Abstract
In the ovary, proliferation and differentiation of granulosa cells (GCs) drive follicular growth. Our immunohistochemical study in a non-human primate, the Rhesus monkey, showed that the mitochondrial activity marker protein cytochrome c oxidase subunit 4 (COX4) increases in GCs in parallel to follicle size, and furthermore, its intracellular localization changes. This suggested that there is mitochondrial biogenesis and trafficking, and implicates the actions of gonadotropins, which regulate follicular growth and ovulation. Human KGN cells, i.e. granulosa tumour cells, were therefore used to study these possibilities. To robustly elevate cAMP, and thereby mimic the actions of gonadotropins, we used forskolin (FSK). FSK increased the cell size and the amount of mitochondrial DNA of KGN cells within 24 h. As revealed by MitoTracker™ experiments and ultrastructural 3D reconstruction, FSK treatment induced the formation of elaborate mitochondrial networks. H89, a protein kinase A (PKA) inhibitor, reduced the network formation. A proteomic analysis indicated that FSK elevated the levels of regulators of the cytoskeleton, among others (data available via ProteomeXchange with identifier PXD032160). The steroidogenic enzyme CYP11A1 (Cytochrome P450 Family 11 Subfamily A Member 1), located in mitochondria, was more than 3-fold increased by FSK, implying that the cAMP/PKA-associated structural changes occur in parallel with the acquisition of steroidogenic competence of mitochondria in KGN cells. In summary, the observations show increases in mitochondria and suggest intracellular trafficking of mitochondria in GCs during follicular growth, and indicate that they may partially be under the control of gonadotropins and cAMP. In line with this, increased cAMP in KGN cells profoundly affected mitochondrial dynamics in a PKA-dependent manner and implicated cytoskeletal changes.
Keywords: ovary, granulosa cells, mitochondrial dynamics, cAMP, KGN
Introduction
Ovarian granulosa cells (GCs) are the major cellular component of the follicle and crucially contribute to the intrafollicular environment. During the development of the ovarian follicle, GCs change their shape and increase dramatically in number and function. Gonadotropins are the major hormonal regulators of these events (Simoni et al., 1997; Jeppesen et al., 2012; Casarini and Crépieux, 2019). After ovulation, GCs form the large luteal cells of the corpus luteum (CL) and acquire the ability for de novo steroid production. The first step of de novo steroid production is located in the mitochondria and starts from cholesterol.
Mitochondria, in general, impact cell survival and cell metabolism and are involved in the initiation of cell death mechanisms by producing ATP, free radicals and releasing apoptotic proteins, respectively (Roger et al., 2017). They are not static cellular organelles but rather dynamic structures, which can divide and fuse. Changes in mitochondrial morphology are thought to be controlled by fusion (mitofusin 1 (MFN1), mitofusin 2 (MFN2) and optic atrophy 1 (OPA1)) and fission proteins (dynamin 1 (DNM1), dynamin 2 (DNM2), dynamin-related protein 1 (DNM1L; also referred to as DRP1), mitochondrial fission factor (MFF) and fission 1 protein (FIS1)) (Hoppins et al., 2007; Kraus et al., 2021). In addition, elements of the cytoskeleton are involved, and the importance of actin cytoskeleton-mediated and microtubule-associated regulation of mitochondrial dynamics is emerging (Illescas et al., 2021; Vona et al., 2021). Mitochondrial biogenesis is a complex process resulting in increased numbers of mitochondria. This is driven by a variety of nuclear genes, which encode mitochondrial proteins that are responsible for the replication and transcription of the mitochondrial genome (Hock and Kralli, 2009; Popov, 2020). Mitochondria and their roles in oocytes are rather well studied (Udagawa and Ishihara, 2020; Zou et al., 2021). In contrast, mitochondria in follicular GCs and luteal cells, and their structure, dynamics and roles are only rudimentarily known, especially in human cells.
There is evidence for mitochondrial changes associated with ageing (May-Panloup et al., 2016; Liu et al., 2017). Electron microscopy studies of GCs derived from IVF patients have indicated that the shape of mitochondria varies from round and oval to elongated, presumably due to age. However, a recent study in cultured human GCs also implicated LH in mitochondrial changes, as high concentrations of LH reduced mitochondrial diameter and size (Wan et al., 2021). A recent ultrastructural study in goat ovaries also provided morphological information. It indicated that a branched mitochondrial network dominated in GCs of follicles, while spherical and tubular mitochondria were typical for large luteal cells. The data also indicated that mitochondrial diameter and volume increased during folliculogenesis (Jiang et al., 2021). Thus, many factors and possible species differences may be involved in determining mitochondrial morphology of GCs.
The human ovary is not readily accessible for investigation. Therefore, the aim of the present study was to examine and visualize aspects of mitochondrial dynamics during normal follicular development, focusing on the ovary of a closely related non-human primate species. To this end, the expression of the mitochondrial marker protein cytochrome c oxidase subunit 4 (COX4), indicating mitochondrial activity, was examined in different stages of follicles ranging from primary to large antral follicles, and in active CL from Rhesus monkey ovaries, employing immunohistochemistry. As an apt model for proliferating human GCs available for cellular studies, human KGN cells were used, i.e. tumour cells derived from a patient’s ovarian granulosa cell tumour (Bagnjuk et al., 2019; Nishi et al., 2001). KGN cells, rather than IVF-derived human GCs from individual patients were studied, because the latter are notoriously heterogeneous and are only used as a model for the peri-ovulatory follicle and CL. Mitochondrial morphology and dynamics in KGN cells were examined after stimulation with forskolin (FSK), employing several experimental approaches, including fluorescence mitochondrial stainings, ultrastructural 3D reconstruction and proteomic analysis.
Materials and methods
Cell culture of KGN cells
KGN cells are a human tumour cell line derived from an ovarian granulosa cell tumour (Nishi et al., 2001), provided by the RIKEN BioResource Center, Japan. KGN cell culture conditions were described in detail previously (Bagnjuk et al., 2019; Buck et al., 2019). In brief, KGN cells were cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12, Thermo Fisher Scientific, Waltham, MA, USA) complemented with 10% foetal calf serum (FCS, Capricorn Scientific GmbH, Ebsdorfergrund, Germany) and 0.1 µg/ml Normocin (InvivoGen Europe, Toulouse, France) and were incubated at 37°C with 5% CO2 and 95% humidity. For the experiments, KGN cells from passage numbers 20–30 were used.
Treatment of KGN cells
One day before any treatment, 5 × 105 KGN cells were seeded onto cell culture dishes (TC Dish 60, Standard, Sarstedt AG & Co KG, Nümbrecht, Germany) for RT-qPCR studies, and 2.5 × 104 cells were seeded on glass cover slips for mitochondrial staining. Treatments were carried out with cells starved on DMEM/F-12 without FCS and antibiotic for 2 h. For FSK and control treatments, 50 µM FSK (Sigma Aldrich, St. Louis, MO, USA) or the equivalent volume of the corresponding solvent, ethanol (EtOH; final concentration 0.1%), was added to the medium for 24 h. The combinational treatment with FSK and the protein kinase A (PKA) inhibitor H89 (Cayman Chemical, Ann Arbor, MI, USA) was assessed after 1 h of starvation by the administration of 30 µM H89 or the equivalent volume of EtOH for the controls. After completion of the 2 h starvation period, 50 µM FSK or solvent control were added either for a period of 3 h (for RT-qPCR studies) or for 24 h (for mitochondrial staining). After any treatment, the medium was aspired and the cells were washed thoroughly with PBS before further processing.
Cell size determination
The CASY® Cell Counter (Schärfe Systems, Reutlingen, Germany) was used to determine the cell number (n = 3) and cellular diameter (n = 6) of trypsinized KGN after 24 h treatment with solvent control or 50 µM FSK. The measurement was performed as described previously (Schell et al., 2010). CASY measures the conductivity between two electrodes separated by a defined pore and counts cells using Electronic Current Exclusion and Pulse Field Analysis enabling the determination of viability without staining or focusing. Viable cells with full cell volume are detected, as the cell membrane provides a barrier for the current, while in dead cells lacking membrane resistance, only the nucleus is detected.
DNA extraction
Total DNA was extracted from 24 h solvent control or 50 µM FSK-treated KGN cells (5 × 105 cells/group) using the Wizard® SV Genomic DNA Purification System (Promega, Fitchburg, WI, USA) according to the manufacturer’s instructions.
Quantification of mitochondrial DNA copy number
Quantification of mitochondrial DNA (mtDNA) copy number in 24 h solvent-control or 50 µM FSK-treated KGN cells was carried out by qPCR (n = 6), as described earlier (Jackson et al., 2012; Schmid et al., 2019). In brief, we used 5 ng DNA and primer sets for nuclear receptor coactivator three (NCOA3) and mtDNA (see Table I). qPCR conditions were: 5 min, 95°C preincubation, then 40 cycles of amplification, including denaturation at 95°C for 10 s, annealing at 60°C for 30 s and a melting step by heating from 65°C to 95°C, followed by a cooling step to 37°C for 30 s in a LightCycler® 96 System (Roche). For mtDNA quantification, the comparative 2−ΔΔCq method was used (Pfaffl, 2001).
Table I.
Oligonucleotide primer sequences and corresponding amplicon size.
| Gene | Ref ID | Nucleotide sequence | Amplicon size (bp) |
|---|---|---|---|
| L19 | NM_000981.3 | 5′-AGG CAC ATG GGC ATA GGT AA-3′ | 199 |
| 5′-CCA TGA GAA TCC GCT TGT TT-3′ | |||
| StAR | NM_000349 | 5′-ACG TGG ATT AAC CAG GTT CG-3′ | 149 |
| 5′-CAG CCC TCT TGG TTG CTA AG-3′ | |||
| CYP19A1 | NM_000103 | 5′-GCT ACC CAG TGA AAA AGG GGA-3′ | 140 |
| 5′-GCC AAA TGG CTG AAA GTA CCT AT-3′ | |||
| CYP11A1 | NM_001099773 | 5′-GGG ACT TCG TCA GTG TCC TG-3′ | 104 |
| 5′-GAT GGA CTC AAA GGC AAA GC-3′ | |||
| OPA1 | NM_130831.2 | 5′-CTC TGC AGG CTC GTC TCA AG-3′ | 108 |
| 5′-CAC ACT GTT CTT GGG TCC GA-3′ | |||
| MFN1 | NM_033540.2 | 5′-AGC TGG CTG TCT TGT ACG TG-3′ | 95 |
| 5′-TGA CAT CTG TGC CTG GAC TG-3′ | |||
| MFN2 | NM_014874.3 | 5′-TCA GAG CCC GAG TAC ATG GA-3′ | 142 |
| 5′-CGT TGA GCA CCT CCT TAG CA-3′ | |||
| DNM1L | NM_001278466.1 | 5′-TAT GCC AGC CAG TCC ACA AA-3′ | 98 |
| 5′-CAC AAT CTC GCT GTT CCC GA-3′ | |||
| COX4 | NM_001318797.1 | 5’-AGC GAG CAA TTT CCA CCT CT-3’ | 90 |
| 5’-TCA CGC CGA TCC ATA TAA GCT-3’ | |||
| mtDNA | NC_012920.1 | 5′-GCC ACA GCA CTT AAA CAC ATC TCT-3′ | 186 |
| 5′-TAG GAT GGG CGG GGG T-3′ | |||
| NCOA3 | NC_000020.11 | 5’-CCTCTGGGCTTTTATTGCGAC-3′’ | 188 |
| 5’-CGGTCATCAGAAGAACAGGTAAGT-3′’ |
RNA extraction and quantitative RT-qPCR
RNA extraction, reverse transcription and RT-qPCR were carried out as described previously (Schmid et al., 2019). The changes in gene expression were normalized to the mean mRNA expression levels of the ribosomal protein L19 (L19) and peptidylprolyl isomerase A (PPIA) as internal controls and subjected to the 2-ΔΔCq method, as described elsewhere (Pfaffl, 2001). Information on oligonucleotide primers is given in Table I.
Focused ion beam/scanning electron microscopy
KGN cells were seeded on laser marked slides, fixed with 2.5% (v/v) glutaraldehyde in 75 mM cacodylate buffer and stained with DAPI (Luckner and Wanner, 2018a). In each case, five representative FSK-treated cells and five solvent-control cells were selected in phase contrast light microscopy and documented with a CCD camera with the corresponding epifluorescence DAPI image. Additionally, either osmium tetroxide or reduced osmium-TCH-osmium (=rOTO) was used for postfixation/staining of the cells, before ultra-thin embedding in epoxy resin (Luckner and Wanner, 2018a,b). For control of the heavy metal staining, rOTO-treated cells were again documented by bright field light microscopy after embedding. Further procedures for high-resolution SEM and focused ion beam/scanning electron microscopy (FIB/SEM) milling have been described in detail (Luckner and Wanner, 2018a). Cytoplasmic areas adjacent to the nucleus were imaged in one solvent-control and one 50 µM FSK-treated cell. The images were recorded at 3072 × 2048 pixels. The data sets were analysed as described previously (Schmid et al., 2019). For FIB/SEM tomography, a voxel size of 4 × 4 × 10 nm was chosen with subsequent 3D reconstruction using Armia™ (Thermo Fisher). Furthermore, the diameters of 11 mitochondria in the solvent-control cell and of 14 mitochondria in the FSK-treated cell were determined (average of smallest and widest cross-section).
Immunohistochemistry
Immunohistochemistry of ovaries was described previously (Blohberger et al., 2015). The paraffin sections stem from adult rhesus monkey ovaries (n = 4) were provided by the Oregon National Primate Research Center (ONPRC) at Oregon Health and Science University (OHSU; Beaverton, OR, USA). The animal husbandry and use were performed according to, and approved by, the institutional animal care and use committee (IACUC) of the ONPRC/OHSU. The samples were used in previous studies (Blohberger et al., 2015). For immunohistochemical staining, polyclonal anti-COX4 antibody (NB110-39115; 1:1000; Novus Biologicals, Wiesbaden Nordenstadt, Germany) was used. This antiserum was raised against a peptide made according to an internal region of human COX4 isoform 1 (within residues 1-100; Swiss-Prot# P13073). For negative controls, the primary antiserum was omitted and replaced by normal goat serum. Non-immune serum or IgG served as further controls. The sections were slightly counterstained with Hematoxilin (Carl Roth, Karlsruhe, Germany) and examined with a Zeiss Axiovert light microscope (Carl Zeiss, Jena, Germany).
Mitochondrial staining
After completed treatment and washing with PBS, cells were incubated with 250 nM MitoTracker™ Orange (Invitrogen, Carlsbad, CA, USA) for 30 min at 37°C, 5% CO2 and 95% humidity in the dark and then fixed with 4% formaldehyde (Carl Roth) and counterstained with DAPI (Carl Roth). Examination was implemented with a confocal microscope, Leica TCS SP8 (Leica Microsystems GmbH, Wetzlar, Germany) or a wide-field microscope Zeiss Axio Observer.Z1 (Carl Zeiss, Oberkochen, Germany). To study consequences of FSK treatment, a total of 526 cells from four experiments were evaluated and for the study of the combinational treatment with FSK and H89, a total of 138 cells from three experiments were analysed for effects on mitochondrial organization. Two investigators evaluated the micrographs independently from each other and came to comparable results.
LC-MS/MS
Samples (KGN treated with 50 µM FSK or solvent control EtOH for 24 h, n = 3) were processed using the Preomics iST-kit as recommended by the manufacturer. For LC-MS purposes, desalted peptides were injected in an Ultimate 3000 RSLCnano system (Thermo) and separated in a 25-cm analytical column (75 µm ID, 1.6 µm C18, IonOpticks) with a 100-min gradient from 2 to 37% acetonitrile in 0.1% formic acid. The effluent from the HPLC was directly electrosprayed into a Qexactive HF (Thermo) operated in data-dependent mode to automatically switch between full scan MS and MS/MS acquisition. Survey full scan MS spectra (from m/z 375–1600) were acquired with resolution R = 60 000 at m/z 400 (AGC target of 3 × 106). The 10 most intense peptide ions with charge states between 2 and 5 were sequentially isolated to a target value of 1 × 105, and fragmented at 27% normalized collision energy. Typical mass spectrometric conditions were: spray voltage, 1.5 kV; no sheath and auxiliary gas flow; heated capillary temperature, 250°C; ion selection threshold, 33.000 counts. MaxQuant 1.6.3.4 was used to identify proteins and quantify by LFQ with the following Parameters: Database, Uniprot_P000005640_Hsapiens_190109.fasta; MS tol, 10 ppm; MS/MS tol, 20 ppm Da; Peptide FDR, 0.1; Protein FDR, 0.01 Min. peptide Length, 7; Variable modifications, Oxidation (M); Fixed modifications, Carbamidomethyl (C); Peptides for protein quantitation, razor and unique; Min. peptides, 1; Min. ratio count, 2. Identified proteins were considered as differential if their MaxQuant log2 fold change LFQ values were higher than 2 log2. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [1] partner repository with the data set identifier PXD032160 (Perez-Riverol et al., 2019).
Data analysis and statistics
Microscopical monitoring during cell culture and recording after any treatment was done using a Leica DM IL LED microscope (Leica Microsystems GmbH, Wetzlar, Germany), equipped with a 10× objective (HI Plan CY ×10/0.25 dry, Leica Microsystems) and a monochrome camera (DFC3000 G, Leica Microsystems) with the corresponding software (Leica Applications Suite X, version 3.7.0.20979, Leica Microsystems). Images were adjusted for brightness and contrast by means of Fiji (open-source image processing package for imageJ). For any quantitative and statistical analysis, Microsoft Excel (2018, Microsoft, Redmond, WA, USA) and GraphPad Prism 7 software (GraphPad Software, San Diego, CA, USA) were used. RT-qPCR data sets were subjected to the Shapiro–Wilk normality test and upon given normal distribution, data from solely FSK-treated cells were subjected to paired two-tailed Students t-test and those from combinational treated ones were subjected to one-way ANOVA. The percental distribution of mitochondrial morphology within the distinct treatment was analysed using ANOVA for matched measures; the percentages of mitochondria ascribed to the defined categories (fragmented, intermediate or network) were compared between the given treatment (EtOH ctrl., 50 µM FSK, 30 µM H89, FSK & H89) and untreated cells. For any test, α was set to 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001), and data are depicted as the mean ± SEM.
Results
Localization of the mitochondrial marker COX4 in GCs of the Rhesus monkey ovary
Immunohistochemistry revealed expression of the mitochondrial marker COX4 in all major ovarian cell types, mainly in GCs, theca cells, oocytes and interstitial cells in the non-human primate ovary (Fig. 1A–C). Staining was specific and all controls (including IgG controls; Fig. 1A, insert) were negative. Intensities of the COX4 stainings changed in GCs, depending on follicular development. COX4 was barely detectable in the pre-granulosa cells of primary follicles, but from the primary stage onwards, staining increased steadily (Fig. 1B). This indicates a connection between follicle growth and mitochondrial activity and biogenesis. In GCs of growing follicles, the staining was typically confined to a restricted localization next to the nucleus. However, from the antral stage onwards, the cells next to the basal lamina showed the strongest staining, which was homogeneously distributed within the cytoplasm (Fig. 1C). In luteinized GCs of the CL, COX4 was also homogeneously distributed (Fig. 1D). The COX4 staining pattern in GCs suggested mitochondrial biogenesis during follicular growth. In antral follicles, which are under the control of FSH, the strong staining of mural GCs, further implicated that FSH might, in part, be involved in mitochondrial biogenesis and/or intracellular network formation in antral follicles. The homogeneous staining of luteal cells may indicate the presence of intracellular networks and may possibly imply a role for LH.
Figure 1.
Immunohistochemical staining of COX4 in Rhesus monkey ovaries. (A) COX4 is readily detected in oocytes (asterisks), granulosa (GC) and thecal cells (TC) of growing follicles, as well as in interstitial cells (IC). Inset: Corresponding control (IgG instead of primary antibody). (B) COX4 protein is barely seen in flat pre-granulosa cells of primordial follicles (1) but increased staining is seen in granulosa cells of primary (2) and secondary follicles (3). Asterisks mark oocytes. (C) COX4 levels in mural granulosa cells (GC; arrows) of an antral follicle were increased. Asterisks mark oocytes. (D) COX4 in luteinized granulosa cells (GLC) and luteinized thecal cells (TLC). Inset: Corresponding control (primary antibody omitted). Sections were slightly counterstained with haematoxylin.
Evaluation of mitochondrial dynamics in KGN cells
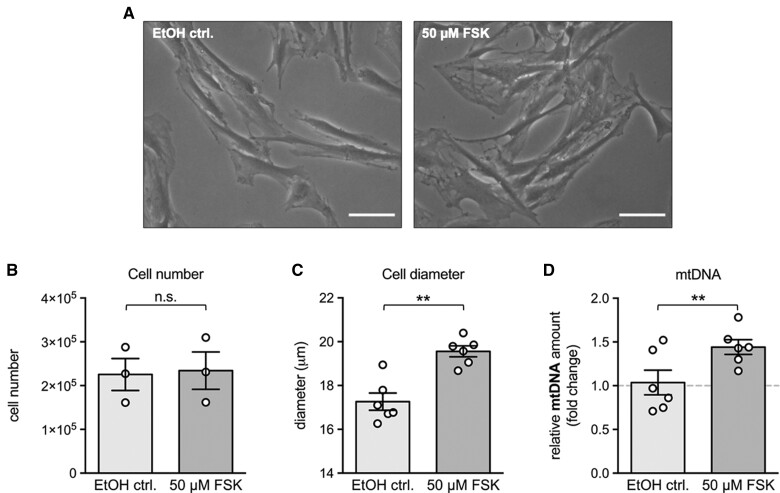
Human KGN cells were used to further examine aspects of mitochondrial dynamics and its regulation. FSH signalling was mimicked using FSK to increase the second messenger, intracellular cAMP. This treatment (24 h) resulted in a significant enlargement of the cell diameter (2.3 ± 0.4 µm) (Fig. 2A and C) and a slight, yet significant increase in mtDNA copy numbers (1.44 ± 0.1-fold) (Fig. 2D), when compared with solvent controls. Cell number was not changed due to the FSK treatment (Fig. 2B) when compared with solvent controls.
Figure 2.
Effects of FSK treatment on morphology, number, diameter and mtDNA amount of granulosa tumour (KGN) cells. (A) Representative microscopic images of KGN cells treated with the solvent (ethanol) control (EtOH ctrl., left panel) or 50 µM FSK (right panel) for 24 h. FSK treatment resulted in altered cellular morphology compared to the solvent control. Scale bar 50 µm. (B) FSK treatment did not affect the cell number (n = 3), but significantly increased both the (C) cellular diameter and the (D) mtDNA amount (n = 6 each). Individual values and means ± SEM are given; **P < 0.01; n.s., not significant; FSK, forskolin.
FSK also induced the formation of an elaborate intracellular mitochondrial network, indicated by confocal microscopy after MitoTracker™ staining (Fig. 3A and B). Examples for defining mitochondrial morphology as ‘fragmented’, ‘intermediate’ or ‘network’ are provided in Fig. 3A. Almost 80% of FSK-treated cells formed mitochondrial networks in contrast to the solvent control cells, in which networks were only observed in about 5% of the cells (Fig. 3B).
Figure 3.
Mitochondrial morphology in granulosa tumour (KGN) cells and effect of FSK treatment. (A) Representative MitoTracker™ Orange images of KGN mitochondria with fragmented (left panel), intermediate (middle panel) or network-like morphology (right panel). (B) Representative MitoTracker™ Orange images of 24 h solvent control or 50 µM FSK-treated KGN cells (B, left). Percentage distribution of the mitochondrial morphology after the treatments (n = 4 individual experiments, 526 evaluated cells). Means ± SEM are given; *P < 0.05; **P < 0.01; n.s., not significant (B, right). For a detailed statistical evaluation, see Supplementary Table SI. (C) FIB/SEM tomography of KGN cells treated with solvent control or 50 µM FSK for 24 h. Selected cells were re-localized with SEM and longitudinally sectioned with FIB. In the control cell, the mitochondria were rather egg-shaped with large diameter (C, top left). The mitochondria of the FSK-treated cell had a small diameter and were roundish (C, top right). 3D reconstruction of the mitochondria confirmed egg-shaped mitochondria in the control cells (C, lower left). Mitochondria of the FSK-treated KGN cell shown were rather thin and elongated (C, lower right). FSK, forskolin.
FIB/SEM tomography of FSK-treated and control cells allowed us to reconstruct mitochondria in perinuclear cytoplasmic regions of KGN cells and to compare the resulting 3D reconstruction with the results of the MitoTracker™ microscopic study (Fig. 3C). In the FSK-treated cell, mitochondria have a small diameter (230 ± 17 nm; n = 14 mitochondria) and are elongated, in contrast to the mitochondria observed in the control cell, which were rather roundish (592 ± 128 nm; n = 11 mitochondria). This result agrees with the results of the MitoTracker™ study and indicates that the MitoTracker™ images adequately represent the morphological change and network formation of mitochondria upon FSK treatment.
Since FSK elevates cAMP, which activates PKA, we tested the effects of the PKA-inhibitor H89 (Fig. 4A and B). The changes in mitochondrial morphology and network formation were blocked by H89, implying cAMP/PKA as the main regulators. Also, the solvent-treated cells were compared in these experiments to medium-only treated cells (Fig. 4) and a slight trend towards increased fragmentation was observed but was not statistically significant.
Figure 4.
Effect of FSK and H89 individually and in combination on mitochondrial morphology in granulosa tumour (KGN) cells. (A) Representative MitoTracker™ Orange images of untreated (untr.), ethanol solvent (EtOH ctrl.), 50 µM FSK, 30 µM H89 or FSK and H89 (in combination) treated KGN cells. (B) Changes in percentage distribution of mitochondrial morphology after 24 h treatment with 50 µM FSK, 30 µM H89, the combination of both, or in untreated cells (n = 3 experiments, 138 evaluated cells). Means ± SEM are given; *P < 0.05; **P < 0.01; n.s., not significant. For detailed statistical evaluation, see Supplementary Table SI. FSK, forskolin; H89, a protein kinase A (PKA) inhibitor.
Results of proteomic analysis and RT-qPCR
A proteomic analysis of FSK-treated KGN cells (n = 3) was performed to identify FSK-regulated proteins. The results revealed 12 significantly increased and 5 significantly decreased proteins, as shown in the Volcano plot (Fig. 5) and in Table II. Proteins with increased abundance upon FSK treatment are involved in microtubular dynamics (RMDN2, Regulator Of Microtubule Dynamics 2) or actin network formation (JMY, Junction Mediating And Regulatory Protein, P53 Cofactor; FAT1, FAT Atypical Cadherin 1), as well as steroidogenesis (CYP11A1, Cytochrome P450 Family 11 Subfamily A Member 1). Mitofusins and OPA1, important mitochondrial fusion factors, were detected among the identified proteins but, of note, not among the proteins altered in abundance (data available via ProteomeXchange with identifier PXD032160).
Figure 5.
Volcano plot of proteomic analysis of granulosa tumour (KGN) cells treated with FSK or solvent control. Proteomic analysis of KGN cells treated with 50 µM FSK or solvent control for 24 h (n = 3). Volcano plot of significantly decreased (left, 1–5) and significantly increased (right, 6–17) proteins due to FSK treatment. Proteins altered in abundance of FSK-treated KGN cells are listed in Table II. FSK, forskolin.
Table II.
Proteins altered in abundance in FSK-treated KGN.
| # | Protein | Gene | Fold change |
|---|---|---|---|
| 1 | Ribonucleoside-diphosphate reductase subunit M2 | RRM2 | −3.05 |
| 2 | Thioredoxin-interacting protein | TXNIP | −2.50 |
| 3 | Ras and Rab interactor 2 | RIN2 | −2.33 |
| 4 | Cellular communication network factor 1 | CCN1 | −2.59 |
| 5 | Calcium-binding and coiled-coil domain-containing protein 1 | CALCOCO1 | −2.05 |
| 6 | Arylacetamide deacetylase | AADAC | 2.27 |
| 7 | Sodium-dependent phosphate transporter 1 | SLC20A1 | 2.07 |
| 8 | Complement decay-accelerating factor | CD55 | 2.76 |
| 9 | Leucine-rich repeat and calponin homology domain-containing protein 1 | LRCH1 | 2.81 |
| 10 | Roundabout homolog 1 | ROBO1 | 3.12 |
| 11 | Cholesterol side-chain cleavage enzyme | CYP11A1 | 3.61 |
| 12 | Protocadherin Fat 1 | FAT1 | 3.75 |
| 13 | Ubiquitin carboxyl-terminal hydrolase | USP19 | 2.16 |
| 14 | Neuropeptide Y receptor type 1 | NPY1R | 2.06 |
| 15 | Junction-mediating and -regulatory protein | JMY | 2.05 |
| 16 | Regulator of microtubule dynamics protein 2 | RMDN2 | 2.08 |
| 17 | Histone H3 | H3-3B | 2.69 |
RT-qPCR studies (Fig. 6) of 24 h solvent-control or FSK-treated KGN cells indicated that even at the transcript level, the mitochondrial fusion genes MFN1, MFN2 and OPA1, as well as the fission gene DNM1L, were not affected by FSK treatment (Fig. 6A). Likewise, COX4 levels were not changed. In contrast, the expression levels of the steroidogenic enzymes StAR, CYP19A1 and CYP11A1 were strongly elevated (Fig. 6B), and this was blocked by the PKA-inhibitor H89 (Fig. 6C).
Figure 6.
Effect of FSK and H89 individually and in combination on mRNA levels in granulosa tumour (KGN) cells. (A, B) 24 h treatment of KGN cells with 50 µM FSK (n = 7) showed no changes in mRNA expression levels of (A) the fusion genes MFN1, MFN2, OPA1 and of the fission gene DNM1L, as well as COX4, but the mRNA levels of (B) the steroidogenic enzymes StAR, CYP19A1 and CYP11A1 were significantly increased. (C) 3 h treatment with 50 µM FSK resulted in significantly increased expression levels of StAR, CYP19A1 and CYP11A1, which could be blocked by 1 h preincubation with 30 µM of the PKA-inhibitor H89 and subsequent treatment with both agents in combination (n = 4). mRNA levels were normalized to solvent treated control cells; individual levels and means ± SEM are given; **P < 0.01: ***P < 0.001; n.s., not significant; FSK, forskolin.
Discussion
A recent study in goat ovaries indicated that mitochondrial diameter and volume increase when follicular GCs develop into luteal cells (Jiang et al., 2021). The mentioned study also followed the formation of the CL in situ and used experimental approaches, which are in part different from ours, i.e. mainly electron microscopy (including COX-labelling) and histochemistry. The authors suggested that mitochondrial enlargement may occur throughout follicular and early CL development.
Few studies have described mitochondrial changes in the rhesus monkey ovary (Gulyas et al., 1976; Dong et al., 2014). Yet, hypophysectomy was shown to result in smaller mitochondria of granulosa lutein cells (Gulyas et al., 1976). Upon repeated hormone-induced superovulation, mitochondrial abnormalities, including cristae degradation in GCs, and a reduced expression of genes involved in steroid hormone synthesis were observed (Dong et al., 2014). Hence, mitochondrial structure, integrity and functionality appear to be closely interlinked and to be hormone-dependent in this non-human primate.
In our study, COX4 immunohistochemistry was used to examine mitochondria in non-human primate ovaries at the light microscopic level. This protein is a component of cytochrome c oxidase, the last enzyme in the mitochondrial electron transport chain driving oxidative phosphorylation. Thus, we reasoned that visualization of COX4 provides an adequate estimate not only of existing, but also of metabolically active, mitochondria. Of note, follicles undergoing atresia (e.g. showing pycnotic nuclei) were not considered. The immunohistochemical results for COX4 immunoreactivity in ovaries of the Rhesus monkey supported the notion that functional mitochondria increase numerically in GCs during follicle growth. The results also suggested that mitochondria are re-located to different intracellular positions within GCs, possibly implying intracellular trafficking. In preantral and small antral follicles, a peri-nuclear localization dominates. In large antral follicles and in mural GCs, the staining was mainly positioned towards the basal lamina, i.e. towards the side of blood and hence towards the oxygen, glucose and hormone supply. Of note, mural GCs possess abundant FSH receptors (Casarini and Crépieux, 2019), hinting to a possible regulatory influence. In luteal cells, which develop from GCs after ovulation, staining appeared evenly distributed within the cells. This homogeneous staining in the cytoplasm of luteal cells might suggest large intracellular networks of mitochondria. Taken together, the observed changes of the mitochondrial marker COX4, first, imply increased numbers and intracellular re-distribution of mitochondria in GCs during follicle growth, and, second, indicate that factors controlling GCs in situ may also regulate mitochondrial dynamics. As the gonadotropins FSH/LH are especially involved in governing growth and differentiation of GCs of large follicles and in the CL, they are likely candidates for such regulatory factors. Both hormones elevate cAMP levels and act mainly via PKA (Riccetti et al., 2018).
As the mechanism underlying mitochondrial dynamics in GCs of non-human primates and humans are not readily accessible to investigation, we reasoned that studies in the human GC line KGN may be instructive, specifically to address the question of whether cAMP may mediate mitochondrial dynamics. KGN cells were also used because we found that under basal culture conditions, different mitochondrial morphologies are present (Fig. 3A). Furthermore, they allow one to exclude interfering factors, such as patient age, medical history or different responses to LH (Liu et al., 2017; Wan et al., 2021), i.e. factors, which were shown to strongly impact mitochondrial morphology and function in human IVF-derived GCs. Finally, in contrast to IVF-derived GCs, KGN cells are an adequate model for proliferating human GCs (Bagnjuk et al., 2019) and may be regarded as a model for the growing follicle. Yet, KGN cells can change their phenotype and growth when propagated in vitro (Imai et al., 2008). It is not known whether this also involves altered FSH receptor signalling. We reasoned that FSK provides a more reliable and far more robust means of increasing intracellular cAMP than FSH.
As mentioned, MitoTracker™ experiments revealed that mitochondria in KGN cells can be observed in different morphological states under basal conditions ranging from fragmented to a network-like state. FSK changed the proportions of the different mitochondrial morphologies and induced a significantly increased mitochondrial network within 24 h. That MitoTracker™ images correspond to mitochondrial shape was confirmed by the results of FIB/SEM tomography and this, therefore, confirmed the action of FSK to induce elongated network-like mitochondria.
H89 is a frequently used cell-permeable and relatively specific PKA inhibitor, as recently reviewed (Liu et al., 2020) but can also inhibit other kinases (e.g. myosin light chain kinase, Ca2+/calmodulin-dependent protein kinase II, protein kinase C, casein kinase I and Rho Kinase II). When added to FSK-treated KGNs, it prevented network formation induced by FSK (Fig. 4A). Hence, it is likely that the mitochondrial changes observed are mainly due to downstream actions of PKA, yet the involvement of other kinases cannot be completely excluded.
FSK did not change the cell number but increased the cell size and amount of mtDNA moderately. The proteomic study performed after 24 h of FSK treatment identified 17 proteins with significant changes in abundance. Among others, the list of the decreased proteins includes CALCOCO1 (Calcium Binding And Coiled-Coil Domain 1), which was identified as a soluble reticulophagy and Golgiphagy-receptor (Nthiga et al., 2020, 2021). Hence, it is possible that the lysosome-mediated macroautophagy degradation pathway is reduced upon FSK-treatment and that this consequently may contribute to the enlargement of KGN cells.
Increased levels of proteins involved in microtubular dynamics (RMDN2) or actin network formation (JMY), as well as the steroidogenic enzyme CYP11A1 were also found. However, mitofusins or OPA1, which are known mitochondrial regulators, were not among the proteins changed in abundance. They were, however, present in the proteomic data of KGN cells and, in case of DNM1L (DRP1), also present in monkey ovarian GCs (Supplementary Fig. S1). Additional RT-qPCR studies indicated that mRNA levels of the mitofusins (MFN1/2), OPA1 and DNM1L were not affected by FSK within 24 h. Hence, it is possible that the observed rearrangement of mitochondria occurring within 24 h in KGN cells may not depend on the levels of these proteins.
It is, however, possible that not the levels, but rather the activities, of some of these proteins may be of importance. The activity of DRP1 (DNM1L), for example, a key mediator of mitochondrial fission, can be regulated by its phosphorylation state. Indeed, DRP1 (DNM1L) has two different sites (Ser616/Ser 637), which are phosphorylated by PKA (Chang and Blackstone, 2007; Cribbs and Strack, 2007; Kraus et al., 2021). DRP1 (DNM1L) was recently studied in bovine luteal cells and a role in basal and LH-induced progesterone production became apparent (Plewes et al., 2020). Unfortunately, that study did not address morphological changes of mitochondria upon LH but rather revealed that LH inhibits colocalization of DRP1 (DNM1L) on mitochondria. A study in MA-10 Leydig cell tumour cells (Park et al., 2019) showed a role of DRP1 (DNM1L) phosphorylation in steroidogenesis and, furthermore, the authors noted that dBcAMP, when added to the cells, significantly affected mitochondrial shape and specifically increased mitochondrial elongation. These results appear comparable to the results of our study in KGN, where FSK was used to elevate cAMP levels. Clearly, a possible role of DRP1 (DNM1L) in morphological changes of mitochondria in GCs/KGN, as well as the roles of mitofusins and other proteins involved in mitochondrial biogenesis and dynamics, remain to be fully evaluated. In this context, a number of potential influences may come into play, since in human GCs, the levels of MFN2 levels were reported to be related to ageing and its regulation appeared to be related to pregnancy, albeit in young patients only (Wang et al., 2019).
The results of the proteome analysis indicate a possible role of the cytoskeleton in mitochondrial dynamics. A role for microtubules and the cytoskeleton in ovarian steroidogenesis has been suggested (Murdoch, 1996). Yet few follow-up studies exist to our knowledge and therefore, this topic remains to be fully examined (Wu and Zhang, 2022). Nevertheless, the role of the cytoskeleton in the regulation of mitochondria is of growing interest beyond the ovary. The importance of actin cytoskeleton-mediated regulation of mitochondrial function and the role of microtubules in the regulation of mitochondrial dynamics are emerging. These topics, to the best of our knowledge, have not yet been examined in the context of the ovary or (human) GCs. The abundance of FAT1, a member of the vertebrate FAT atypical cadherins, was increased upon FSK treatment in KGN cells. Its roles are not well established, yet FAT1 is linked to the regulation of the cytoskeleton (Tanoue and Takeichi, 2004; Hatakeyama et al., 2021) and has been implicated in cell remodelling. Additional studies have shown that it acts as a molecular brake on mitochondrial respiration (Cao et al., 2016). Whether similar modes of action may exist in KGN cells and in ovarian GCs, e.g. upon ovulation, and their possible links to mitochondria remain to be studied. Such roles may also include regulation of apoptosis, as shown in porcine ovarian cumulus cells, i.e. GCs surrounding the oocyte (Wu et al., 2017).
Upon ovulation, GCs differentiate and express CPY11A1 and thus acquire the ability to perform de novo synthesis of steroids. Our proteomics data and RT-qPCR data indicate that this is also a consequence of FSK stimulation in KGN cells. Whether and how CYP11A1 may be involved in the parallel mitochondrial shape change remain to be shown. An earlier study has suggested such a link (Yoshinaga-Hirabayashi and Yoneda, 2001). Murine NIH/3T3 cells under basal conditions had filamentous and elongated mitochondria. When transfected with CPY11A1, globular and round mitochondria were detected. While the results indicate an influence of this important steroidogenic factor in mitochondrial morphology, they are not in agreement with our results. Whether species differences, different time points of observation or other factors, e.g. cell type-related ones, are involved remains to be studied.
In conclusion, our results indicate that in vivo follicular growth is associated with increasing numbers of active mitochondria in GCs in a non-human primate species. In human KGN cells, elevation of cAMP, via PKA, elevated the mtDNA copy number and regulated the mitochondrial network formation in vitro. Our study also indicates that in KGN cells, regulation of mitochondria may be linked to the cytoskeleton. A limitation of the present study is that KGN cells, due to their tumour nature, may not fully mirror the situation in the human ovary. Furthermore, only short-term experiments with KGN cells were performed. However, within the ovary, where follicular growth is associated with increased numbers of mitochondria, these results may imply a fundamental role of cAMP, and presumably gonadotropins, in the regulation of mitochondrial dynamics in GCs and luteal cells.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Supplementary Material
Acknowledgements
We gratefully acknowledge the expert technical assistance of Nicole Kreitmair, Carola Herrmann and Astrid Tiefenbacher. We especially thank Toshihiko Yanase, Fukuoka University, Japan, for their permission to use KGN cells.
Authors’ roles
M.K., K.G. and K.E. performed the cellular experiments and evaluated the results. G.A.D. provided monkey tissue and conceptual input. N.S. and G.W. performed the FIB/SEM tomography and 3D reconstruction. I.F. and A.I. performed the proteomic analysis and evaluation of the study. N.S., K.E. and A.M. drafted the manuscript. A.M.-T. and M.H. gave guidance and suggestions. A.M. supervised and conceived of the manuscript. All authors contributed to the final version of the manuscript.
Funding
The studies were supported in part by grants from the German Research Foundation (DFG) (project number 456828204 to A.M. and project number 413985647 to M.H.).
Conflict of interest
The authors declare no conflict or financial interest.
Contributor Information
Melanie Kaseder, Biomedical Center Munich (BMC), Cell Biology, Anatomy III, Faculty of Medicine, Ludwig Maximilian University of Munich, Planegg-Martinsried, Germany.
Nina Schmid, Biomedical Center Munich (BMC), Cell Biology, Anatomy III, Faculty of Medicine, Ludwig Maximilian University of Munich, Planegg-Martinsried, Germany.
Katja Eubler, Biomedical Center Munich (BMC), Cell Biology, Anatomy III, Faculty of Medicine, Ludwig Maximilian University of Munich, Planegg-Martinsried, Germany.
Katharina Goetz, Biomedical Center Munich (BMC), Cell Biology, Anatomy III, Faculty of Medicine, Ludwig Maximilian University of Munich, Planegg-Martinsried, Germany.
Annette Müller-Taubenberger, Biomedical Center Munich (BMC), Cell Biology, Anatomy III, Faculty of Medicine, Ludwig Maximilian University of Munich, Planegg-Martinsried, Germany.
Gregory A Dissen, Molecular Virology Core, Oregon Health & Science University Oregon National Primate Research Center, Beaverton, OR, USA.
Max Harner, Biomedical Center Munich (BMC), Cell Biology, Anatomy III, Faculty of Medicine, Ludwig Maximilian University of Munich, Planegg-Martinsried, Germany.
Gerhard Wanner, Ultrastructural Research, Department Biology I, Ludwig Maximilian University (LMU), Planegg-Martinsried, Germany.
Axel Imhof, Biomedical Center Munich (BMC), Protein Analysis Unit, Faculty of Medicine, Ludwig Maximilian University (LMU), Planegg-Martinsried, Germany.
Ignasi Forne, Biomedical Center Munich (BMC), Protein Analysis Unit, Faculty of Medicine, Ludwig Maximilian University (LMU), Planegg-Martinsried, Germany.
Artur Mayerhofer, Biomedical Center Munich (BMC), Cell Biology, Anatomy III, Faculty of Medicine, Ludwig Maximilian University of Munich, Planegg-Martinsried, Germany.
Data Availability
All relevant data for the study are included in the article. The proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [1] partner repository with the data set identifier PXD032160.
References
- Bagnjuk K, Kast VJ, Tiefenbacher A, Kaseder M, Yanase T, Burges A, Kunz L, Mayr D, Mayerhofer A.. Inhibitor of apoptosis proteins are potential targets for treatment of granulosa cell tumors—implications from studies in KGN. J Ovarian Res 2019;12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blohberger J, Kunz L, Einwang D, Berg U, Berg D, Ojeda SR, Dissen GA, Fröhlich T, Arnold GJ, Soreq H. et al. Readthrough acetylcholinesterase (AChE-R) and regulated necrosis: pharmacological targets for the regulation of ovarian functions? Cell Death Dis 2015;6:e1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck T, Hack CT, Berg D, Berg U, Kunz L, Mayerhofer A.. The NADPH oxidase 4 is a major source of hydrogen peroxide in human granulosa-lutein and granulosa tumor cells. Sci Rep 2019;9:3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao LL, Riascos-Bernal DF, Chinnasamy P, Dunaway CM, Hou R, Pujato MA, O'Rourke BP, Miskolci V, Guo L, Hodgson L. et al. Control of mitochondrial function and cell growth by the atypical cadherin Fat1. Nature 2016;539:575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarini L, Crépieux P.. Molecular mechanisms of action of FSH. Front Endocrinol (Lausanne) 2019;10:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CR, Blackstone C.. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 2007;282:21583–21587. [DOI] [PubMed] [Google Scholar]
- Cribbs JT, Strack S.. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 2007;8:939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Guo Y, Cao H, Zhou T, Zhou Z, Sha J, Guo X, Zhu H.. Long-term effects of repeated superovulation on ovarian structure and function in rhesus monkeys. Fertil Steril 2014;102:1452–1457.e1. [DOI] [PubMed] [Google Scholar]
- Gulyas BJ, Yuan L, Tullner WW, Hodgen GD.. The fine structure of corpus luteum from intact, hypophysectomized and fetectomized pregnant monkeys (Macaca mulatta) at term. Biol Reprod 1976;14:613–626. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Nomura M, Wakimoto Y, Inoue S, Li C, Takamura D, Akisue T, Moriyama H.. Effects of cyclic tensile strain and microgravity on the distribution of actin fiber and Fat1 cadherin in murine articular chondrocytes. J Biomech 2021;129:110774. [DOI] [PubMed] [Google Scholar]
- Hock MB, Kralli A.. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 2009;71:177–203. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J.. The machines that divide and fuse mitochondria. Annu Rev Biochem 2007;76:751–780. [DOI] [PubMed] [Google Scholar]
- Illescas M, Peñas A, Arenas J, Martín MA, Ugalde C.. Regulation of mitochondrial function by the actin cytoskeleton. Front Cell Dev Biol 2021;9:795838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Muraki M, Takamatsu K, Saito H, Seiki M, Takahashi Y.. Spontaneous transformation of human granulosa cell tumours into an aggressive phenotype: a metastasis model cell line. BMC Cancer 2008;8:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CB, Gallati S, Schaller A.. qPCR-based mitochondrial DNA quantification: influence of template DNA fragmentation on accuracy. Biochem Biophys Res Commun 2012;423:441–447. [DOI] [PubMed] [Google Scholar]
- Jeppesen JV, Kristensen SG, Nielsen ME, Humaidan P, Dal Canto M, Fadini R, Schmidt KT, Ernst E, Yding Andersen C.. LH-receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab 2012;97:E1524–E1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YF, Yu PH, Budi YP, Chiu CH, Fu CY.. Dynamic changes in mitochondrial 3D structure during folliculogenesis and luteal formation in the goat large luteal cell lineage. Sci Rep 2021;11:15564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus F, Roy K, Pucadyil TJ, Ryan MT.. Function and regulation of the divisome for mitochondrial fission. Nature 2021;590:57–66. [DOI] [PubMed] [Google Scholar]
- Liu C, Ke P, Zhang J, Zhang X, Chen X.. Protein kinase inhibitor peptide as a tool to specifically inhibit protein kinase A. Front Physiol 2020;11:574030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Han M, Li X, Wang H, Ma M, Zhang S, Guo Y, Wang S, Wang Y, Duan N. et al. Age-related changes in the mitochondria of human mural granulosa cells. Hum Reprod 2017;32:2465–2473. [DOI] [PubMed] [Google Scholar]
- Luckner M, Wanner G.. From light microscopy to analytical scanning electron microscopy (SEM) and focused ion beam (FIB)/SEM in biology: fixed coordinates, flat embedding. Microsc Microanal 2018a;24:526–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckner M, Wanner G.. Precise and economic FIB/SEM for CLEM: with 2 nm voxels through mitosis. Histochem Cell Biol 2018b;150:149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferré-L'Hotellier V, Morinière C, Descamps P, Procaccio V, Reynier P.. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update 2016;22:725–743. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ. Microtubular dynamics in granulosa cells of periovulatory follicles and granulosa-derived (large) lutein cells of sheep: relationships to the steroidogenic folliculo-luteal shift and functional luteolysis. Biol Reprod 1996;54:1135–1140. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K. et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001;142:437–445. [DOI] [PubMed] [Google Scholar]
- Nthiga TM, Kumar Shrestha B, Lamark T, Johansen T.. The soluble reticulophagy receptor CALCOCO1 is also a Golgiphagy receptor. Autophagy 2021;17:2051–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nthiga TM, Shrestha BK, Lamark T, Johansen T.. CALCOCO1 is a soluble reticulophagy receptor. Autophagy 2020;16:1729–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Kim YJ, Lee SG, Kim JY, Chung JY, Jeong SY, Koh H, Yun J, Park HT, Yoo YH. et al. Drp1 phosphorylation is indispensable for steroidogenesis in leydig cells. Endocrinology 2019;160:729–743. [DOI] [PubMed] [Google Scholar]
- Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J, Mayer G, Eisenacher M. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 2019;47:D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewes MR, Hou X, Talbott HA, Zhang P, Wood JR, Cupp AS, Davis JS.. Luteinizing hormone regulates the phosphorylation and localization of the mitochondrial effector dynamin-related protein-1 (DRP1) and steroidogenesis in the bovine corpus luteum. FASEB J 2020;34:5299–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov LD. Mitochondrial biogenesis: an update. J Cell Mol Med 2020;24:4892–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccetti L, Sperduti S, Lazzaretti C, Casarini L, Simoni M.. The cAMP/PKA pathway: steroidogenesis of the antral follicular stage. Minerva Ginecol 2018;70:516–524. [DOI] [PubMed] [Google Scholar]
- Roger AJ, Muñoz-Gómez SA, Kamikawa R.. The origin and diversification of mitochondria. Curr Biol 2017;27:R1177–R1192. [DOI] [PubMed] [Google Scholar]
- Schell C, Albrecht M, Spillner S, Mayer C, Kunz L, Kohn FM, Schwarzer U, Mayerhofer A.. 15-Deoxy-delta 12-14-prostaglandin-J2 induces hypertrophy and loss of contractility in human testicular peritubular cells: implications for human male fertility. Endocrinology 2010;151:1257–1268. [DOI] [PubMed] [Google Scholar]
- Schmid N, Flenkenthaler F, Stockl JB, Dietrich KG, Kohn FM, Schwarzer JU, Kunz L, Luckner M, Wanner G, Arnold GJ. et al. Insights into replicative senescence of human testicular peritubular cells. Sci Rep 2019;9:15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E.. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 1997;18:739–773. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Takeichi M.. Mammalian Fat1 cadherin regulates actin dynamics and cell-cell contact. J Cell Biol 2004;165:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa O, Ishihara N.. Mitochondrial dynamics and interorganellar communication in the development and dysmorphism of mammalian oocytes. J Biochem 2020;167:257–266. [DOI] [PubMed] [Google Scholar]
- Vona R, Mileo AM, Matarrese P.. Microtubule-based mitochondrial dynamics as a valuable therapeutic target in cancer. Cancers (Basel) 2021;13:5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YT, Liu S, Zhao SK, Luo YY, Lv YS, Qu DN, Liu MH, Li Y.. Effect of luteinizing hormone concentration on transcriptome and subcellular organelle phenotype of ovarian granulosa cells. J Assist Reprod Genet 2021;38:809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Song S, Liu X, Zhang M, Xiang W.. Low MFN2 expression related to ageing in granulosa cells is associated with assisted reproductive technology outcome. Reprod Biomed Online 2019;38:152–158. [DOI] [PubMed] [Google Scholar]
- Wu X, Fu Y, Sun X, Liu C, Chai M, Chen C, Dai L, Gao Y, Jiang H, Zhang J.. The possible FAT1-mediated apoptotic pathways in porcine cumulus cells. Cell Biol Int 2017;41:24–32. [DOI] [PubMed] [Google Scholar]
- Wu Z, Zhang C.. Role of the cytoskeleton in steroidogenesis. Endocr Metab Immune Disord Drug Targets 2022;22:549–557. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Hirabayashi T, Yoneda Y.. Expression of SCC in ovarian granulosa cells and cultured cells, induced rapid structural changes in mitochondria. Ital J Anat Embryol 2001;106:51–57. [PubMed] [Google Scholar]
- Zou W, Ji D, Zhang Z, Yang L, Cao Y.. Players in mitochondrial dynamics and female reproduction. Front Mol Biosci 2021;8:717328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data for the study are included in the article. The proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [1] partner repository with the data set identifier PXD032160.