Abstract
Background
Inflammatory bowel disease (IBD) patients may develop anterior uveitis.
Methods
An observational cohort of IBD patients followed new users of (1) tumor necrosis factor inhibitor versus nonbiologic agents or (2) adalimumab versus infliximab until occurrence of anterior uveitis or treatment change/discontinuation. Cox-proportional hazards models estimated hazard ratios in propensity score-matched cohorts of Crohn disease or ulcerative colitis patients.
Results
No statistically significant differences in the risk of uveitis were observed between initiators of nonbiologics and tumor necrosis factor inhibitor. Effect estimates for adalimumab versus infliximab were highly imprecise due to limited outcomes.
Conclusions
Uveitis risk was not different between IBD patients treated with immunosuppressives.
Keywords: adalimumab, inflammatory bowel disease, infliximab, uveitis
INTRODUCTION
Inflammatory bowel disease (IBD) is estimated to affect 1–1.3 million people in the United States1, 2 and is associated with substantial societal burden and high healthcare costs.3, 4 More than one third of patients with IBD may be affected by manifestations outside of the gastrointestinal tract,5 which most commonly occur in the joints, skin, and eyes.6 Estimated incidence of ocular complications has varied, ranging between 4% and 30% of patients with IBD, with reports indicating higher incidence in patients with Crohn disease (CD) compared with ulcerative colitis (UC).7–12 Uveitis is among the most common ocular manifestations, accounting for 4%–6% of complications in IBD13–15, and can result in poor vision and blindness.16
Nonbiologic and biologic immunosuppressive agents are used to induce and maintain remission in IBD.17, 18 Nonbiologics immunosuppressives, such as azathioprine, 6-mercaptopurine, and methotrexate, have general immunomodulatory properties,19 whereas biologics target specific components of the immune system. For instance, the tumor necrosis factor inhibitors (TNFis) bind to the proinflammatory TNF-α proteins and are very effective in reducing inflammation in IBD patients.20 In addition to IBD, immunosuppressive agents are indicated for managing many other immune-mediated diseases such as rheumatoid arthritis and ankylosing spondylitis.21
Nonbiologics and TNFis are often prescribed to IBD patients with uveitis when inflammation in the eyes fail to resolve with the regular course of steroids.22 Recently, in a large randomized controlled trial,23 adalimumab was reported to be effective in reducing risk of flares and visual impairment in patients with noninfectious uveitis. Many small studies have suggested that TNFis, particularly infliximab, are effective at suppressing uveitis associated with various immune-mediated diseases.24–34 In a large systematic review, treatment with nonbiologic and biologic immunosuppressive agents was noted to be effective in controlling autoimmune uveitis.35 As uveitis secondary to IBD has immune-mediated origin, it is plausible to hypothesize that immunosuppressive treatment may be able to reduce risk of uveitis in IBD. No large-scale studies have evaluated the comparative risk of uveitis in IBD patients treated with different immunosuppressive agents.
The objective of this study was to compare the risk of developing uveitis in patients with IBD who were newly initiated on immunosuppressive medications: (1) nonbiologic immunomodulators versus TNFis and (2) adalimumab versus infliximab.
MATERIALS AND METHODS
Study Design and Data Source
We conducted an observational cohort study using health insurance claims data from the Truven MarketScan database, which captures longitudinal, individual-level administrative claims data from large employers, health plans, and public organizations in the United States. This database contains information on 179.2 million enrollees between 2003 and 2015. The following database tables were available for analysis: Enrollment Detail, Inpatient Admissions, Inpatient Services, Outpatient Services, Outpatient Pharmaceutical Claims, and Long-Term Care.
Cohort Creation
We identified patients with IBD based on an International Classification of Diseases, 9th Revision [ICD-9] diagnosis code for CD (ICD-9 code 555.xx) or ulcerative colitis (ICD-9 code 556.xx). We considered these classifications mutually exclusive and patients with both diagnoses were not included.
We required the IBD diagnosis code to be followed by at least one filled prescription for either a TNF-α inhibitor (infliximab, adalimumab, certolizumab, or golimumab) or a nonbiologic agent (mercaptopurine, azathioprine, methotrexate) during a period of at least 180 days of continuous health plan enrollment between January 1, 2003 and September 30, 2015. Combining diagnosis codes with IBD specific treatment dispensing was shown to have a positive predicted value of 90% in identifying cases from insurance claims in a previous validation study.36 We followed a new-user design, requiring all patients be incident users with respect to both drug classes within each comparison, with a wash-out period of 180 days. The date of filling the new prescription was defined as the index date. Patients initiating an agent from both exposure groups within a comparison on the same index date were excluded.
Patients were excluded from the adalimumab versus infliximab analysis if they had prior use of other TNFi drugs, certolizumab or golimumab, in the 180 days prior to index. Patients were excluded from the nonbiologics versus TNFi analysis if they had prior use of cyclosporine in the 180 days prior to index and if their TNFi prescription began during or within 14 days following a hospitalization to exclude IBD patients who may have initiated therapy while in hospital for whom the index date would be inaccurate (described below) and with very high disease activity.
Outcome Measurement
The outcome of interest was occurrence anterior noninfectious uveitis (ICD-9 codes 364.00, 364.01, 364.04) recorded in the inpatient or outpatient setting by an ophthalmologist, with a prescription for prednisolone acetate or difluprednate eye drops within 30 days before or after the diagnosis. Patients with a previous diagnosis of uveitis during the preindex period were not excluded from the primary analysis to capture all recurring acute episodes in addition to incident episodes. As a sensitivity analysis, we changed our primary outcome definition in 2 ways: (1) the requirement for uveitis diagnosis by an ophthalmologist was removed and (2) expansion of the uveitis diagnosis codes list to include additional ocular manifestations (iridocyclitis [364.3], posterior uveitis [363.2x, 363.0x, 363.10-.13, 363.15],37 and other disorders of the eye [379.xx]).
Follow-up Period
Patients were followed-up beginning the day after the index date. Follow-up was truncated at the earliest occurrence of the uveitis outcome, disenrollment from the health plan, death, end of data availability, and study end date September 30, 2015. Our primary analysis used an “as-treated” follow-up scheme, in which patients were only allowed to have a single exposure such that follow-up ceased for patients who filled a prescription for a drug in the other exposure group or discontinued the index drug (defined as not filling a subsequent prescription for 90 successive days following the day supply end of the most recent prescription). We pursued an “intention-to-treat” follow-up scheme in a sensitivity analysis, in which patients were followed-up regardless of index drug discontinuation or switching (but retained within their original exposure category). Follow-up for all patients in this scheme was truncated at 365 days to limit potential for exposed person-time misclassification.
Covariates
We evaluated baseline covariates during the 180 days prior to the index date. This included patient demographic characteristics, comorbid conditions (including competing indications for immunosuppressive medications), concomitant use of other medications, markers of healthcare utilization, and proxy measures of IBD severity. In order to capture IBD severity, the following variables for comorbid diagnoses and IBD-related healthcare services during the baseline period were defined: volume depletion, anemia, malnutrition, active fistulizing or internal penetrating disease, obstructing or structuring disease, total parenteral nutrition, blood transfusions, intra-abdominal surgeries, number of gastroenterologist visits, IBD hospitalization recency, colonoscopy recency, sigmoidoscopy recency, magnetic resonance imaging (MRI) of the abdomen and/or pelvis, computed tomography of the abdomen and/or pelvis, and clostridium difficile testing performed. The full list of covariates is presented in Table 1. In a sensitivity analysis, the preindex period of covariate assessment was expanded to 365 days.
Table 1.
Patient Characteristics Stratified by Exposure in the Propensity Score-Matched Population
| Crohn Disease | Ulcerative Colitis | Crohn Disease | Ulcerative Colitis | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | TNF Inhibitors | Nonbiologics | TNF Inhibitors | Nonbiologics | Infliximab | Adalimumab | Infliximab | Adalimumab |
| Number of patients | 14,044 | 14,044 | 7045 | 7045 | 9200 | 9200 | 2984 | 2984 |
| Demographics | ||||||||
| Age | ||||||||
| Mean (SD) | 39.00 (16.06) | 38.93 (16.95) | 42.34 (15.94) | 42.14 (16.63) | 37.51 (17.43) | 37.67 (14.51) | 41.88 (16.49) | 42.18 (14.89) |
| Median [IQR] | 38.00 [26.00, 52.00] | 39.00 [25.00, 52.00] | 42.00 [30.00, 54.00] | 42.00 [29.00, 55.00] | 37.00 [22.00, 51.00] | 36.00 [26.00, 49.00] | 42.00 [29.00, 55.00] | 42.00 [30.00, 54.00] |
| Gender | ||||||||
| Male | 6404 (45.6%) | 6359 (45.3%) | 3591 (51.0%) | 3562 (50.6%) | 4246 (46.2%) | 4255 (46.2%) | 1520 (50.9%) | 1520 (50.9%) |
| Female | 7640 (54.4%) | 7685 (54.7%) | 3454 (49.0%) | 3483 (49.4%) | 4954 (53.8%) | 4945 (53.8%) | 1464 (49.1%) | 1464 (49.1%) |
| Region | ||||||||
| Northeast | 2112 (15.0%) | 2142 (15.3%) | 1236 (17.5%) | 1231 (17.5%) | 1770 (19.2%) | 1772 (19.3%) | 542 (18.2%) | 568 (19.0%) |
| North Central | 4354 (31.0%) | 4314 (30.7%) | 1687 (23.9%) | 1645 (23.3%) | 2553 (27.8%) | 2543 (27.6%) | 725 (24.3%) | 715 (24.0%) |
| South | 5215 (37.1%) | 5200 (37.0%) | 2765 (39.2%) | 2812 (39.9%) | 3387 (36.8%) | 3426 (37.2%) | 1148 (38.5%) | 1114 (37.3%) |
| West | 1993 (14.2%) | 1991 (14.2%) | 1177 (16.7%) | 1180 (16.7%) | 1260 (13.7%) | 1223 (13.3%) | 506 (17.0%) | 525 (17.6%) |
| Unknown | 370 (2.6%) | 397 (2.8%) | 180 (2.6%) | 177 (2.5%) | 230 (2.5%) | 236 (2.6%) | 63 (2.1%) | 62 (2.1%) |
| Year of cohort entry date | ||||||||
| 2003 | 201 (1.4%) | 204 (1.5%) | 18 (0.3%) | 22 (0.3%) | 8 (0.1%) | 8 (0.1%) | 3 (0.1%) | 3 (0.1%) |
| 2004 | 499 (3.6%) | 476 (3.4%) | 49 (0.7%) | 55 (0.8%) | 17 (0.2%) | 18 (0.2%) | 3 (0.1%) | 3 (0.1%) |
| 2005 | 599 (4.3%) | 597 (4.3%) | 126 (1.8%) | 130 (1.8%) | 57 (0.6%) | 55 (0.6%) | 15 (0.5%) | 17 (0.6%) |
| 2006 | 633 (4.5%) | 638 (4.5%) | 275 (3.9%) | 249 (3.5%) | 69 (0.8%) | 71 (0.8%) | 10 (0.3%) | 16 (0.5%) |
| 2007 | 863 (6.1%) | 874 (6.2%) | 347 (4.9%) | 341 (4.8%) | 464 (5.0%) | 512 (5.6%) | 70 (2.3%) | 71 (2.4%) |
| 2008 | 1398 (10.0%) | 1406 (10.0%) | 525 (7.5%) | 531 (7.5%) | 895 (9.7%) | 897 (9.8%) | 156 (5.2%) | 165 (5.5%) |
| 2009 | 1527 (10.9%) | 1507 (10.7%) | 729 (10.3%) | 720 (10.2%) | 1028 (11.2%) | 1032 (11.2%) | 225 (7.5%) | 214 (7.2%) |
| 2010 | 1430 (10.2%) | 1445 (10.3%) | 706 (10.0%) | 691 (9.8%) | 954 (10.4%) | 938 (10.2%) | 236 (7.9%) | 224 (7.5%) |
| 2011 | 1628 (11.6%) | 1669 (11.9%) | 907 (12.9%) | 904 (12.8%) | 1212 (13.2%) | 1156 (12.6%) | 237 (7.9%) | 237 (7.9%) |
| 2012 | 1714 (12.2%) | 1710 (12.2%) | 988 (14.0%) | 1030 (14.6%) | 1407 (15.3%) | 1403 (15.2%) | 396 (13.3%) | 413 (13.8%) |
| 2013 | 1401 (10.0%) | 1420 (10.1%) | 862 (12.2%) | 864 (12.3%) | 1119 (12.2%) | 1089 (11.8%) | 553 (18.5%) | 559 (18.7%) |
| 2014 | 1368 (9.7%) | 1329 (9.5%) | 964 (13.7%) | 948 (13.5%) | 1298 (14.1%) | 1301 (14.1%) | 679 (22.8%) | 662 (22.2%) |
| 2015 | 783 (5.6%) | 769 (5.5%) | 549 (7.8%) | 560 (7.9%) | 672 (7.3%) | 720 (7.8%) | 401 (13.4%) | 400 (13.4%) |
| IBD severity-related factors | ||||||||
| Volume depletion | 1154 (8.2%) | 1117 (8.0%) | 676 (9.6%) | 677 (9.6%) | 903 (9.8%) | 932 (10.1%) | 311 (10.4%) | 323 (10.8%) |
| Anemia | 1346 (9.6%) | 1340 (9.5%) | 726 (10.3%) | 724 (10.3%) | 998 (10.8%) | 1033 (11.2%) | 334 (11.2%) | 357 (12.0%) |
| Malnutrition | 235 (1.7%) | 238 (1.7%) | 97 (1.4%) | 91 (1.3%) | 210 (2.3%) | 210 (2.3%) | 46 (1.5%) | 51 (1.7%) |
| Active fistulizing or internal penetrating disease | 1756 (12.5%) | 1751 (12.5%) | 391 (5.6%) | 404 (5.7%) | 1373 (14.9%) | 1390 (15.1%) | 188 (6.3%) | 183 (6.1%) |
| Obstructing or structuring disease | 1631 (11.6%) | 1589 (11.3%) | 110 (1.6%) | 120 (1.7%) | 1305 (14.2%) | 1326 (14.4%) | 53 (1.8%) | 49 (1.6%) |
| Total parenteral nutrition | 154 (1.1%) | 155 (1.1%) | 47 (0.7%) | 53 (0.8%) | 149 (1.6%) | 158 (1.7%) | 14 (0.5%) | 18 (0.6%) |
| Blood transfusions | 140 (1.0%) | 150 (1.1%) | 142 (2.0%) | 144 (2.0%) | 98 (1.1%) | 103 (1.1%) | 58 (1.9%) | 62 (2.1%) |
| Intra-abdominal surgeries | 452 (3.2%) | 426 (3.0%) | 20 (0.3%) | 22 (0.3%) | 295 (3.2%) | 304 (3.3%) | 9 (0.3%) | 14 (0.5%) |
| Number of gastroenterologist visits | ||||||||
| Mean (SD) | 2.23 (3.62) | 2.16 (3.91) | 2.87 (3.93) | 2.80 (4.46) | 2.95 (5.31) | 2.92 (5.82) | 3.43 (4.74) | 3.40 (5.36) |
| Median [IQR] | 1.00 [0.00, 3.10] | 0.74 [0.00, 2.86] | 1.69 [0.00, 4.14] | 1.36 [0.00, 3.86] | 1.11 [0.00, 3.80] | 1.32 [0.00, 3.81] | 2.00 [0.00, 4.80] | 1.98 [0.03, 4.64] |
| IBD hospitalization recency | ||||||||
| None during baseline | 12,311 (87.7%) | 12,312 (87.7%) | 6264 (88.9%) | 6267 (89.0%) | 7786 (84.6%) | 7737 (84.1%) | 2625 (88.0%) | 2606 (87.3%) |
| Recent (30 d pre-index) | 751 (5.3%) | 740 (5.3%) | 381 (5.4%) | 377 (5.4%) | 652 (7.1%) | 694 (7.5%) | 203 (6.8%) | 210 (7.0%) |
| Nonrecent (31–180 d pre-index) | 982 (7.0%) | 992 (7.1%) | 400 (5.7%) | 401 (5.7%) | 762 (8.3%) | 769 (8.4%) | 156 (5.2%) | 168 (5.6%) |
| Colonoscopy recency | ||||||||
| None during baseline | 8201 (58.4%) | 8228 (58.6%) | 3639 (51.7%) | 3621 (51.4%) | 5297 (57.6%) | 5202 (56.5%) | 1528 (51.2%) | 1513 (50.7%) |
| Recent (30 d pre-index) | 2228 (15.9%) | 2209 (15.7%) | 1199 (17.0%) | 1222 (17.3%) | 1302 (14.2%) | 1358 (14.8%) | 508 (17.0%) | 526 (17.6%) |
| Nonrecent (31–180 d pre-index) | 3615 (25.7%) | 3607 (25.7%) | 2207 (31.3%) | 2202 (31.3%) | 2601 (28.3%) | 2640 (28.7%) | 948 (31.8%) | 945 (31.7%) |
| Sigmoidoscopy recency | ||||||||
| None during baseline | 13,628 (97.0%) | 13,636 (97.1%) | 6196 (87.9%) | 6204 (88.1%) | 8919 (96.9%) | 8906 (96.8%) | 2578 (86.4%) | 2565 (86.0%) |
| Recent (30 d pre-index) | 141 (1.0%) | 144 (1.0%) | 375 (5.3%) | 367 (5.2%) | 92 (1.0%) | 102 (1.1%) | 175 (5.9%) | 187 (6.3%) |
| Nonrecent (31–180 d preindex) | 275 (2.0%) | 264 (1.9%) | 474 (6.7%) | 474 (6.7%) | 189 (2.1%) | 192 (2.1%) | 231 (7.7%) | 232 (7.8%) |
| MRI of abdomen and/or pelvis | 890 (6.3%) | 897 (6.4%) | 203 (2.9%) | 207 (2.9%) | 858 (9.3%) | 866 (9.4%) | 112 (3.8%) | 105 (3.5%) |
| CT of abdomen and/or pelvis | 4506 (32.1%) | 4515 (32.1%) | 1218 (17.3%) | 1250 (17.7%) | 3291 (35.8%) | 3368 (36.6%) | 537 (18.0%) | 557 (18.7%) |
| Clostridium difficile testing performed | 1585 (11.3%) | 1577 (11.2%) | 1714 (24.3%) | 1759 (25.0%) | 1130 (12.3%) | 1196 (13.0%) | 841 (28.2%) | 806 (27.0%) |
| Index TNF prescribed during hospitalization | 459 (5.0%) | 483 (5.2%) | 148 (5.0%) | 159 (5.3%) | ||||
| Comorbid conditions | ||||||||
| Diabetes | 743 (5.3%) | 723 (5.1%) | 491 (7.0%) | 497 (7.1%) | 431 (4.7%) | 433 (4.7%) | 221 (7.4%) | 227 (7.6%) |
| Obesity | 416 (3.0%) | 429 (3.1%) | 253 (3.6%) | 252 (3.6%) | 300 (3.3%) | 299 (3.2%) | 129 (4.3%) | 129 (4.3%) |
| Smoking | 883 (6.3%) | 860 (6.1%) | 221 (3.1%) | 220 (3.1%) | 625 (6.8%) | 644 (7.0%) | 111 (3.7%) | 104 (3.5%) |
| Multiple sclerosis and variants of multiple sclerosis | 39 (0.3%) | 41 (0.3%) | 14 (0.2%) | 13 (0.2%) | 13 (0.1%) | 12 (0.1%) | 3 (0.1%) | 3 (0.1%) |
| Rheumatoid arthritis | 416 (3.0%) | 412 (2.9%) | 309 (4.4%) | 320 (4.5%) | 275 (3.0%) | 285 (3.1%) | 180 (6.0%) | 199 (6.7%) |
| Psoriatic arthritis | 73 (0.5%) | 68 (0.5%) | 76 (1.1%) | 69 (1.0%) | 44 (0.5%) | 55 (0.6%) | 38 (1.3%) | 50 (1.7%) |
| History of hospitalization with serious bacterial infections | 362 (2.6%) | 372 (2.6%) | 111 (1.6%) | 104 (1.5%) | 287 (3.1%) | 281 (3.1%) | 57 (1.9%) | 61 (2.0%) |
| History of hospitalization with opportunistic infections | 34 (0.2%) | 29 (0.2%) | 13 (0.2%) | 15 (0.2%) | 31 (0.3%) | 26 (0.3%) | 11 (0.4%) | 8 (0.3%) |
| Previous diagnosis of oveitis | 56 (0.4%) | 58 (0.4%) | 32 (0.5%) | 36 (0.5%) | 50 (0.5%) | 50 (0.5%) | 20 (0.7%) | 18 (0.6%) |
| Comedications | ||||||||
| Corticosteroids use recency | ||||||||
| Never during baseline | 5991 (42.7%) | 6012 (42.8%) | 2149 (30.5%) | 2135 (30.3%) | 3667 (39.9%) | 3736 (40.6%) | 665 (22.3%) | 676 (22.7%) |
| Recent (30 d pre-index) | 5750 (40.9%) | 5760 (41.0%) | 3679 (52.2%) | 3706 (52.6%) | 3869 (42.1%) | 3871 (42.1%) | 1784 (59.8%) | 1755 (58.8%) |
| Nonrecent (31–180 d preindex) | 2303 (16.4%) | 2272 (16.2%) | 1217 (17.3%) | 1204 (17.1%) | 1664 (18.1%) | 1593 (17.3%) | 535 (17.9%) | 553 (18.5%) |
| ASA compounds | 6091 (43.4%) | 6068 (43.2%) | 4819 (68.4%) | 4807 (68.2%) | 3637 (39.5%) | 3600 (39.1%) | 2228 (74.7%) | 2200 (73.7%) |
| Natalizumab | 10 (0.1%) | 12 (0.1%) | 0 (0.0%) | 0 (0.0%) | 4 (0.0%) | 3 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Insulin | 142 (1.0%) | 134 (1.0%) | 105 (1.5%) | 98 (1.4%) | 88 (1.0%) | 85 (0.9%) | 57 (1.9%) | 63 (2.1%) |
| Noninsulin antidiabetics | 457 (3.3%) | 448 (3.2%) | 284 (4.0%) | 301 (4.3%) | 262 (2.8%) | 271 (2.9%) | 143 (4.8%) | 140 (4.7%) |
| NSAIDs and Coxibs | 1257 (9.0%) | 1290 (9.2%) | 637 (9.0%) | 627 (8.9%) | 674 (7.3%) | 736 (8.0%) | 308 (10.3%) | 319 (10.7%) |
| Opioids | 4694 (33.4%) | 4657 (33.2%) | 1782 (25.3%) | 1836 (26.1%) | 3154 (34.3%) | 3200 (34.8%) | 884 (29.6%) | 895 (30.0%) |
| Biphosphonates | 397 (2.8%) | 391 (2.8%) | 202 (2.9%) | 217 (3.1%) | 219 (2.4%) | 237 (2.6%) | 96 (3.2%) | 92 (3.1%) |
| Nonbiologics | . | . | . | . | 2081 (22.6%) | 2020 (22.0%) | 767 (25.7%) | 760 (25.5%) |
| Healthcare utilization variables | ||||||||
| Number of hospitalizations not for IBD | ||||||||
| Mean (SD) | 0.20 (0.56) | 0.19 (0.58) | 0.15 (0.45) | 0.15 (0.49) | 0.20 (0.54) | 0.20 (0.58) | 0.15 (0.42) | 0.15 (0.45) |
| Median [IQR] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] |
| Number of ED visits | ||||||||
| Mean (SD) | 0.75 (1.67) | 0.76 (2.12) | 0.65 (1.44) | 0.65 (1.50) | 0.86 (1.79) | 0.87 (2.04) | 0.68 (1.37) | 0.68 (1.46) |
| Median [IQR] | 0.00 [0.00, 1.00] | 0.00 [0.00, 0.97] | 0.00 [0.00, 0.82] | 0.00 [0.00, 0.80] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 0.99] | 0.00 [0.00, 0.89] |
| Number of distinct medication prescriptions | ||||||||
| Mean (SD) | 6.09 (5.38) | 6.04 (4.92) | 6.33 (5.17) | 6.35 (4.80) | 6.24 (5.35) | 6.39 (4.88) | 7.46 (5.47) | 7.49 (4.84) |
| Median [IQR] | 5.00 [2.00, 9.00] | 5.00 [2.00, 8.00] | 6.00 [3.00, 9.00] | 5.00 [3.00, 9.00] | 5.00 [2.00, 9.00] | 5.00 [3.00, 9.00] | 7.00 [4.00, 10.00] | 7.00 [4.00, 10.00] |
ASA, aminosalicylates; CT, computed tomography; IQR, interquartile range; NSAIDs: nonsteroidal anti-inflammatory drugs; ED, emergency department.
Statistical Analyses
Crude incidence rates of uveitis were reported for both exposure groups. Crude incidence rate differences and crude incidence rate ratios, along with 95% confidence intervals, were presented to compare the unadjusted rate of uveitis in (1) nonbiologic immunosuppressive drug-treated versus TNFi-treated patients and (2) adalimumab-treated versus infliximab-treated patients.
We used propensity score (PS) methods, conducted separately for each comparison and by IBD subtype, to account for potential confounding. PSs were defined as the predicted probability of exposure using multivariable logistic regression models including the covariates described above. We used 1:1 matching such that each exposed patient was matched to one referent patient, with a maximum matching caliper of 0.01 on the probability scale. We evaluated balance achieved after matching using standardized differences with values greater than 0.1 indicating substantial imbalance between the 2 groups.
Cox-proportional hazards regression models were used to estimate hazard ratios (HR) for both comparisons, separately for IBD subtype, before and after PS matching. Stratification based on IBD subtype was considered to appropriately account for confounding because characteristics and treatment patterns differ between those with CD and UC.38 Additionally, it has been suggested that the incidence of ocular complications including uveitis differs between patients with CD and UC.7–12
PS-matched HRs for CD and UC were pooled to produce overall IBD estimates, based on DerSimonian and Laird random-effects estimates.
RESULTS
Study Cohort Selection
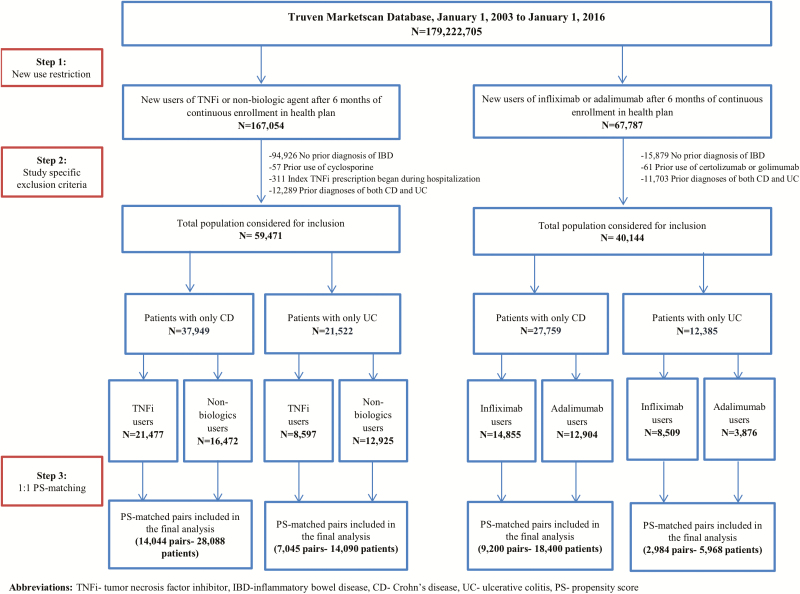
The flow chart of patient selection is shown separately for each cohort in Figure 1. Of the 773,663 patients filling at least one nonbiologic or TNFi prescription, 59,471 met inclusion criteria and were included in the analytic cohort for that comparison (37,949 or 63.8% with CD; 21,522 or 36.2% with UC). Of the 272,076 patients with at least one prescription for adalimumab or infliximab, 40,144 patients met inclusion criteria and were included in the analytic cohort (27,759 or 69.1% with CD; 12,385 or 30.9% with UC).
Figure 1.
Flow chart for patient selection, shown separately for (A) nonbiologic immunosuppressive drug-exposed versus TNFi-exposed patients and (B) adalimumab-exposed versus infliximab-exposed patients.
Patient Characteristics
We examined characteristics of patients in the unmatched population (see Supplementary Table in Data Content 1). IBD patients newly prescribed nonbiologics were older than those prescribed TNFi (mean age: 39.63 vs. 37.89 for CD, 43.68 vs. 41.62 for UC). In both CD and UC, TNFi users had higher percentages of several markers of IBD severity during the baseline period (e.g., MRI and computed tomography of the abdomen and/or pelvis, anemia, active fistulizing or internal penetrating disease). TNFi new users also had more emergency department visits during the baseline period than nonbiologics new users. Nonbiologics new users were more likely to have been prescribed several comedications during the baseline period compared with TNFi new users (e.g., corticosteroids, aminosalicylates, noninsulin drugs for diabetes, nonsteroidal anti-inflammatory drug and coxib, opioids, bisphosphonates) and similarly had a higher average number of distinct prescription medications.
IBD patients newly prescribed adalimumab were older than those newly prescribed infliximab (mean age: 39.47 vs. 35.89 for CD, 42.95 vs. 40.67 for UC), and were more commonly female (55.6% vs. 52.4% for CD, 50.3% vs. 48.1% for UC). In both patients with CD and with UC, the percentage of patients with several key markers of IBD severity (e.g., IBD hospitalization, colonoscopies, MRI of the abdomen and/or pelvis) during the baseline period was observed to be significantly higher in new users of infliximab as compared to those of adalimumab. However, adalimumab initiators used other medications including steroids, opioids, and nonsteroidal anti-inflammatory drugs more frequently during the baseline period when compared with infliximab initiators.
The PS matching, conducted separately for each cohort and by disease (CD or UC), was successful in achieving balance between the exposure groups in all measured covariates (see Table 1 for patient characteristics in the PS-matched population; standardized differences before and after matching presented in Supplementary Figure in Data Content 2).
Risk of Uveitis
The total number of uveitis events, total follow-up time, rate ratios, rate differences, and hazard ratios are presented in Table 2, both before and after PS matching, separately for CD and UC.
Table 2.
Event Rates by Exposure Status
| Crohn Disease | Ulcerative Colitis | Crohn Disease | Ulcerative Colitis | |||||
|---|---|---|---|---|---|---|---|---|
| TNF Inhibitors | Nonbiologics | TNF Inhibitors | Nonbiologics | Infliximab | Adalimumab | Infliximab | Adalimumab | |
| Before matching | ||||||||
| Number of patients | 21,477 | 16,472 | 8597 | 12,925 | 14,855 | 12,904 | 8509 | 3876 |
| Number of person-years | 22,781 | 13,910 | 8034 | 12,151 | 15,756 | 14,550 | 8745 | 3328 |
| Number of events | 25 | 26 | 13 | 18 | 10 | 17 | 4 | 8 |
| Rate per 1000 person-years | 1.1 (0.7, 1.6) | 1.9 (1.2, 2.7) | 1.6 (0.9, 2.8) | 1.5 (0.9, 2.3) | 0.6 (0.3, 1.2) | 1.2 (0.7, 1.9) | 0.5 (0.1, 1.2) | 2.4 (1.0, 4.7) |
| Rate difference per 1000 person-years (vs. referent; 95% CI) | Referent | 0.77 (−0.07, 1.61) | Referent | −0.14 (−1.25, 0.98) | Referent | 0.53 (−0.15, 1.21) | Referent | 1.95 (0.22, 3.67) |
| Hazard ratio (95% CI) | Referent | 1.75 (0.95, 3.22) | Referent | 1.01 (0.43, 2.38) | Referent | 3.18 (1.20, 8.41) | Referent | 1.46 (0.39, 5.51) |
| After propensity score matching | ||||||||
| Number of patients | 14,044 | 14,044 | 7045 | 7045 | 9200 | 9200 | 2984 | 2984 |
| Number of person-years | 15,606 | 11,399 | 6840 | 5965 | 9570 | 10,762 | 2639 | 2708 |
| Number of events | 20 | 18 | 10 | 12 | 6 | 14 | 2 | 5 |
| Rate per 1000 person-years | 1.28 | 1.58 | 1.46 | 2.01 | 0.63 | 1.30 | 0.76 | 1.85 |
| Rate difference per 1000 person-years (vs. referent; 95% CI) | Referent | 0.30 (−0.62, 1.22) | Referent | 0.55 (−0.90, 2.00) | Referent | 0.67 (−0.17, 1.52) | Referent | 1.09 (−0.84, 3.02) |
| Hazard ratio (95% CI) | Referent | 1.22 (0.64, 2.30) | Referent | 1.35 (0.58, 3.12) | Referent | 2.10 (0.81, 5.46) | Referent | 2.45 (0.48, 12.62) |
Crohn Disease
Within the nonbiologics versus TNFi cohort, a total of 51 events were observed among CD patients. The crude incidence rates per 1000 person-years were 1.9 (95% CI 1.2–2.7) among nonbiologic initiators and 1.1 (95% CI 0.7–1.6) among TNFi initiators. After PS matching, no differences in the risk of uveitis were noted when comparing nonbiologic initiators to TNFi with CD (HR 1.22, 95% CI 0.64–2.30).
Within the adalimumab versus infliximab cohort, a total of 27 events were observed among CD patients. The corresponding incidence rates per 1000 person-years among adalimumab and infliximab initiators were 1.2 (95% CI 0.7–1.9) and 0.6 (95% CI 0.3–1.2), respectively. Due to small event counts, the CIs were wide for this comparison and included the null value after PS matching (HR 2.10 [0.81–5.46]).
Ulcerative Colitis
Within the nonbiologics versus TNFi cohort, a total of 31 events were observed among UC patients. The crude incidence rates per 1000 person-years were 1.5 (95% CI 0.9–2.3) among nonbiologic initiators and 1.6 (95% CI 0.9–2.8) among TNFi initiators. After PS matching, no differences in the risk of uveitis were noted when comparing nonbiologic initiators to TNFi with UC (HR 1.35, 95% CI 0.58–3.12).
Within the adalimumab versus infliximab cohort, a total of 12 events were observed among UC patients. The corresponding incidence rates per 1000 person-years among adalimumab and infliximab initiators were 2.4 (95% CI 1.0–4.7) and 0.5 (95% CI 0.1–1.2), respectively. Due to small event counts, the CIs were wide for this comparison and included the null value after PS matching (2.45 [95% CI 0.48–12.62]).
Overall IBD
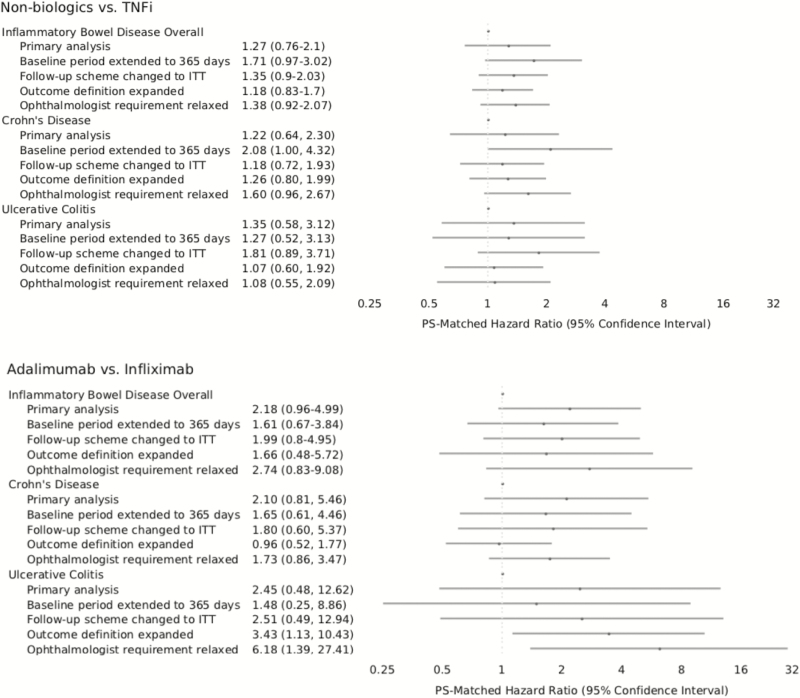
The PS-matched HRs obtained for patients with CD and UC were pooled to obtained overall IBD estimates (Fig. 2). For the nonbiologics versus TNFi comparison, the pooled HR was 1.27 (95% CI 0.76–2.1). For the adalimumab versus infliximab comparison, the pooled HR was 2.18 (95% CI 0.96–4.99).
Figure 2.
Hazard ratios and 95% confidence intervals for the outcome of anterior noninfectious uveitis in the propensity score-matched cohorts: (1) nonbiologic immunosuppressive drug-exposed versus TNFi-exposed patients and (2) adalimumab-exposed versus infliximab-exposed patients.
Sensitivity Analyses
HRs in the PS-matched cohorts are presented for all sensitivity analyses in Figure 2. Overall, sensitivity analyses where we changed the follow-up scheme to ITT expanded outcome definition to include additional ocular manifestations, changed the outcome definition to be more sensitive (less specific) by relaxing the requirement for uveitis diagnosis (with any of the ICD-9 codes from the original outcome definition) by an ophthalmologist, and extended the baseline period to 365 days provided results that were qualitatively consistent with the primary analysis for both comparisons with widely overlapping confidence intervals. However, the analysis with more sensitive outcome definition as well as the analysis including additional ocular manifestations reached statistical significance suggesting a higher risk of uveitis with adalimumab versus infliximab in UC patients (HR 6.18, 95% CI 1.39–27.41 and 3.23, 95% CI 1.05–9.90). The analysis where preindex period was extended suggested a higher risk of incident uveitis with nonbiologics versus TNFi in CD patients (HR 2.08, 95% CI 1.00–4.32).
DISCUSSION
In this large cohort study, we noted crude rates of uveitis in patients with IBD initiating nonbiologic agents or TNFis ranged from 1.1 to 1.9 per 1000 person-years. Among patients in the adalimumab versus infliximab comparison, the crude incidence rate of uveitis per 1000 person-years ranged from 0.5 to 2.4. No differences in risk of uveitis were observed after PS matching between nonbiologic initiators and TNFi initiators with CD (HR 1.22, 95% CI 0.64–2.30) or UC (HR 1.35, 95% CI 0.58–3.12). For the adalimumab versus infliximab comparison, the effect estimates were highly imprecise due to limited number of outcome events and were sensitive to variation in outcome definition, which precluded a definitive conclusion.
This study is the first large-scale observational cohort to investigate the comparative risk of uveitis in patients with IBD newly treated with different immunosuppressive agents. Thus, we draw on a wider body of literature to put our results into context. Previous reports have estimated uveitis to occur as a complication in 4%–6% of patients with IBD.13–15 In this study, we observed a crude incidence proportion of uveitis ranging from 0.05% to 0.21%. It is possible that our observed percentage of patients that develop uveitis may be lower than those previously reported because study time period may not have been long enough to capture all eventual cases of uveitis (average of follow-up time ranged from 0.84 to 1.13 years per patient). The prior literature13–15 examined the prevalence of extraintestinal manifestations over periods of 1014, 15 and 2513 years, in cohorts of patients enrolled from IBD databases15 and referral centers,13 which would probably explain the higher frequencies reported in those studies. As the present study was conducted in a population-based cohort with an average follow-up of about 1 year, it is unsurprising that our observed rates are much smaller than those reported in the literature. It is possible that our results are more accurate in capturing the rates of uveitis in treated, contemporary cohorts of IBD patients.
Previous studies have suggested that TNFi treatment may be effective in controlling uveitis recurrence in patients with immune-mediated diseases24–34 via treatment of ocular inflammation that may be resistant to steroid treatment. However, in our study, we observed no differences in the risk of uveitis between nonbiologic initiators and TNFi initiators or between adalimumab and infliximab initiators. Our results may be explained by several factors. First, although hazard ratios indicated a 2-fold higher risk of uveitis among adalimumab versus infliximab initiators, the small event counts provided imprecise, nonsignificant estimates. Therefore, it is possible that our study may not have the statistical power to detect a difference with small magnitude. Second, it is possible that the lack of significant difference in this study may be due to our choice of active comparators. The small studies33, 24, 25, 27, 29–31, 28 and a recent large randomized controlled trial23 that have examined uveitis risk in association with TNFi do not involve active comparators. A large systematic review concluded treatment with nonbiologic and biologic immunosuppressive agents to both be effective in suppressing autoimmune uveitis.35 It is possible that reduction of systemic inflammation in IBD patients with a nonbiologic agent versus a biologic agent may lead to similarly reduced risk of uveitis.
This study has several key strengths. First, the use of the Truven MarketScan database allowed us to have a large sample size. Use of this database avoids potential bias of studies set exclusively in referral centers, particularly when considering the estimated incidence of uveitis among a population of patients with IBD. Next, the active comparison new-user study design used in this study provide protection against confounding by indication and confounding by treatment duration. Furthermore, we accounted for many important measured confounders with PS matching. Finally, we undertook rigorous sensitivity analyses varying key assumptions of this study to assess robustness of our findings.
Our study has several limitations. There is potential for residual confounding by indication due to our lack of ability to account for IBD-related disease activity as this information is unavailable in insurance claims data. However, many IBD-related ICD-9 codes were used as proxy variables and adjusted for in our analyses. Next, our inability to differentiate between patients with CD and UC in our database led to exclusion of many patients who had ICD-9 codes for both conditions recorded. Previous reports have indicated a higher incidence of uveitis in patients with CD when compared with those with UC.7–12 In the present study, we found the risk of uveitis to be similar among patients with CD and UC in both comparisons after PS matching. It is also important to note that this analysis did not exclude patients with a previous diagnosis of uveitis during the preindex period, with the goal of capturing all clinically relevant recurring acute episodes in addition to incident episodes of uveitis. A sensitivity analysis in which the analytic cohorts were restricted to incident episodes of uveitis was considered, but due to small event counts (among CD patients, 22 incident cases in the nonbiologics vs. TNFi comparison and 6 incident cases in the adalimumab vs. infliximab comparison; among UC patients, 11 incident cases in the nonbiologics vs. TNFi comparison and 5 incident cases in the adalimumab vs. infliximab comparison), the analysis was not pursued. It is also important to note that a lack of data availability from more recent years precluded our ability to conduct additional comparison of newer biologics, which we recognize as an intriguing avenue for future research. Finally, results from several sensitivity analyses, where the outcome definition was varied, which were consistent in directionality with the primary analysis, indicated statistically significant differences between adalimumab and infliximab groups, suggesting that the primary analysis may have had limited power to detect statistically significant differences. Although the additional outcome definitions were tested to evaluate robustness of our results, we believe that the primary outcome definition reflects the most clinically relevant events (anterior noninfectious uveitis cases only) and possesses higher validity because it only includes cases where uveitis was diagnosed by an ophthalmologist. However, future studies with larger event counts may be needed to rule out residual uncertainty regarding the equivalence in risk of uveitis between infliximab and adalimumab.
CONCLUSIONS
In this large observational cohort study of patients with IBD initiating treatment with different immunosuppressive agents, we observed crude incidence of uveitis per 1000 person-years ranging from 0.5 to 2.4 across all treatment groups. After adjustment for potential confounding factors, no significant differences in the risk of uveitis were observed between nonbiologic and TNFi initiators, suggesting that the effect of immunosuppressive treatment on uveitis risk may not be differential. Despite the numerically elevated risk, due to imprecision attributable to small event counts and some inconsistency observed in sensitivity analyses, no definitive conclusion could be drawn for the adalimumab versus infliximab comparison.
Supplementary Material
Funding: This study was funded through internal sources of the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School. No external funding was received.
Conflict of Interest: R.J.D. serves as PI on research grants from Merck, Bayer, and Vertex to the Brigham and Women’s Hospital for unrelated studies. S.C.K. has received research grants from Pfizer, Bristol-Myers Squibb, and Roche to the Brigham and Women’s Hospital for unrelated studies. J.D.L. reports grants and personal fees from Nestle Health Science, personal fees from Johnson & Johnson Consumer Inc, grants and personal fees from Janssen Pharmaceuticals, grants and personal fees from Takeda, personal fees and nonfinancial support from AbbVie, personal fees from Merck, personal fees from Celgene, personal fees from Eli Lilly and Company, personal fees from Samsung Bioepis, personal fees from Bridge Biotherapeutics Inc, personal fees from Gilead, personal fees from Pfizer, personal fees from Bristol-Myers Squibb, personal fees from UCB, outside the submitted work. In addition, Dr. Lewis has a patent 15/090,609 issued.
References
- 1. Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504–1517. [DOI] [PubMed] [Google Scholar]
- 2. Kappelman MD, Rifas–Shiman SL, Kleinman K, et al. . The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–9. [DOI] [PubMed] [Google Scholar]
- 3. Burisch J, Jess T, Martinato M, et al. ; ECCO-EpiCom . The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013;7:322–337. [DOI] [PubMed] [Google Scholar]
- 4. Longobardi T, Jacobs P, Wu L, Bernstein CN. Work losses related to inflammatory bowel disease in Canada: results from a National Population Health Survey. Am J Gastroenterol. 2003;98:844–849. [DOI] [PubMed] [Google Scholar]
- 5. Karmiris K, Avgerinos A, Tavernaraki A, et al. . Prevalence and characteristics of extra-intestinal manifestations in a large cohort of Greek patients with inflammatory bowel disease. J Crohns Colitis 2016;10:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585–595. [DOI] [PubMed] [Google Scholar]
- 7. Hopkins DJ, Horan E, Burton IL, et al. . Ocular disorders in a series of 332 patients with Crohn’s disease. Br J Ophthalmol. 1974;58:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rankin GB, Watts HD, Melnyk CS, Kelley ML Jr. National Cooperative Crohn’s Disease Study: extraintestinal manifestations and perianal complications. Gastroenterology 1979;77:914–920. [PubMed] [Google Scholar]
- 9. Wright R, Lumsden K, Luntz MH, et al. . Abnormalities of the sacro-iliac joints and uveitis in ulcerative colitis. Q J Med. 1965;34:229–236. [PubMed] [Google Scholar]
- 10. Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore) 1976;55:401–412. [DOI] [PubMed] [Google Scholar]
- 11. Cloché V, Buisson A, Tréchot F, et al. . Ocular symptoms are not predictive of ophthalmologic inflammation in inflammatory bowel disease. Dig Liver Dis. 2013;45:195–199. [DOI] [PubMed] [Google Scholar]
- 12. Mady R, Grover W, Butrus S. Ocular complications of inflammatory bowel disease. Scientificworldjournal 2015;2015:438402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lakatos L, Pandur T, David G, et al. . Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9:2300–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernstein CN, Blanchard JF, Rawsthorne P, et al. . The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001;96:1116–1122. [DOI] [PubMed] [Google Scholar]
- 15. Vavricka SR, Brun L, Ballabeni P, et al. . Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110–119. [DOI] [PubMed] [Google Scholar]
- 16. Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80:844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bressler B, Marshall JK, Bernstein CN, et al. ; Toronto Ulcerative Colitis Consensus Group . Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology 2015;148:1035–1058.e3. [DOI] [PubMed] [Google Scholar]
- 18. Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology . Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–83; quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 19. Center IMSRRIR. Immunomodulators. In: Fact Sheet: News from the IBD Help Center. Crohn’s & Colitis Foundation. 2017. http://www.crohnscolitisfoundation.org/assets/pdfs/immunomodulators.pdf (May 2018, date last accessed). [Google Scholar]
- 20. Center IMSRRIR. Biologics. In: Fact Sheet: News from the IBD Help Center. Crohn’s & Colitis Foundation. 2017. http://www.crohnscolitisfoundation.org/assets/pdfs/medications-biologic-therapy.pdf (May 2018, date last accessed). [Google Scholar]
- 21. Singh JA, Saag KG, Bridges SL Jr, et al. . 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 22. Levy-Clarke G, Jabs DA, et al. . Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology 2014;121:785–796.e3. [DOI] [PubMed] [Google Scholar]
- 23. Jaffe GJ, Dick AD, Brézin AP, et al. . Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375:932–943. [DOI] [PubMed] [Google Scholar]
- 24. Ardoin SP, Kredich D, Rabinovich E, et al. . Infliximab to treat chronic noninfectious uveitis in children: retrospective case series with long-term follow-up. Am J Ophthalmol. 2007;144:844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobrin L, Kim EC, Christen W, et al. . Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol. 2007;125:895–900. [DOI] [PubMed] [Google Scholar]
- 26. Galor A, Perez VL, Hammel JP, et al. . Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology 2006;113:2317–2323. [DOI] [PubMed] [Google Scholar]
- 27. Kahn P, Weiss M, Imundo LF, et al. . Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology 2006;113:860–4.e2. [DOI] [PubMed] [Google Scholar]
- 28. Saurenmann RK, Levin AV, Rose JB, et al. . Tumour necrosis factor α inhibitors in the treatment of childhood uveitis. Rheumatology 2006;45:982–989. [DOI] [PubMed] [Google Scholar]
- 29. Baughman RP, Bradley DA, Lower EE. Infliximab in chronic ocular inflammation. Int J Clin Pharmacol Ther. 2005;43:7–11. [DOI] [PubMed] [Google Scholar]
- 30. Lindstedt EW, Baarsma GS, Kuijpers RWAM, et al. . Anti-TNF-α therapy for sight threatening uveitis. Br J Ophthalmol. 2005;89:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rispo A, Scarpa R, Di Girolamo E, et al. . Infliximab in the treatment of extra‐intestinal manifestations of Crohn’s disease. Scand J Rheumatol. 2005;34:387–91. doi: 10.1080/03009740510026698. [DOI] [PubMed] [Google Scholar]
- 32. Suhler EB, Smith JR, Wertheim MS, et al. . A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol. 2005;123:903–912. [DOI] [PubMed] [Google Scholar]
- 33. Smith Justine R, Levinson Ralph D, Holland Gary N, et al. . Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Care Res. 2001;45: 252–257. [DOI] [PubMed] [Google Scholar]
- 34. Cordero-Coma M, Yilmaz T, Onal S. Systematic review of anti-tumor necrosis factor-alpha therapy for treatment of immune-mediated uveitis. Ocul Immunol Inflamm. 2013;21:19–27. [DOI] [PubMed] [Google Scholar]
- 35. Pato E, Muñoz-Fernández S, Francisco F, et al. ; Uveitis Working Group from Spanish Society of Rheumatology . Systematic review on the effectiveness of immunosuppressants and biological therapies in the treatment of autoimmune posterior uveitis. Semin Arthritis Rheum. 2011;40:314–323. [DOI] [PubMed] [Google Scholar]
- 36. Liu L, Allison JE, Herrinton LJ. Validity of computerized diagnoses, procedures, and drugs for inflammatory bowel disease in a northern California managed care organization. Pharmacoepidemiol Drug Saf. 2009;18:1086–1093. [DOI] [PubMed] [Google Scholar]
- 37. Dick AD, Tundia N, Sorg R, et al. . Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology 2016;123:655–662. [DOI] [PubMed] [Google Scholar]
- 38. Geboes K. Crohn’s disease, ulcerative colitis or indeterminate colitis – how important is it to differentiate? Acta Gastroenterol Belg. 2001;64:197–200. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.