Abstract
Background
Endoscopy plays a fundamental role in the management of patients with inflammatory bowel disease (IBD). The aim of this study was to prospectively evaluate the tolerability and efficacy of bowel preparation and colonoscopy in ulcerative colitis (UC) and Crohn’s disease (CD) patients compared to subjects participating in a colorectal cancer population screening program.
Methods
Consecutive enrolment of CD and UC patients and screening subjects (SS) undergoing colonoscopy. Bowel preparation was done by split dose of 2 L PEG-ELS + simethicone. We recorded endoscopic, clinical, and demographic features; cleanliness rating using the Boston Bowel Preparation Scale (BBPS); and sedation doses. Bowel-preparation tolerability, discomfort, and pain during colonoscopy were assessed using a Visual Analogue Scale from 0 to 100 mm.
Results
Sixty-three UC (mean age 49.9 ± 14.9 years), 63 CD (mean age 44.0 ± 14.0 years), and 63 SS (mean age 59.9 ± 6.3 years) patients were enrolled. Bowel preparation was similarly tolerated in UC, CD, and SS (P = 0.397). A complete colonoscopy was similarly performed in UC (59/63, 93.7%), CD (58/63, 92.1%), and SS (60/63, 95.2%) (P = 0.364). The BBPS did not show significant differences between UC (6.2 ± 1.6), CD (6.1 ± 1.3), and SS (6.2 ± 1.4) (P = 0.824). The need to increase sedation doses was significantly higher in CD (24/63, 38.1%) and UC (16/63, 25.4%) than in SS (4/63, 6.3%) (P < 0.0001).
Conclusions
Bowel preparation is equally tolerated and efficacious in IBD patients and in healthy SS. In IBD, higher sedation doses are needed to guarantee an equally tolerated colonoscopy.
Keywords: Crohn’s disease, ulcerative colitis, endoscopy, sedation, bowel preparation
INTRODUCTION
Endoscopy plays a fundamental role in the management of patients with inflammatory bowel disease (IBD). Ileocolonoscopy with biopsies is essential for IBD diagnosis, colorectal cancer (CCR) surveillance and evaluation of endoscopic activity, postsurgical recurrence of Crohn’s disease (CD), and endoscopic response to treatment.1, 2 In particular, mucosal healing (MH) represents a treatment goal since it is associated with prolonged remission times, lower complication rates, and reduced hospitalization and surgery.3, 4
Considering the multiple indications for colonoscopy and an incidence peak that occurs at a young age, IBD patients will undergo a high number of endoscopic procedures during their lifetime. Endoscopy is perceived as useful by IBD patients but, mainly because of bowel preparation, is among the less-appreciated procedures.5, 6 Poor tolerability of intestinal preparations represents the main factor in patients failing to adhere to recommended surveillance in long-standing IBD.7
Adequate bowel cleanliness is fundamental for a high-quality colonoscopy8 and even more so in IBD patients, especially in case of surveillance for dysplasia.9
IBD patients experience more pain and discomfort than subjects undergoing colonoscopy for other indications.6, 10 Moreover, IBD patients are young, with high levels of preprocedural anxiety and have already undergone a high number of previous colonoscopies: these characteristics are known risk factors for intolerance to colonoscopy.11–13
However, despite the relevance of these multiple aspects connected to colonoscopy in patients with IBD, there is only a small amount of low-quality available data.14
The aims of this prospective study were to evaluate the acceptability and the adequacy of bowel preparation and the tolerability of colonoscopy in patients with a diagnosis of ulcerative colitis (UC) or CD and in subjects undergoing screening colonoscopies for colorectal cancer prevention.
METHODS
Population
We consecutively enrolled patients with UC or CD and subjects undergoing colonoscopy at the Rho Hospital Gastroenterology Unit between August 2017 and August 2019. As controls, we enrolled screening subjects (SS) who underwent colonoscopy as part of the regional CCR screening program.
For the IBD patients, we considered data on diagnosis, extension/localization of disease according to the Montreal classification,15 clinical activity (according to partial Mayo score for UC16 and Harvey-Bradshaw Index for CD17), previous surgery for IBD, and ongoing therapy. This information was already included in the clinic notes for each patient by the respective gastroenterologists.
Procedure
All patients enrolled received the same split-dose low-volume bowel preparation (2 L polyethylene glycol + simethicone + electrolytes).
All endoscopic examinations were performed by the same experienced endoscopist with the same model of colonoscope, using a combination of intravenous midazolam and fentanyl at a starting dose of 3 mg and 50 µg, respectively.
We used specifically designed questionnaires to collect data on the tolerability of the bowel preparation and endoscopic procedure; they were administered by a blinded endoscopy nurse. Within 1 hour before the procedure, subjects were asked to fill in a questionnaire to evaluate bowel preparation tolerability; immediately after the procedure, when woken up, they were asked to complete the questionnaire to assess the tolerability of the endoscopic procedure. Bowel preparation tolerability, abdominal pain, and discomfort experienced during colonoscopy, and overall endoscopic procedure tolerability was measured using a Visual Analogue Scale (VAS) from 0 to 100 mm. VAS was recorded by the same endoscopy nurse trained by a single education module, to provide objective, consistent evaluation across all CD/UC/SS subjects.
For each colonoscopy, we recorded indication, examination completion (defined as terminal ileum intubation), disease activity, using a Mayo endoscopic score for UC16 and Simple Endoscopic Score for Crohn’s Disease (SES-CD) for CD,18 adjustment to sedation dose, and the amount of additional midazolam and fentanyl. Quality of bowel preparation was assessed by Boston Bowel Preparation Scale (BBPS)19: adequate if BBPS BP6, with a minimum of 2 for each segment. Well-tolerated bowel preparation and endoscopic procedure were defined by a VAS > 70 mm.
Statistical Analysis
Supposing that patients with UC and CD have a poor tolerability of bowel preparation and endoscopic examination and considering a statistical test power of 80%, a probability of 0.05 for type I error and assuming a 2-tailed test, we calculated a sample size of 63 subjects for each group to find a difference of about 15% in tolerability (primary outcome of tolerability of bowel preparation and endoscopic procedure using VAS: 70 ± 15 mm vs 60 ± 15).
We reported arithmetic mean and standard deviation as continuous variables and absolute frequency and percentage as discrete variables. Comparisons between groups (UC, CD, and SS) were made using the analysis of variance and Tukey’s range test for quantitative variables and the chi-square test for qualitative variables. All tests have to be considered as 2 tailed with statistical significance set at 0.05.
Ethical Considerations
The study was approved by the local ethics committee and then has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
RESULTS
We enrolled 189 patients: 63 with UC (26 women, mean age 49.9 ± 14.9 years), 63 with CD (29 women, mean age 44.0 ± 14.0 years), and 63 subjects undergoing screening colonoscopy (32 women, mean age 59.9 ± 6.3 years). The demographic and clinical features of the enrolled populations are shown in Table 1. Indications to colonoscopy in IBD patients were the following: disease flare in 38/63 CD (60.3%) and 29/63 (46.0%), assessment of MH in 22/63 CD (34.9%) and in 8/63 UC (12.7%), and surveillance in 3/63 CD (4.8%) and in 26/63 UC (41.3%). Endoscopic and clinical activity of IBD patients is shown in Table 2.
Table 1.
Demographic and Clinical Features of IBD Population
| CD (n = 63) | UC (n = 63) | SS (n = 63) | P | |
|---|---|---|---|---|
| Women, n (%) | 29 (46.0) | 26 (41.3) | 32 (50.8) | 0.563 |
| Mean age ± SD, years | 44.0 ± 14.0 | 49.9 ± 14.9 | 59.9 ± 6.3 | <0.0001* |
| Previous abdominal surgery†, n (%) | 25 (39.7) | 2 (3.2) | 3 (4.8) | <0.0001 |
| Previous colonoscopies > 3‡ | 36 (57.1) | 34 (54.0) | 5 (7.9) | <0.0001 |
| Location | ||||
| L1 | 29 (46.0%) | — | — | — |
| L2 | 15 (23.8%) | — | — | — |
| L3 | 19 (30.2%) | — | — | — |
| Perianal | 7 (11.1%) | — | — | — |
| Extension | — | — | ||
| E1 | — | 7 (11.1%) | — | — |
| E2 | — | 33 (52.4%) | — | — |
| E3 | — | 23 (36.5%) | — | — |
| Therapies | — | — | ||
| Mesalamine | 12 (19.0%) | 55 (87.3%) | — | — |
| Thiopurines | 11 (17.5%) | 9 (14.3%) | — | — |
| Biologics | 23 (36.5%) | 14 (22.2%) | — | — |
| Low-bioavailability steroids | 2 (3.2%) | 2 (3.2%) | — | — |
| Systemic steroids | 6 (9.5%) | 3 (4.8%) | — | — |
| Topical therapy | 1 (1.6%) | 14 (22.2%) | — | — |
*UC versus CD P < 0.05, UC versus SS P < 0.01, CD versus SS P < 0.01.
†in CD: 18 ileo-caecal resection, 3 ileal resection, 2 fistulectomy, 1 ileo-caecal resection + right ovariectomy, 1 ileal resection + prostatectomy; in UC: 1 cystectomy + prostatectomy, 1 nephrectomy; and in SS: 2 hysterectomy, 1 hysteroannessiectomy.
‡3 is the median value in CD and UC populations.
Table 2.
Endoscopic and Clinical Activity of IBD Population
| CD (n = 63) | UC (n = 63) | |
|---|---|---|
| Clinical activity* | ||
| Remission | 44 (69.8%) | 32 (50.8%) |
| Mild | 9 (14.3%) | 23 (36.5%) |
| Moderate | 10 (15.9%) | 7 (11.1%) |
| Severe | 0 (0.0%) | 1 (1.6%) |
| Endoscopic activity† | ||
| Remission | 24 (38.1%) | 23 (36.5%) |
| Mild | 17 (27.0%) | 7 (11.1%) |
| Moderate | 17 (27.0%) | 18 (28.6) |
| Severe | 5 (7.9%) | 15 (23.8%) |
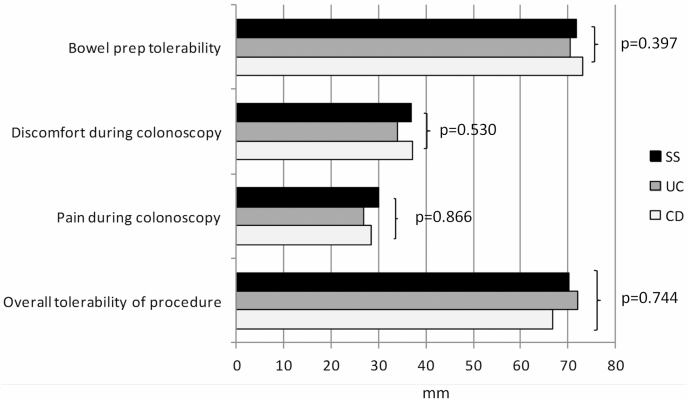
Bowel preparation tolerability, measured by VAS, was similar in UC (70.5 ± 18.1 mm), CD (73.1 ± 12.9 mm), and SS (71.9 ± 13.4 mm) (P = 0.397; Figure 1). With regard to symptoms (headache, insomnia, nausea/vomiting, bloating/abdominal distension, abdominal pain, unpleasant taste in mouth, and feeling of fullness), no statistical difference was observed between UC, CD, and SS groups (Supplementary Table 1).
Figure 1.
Feeling about bowel preparation and colonoscopy assessed by VAS (for bowel preparation tolerability and overall procedure tolerability: from 0 mm [the worst] to 100 mm [the best]; for discomfort and pain during colonoscopy: from 0 mm [none] to 100 [the maximum]).
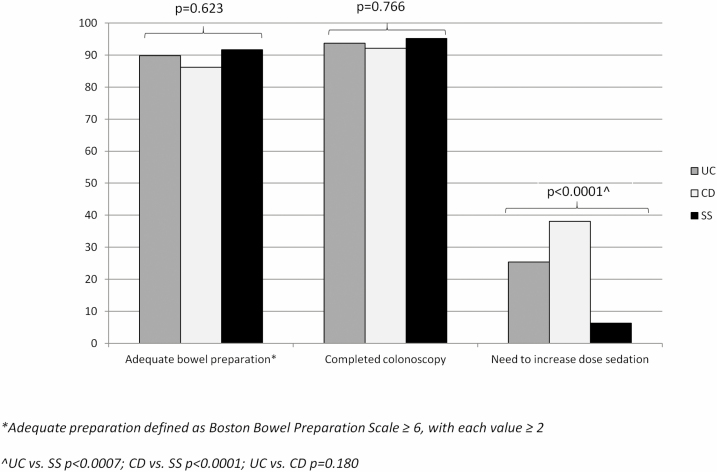
No difference was observed in bowel preparation quality between UC (mean BBPS ± SD 6.2 ±1.6), CD (6.1 ± 1.3), and SS (6.2 ± 1.4) (P = 0.940). Likewise, bowel preparation was adequate with similar rates between UC (53/59, 89.8%), CD (50/58, 86.2%), and SS (55/60, 91.7%) groups (P = 0.623; Figure 2).
Figure 2.
Quality parameters of colonoscopy.
Colonoscopy was completed with similar rates between UC (59/63, 93.7%), CD (58/63, 92.1%), and SS (60/63, 95.2%) (P = 0.766). We observed a statistical difference between the groups in the number of procedures where an adjusted sedation dose was required: 24/63 (38.1%) in CD, 16/63 (25.4%) in UC, and 4/63 (6.3%) in SS (P < 0.0001), with no significant difference between UC and CD (Figure 2).
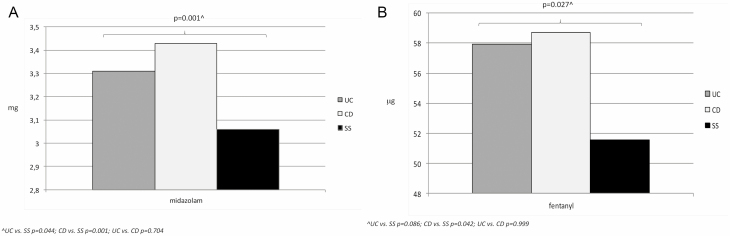
The mean dosage of midazolam and fentanyl was statistically different between the 3 groups: 3.43 ± 0.67 mg and 58.73 ± 19.13 mcg, respectively in CD patients, 3.31 ± 0.64 mg and 57.94 ± 18.42 mcg in UC patients, and 3.06 ± 0.30 mg and 51.59 ± 8.84 mcg in SS (Figure 3; overall P = 0.001 and P = 0.027, respectively), with no statistical difference between UC and CD groups (Figure 3).
Figure 3.
Mean total doses of midazolam (a) and fentanyl (b) during colonoscopy.
The abdominal discomfort and pain experienced during colonoscopy were similar in patients with UC (34.1 ± 22.8 mm and 26.9 ± 24.5 mm, respectively), CD (37.1 ± 21.8 mm and 28.4 ± 22.5 mm), and SS (36.9 ± 20.3 mm and 30.1 ± 22.3 mm) (P = 0.530 and P = 0.866, respectively; Figure 1).
Overall procedure tolerability (bowel preparation + endoscopic examination) was comparable in patients with UC (72.1 ± 22.2 mm), CD (66.8 ± 23.1 mm), and SS (70.2 ± 19.7 mm) (P = 0.744; Figure 1).
No significant associations were found between tolerability of bowel preparation and colonoscopy and clinical and demographic features: sex, disease activity (clinical and endoscopic), previous number of colonoscopies, indications, and previous surgery.
DISCUSSION
Our findings clearly and unequivocally demonstrate that patients with UC and CD do not show statistical differences in terms of tolerability and efficacy of bowel preparation in comparison with healthy subjects. Colonoscopy completion rates and overall procedure tolerability are similar between UC, CD, and control group. However, it is essential to highlight that in IBD patients, especially those with CD, higher doses of midazolam and fentanyl are needed compared to the control population.
The fact that UC and CD patients satisfactorily tolerate a split-dose low-volume PEG preparation confirms previous observations about better tolerability of low-volume bowel preparations in patients with IBD.20, 21 Moreover, we found that no demographic and clinical features of IBD patients are associated with a lower efficacy of bowel preparation. This finding rebuts previous observations who indicated disease activity and surgical intestinal resections as factors associated with poor bowel preparation.22, 23
The need of higher sedation doses in IBD patients is consistent with other results from previous studies6, 24 and was not associated with any demographic and clinical features.
Patients with IBD will undergo many endoscopies during their lifetime. Furthermore, UC and CD are chronic diseases characterized, even during remission, by intestinal symptoms, a need for surgery, and strong emotional impact. All these factors contribute to the idea that the overall tolerability of endoscopic procedures in IBD patients is inferior with respect to the general population. This may apply both to bowel preparation (in terms of tolerability and efficacy) and toendoscopic examination (in terms of completion rates and tolerability).
However, although highly relevant, there are very little data on these issues in the literature and, when available, they come mostly from retrospective studies, in which the type of bowel preparation, the outcomes and evaluation methods are not always clearly defined, or do not specifically refer to this group of patients.14 Moreover, these studies include patients with diagnosis of IBD, without distinguishing between UC and CD that are actually 2 distinct diseases in terms of clinical course, anatomical involvement, and need for surgery.
The limitations of this study are the choice of SS as control population, which did not allow for balanced groups in terms of age, and being a referral center for IBDs which may limit the generalizability of our findings to different clinical contexts. While the assessment questionnaires used in the study are not standardized and validated tools, they were specifically designed in order to obtain solid data. Moreover, we cannot exclude that retrograde and anterograde amnesia caused by procedural sedation could have been impaired the actual perceptions of subjects undergoing colonoscopy. The strengths of this study are the prospective case–control design, the distinction between UC and CD, and all endoscopic examinations carried out by the same endoscopist.
In conclusion, a split-dose low-volume PEG + simethicone bowel preparation is a valid option for patients with UC and CD who need to undergo colonoscopy for any indication. Using higher initial doses of sedation in patients with IBD can be necessary in order to obtain colonoscopy completion rates and overall procedure tolerability similar to those of the general population.
Future studies could also assess tolerability, safety, and efficacy in IBD patients of the new very low-volume colon preparations, such as NER1006.25 Indeed, they may further improve the acceptability of colonoscopy and then the adherence to endoscopic indications, a critical issue in this peculiar setting.7
Supplementary Material
ACKNOWLEDGMENT
We thank you Ms Victoria J. Miller for her invaluable help in editing the manuscript.
Conflict of Interest: C.B. received lecture fees from Takeda, AbbVie, and Janssen. S.S. received lecture fees from Takeda Pharmaceuticals and Janssen Pharmaceuticals and served as a consultant and a member of Advisory Boards for AbbVie and Janssen Pharmaceuticals. The other authors have no financial interests to disclose.
Funding: None declared.
Data Availability: The data that support the findings of this study are not public; they are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. Maaser C, Sturm A, Vavricka SR, et al. ; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR] . ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164. [DOI] [PubMed] [Google Scholar]
- 2. Annese V, Daperno M, Rutter MD, et al. ; European Crohn’s and Colitis Organisation . European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. [DOI] [PubMed] [Google Scholar]
- 3. Baert F, Moortgat L, Van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club . Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–8; quiz e10. [DOI] [PubMed] [Google Scholar]
- 4. Vaughn BP, Shah S, Cheifetz AS. The role of mucosal healing in the treatment of patients with inflammatory bowel disease. Curr Treat Options Gastroenterol. 2014;12:103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buisson A, Gonzalez F, Poullenot F, et al. ; ACCEPT study group . Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1425–1433. [DOI] [PubMed] [Google Scholar]
- 6. Denters MJ, Schreuder M, Depla AC, et al. Patients’ perception of colonoscopy: patients with inflammatory bowel disease and irritable bowel syndrome experience the largest burden. Eur J Gastroenterol Hepatol. 2013;25:964–972. [DOI] [PubMed] [Google Scholar]
- 7. Friedman S, Cheifetz AS, Farraye FA, et al. Factors that affect adherence to surveillance colonoscopy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:534–539. [DOI] [PubMed] [Google Scholar]
- 8. Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. [DOI] [PubMed] [Google Scholar]
- 9. Mowat C, Cole A, Windsor A, et al. ; IBD Section of the British Society of Gastroenterology . Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- 10. Weber AT, Ather N, Tran V, et al. Higher sedation requirements along inflammatory bowel disease patients undergoing colonoscopy for disease activity assessment or dysplasia surveillance. Crohn’s & Colitis 360. 2019;1:otz006. [Google Scholar]
- 11. Bessissow T, Van Keerberghen CA, Van Oudenhove L, et al. Anxiety is associated with impaired tolerance of colonoscopy preparation in inflammatory bowel disease and controls. J Crohns Colitis. 2013;7:e580–e587. [DOI] [PubMed] [Google Scholar]
- 12. Paggi S, Radaelli F, Amato A, et al. Unsedated colonoscopy: an option for some but not for all. Gastrointest Endosc. 2012;75:392–398. [DOI] [PubMed] [Google Scholar]
- 13. Terruzzi V, Paggi S, Amato A, Radaelli F. Unsedated colonoscopy: a neverending story. World J Gastrointest Endosc. 2012;4:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bezzio C, Andreozzi P, Casini V, et al. Endoscopy for patients affected by inflammatory bowel disease: bowel preparation and sedation. Expert Rev Gastroenterol Hepatol. 2018;12:119–124. [DOI] [PubMed] [Google Scholar]
- 15. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 16. D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. [DOI] [PubMed] [Google Scholar]
- 17. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 18. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 19. Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy oriented research. Gastrointest Endosc. 2009;69:620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manes G, Fontana P, de Nucci G, et al. Colon cleansing for colonoscopy in patients with ulcerative colitis: efficacy and acceptability of a 2-L PEG plus bisacodyl versus 4-L PEG. Inflamm Bowel Dis. 2015;21:2137–2144. [DOI] [PubMed] [Google Scholar]
- 21. Briot C, Faure P, Parmentier AL, et al. ; CLEAN Study Group . Efficacy, tolerability, and safety of low-volume bowel preparations for patients with inflammatory bowel diseases: the French multicentre CLEAN study. J Crohns Colitis. 2019;13:1121–1130. [DOI] [PubMed] [Google Scholar]
- 22. Hassan C, Fuccio L, Bruno M, et al. A predictive model identifies patients most likely to have inadequate bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2012;10:501–506. [DOI] [PubMed] [Google Scholar]
- 23. Dik VK, Moons LM, Hüyük M, et al. ; Colonoscopy Quality Initiative . Predicting inadequate bowel preparation for colonoscopy in participants receiving split-dose bowel preparation: development and validation of a prediction score. Gastrointest Endosc. 2015;81:665–672. [DOI] [PubMed] [Google Scholar]
- 24. Weber AT, Ather N, Tran V, et al. Higher sedation requirements among inflammatory bowel disease patients undergoing colonoscopy for disease activity assessment or dysplasia surveillance. Crohn’s & Colitis 360 2019;1(1). doi: 10.1093/crocol/otz006 [DOI] [Google Scholar]
- 25. Schettino M, Saibeni S, Bezzio C, et al. Efficacy, safety and tolerability, the imperfect triangle arising from the new low volume colon preparations. Dig Liver Dis. 2020;52:840–841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.