti WB2 line with a wAlbB infection
To examine the long-term stability of wAlbB in Ae. aegypti, we generated an identical wAlbB transinfection in Ae. aegypti to compare it with the WB1 line, which was established in 2005 and had the US (Waco, TX, USA) genetic background (17). The wild-type Ae. albopictus Houston (HOU) line was used as the donor, and the cytoplasm of each individual HOU embryo was transferred to wild-type Ae. aegypti by embryogenic microinjection. Experiments were repeated three times, in each case with approximately 200 to 400 embryos injected. Females (G0) developing from the embryos that survived the microinjection were mated with wild-type males (Waco), then allowed to blood-feed. After their offspring (G1) were produced, the G0 females were sacrificed and screened for Wolbachia infection by PCR assay. In the third experiment, three of the five surviving females were positive for wAlbB infection (Fig. S1a). Only the progeny from the positive females were selected for outcross with Waco males to produce the next generation. Six of the 21 G1 isofemales (28.5%) were observed to carry the wAlbB infection. At G2, 16 of 45 females had wAlbB infections. From them, seven females that showed strong infections were selected to establish the next generation (G3). The subsequent PCR assay showed that all the tested G3 individuals (n = 10) carried wAlbB. This transinfected line is hereafter referred to as the WB2 line. Subsequently, we randomly selected 10 to 20 individuals from G4 to G8 for PCR assay, and all were positive for wAlbB, indicating 100% maternal transmission efficiency (Fig. S1b). Although initial egg hatch rates were low (29% to 50%), they recovered to a level close to 70% from G5 onward (Fig. S1b), likely thanks to the removal of inbreeding effects through repeated outcrosses of WB2 females with Waco males. This new transinfected line provided us the opportunity to study phenotypic effect of wAlbB on hosts over time.
No difference in CI induction between the WB1 and WB2 lines
To determine whether there was any difference in wAlbB-induced CI between the WB1 and WB2 lines, we set up crosses involving the WB1, WB2, and Waco lines. The self-crosses of WB1, WB2, and Waco yielded hatch rates of 47.9% (95% interval = 36.1% to 59.7%), 51.5% (95% interval = 50.2% to 52.8%), and 53.6% (95% interval = 43.6% to 63.5%), respectively. The WB1 males were compatible with WB2 females, with an average hatch rate of 52.4% (95% interval = 34.3% to 70.4%). Similarly, WB2 males were also compatible with WB1 females, with a hatch rate of 50.8% (95% interval = 49.6% to 52.0%). Both WB1 and WB2 males induced a 100% CI when crossed with Waco females (95% interval = 0% to 0%) (Fig. 1). The results indicate that WB1 and WB2 are compatible with each other but incompatible with wild-type Ae. aegypti, pointing to lack of host effects on wAlbB-induced CI after its transfer into Ae. aegypti for 15 years (or approximately 180 generations).
Fig. 1.
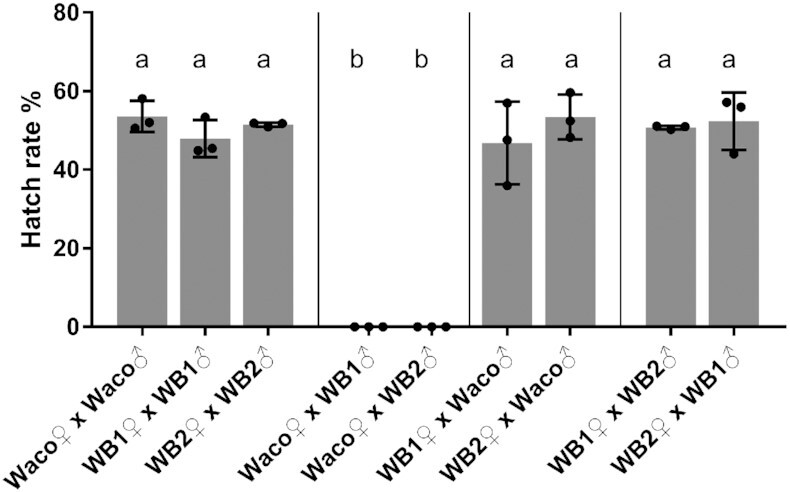
CI crosses involving wild-type Waco, WB1, and WB2 mosquitoes. The results are expressed as the mean of three replicates for each cross involving 10 females and 10 males. Dots indicate the sample values. Error bars indicate the SD. The letters above the columns indicate significant differences: P < 0.0001 by ANOVA-Tukey’s multiple comparison test.
No difference in wAlbB density in female whole bodies and male testes between the WB1 and WB2 lines
To examine the impact of long-term association with Ae. aegypti on the tissue distribution of wAlbB in a more natural host, rather than an inbred line, we compared the densities of wAlbB in whole bodies and reproductive tissues, including ovaries and testes, between the WB1 and WB2 lines after both were outcrossed with Waco for seven generations to homogenize the host genetic background (Fig. S2). The results showed no difference in wAlbB density in the female whole bodies and male testes between the WB1 and WB2 lines. However, WB2 ovaries showed a significantly higher Wolbachia density than did the WB1 ovaries (Fig. 2). These results indicate that wAlbB maintains a stable density in an outbred genetic background after associated with Ae. aegypti at the laboratory conditions for 15 years, which is consistent with a recent report showing a stable wAlbB in transinfected Ae. aegypti under two different host backgrounds (43).
Fig. 2.
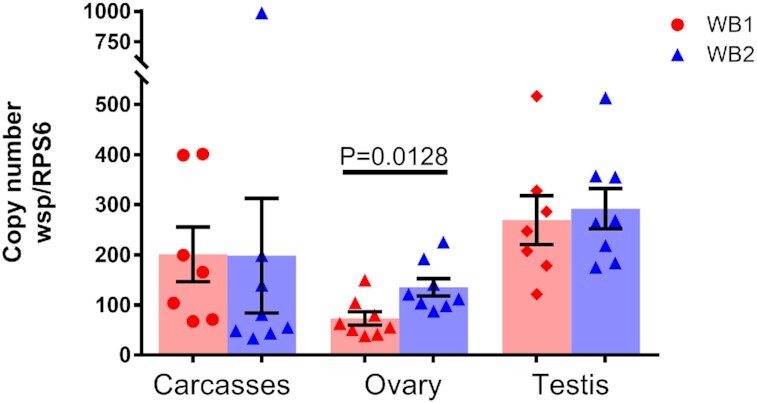
The density of wAlbB in WB1 and WB2 mosquitoes. The copy number of the Wolbachia wsp gene was normalized by the mosquito rps6 gene. Each point represents an individual tissue. Data are shown as the mean of eight replicates ± SE. Error bars indicate the SE. P = 0.0128, Student’s t-test.
Variation among different arboviruses in the strength of virus blocking by wAlbB
Vector competence assays were performed to measure the wAlbB-mediated blocking effects on DENV, CHIKV, and ZIKV in Ae. aegypti with a Singaporean genetic background. After mosquitoes were fed with an infectious bloodmeal, midguts and salivary glands were collected at 6 and 13 d post-infection (DPI) to measure viral titers and infection rates. We observed 100% infection rates in the midguts and salivary glands of wild-type mosquitoes in all the infection assays with the three viruses, except for an 80% DENV infection rate in the salivary glands at 6 DPI (Fig. 3). In the assays with DENV serotype 2 (DENV-2) at 6 DPI, wAlbB significantly inhibited viral loads in the salivary glands (95% interval = 1.87 to 2.39) as compared to the wild-type mosquitoes (95% interval = 2.30 to 4.21, P = 0.0057), whereas both WB2 and wild-type had a similar viral loads in the midgut (P = 0.13). At 13 DPI, wAlbB significantly inhibited viral loads in both midguts (95% interval = 3.16 to 4.62) and salivary glands (95% interval = 0.81 to 3.66) as compared to their wild-type counterparts (midguts, 95% interval = 4.28 to 5.28, P = 0.049; salivary glands, 95% interval = 5.79 to 6.34, P < 0.0001) (Fig. 3a). In the ZIKV infection assays, although the salivary gland infection rates were about the same for wild-type and WB2 mosquitoes, the viral titers in the salivary glands of the WB2 mosquitoes were significantly reduced at both 6 (95% interval = 3.02 to 4.84) and 13 DPI (95% interval = 3.77 to 6.72), respectively, when compared to those of wild-type mosquitoes (6 DPI, 95% interval = 5.25 to 6.42, P = 0.0008; 13 DPI, 95% interval = 6.51 to 7.45, P = 0.0044). Although not observed in midguts at 13 DPI, a reduction in viral titers was also observed in the WB2 midguts at 6 DPI (95% interval = 6.52 to 7.27) as compared to the wild-type mosquitoes (95% interval = 5.82 to 6.60, P = 0.01) (Fig. 3b). In the CHIKV infection assays of the midguts sampled at both time points, wAlbB reduced the viral titers significantly in the WB2 mosquitoes (6 DPI, 95% interval = 2.37 to 4.56; 13 DPI, 95% interval = 1.53 to 3.31) as compared to the wild-type mosquitoes (6 DPI, 95% interval = 6.17 to 6.82, P < 0.0001; 13 DPI, 95% interval = 4.05 to 5.40, P = 0.0002). In the salivary glands at both time points, wAlbB decreased the infection rates by 90% (P = 0.0001) (Fig. 3C). These results indicate that wAlbB induced resistance to all three arboviruses in Ae. aegypti, with the strength of the viral blocking varied among them. Although wAlbB-mediated suppression of dengue virus has been reported previously (19) and a suppression of ZIKV was published (40) while this manuscript was in review, this is the first showing suppression of CHIKV by wAlbB, indicating its spectrum of antiviral activity similar to wMel (49).
Fig. 3.
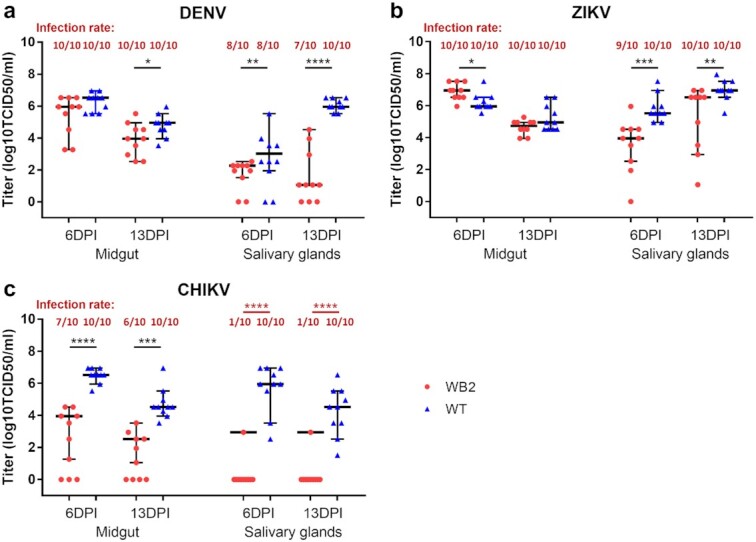
Vector competence for DENV, ZIKV, and CHIKV of wAlbB-infected Ae. aegypti on the Singaporean genetic background. After the outcrossed WB2 and wild-type (WT) mosquitoes, both on a Singaporean genetic background, had been fed with blood spiked with either DENV-2 at 7.74 log10TCID50/ml (a), ZIKV (MR766 strain) at 8.74 log10TCID50/ml (b), or CHIKV (EHIKJ71albY08 strain) at 6.24 log10TCID50/ml (c), the midguts and salivary glands were collected at 6 and 13 DPI and assayed for viral infection. Virus infection levels were determined using a viral titration assay and expressed as log10TCID50/ml. Lines and error bars denote medians ± 95% CIs of viral titers with nonzero values, and each point represents an individual midgut/salivary gland. Significant differences in viral titers and infection rate (prevalence) were determined using Mann–Whitney test and two-sided Fisher’s exact test, respectively. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Variation in the strength of virus blocking by wAlbB on different host genetic backgrounds
We then compared the ability of wAlbB to block two ZIKV lineages, an Asian and a South American lineage, in Ae. aegypti with genetic backgrounds from Mexico or Singapore. The ZIKV infections were measured in both midguts and salivary glands at 7 and 14 DPI. For both the Mexican and Singaporean mosquito genetic backgrounds, wAlbB exhibited virus inhibition at both time points (Fig. 4). For the Asian lineage, virus loads were significantly affected due to Wolbachia (generalized linear model [GLM]: F = 26.62, df = 1, P < 0.0001), mosquito genetic background (GLM: F = 135.41, df = 1, P < 0.0001) and their interactions (GLM: F = 8.42, df = 1, P = 0.004). There were significant effects of mosquito genetic background on virus loads in both wAlbB-infected midguts and salivary glands (GLM: F = 14.05, df = 1, P = 0.0003), whereas similar effects were not observed in wild-type mosquitoes (GLM: F = 1.92, df = 1, P = 0.17). Indeed, there was a consistent pattern that wAlbB-infected Ae. aegypti on the Singaporean genetic background induced stronger viral blocking than did those on the Mexican genetic background as described below. For the Singaporean background, virus titers were significantly lower in the midguts of wAlbB-infected mosquitoes at both 7 DPI (95% interval = 3.36 to 4.44) and 14 DPI (95% interval = 3.39 to 4.27) than those of wild-type mosquitoes (7 DPI, 95% interval = 4.84 to 5.85, P < 0.0001; 14 DPI, 95% interval = 4.59 to 5.13, P < 0.0001), respectively. However, a similar difference was not observed in midguts for mosquitoes on the Mexican background at both time points (Fig 4a). In salivary glands at 7 DPI, a complete virus blocking was observed in wAlbB-infected mosquitoes on the Singaporean background as compared to a 90% infection rate in wild-type mosquitoes (P < 0.0001), whereas wAlbB induced partial virus blocking, with a 50% infection rate, on the Mexican background as compared to a 95% infection rate in the wild-type mosquitoes (P = 0.003) (Fig. 4b). In salivary glands at 14 DPI, virus infection rates significantly decreased in wAlbB-infected mosquito on both genetic backgrounds as compared to wild-type mosquitoes (Singaporean, P = 0.002; Mexican, P = 0.001). While similar effects were not observed in mosquitoes on the Mexican background, wAlbB significantly decreased the median viral titers by 2.29 log10TCID50/ml in mosquitoes on the Singaporean background (95% interval = 2.08 to 4.58) as compared to their Wolbachia-free counterparts (95% interval = 4.81 to 5.95, P = 0.002) (Fig. 4b). For the South American lineage, a complete virus blocking was observed in the WB2 midguts on the Singaporean backgrounds at both 7 and 14 DPI as compared to their Wolbachia-free counterparts (7 DPI, P = 0.047; 14 DPI, P = 0.0083) (Fig. 4c), perhaps because of the low viral titer in the infectious bloodmeal, whereas only partial virus blocking occurred in salivary glands of mosquitoes on the Mexican background at 14 DPI (P = 0.043) (Fig. 4d). These results demonstrate that the host’s genetic background can modulate the strength of wAlbB-induced virus blocking in Ae. aegypti, which is consistent with the previous studies showing that variations of mosquito genes affect wMel-mediated dengue blocking (33).
Fig. 4.
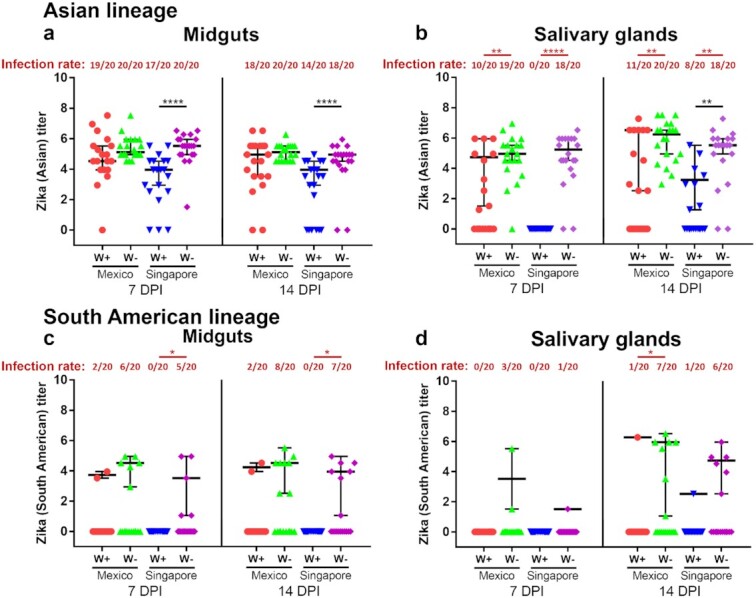
Vector competence of wAlbB-infected Ae. aegypti, on either the Mexican or Singaporean genetic background, for ZIKV. The infection rates and titers of ZIKV LP0210Y17 strain, an Asian lineage (a, b), and SG(EHI)ZIKV/33164Y17, a South American lineage (c, d), in the midguts (a, c) and salivary glands (b, d) at 7 and 14 DPI. The virus titers were calculated as log10TCID50/ml. W+, wAlbB-infected mosquitoes on either the Mexican genetic background (WBM) or Singaporean genetic background (WBSG); W-, wild-type Ae. aegypti (Wolbachia free) on either the Mexican genetic background (AFM) or Singaporean genetic background (WTSG). Lines and error bars denote medians ± 95% CIs of viral titers with nonzero values, and each point represents an individual midgut/salivary gland. Significant differences in viral titers and infection rate (prevalence) were determined using Mann–Whitney test and two-sided Fisher’s exact test, respectively. *P < 0.05, **P < 0.01, ****P < 0.0001.
Variation in mating competitiveness between WB2 and wild-type males on the Mexico genetic background
As an effort to develop a WB2 mosquito line for population suppression in Mexico (12, 44), male mating competitiveness assays were carried out to measure the ability of the outcrossed WB2 (WBM) males on the Mexican genetic background to compete with the Mexican wild-type (AFM) males for mating with wild-type females. Five cages containing different ratios of WBM males to AFM males to AFM females (0:1:1, 1:0:1, 1:1:1, 5:1:1 and 10:1:1) were set up. As expected, when only WBM males were present, none of eggs hatched (Table 1). In the cage with the ratio of 5:1:1, the observed egg hatch rate was not significantly different from the expected values assuming an equal competitiveness of WBM and AFM males and random mating (P = 0.12). For the other two ratios, however, the observed egg hatch rates were higher than the expected values (1:1:1, P = 0.02; 10:1:1, P = 0.01). These results indicate that the mating competitiveness of WB2 males on the Mexican genetic background varies by ratios and may be slightly reduced compared to wild-type males, which is consistent with the previous observation of mating competitiveness of WB2 males on two different genetic backgrounds (47).
Table 1.
Mating competitiveness of Ae. aegypti males on the Mexican genetic background at various release ratios.
| WBM♂:AFM♂:AFM♀ | Number of eggs | Observed egg hatch rate | Expected egg hatch rate | Competitiveness index | P -value* |
|---|---|---|---|---|---|
| 0:1:1 | 303 | 0.77 | |||
| 1:0:1 | 331 | 0.00 (0) | 0.00 | >0.99 | |
| 1:1:1 | 1,071 | 0.42 (449) | 0.39 | 0.84 | 0.02 |
| 5:1:1 | 494 | 0.10 (51) | 0.13 | 1.30 | 0.12 |
| 10:1:1 | 340 | 0.11 (36) | 0.07 | 0.63 | 0.01 |
*Comparisons between the observed and expected egg hatch through exact binomial test. WBM: outcrossed wAlbB-infected Ae. aegypti on the Mexican genetic background; AFM: wild type Wolbachia-free Ae. aegypti on the Mexican genetic background.
Discussion
The success of the Wolbachia technology in suppressing an Ae. aegypti mosquito population or reducing its ability to transmit arboviruses hinges on the long-term stability of the Wolbachia infection, the Wolbachia-induced antiviral properties, and strong CI expression. In this work, we have demonstrated the successful establishment of another wAlbB-infected Ae. aegypti WB2 line with a stable 100% maternal transmission efficiency. Comparison of the WB2 line to the WB1 line, generated 15 years ago, has demonstrated a long-term stability of the wAlbB-Ae. aegypti association, with neither alteration of CI expression nor attenuation of the Wolbachia titer in female whole bodies and male testes. wAlbB induced resistance to DENV, ZIKV, and CHIKV. In addition, it conferred a strong resistance to both South American and Asian lineages of ZIKV in WB2 mosquitoes on either a Mexican or Singaporean genetic background. We also found that the outcrossed WB2 males on the Mexican genetic background had a mating competitiveness that was comparable to that of their counterpart wild-type males. These results support the feasibility of developing wAlbB-based population replacement and suppression strategies for controlling DENV, ZIKV, and CHIKV in disease-endemic countries.
Control of mosquito-borne diseases through Wolbachia-based population replacement relies on the long-term stability of the Wolbachia-mediated viral blocking properties in the transinfected line, in which the Wolbachia-mosquito association may co-evolve over time. Establishment of the WB2 line has enabled us to examine the stability of artificial wAlbB infection in Ae. aegypti over 15 years. We previously discovered that the density of the native wAlbB in the donor species Ae. albopictus is much lower than that in the transinfected WB1 mosquitoes, raising a concern that the titer of wAlbB may become attenuated after co-adaptation occurs between the symbiont and its host over a long period of time (25). Since the density of Wolbachia is often associated with the strength of its induced viral inhibition, such attenuation could result in the reduction or even loss of its viral-blocking properties (25). Our results show that, in an outbred genetic background, WB1 and WB2 had similar densities in both female whole bodies and male testes except for in the ovaries, where WB2 had higher density than did WB1. A similar observation was made in a recent study that compared the density of wMel between a recently transinfected line and another line generated 10 year ago (50). It is predicated that CI genes would degrade over time in selectively neutral populations, starting with the sperm modification factor cifB, followed by the rescue factor cifA (51). The fact that wMel induces weak CI in Drosophila melanogaster but complete CI in mosquito also provides a direct evidence of a strong host effect that supports the existence of segregating host suppressors in natural hosts. We observed that WB1 and WB2 were bidirectionally compatible and induced the same CI pattern, indicating that the phenotypic expression of CI factors remained the same, which is different from natural systems (e.g. D. melanogaster and D. yakuba) where host factors are known to modify CI strength (52, 53). These results indicate that wAlbB transinfection has been stable for >15 years under laboratory conditions, consistent with a recent report showing few genetic changes occurring during this period (43). Although it may eventually evolve to a low density as observed for Ae. albopictus, this decrease probably will be a long-term process and should not affect the efficacy of disease control through viral blocking in the short- or middle-term. This is supported by a recent study showing that wAlbB maintains high density and dengue inhibition at least over 1 year after introduced into the field (54). Further studies are needed to determine whether real-world field conditions will facilitate the co-adaptation of wAlbB and Ae. aegypti to accelerate the process. It is worthy to note that we have compared Wolbachia densities between WB1 and WB2 after outbred with Waco because such an outcross with a local genetic background is the first step when a transinfected line is deployed to a country/region for disease control. Evaluation of wAlbB’s capacity to form a stable association in the outbred line would provide more direct implication for implementation than that in the inbred line. However, a comparison of WB2 with the inbred WB1 line through reciprocal crosses would allow to examine how wAlbB and Ae. aegypti differentiate during the co-adaptation.
In our study, wAlbB was able to limit CHIKV, DENV, and ZIKV replication and/or dissemination in transinfected Ae. aegypti when compared to wild-type mosquitoes. In wild-type mosquitoes, the infection rates were 100% for all three viruses in the midguts and salivary glands at 6 and 13 DPI, respectively, indicating that the infection doses were sufficient to develop transmissible infection in the mosquitoes. In comparison to wild-type mosquitoes, CHIKV infection rates in WB2 mosquitoes were reduced by 90% in the salivary glands at both 6 and 13 DPI. For DENV-2 and ZIKV, viral titers were significantly lower in WB2 salivary glands at both time points as compared to their wild-type counterparts, but infection rates were not statistically significant. This variation in virus blocking is probably a result of differences in their viral genomes, life cycles, and required host factors between the alphavirus (CHIKV) and the flaviviruses (DENV and ZIKV). Previous studies also observed variations in Wolbachia-mediated virus blocking across different DENV serotypes with underlined mechanisms unclear (21, 30, 34). We cannot rule out the possibility that differences in the infectious bloodmeal viral titers may have contributed to the degree or magnitude of the blocking effects observed. Our results also show that viral inhibition was much stronger in the salivary glands than in the midguts, highlighting the potential for underestimating the Wolbachia-mediated virus blocking effect if a viral assay is only conducted on midguts.
It has been shown that the genetic variations among different geographic Ae. aegypti populations can influence their competence to transmit various viral pathogens (55). Previous studies have also shown that genetic variation in mosquitoes affects wMel-mediated dengue blocking (33). Therefore, it is expected that the host genetic background may also influence the strength of the wAlbB-mediated inhibition of ZIKV. In the present study, we observed a consistent pattern that wAlbB induced a stronger inhibition of the Asian lineage of ZIKV in mosquitoes on the Singaporean genetic background than in those on the Mexican genetic background. It is unknown whether this variation is caused by a direct impact on viral interference or an indirect impact on the modulation of Wolbachia density. A previous study in Drosophila has reported that the Wolbachia genome has a much greater influence on the level of antiviral protection than does the host genome and that it is Wolbachia rather than the host that controls the Wolbachia density (56). In our study, the hosts’ genetic background may have played a significant role in the wAlbB-mediated viral blocking effect, since the replication and dissemination of both ZIKV lineages were, overall, suppressed to much lower levels in the WB2 mosquitoes on the Singaporean genetic background than in those on the Mexican genetic background. Consistent with our results, a recent study has reported that wAlbB inhibits ZIKV in Ae. aegypti on an Australian background, a line that was also derived from WB2 through outcrosses (40); interestingly, the authors used a Zika virus strain from Brazil for their vector competence assay and found that wAlbB reduced the ZIKV prevalence in saliva by 6- to 7-fold, a similar level of inhibition of ZIKV with a South America lineage in salivary glands that we observed here.
The discovery of variation in Wolbachia-mediated viral blocking among various arboviruses and various host genetic backgrounds highlights a caution with regard to deploying Wolbachia for disease control in endemic countries in which it will encounter diversity in mosquitoes and arboviruses. The same Wolbachia strain may have a high efficacy in reducing viral transmission in one country but produce a different outcome in another country because of differences in terms of local mosquitoes’ genetic background and locally circulating viruses. It is likely that the Wolbachia-mediated virus-blocking effect can also be affected by differences in the environmental conditions into which the Wolbachia-infected mosquitoes are released. In Drosophila, cool temperature is reported to reduce the native wMel oocyte abundance and maternal transmission and this temperature-dependent transmission can explain the fluctuation of continent-wide wMel frequency (57). While some strains are maintained at high, stable equilibria, such as wRi in D. simulans (58), host-wMel combinations from the temperate produced a higher rate of transmission in the cold that than tropical genotypes (57). Accordingly, an impact of environmental conditions on maternal transmission, CI, and invasion of Wolbachia into field populations has recently been documented in transinfected Ae. aegypti (59, 60). The high sensitivity of wMel to heat stress and other factors makes the transinfected mosquito difficult to establish in some areas in Vietnam and Brazil, where wAlbB and a heat-resistant wMelM variant have been proposed as an alternative because of their stability in extreme temperatures (41, 50, 61, 62). When Wolbachia is to be deployed globally for arbovirus disease control, different Wolbachia strains may be required for regions with specific environmental or ecological conditions. Thus, a comprehensive profile of various Wolbachia strains on the local mosquito genetic background should facilitate and guide future implementation of Wolbachia for arbovirus disease control in various field settings. It is expected that such a profile would include an evaluation of the stability of the Wolbachia infection under various environmental conditions and with various levels of maternal transmission, CI expression, and pathogen blocking against contemporary viruses.
Since it was generated, the WB2 line has been outcrossed to local Ae. aegypti from Singapore, Mexico, and Australia for population suppression field trials (5, 11, 44), and WB1 was released in the United States for the same purpose (6, 63). The slightly reduced male mating competitiveness (index values ranging from 0.63 to 1.30) of WB2 on a Mexican genetic background that we describe here is similar to what was found in a previous study using WB2 outcrossed with a different Mexican mosquito population (index values ranging from 0.57 to 0.79) (47) and is also consistent with the high performance of released males and successful population suppression observed under both semifield and field conditions (5, 6, 11, 46, 64). The WB2 line outcrossed with Singaporean Ae. aegypti mosquitoes also showed comparable mating competitiveness with local Ae. aegypti mosquitoes and demonstrated for the first time that the Wolbachia-based suppression approach is able to reduce the wild-type Ae. aegypti mosquito population in an urban landscape (11).
In addition to comparable mating competitiveness with wild-type males, the success of population suppression strategy requires for the stability of Wolbachia infection and perfect maternal transmission that are critical for large-scale production of male Wolbachia-infected mosquitoes for release. Results from the present study show that wAlbB can retain complete CI, a high density in mosquitoes, and 100% maternal transmission over 15 years. These findings are consistent with results obtained for wAlbB that has been introgressed onto a Singaporean genetic background since 2016 and is currently being extensively trialed to evaluate its effectiveness in suppressing Ae. aegypti mosquito populations (11). As part of the quality assurance evaluation for large-scale production of male wAlbB-infected Ae. aegypti for release, regular screening and testing of wAlbB have shown that Wolbachia infections remain stable, with no loss of the bacterium detected, and there has been complete CI and 100% maternal transmission since Singapore’s Project Wolbachia field study started 6 years ago. Ross and colleagues have also demonstrated that wAlbB genome is stable with very few changes over 15 years and showed perfect maternal transmission and CI in both Australian and Malaysian host backgrounds (43). These results indicate that releases of wAlbB-infected males for suppression of Ae. aegypti mosquito populations are likely to remain effective.
In conclusion, we have established a recent wAlbB transinfection in Ae. aegypti and shown that wAlbB can reduce the potential of Ae. aegypti to transmit DENV, ZIKV, and CHIKV. Together with perfect maternal transmission, complete CI, and high male mating competitiveness, these results support the feasibility of scaling up WB2 release for controlling arboviruses in Singapore and Mexico, as is currently underway (11, 12). The stability of the wAlbB–Ae. aegypti association is expected to further boost cost-effectiveness and sustainability of both population replacement and suppression strategies. Further studies should include identifying the key factors affecting the long-term stability of Wolbachia-host associations under mass-rearing conditions and in the field in disease-endemic countries.
Materials and methods
Mosquito lines and maintenance
The wild-type Ae. albopictus HOU line carries a native superinfection of wAlbA and wAlbB (24). Waco is a wild-type Ae. aegypti line that does not carry a native Wolbachia infection. WB1 is a wAlbB-infected line that was developed previously (17). AFM is a wild-type Ae. aegypti line that was recently established in the laboratory using eggs collected in the field in Merida, Mexico. WBM denotes the wAlbB-infected Ae. aegypti derived by repeated outcrossing of WB2 females with AFM males for seven generations. WTSG is a wild-type Ae. aegypti line from Singapore and established as previously described (49), and WBSG is the wAlbB-infected Ae. aegypti line derived by repeated outcrossing of WB2 females with WTSG mosquitoes for seven generations. All the mosquito lines were maintained on 10% sugar solution at 27 ± 1°C and 80 ± 10% relative humidity (RH), with a 12:12 h light:dark photoperiod, according to standard rearing procedures. For routine colony maintenance and experimental studies, female mosquitoes were provided with sheep or swine blood at day 7 post-eclosion, and eggs were collected at 2-d post-bloodmeal.
Transinfection to generate the WB2 line
The WB2 line was generated by transferring wAlbB from HOU to Waco using embryonic microinjection according to the approach described previously (17). Thus, the same donor and recipient mosquitoes were used to generate the WB1 and WB2 lines. In brief, cytoplasm from each donor embryo was transferred to the posterior of a recipient embryo (60 to 90 min old) by using an IM300 microinjector (Narishige Scientific). After injection, the embryos were incubated at 85% RH and 27°C for 1 h and transferred to wet filter paper. They were then allowed to mature for 5 to 7 d before being hatched. Females (G0) developing from the surviving embryos were isolated and mated with Waco males. After blood-feeding and oviposition, the G0 females were tested for wAlbB infection by PCR using the strain-specific primers described below. G1 females were again crossed with Waco males, blood-fed, isolated, and allowed to oviposit. The offspring from the wAlbB-positive G1 were selected for the next screen, and this process was repeated until the wAlbB maternal transmission rate reached 100%. The wAlbB-positive females also assayed for the presence of wAlbA by PCR, and none of them tested positive for wAlbA infection.
PCR assay of Wolbachia infection
Genomic DNA was extracted from whole bodies, ovaries, or testes of 7-d-old mosquitoes, with 7 to 8 replicates for each treatment, using a Thermo Scientific Phire Animal Tissue Direct PCR Kit (F-140WH). All mosquitoes were reared in 30 × 30 × 30 cm standard cages under controlled condition to ensure collected mosquitoes have similar size. Samples were pretreated in 20 µl dilution buffer with 0.5 µl DNARelease Additive. The reaction mixture contained 10 µl 2X Phire Animal Tissue PCR Buffer, 0.4 µl Phire Hot Start II DNA Polymerase, 0.2 µl of both the forward and reverse primers, and 7.2 µl distilled H2O. The regular PCR conditions were: initial denaturation at 98°C for 6 min, followed by 40 cycles of 5 s at 98°C, 5 s at 56°C, and 45 s at 72°C. qPCR was performed using a QuantiTect SYBR Green PCR Kit (Qiagen) and ABI Detection System ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA). The primers for wAlbA, wAlbB, and mosquito rps6 were used as previously described (65). Standard curves were generated for each of the above genes to convert the Ct value for the qPCR into the copy number for each target sequence.
CI crosses
CI crosses were conducted as previously described (17). Ten virgin males were mated with ten virgin females, with three replicate cages for each cross. A bloodmeal was provided to the females at day 7 post-eclosion. Two days after the bloodmeal, eggs were collected into oviposition cups containing wet filter paper, which was subsequently desiccated for 7 d at 27°C and 80% RH. Eggs were counted and then hatched in water containing 6% m/v bovine liver powder. Larvae were counted at the L2–L3 stage to record the hatch rate.
Outcrosses to develop WB2 lines with a Mexican or Singaporean genetic background
To introduce the Mexican Ae. aegypti genetic background into the WB2 line, we crossed the WB2 line with wild-type mosquitoes collected from the field in Merida, Mexico (AFM) for seven generations (Figs. S2 and S3). During each cross, 100 virgin WB2 females and 100 AFM males were randomly selected. The offspring from each cross were tested for maternal transmission rate. The maternal transmission rates of the outcrossed Mexican WBM line were maintained at 100% during the crosses (Fig. S3). The same procedure was performed to introduce the Singaporean genetic background into WB2, with 100% maternal transmission rates of wAlbB maintained at each generation. These outcrossed mosquitoes were subsequently used for the vector competence and mating competitiveness assays described below.
Vector competence assay
The viruses listed below were used for comparison of wAlbB-mediated blocking effects on DENV, CHIKV, and ZIKV: DENV-2 EHIE18944Y13 (KR779784), ZIKV MR766 strain (ATCC), and CHIKV EHIKJ71albY08 (66). After propagation in Vero cells (ATCC CCL-81), the supernatants were mixed with an equal part of swine packed red blood cells. Adenosine triphosphate (ThermoFisher Scientific, USA) was added to the infectious bloodmeal as a phagostimulant at a final concentration of 3 mM. The virus titers used in the infectious bloodmeal were 6.24, 8.74, and 7.74 log10TCID50/ml for CHIKV, ZIKV, and DENV-2, respectively, before mosquito feeding. Two ZIKV lineages, SG(EHI)ZIKV/33164Y17, a South American lineage (GenBank accession no. MF988734) (67); and LP0210Y17, an Asian lineage (not submitted to GenBank) were used to compare wAlbB-mediated blocking effects in Ae. aegypti on either a Singaporean or Mexican genetic background. Both lineages were isolated from clinical samples in 2017 and had been passaged three times in Vero cells (ATCC, USA) prior to oral infection of the mosquitoes with viral titers of 4.95 and 6.52 log10TCID50/ml for the South American and Asian lineages, respectively. All mosquitoes in Fig. 4 were tested at the same time with the same virus titration assay in randomized designs. One experimental replication was performed for each vector competence assay and there were no blocks in the experiments. The mosquitoes (5 to 7 d old) were fed on a virus-spiked bloodmeal for 45 min. At 6 to 7 and 13 to 14 DPI, the midgut and salivary glands were sampled to measure viral titers. The virus levels were determined using a viral titration assay and expressed as log10TCID50/ml (49).
Mating competitiveness assay
Fifty AFM females, 50 AFM males and varying numbers of WBM males (0, 50, 250, or 500) were placed in adult cages. Additional cages with either 50 AFM females and 50 WBM males or 50 AFM females and 50 AFM males were set up as the control groups for sterile or fertile mating, respectively. Mosquitoes were allowed to mate for 2 d before bloodfeeding for 20 min. Two days after the bloodfeeding, egg cups were placed in the cages for egg collection. The eggs were then hatched, and the hatch rates were calculated as described for the CI crosses. The egg hatch rate was compared to the expected hatch rate assuming: (i) random mating and equal mating competitiveness between WBM and AFM males, and (ii) complete unidirectional cytoplasmic incompatibility between WBM males and AFM females (8, 68). Male mating competitiveness index was calculated as described previously (8).
Statistical analysis
Differences between Wolbachia density and virus titer were analyzed using a Student’s t-test or one-way ANOVA, with P values < 0.05 considered significant. Prior to analyses, the normality of the data sets was checked using the D’Agostino and Pearson omnibus normality test. If they were not normally distributed, the Mann–Whittney U-test was used for analysis. Virus-negative samples were not included in determining the medians ± 95% CIs and significant difference in viral titers. Differences in the infection rate were evaluated using two-tailed Fisher’s exact test. Variation in virus loads was assessed with a GLM including genetical background, Wolbachia, tissue, and their interactions using SAS 9.1. All other analyses were performed in GraphPad Prism v. 7 (GraphPad Software, San Diego, CA, USA).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Prof. Elizabeth A. McGraw and Jason L. Rasgon for their comments and suggestions and Dr. Deborah McClellan for editorial assistance.
Notes
Competing Interest: Z. X. is affiliated with Guangzhou Wolbaki Biotech Co., Ltd.
Contributor Information
Xiao Liang, Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI 48824, USA.
Cheong Huat Tan, Environmental Health Institute, National Environment Agency, Singapore 138667.
Qiang Sun, Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI 48824, USA.
Meichun Zhang, Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI 48824, USA.
Pei Sze Jeslyn Wong, Environmental Health Institute, National Environment Agency, Singapore 138667.
Meizhi Irene Li, Environmental Health Institute, National Environment Agency, Singapore 138667.
Keng Wai Mak, Environmental Health Institute, National Environment Agency, Singapore 138667.
Abdiel Martín-Park, Laboratorio para el Control Biologico de Aedes aegypti (LCB-UADY), Unidad Colaborativa para Bioensayos Entomologicos, Campus de Ciencias Biologicas y Agropecuarias, Universidad Autonoma de Yucatan, Mérida, Yucatán CP 97315, Mexico.
Yamili Contreras-Perera, Laboratorio para el Control Biologico de Aedes aegypti (LCB-UADY), Unidad Colaborativa para Bioensayos Entomologicos, Campus de Ciencias Biologicas y Agropecuarias, Universidad Autonoma de Yucatan, Mérida, Yucatán CP 97315, Mexico.
Henry Puerta-Guardo, Laboratorio para el Control Biologico de Aedes aegypti (LCB-UADY), Unidad Colaborativa para Bioensayos Entomologicos, Campus de Ciencias Biologicas y Agropecuarias, Universidad Autonoma de Yucatan, Mérida, Yucatán CP 97315, Mexico.
Pablo Manrique-Saide, Laboratorio para el Control Biologico de Aedes aegypti (LCB-UADY), Unidad Colaborativa para Bioensayos Entomologicos, Campus de Ciencias Biologicas y Agropecuarias, Universidad Autonoma de Yucatan, Mérida, Yucatán CP 97315, Mexico.
Lee Ching Ng, Environmental Health Institute, National Environment Agency, Singapore 138667; School of Biological Sciences, Nanyang Technological Institute, Singapore 637551.
Zhiyong Xi, Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI 48824, USA.
Funding
This work was supported by the U.S. Agency for International Development (USAID) (AID-OAA-F-16–00082) to ZX and PMS, Singapore’s National Environment Agency, Ministry of Sustainability and the Environment to LCN, and the Fondo Mixto Consejo Nacional de Ciencia y Tecnologia (CONACYT) (Mexico)-Gobierno del Estado de Yucatán (YUC2017-03-01-556) to PMS and AMP. AMP is supported by the Investigadoras e Investigadores por Mexico - CONACYT program.
Authors' Contributions
Z.X.: designed research; contributed new reagents/analytic tools; analyzed data; wrote the paper. X.L.: designed research; performed research; analyzed data; wrote the paper. C.T.: designed research; performed research; contributed new reagents/analytic tools; analyzed data; wrote the paper. Q.S.: Performed research; contributed new reagents/analytic tools. M.Z.: performed research; contributed new reagents/analytic tools. P.J.W.: performed research. M.L.: performed research; wrote the paper. K.W.M., A.M.P., Y.C.P., and H.P.G.: performed research. P.M.S.: performed research; wrote the paper. L.C.N.: designed research; wrote the paper.
Data Availability
All data are included in the manuscript and/or Supplementary Material.
References
- 1. Messina JP, et al. 2019. The current and future global distribution and population at risk of dengue. Nat Microbiol. 4:1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Organization WH . 2012. Global strategy for dengue prevention and control 2012–2020.[Last accessed date 12 Sep, 2022] https://apps.who.int/iris/handle/10665/75303 [Google Scholar]
- 3. Russell RC. 2009. Mosquito-borne disease and climate change in Australia: time for a reality check. Aust J Entomol. 48:1–7. [Google Scholar]
- 4. Utarini A, et al. 2021. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med. 384:2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beebe NW, et al. 2021. Releasing incompatible males drives strong suppression across populations of wild and Wolbachia-carrying Aedes aegypti in Australia. Proc Natl Acad Sci USA. 118:e2106828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crawford JE, et al. 2020. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat Biotechnol. 38:482–492. [DOI] [PubMed] [Google Scholar]
- 7. Pinto SB, et al. 2021. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niteroi, Brazil: a quasi-experimental study. PLoS Negl Trop Dis. 15:e0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng X, et al. 2019. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 572:56–61. [DOI] [PubMed] [Google Scholar]
- 9. Ryan PA, et al. 2020. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 3:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crawford JE, et al. 2020. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat Biotechnol. 38:482–492. [DOI] [PubMed] [Google Scholar]
- 11. Consortium PWS. 2021. Wolbachia-mediated sterility suppresses Aedes aegypti populations in the urban tropics. medRxiv 2021.06.16.21257922.[Last accessed date 12 Sep, 2022] https://www.medrxiv.org/content/10.1101/2021.06.16.21257922v1 10.1101/2021.06.16.21257922 [DOI] [Google Scholar]
- 12. Martin-Park A, et al. 2022. Pilot trial using mass field-releases of sterile males produced with the incompatible and sterile insect techniques as part of integrated Aedes aegypti control in Mexico. PLoS Negl Trop Dis. 16:e0010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Werren JH, Baldo L, Clark ME.. 2008. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 6:741–751. [DOI] [PubMed] [Google Scholar]
- 14. LePage DP, et al. 2017. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature. 543:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barton NH, Turelli M.. 2011. Spatial waves of advance with bistable dynamics: cytoplasmic and genetic analogues of Allee effects. Am Nat. 178:E48–E75. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann AA, et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 476:454–457. [DOI] [PubMed] [Google Scholar]
- 17. Xi Z, Khoo CC, Dobson SL.. 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 310:326–328. [DOI] [PubMed] [Google Scholar]
- 18. Xi Z, Joshi D.. 2016. Genetic control of malaria and dengue using Wolbachia. In Adelman ZN, editor. Genetic control of malaria and dengue. San Diego, CA 92101-4495, USA. Elsevier Inc. pp. 305–333. [Google Scholar]
- 19. Bian G, Xu Y, Lu P, Xie Y, Xi Z.. 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 6:e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moreira LA, et al. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 139:1268–1278. [DOI] [PubMed] [Google Scholar]
- 21. Flores HA, et al. 2020. Multiple Wolbachia strains provide comparative levels of protection against dengue virus infection in Aedes aegypti. PLoS Pathog. 16:e1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schultz MJ, et al. 2018. Wolbachia wStri blocks Zika virus growth at two independent stages of viral replication. mBio. 9:e00738–e00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ross PA, et al. 2020. An elusive endosymbiont: does Wolbachia occur naturally in Aedes aegypti?. Ecol Evol. 10:1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sinkins SP, Braig HR, O'Neill SL.. 1995. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc Biol Sci. 261:325–330. [DOI] [PubMed] [Google Scholar]
- 25. Lu P, Bian G, Pan X, Xi Z.. 2012. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl Trop Dis. 6:e1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan X, et al. 2012. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 109:E23–E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caragata EP, et al. 2013. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 9:e1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fraser JE, et al. 2017. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 13:e1006751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fraser JE, et al. 2020. Novel phenotype of Wolbachia strain wPip in Aedes aegypti challenges assumptions on mechanisms of Wolbachia-mediated dengue virus inhibition. PLoS Pathog. 16:e1008410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferguson NM, et al. 2015. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 7:279ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walker T, et al. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 476:450–453. [DOI] [PubMed] [Google Scholar]
- 32. Shropshire JD, Hamant E, Cooper BS. 2021. Male age and Wolbachia dynamics: investigating how fast and why bacterial densities and cytoplasmic incompatibility strengths vary. mBio. 12:e0299821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ford SA, et al. 2019. Selection on Aedes aegypti alters Wolbachia-mediated dengue virus blocking and fitness. Nat Microbiol. 4:1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carrington LB, et al. 2018. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 115:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joubert DA, et al. 2016. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog. 12:e1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Souza-Neto JA, Powell JR, Bonizzoni M.. 2019. Aedes aegypti vector competence studies: a review. Infect Genet Evol. 67:191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gloria-Soria A, et al. 2016. Global genetic diversity of Aedes aegypti. Mol Ecol. 25:5377–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xi Z, Dean JL, Khoo C, Dobson SL.. 2005. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem Mol Biol. 35:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bian G, et al. 2013. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 340:748–751. [DOI] [PubMed] [Google Scholar]
- 40. Hugo LE, et al. 2022. . bioRxiv 2022.03.22.485408.[Last accessed date 12 Sep, 2022] https://www.biorxiv.org/content/10.1101/2022.03.22.485408v1 10.1101/2022.03.22.485408:2022.03.22.485408 [DOI] [Google Scholar]
- 41. Hien N, et al. 2021. Environmental factors influence the local establishment of Wolbachia in Aedes aegypti mosquitoes in two small communities in Central Vietnam. Gates Open Res. 5:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mancini MV, et al. 2021. High temperature cycles result in maternal transmission and dengue infection differences between Wolbachia strains in Aedes aegypti. mBio. 12:e0025021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ross PA, et al. 2021. A wAlbB Wolbachia transinfection displays stable phenotypic effects across divergent Aedes aegypti mosquito backgrounds. Appl Environ Microbiol. 87:e0126421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Che-Mendoza A, et al. 2021. Abundance and seasonality of Aedes aegypti (Diptera: Culicidae) in two suburban localities of South Mexico, with implications for Wolbachia (Rickettsiales: Rickettsiaceae)-carrying male releases for population suppression. J Med Entomol. 58:1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nazni WA, et al. 2019. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr Biol. 29:4241–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu W-L, et al. 2022. Lab-scale characterization and semi-field trials of Wolbachia strain wAlbB in a Taiwan Wolbachia introgressed Ae. aegypti strain. PLoS Negl Trop Dis. 16:e0010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carvalho DO, et al. 2020. Aedes aegypti lines for combined sterile insect technique and incompatible insect technique applications: the importance of host genomic background. Entomol Exp Appl. 168:560–572. [Google Scholar]
- 48. Ross PA, et al. 2022. A decade of stability for wMel Wolbachia in natural Aedes aegypti populations. PLoS Pathog. 18:e1010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tan CH, et al. 2017. wMel limits zika and chikungunya virus infection in a SingaporeWolbachia-introgressed Ae. aegypti strain, wMel-Sg. PLoS Negl Trop Dis. 11:e0005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gu X, et al. 2022. A wMel Wolbachia variant in Aedes aegypti from field-collected Drosophila melanogaster with increased phenotypic stability under heat stress. Environ Microbiol. 24:2119–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martinez J, Klasson L, Welch JJ, Jiggins FM.. 2021. Life and death of selfish genes: comparative genomics reveals the dynamic evolution of cytoplasmic incompatibility. Mol Biol Evol. 38:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cooper BS, Ginsberg PS, Turelli M, Matute DR.. 2017. Wolbachia in the Drosophila yakuba complex: pervasive frequency variation and weak cytoplasmic incompatibility, but no apparent effect on reproductive isolation. Genetics. 205:333–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Poinsot D, Bourtzis K, Markakis G, Savakis C, Mercot H.. 1998. Wolbachia transfer from Drosophila melanogaster into D. simulans: Host effect and cytoplasmic incompatibility relationships. Genetics. 150:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ahmad NA, et al. 2021. Wolbachia strain wAlbB maintains high density and dengue inhibition following introduction into a field population of Aedes aegypti. Philos Trans R Soc Lond B Biol Sci. 376:20190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sim S, et al. 2013. Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in dengue virus-refractory populations and identifies novel virus-vector molecular interactions. PLoS Negl Trop Dis. 7:e2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martinez J, et al. 2017. Symbiont strain is the main determinant of variation in Wolbachia-mediated protection against viruses across Drosophila species. Mol Ecol. 26:4072–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hague MTJ, et al. 2022. Temperature effects on cellular host–microbe interactions explain continent-wide endosymbiont prevalence. Curr Biol. 32:878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR.. 2013. Rapid sequential spread of two Wolbachiavariants in Drosophila simulans. PLoS Pathog. 9:e1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ross PA, et al. 2020. Heatwaves cause fluctuations in wMel Wolbachia densities and frequencies in Aedes aegypti. PLoS Negl Trop Dis. 14:e0007958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ross PA, Ritchie SA, Axford JK, Hoffmann AA.. 2019. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegyptiunder field conditions. PLoS Negl Trop Dis. 13:e0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ross PA, et al. 2017. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 13:e1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ross PA, Hoffmann AA.. 2018. Continued susceptibility of the wMel Wolbachia infection in Aedes aegypti to heat stress following field deployment and selection. Insects. 9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mains JW, Kelly PH, Dobson KL, Petrie WD, Dobson SL.. 2019. Localized control of Aedes aegypti(Diptera: Culicidae) in Miami, FL, via inundative releases of Wolbachia-infected male mosquitoes. J Med Entomol. 56:1296–1303. [DOI] [PubMed] [Google Scholar]
- 64. Manrique-Saide P, et al. 2022. Pilot trial using mass field-releases of sterile males produced with the incompatible and sterile insect techniques as part of integrated Aedes aegypti control in Mexico. PLoS Negl Trop Dis.16:e0010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liang X, Liu J, Bian G, Xi Z.. 2020. Wolbachia inter-strain competition and inhibition of expression of cytoplasmic incompatibility in mosquito. Front Microbiol. 11: 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tan CH, et al. 2011. Entomological investigation and control of a chikungunya cluster in Singapore. Vector Borne Zoonotic Dis. 11:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tan CH, et al. 2018. Viral and antibody kinetics, and mosquito infectivity of an imported case of Zika fever gue to Asian genotype (American strain) in Singapore. Viruses. 10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Atyame CM, et al. 2011. Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Negl Trop Dis. 5:e1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and/or Supplementary Material.


