Abstract
Background:
Breast cancer is the most common cancer among women in the US, and women of low socioeconomic status (SES) show markedly poorer outcomes than those of high SES. SES may influence health through inflammation, although links between SES and inflammatory biomarkers have not been investigated in women with breast cancer. This study tested the hypothesis that breast cancer patients of lower SES would show higher levels of inflammation than those of higher SES. BMI was examined as a mediator of this association.
Methods:
Women recently diagnosed with early-stage breast cancer (N=194) were recruited before neoadjuvant or adjuvant therapy. Participants completed questionnaires and provided blood samples for immune assessment. SES was indexed by participants’ self-reported education and annual household income, BMI was determined by height and weight measurements, and blood was assayed for inflammatory biomarkers linked with cancer outcomes: IL-6, CRP, TNF-α, and sTNF-RII. General linear models tested associations between SES and inflammation, and mediation models examined indirect effects through BMI.
Results:
Consistent with hypotheses, education status was associated with CRP, (F(2,185) = 4.72, p = 0.001), and sTNF-RII, (F(2,185) = 4.19, p = 0.02), such that lower education was associated with higher levels of both biomarkers. Further, BMI mediated the associations between education and CRP, (95% CIs [−0.62, −0.11; −0.76, −0.21]), sTNF-RII, (95% CIs [−0.09, −0.01; −0.10, −0.02]), and IL-6, (95% CIs [−0.32, −0.05; −0.38, −0.09]). Annual household income was not significantly associated with inflammation (ps > 0.25), and indirect effects on inflammation through BMI were not significant.
Conclusions:
Lower education was associated with higher levels of inflammation in this sample, which may presage poor breast cancer-related and clinical outcomes. SES should inform the development of interventions targeting BMI and inflammation in breast cancer.
Keywords: breast cancer, socioeconomic status, inflammation, body mass index
1. Introduction
Striking evidence reveals that socioeconomic status (SES) is associated with mental and physical health outcomes across the lifespan. SES is defined as a composite of economic, social, and work status and can be measured using income, education, and occupational status (Adler et al., 1994a). In particular, education and income are commonly used to measure SES given that these variables are easy to report and allow researchers to construct meaningful groups to categorize participants (Muscatell, 2018). Research findings indicate an inverse linear relationship between SES and health such that individuals of low SES have an elevated risk of morbidity and mortality across a variety of diseases, including breast cancer (Adler and Ostrove, 1999; Chen et al., 2002; Marmot et al., 1991; Matthews and Gallo, 2011). Indeed, empirical evidence from a population-based study showed that among women with breast cancer, those in the highest income category had more favorable breast cancer-related and overall survival rates than those in the lowest income category (Ji et al., 2020). Similarly, higher mean neighborhood income was associated with better overall survival in women with breast cancer, controlling for demographic and treatment-related factors (Kumachev et al., 2016). Researchers have identified biological pathways to develop a better understanding of disparities in health outcomes linked to SES (Chen and Miller, 2013; Matthews and Gallo, 2011; Seeman et al., 2010). In the breast cancer population, however, there is a paucity of research that examines pathways that may elucidate associations between SES and health outcomes.
Systemic chronic inflammation is linked to heightened risk for various diseases (Furman et al., 2019), including cancer onset and progression (Grivennikov et al., 2010; Hanahan and Weinberg, 2011). In women with breast cancer, inflammation is associated with poor behavioral and physical health outcomes, including depression (Bortolato et al., 2017), fatigue (Bower, 2019; Schubert et al., 2007), and increased risk for breast cancer recurrence and mortality (Pierce et al., 2009). Importantly, studies have consistently shown that SES is associated with alterations in inflammation. In a recent meta-analysis, lower income and education were associated with higher levels of interleukin-6 (IL-6) and C-reactive protein (CRP) in non-clinical samples (Muscatell et al., 2018), and studies assessing other indicators of SES show similar relationships (Liu et al., 2017; O’Connor et al., 2009). However, to our knowledge, no studies have investigated the link between SES and inflammation in women with breast cancer, despite potential relevance for long-term health and well-being in this population.
Researchers have begun to examine biobehavioral pathways through which SES may influence inflammation, with recent studies focusing on obesity. Obesity, defined as a body mass index (BMI) ≥ 30kg/m2, is a risk factor for a range of poor health outcomes (James, 2004). Higher BMI is associated with higher levels of inflammatory cytokines (e.g., IL-6) (Fantuzzi, 2005) and predicts worse clinical outcomes in women with breast cancer, including higher risk for breast cancer recurrence and mortality (Jiralerspong & Goodwin, 2016; Picon-Ruiz, Morata-Tarifa, Valle-Goffin, Friedman, & Slingerland, 2017). Research also indicates that SES is associated with BMI, with lower educational attainment and income linked to higher obesity rates among women in the United States in non-breast cancer (Drewnowski and Specter, 2004) and breast cancer samples (Cheng et al., 2015; Hastert et al., 2016). A few studies have directly tested the mediating role of BMI in the association between SES and inflammation and found that BMI mediates associations with CRP (Hagger-Johnson et al., 2012; Matthews et al., 2016) and IL-6 (Gallo et al., 2012). Of note, researchers have also shown that SES can moderate the effect of behavioral interventions, with women of low income showing greater improvements in physical function following a physical activity intervention than women of higher income (Rogers et al., 2016). Despite evidence showing that there are links between SES, BMI, and inflammation, these relationships have not been examined in the breast cancer population. Examination of this mechanism may facilitate the identification of modifiable targets to improve health outcomes in this population. This is particularly important given that education and income are more likely to be fixed indicators of social status for women older than age 40, the age at which breast cancer incidence increases (Howlader et al., 2019).
The goal of this study was to examine the association between two key indicators of SES, education and income, and markers of inflammation among women recently diagnosed with early-stage breast cancer. We focused on women early in the cancer trajectory, after diagnosis but prior to onset of adjuvant therapy, to avoid potential confounding effects of radiation and chemotherapy on inflammatory processes. Based on previous research linking SES and inflammation in other samples, we hypothesized that lower SES would be associated with higher levels of inflammation (Muscatell et al., 2018). Furthermore, we hypothesized that the relationship between SES and inflammation would be mediated by BMI given links between SES and BMI (Hastert et al., 2016) and between BMI and inflammatory biology (Choi et al., 2013; Deng et al., 2016).
2. Material and methods
2.1. Participants
Women were recruited from oncology practices to participate in a longitudinal, observational study designed to identify predictors of cancer-related fatigue (RISE study) (Bower et al., 2019). Women who were recently diagnosed with Stage 0-IIIA breast cancer and had not yet started adjuvant or neoadjuvant therapy with radiation, chemotherapy, or endocrine therapy were eligible to participate. Exclusion criteria included diagnosis with metastatic breast cancer and initiation of neoadjuvant or adjuvant therapy prior to study enrollment. UCLA and Cedars Sinai Medical Center were the primary recruitment sites, and the Institutional Review Boards from each site approved the study. The current study focused on the initial (i.e., baseline) study visit. Of the 270 women who were recruited to participate in the study, 194 women who provided blood samples for immune assessment were included in analyses.
2.2. Procedures
Baseline assessments for the RISE study were conducted after diagnosis, but before onset of adjuvant or neoadjuvant therapy with radiation, chemotherapy and/or endocrine therapy. Trained research staff administered questionnaires, collected blood samples, and measured height and weight. Blood draws were scheduled to coincide with clinic visits, when possible, and typically took place before noon.
2.3. Measures
2.3.1. Demographics.
Participants self-reported socioeconomic status including highest level of educational attainment and annual household income, two key components of SES (Adler et al., 1993). Educational categories on the questionnaire included: grade school, some high school, high school graduate, vocational or training school after high school graduation, some college, associate degree, college graduate, some college or professional school after college graduation, completed master’s degree, completed doctoral degree. Annual household income categories included: <$15,000, $15,000–30,000, $30,001-$60,000, $60,001-$100,000, >$100,000. Other demographic characteristics included age, race/ethnicity, and marital status.
2.3.2. Disease and treatment-related information.
Medical records were used to obtain participants’ breast cancer stage at diagnosis (i.e., Stage 0-IIIA) which was determined by the 7th edition of the American Joint Committee on Cancer staging manual. Type of surgery received (i.e., lumpectomy, mastectomy) was also determined from medical records.
2.3.3. Body mass index.
At study entry, participants’ height and weight were assessed to calculate body mass index (BMI). Guidelines specify four BMI categories: underweight (i.e., < 18.5kg/m2), normal weight (i.e., 18.5 – 24.9kg/m2), overweight (25.0 – < 29.9 kg/m2), and obese (i.e., ≥ 30kg/m2) (Centers for Disease Control and Prevention (CDC), 2020).
2.3.4. Inflammatory Biomarkers.
Blood was collected to assess circulating concentrations of inflammatory biomarkers relevant for breast cancer survivorship: IL-6, TNF-α, CRP, and sTNF-RII. IL-6 and TNF-α are canonical proinflammatory cytokines that play distinct roles in the inflammatory cascade and are each linked with breast cancer outcomes. In particular, empirical evidence suggests that IL-6 is a negative prognostic marker in breast cancer (Knupfer and Preib, 2007), with studies showing that high circulating levels of IL-6 predict shorter survival (Bachelot et al., 2003; Salgado et al., 2003). Similarly, there is evidence that TNF-α plays a pro-metastatic role in the context of breast cancer (Cruceriu et al., 2020; Martínez-Reza et al., 2017). CRP and sTNF-RII were also of interest as downstream markers of IL-6 and TNF-α activity. CRP is an acute phase protein produced by the liver in response to stimulation by IL-6 (Genest, 2010), and sTNFR-II is shed from the cell surface after stimulation by TNF-α (Diez-Ruiz et al., 2009). These markers may provide a more accurate and stable representation of the cytokines that induce their production than the cytokines themselves. In addition, these markers are associated with prognosis and behavioral symptoms in breast cancer. Specifically, CRP is associated with breast cancer recurrence (McAndrew et al., 2021) and shorter survival in breast cancer survivors (Pierce et al., 2009; Villasenor et al., 2014) and has been linked with cancer-related fatigue (Bower et al., 2009; Pertl et al., 2013). Further, studies have shown an association between sTNF-II and fatigue in women with breast cancer (Bower et al., 2011, 2002; Xiao et al., 2017).
Blood samples for biomarkers were collected by venipuncture into EDTA tubes and placed on wet ice, and plasma aliquots were prepared and frozen at −80°C prior to batch testing. All samples were assayed in duplicate, and an internal quality control sample was included on every plate. CRP and sTNF-RII were measured by Human Quantikine ELISA (R& D Systems, Minneapolis, MN) according to the manufacturer’s protocols, with some modifications. Samples with CRP concentrations above the range of the standard curve (25 mg/L) were estimated using extrapolated values. IL-6 and TNF-α were measured in a multiplex assay utilizing a V-PLEX Custom Human Cytokine Proinflammatory Panel on the Meso Scale Discovery (MSD) electrochemiluminesence platform and Discovery Workbench software (MSD, Rockville, MD). For all plasma biomarkers, inter-assay coefficients of variation were less than or equal to 10% and mean intra-assay coefficients of variation were less than 5%. The distributions for IL-6, CRP, TNF-⍺, and sTNF-RII were skewed; natural logs of each inflammatory biomarker were included in all analyses.
2.3.5. Behavioral Symptoms.
Participants completed questionnaires assessing behavioral symptoms that have been linked to inflammation, including depressive symptoms, perceived stress, and sleep disturbance (Bower et al., 2007; Liu et al., 2012; Osimo et al., 2020). The Center for Epidemiologic Studies Depression Scale (CES-D) was used to assess depressive symptoms in the past two weeks (Radloff, 1977), the Perceived Stress Scale (PSS) was used to assess stress over the past month (Cohen et al., 1983), and the Pittsburgh Sleep Quality Index (PSQI) was used to assess subjective sleep quality over the past month (Buysse et al., 1991).
2.3.6. Comorbidities.
Participants completed the Charlson Comorbidity Index, a reliable and valid measure, to report precancer medical comorbidities. This questionnaire includes various chronic diseases, including asthma, diabetes, and autoimmune disease (Katz et al., 1996).
2.3.7. Health Behavior.
Participants reported smoking habits, a health behavior that has been linked with SES (Department of Health and Services, 2014) and inflammation (O’Connor et al., 2009), as a potential confound. Participants responded to two items to indicate lifetime and current smoking behaviors (i.e., “Have you smoked at least 100 cigarettes in your entire life?”; “Do you smoke now?”).
3. Statistical Analysis
Previous research has shown differences in health outcomes along the SES continuum with unique indicators of SES (e.g., occupation, education) (Adler et al., 1994b). Thus, we were interested in assessing graded associations between individual indicators of SES and levels of inflammation. Levels of education and income were divided into three groups of approximately equal size: ‘less than college degree’ (i.e., grade school, high school vocational school, some college, associate degree), ‘college degree’ (i.e., college degree, some professional school after college graduation), and ‘post-graduate degree’ (i.e., completed masters’ degree, completed doctoral degree) for education; ‘<$60K, ‘$60K-$100K’, and ‘>$100K’ for income.
Bivariate associations between demographic and cancer-specific variables were examined using the Pearson correlation coefficient for continuous variables and the χ2 test of independence for categorical variables. General linear models (GLMs) were fit to compare differences in inflammatory biomarkers among women in the low, middle, and high education and income groups.
The PROCESS macro for SPSS (Hayes, 2017) was used to test BMI as a mediator of the association between indicators of SES and inflammatory markers. This statistical approach examines associations between each independent variable (educational attainment or annual income), the mediator (BMI), and the dependent variable (biomarkers of inflammation). Regression coefficients correspond to distinct pathways that characterize: 1) the total effect of the independent variable on the dependent variable (c path); 2) the direct effect of the independent variable on the dependent variable when the mediator is included in the model (c’ path); 3) the effect of the independent variable on the mediator (a path); 4) the effect of the mediator on the dependent variable (b path); and 5) the indirect effect of the mediator on the association between the independent and dependent variables (ab path). The primary test of our hypothesis was the significance of the ab path. The PROCESS macro generates 95% bootstrap confidence intervals for the indirect effects using 10,000 bootstrap samples. If zero is not included in these bootstrap confidence intervals, the indirect effect can be considered significant and mediation – by BMI in this study– can be inferred. In each model, ‘less than college degree’ was used as the reference group to compare levels of inflammation among individuals in the ‘low’ (i.e., less than college degree), ‘middle’ (i.e., college degree), and ‘high’ (i.e., post-graduate degree) education groups. Similarly, ‘< $60K’ was used as the reference group to compare levels of inflammation among individuals in the ‘low’ (i.e., < $60K), ‘middle’ (i.e., $60K-$100K), and ‘high’ (i.e., >$100K) income groups.
Adjusted models controlled for demographic and disease-related factors as potential confounds, including race/ethnicity, age, marital status, breast cancer stage, type of breast cancer surgery, and smoking status, given potential links with key predictor and outcome variables (Knupfer and Preib, 2007; O’Connor et al., 2009). Additional models controlled for demographic, disease-related, and behavioral factors, including race/ethnicity, age, marital status, breast cancer stage, type of breast cancer surgery, smoking status, comorbidities, perceived stress, depressive symptoms, and sleep quality. Categorical variables were created for race (non-Hispanic White, other race/ethnicity), smoking status (current/former, never smoker), surgery type (no surgery, lumpectomy, mastectomy), and breast cancer stage at diagnosis (Stage 0/I, Stage II/III), and comorbidities (0, 1, 2, 3). Non-Hispanic White, current smoker, no surgery, Stage 0/I, and 0 were the reference groups for each of these variables. IBM SPSS Version 25 was used to conduct all analyses.
4. Results
4.1. Demographics, Disease- and Treatment-Related Characteristics
Patient characteristics are shown in Table 1. The average age of women at study enrollment was 55.3 years, and approximately 75% of participants were non-Hispanic White. The majority of participants were diagnosed with Stage I or Stage II breast cancer and had undergone a lumpectomy. A small percentage (9.8%) of women were scheduled to undergo neoadjuvant chemotherapy before surgery and thus had not undergone surgery prior to enrollment. In addition, the average BMI of participants was 25.4 kg/m2, a score that is defined as overweight (Centers for Disease Control and Prevention (CDC), 2020). On average, participants reported elevated scores on the PSQI, indicating poor sleep quality (Buysse et al., 1988). The average score for depressive symptoms was 13.2, which is below the cut off score for clinical depression (Radloff, 1977).
Table 1.
Characteristics of study participants (N=194)
| Demographic, Behavioral, and Cancer-Related Characteristics | |
|---|---|
| Age: Mean (SD) [range], years | 55.3 (11.2) [27—83] |
| Race/ethnicity: N (%) | |
| White, non-Hispanic | 139 (71.6) |
| Asian | 21 (10.8) |
| Hispanic | 20 (10.3) |
| Black | 8 (4.1) |
| Other | 6 (3.1) |
| Marital Status: N (%) | |
| Married/Living as married | 126 (64.9) |
| Divorced/separated | 33 (17.0 |
| Widowed | 8 (4.1) |
| Single (never married) | 27 (13.9) |
| Educational Attainment: N (%) | |
| Less than college | 57 (29.4) |
| College degree | 76 (39.2) |
| Post-graduate degree | 61 (31.4) |
| Annual Household Income: N (%) | |
| ≤ $60,000 | 48 (24.7) |
| $60,001–$100,000 | 40 (20.6) |
| ≥$100,000 | 103 (53.1) |
| Smoking Status: N (%) | |
| Smoked | 62 (32.0) |
| Never Smoked | 132 (68.0) |
| Depressive Symptoms (CES-D): Mean (SD) | 13.2 (10.3) |
| Perceived Stress Scale (PSS): Mean (SD) | 15.5 (6.7) |
| Sleep Quality (PSQI): Mean (SD) | 7.53 (4.1) |
| Charlson Comorbidity Index: Mean (SD) | 0.25 (0.6) |
| Cancer Stage: N (%) | |
| 0 | 25 (12.9) |
| I | 90 (46.4) |
| II | 49 (25.3) |
| IIIA | 7 (3.6) |
| Indeterminable (neoadjuvant or missing pathological information) |
19 (9.8) |
| Surgery type: N (%) | |
| No surgery prior to enrollment | 19 (9.8) |
| Lumpectomy | 116 (59.8) |
| Mastectomy | 59 (30.4) |
| BMI: Mean (SD) [range], kg/m2 | 25.4 (5.8) [14.9—45.6] |
| Categories of BMI: N (%) | |
| Underweight (<18.5 kg/m2) | 10 (5.2) |
| Normal (18.5 to <25 kg/m2) | 97 (50.0) |
| Overweight (25 to <30 kg/m2) | 52 (26.8) |
| Obese (>30 kg/m2) | 35 (18.0) |
| Inflammatory Biomarkers: Median (25th, 75th percentile) | |
| IL-6, pg/mL | 0.6 (0.4, 1.0) |
| CRP, mg/L | 1.5 (0.6, 3.8) |
| TNF-α, pg/mL | 1.90 (1.6, 2.4) |
| sTNF-RII, pg/mL | 1973 (1606, 2479) |
Note. Data for breast cancer stage at diagnosis were unavailable for four participants.
Note. Income data were unavailable for three participants.
Abbreviations. BMI = body mass index. IL = interleukin. CRP = C-reactive protein. TNF-α = tumor necrosis factor alpha. sTNF-RII = soluble tumor necrosis factor receptor II.
The sample was well-educated on average – 70% had completed college – though there was considerable variability in educational attainment, which ranged from grade school to completed doctoral degree. Annual household income was relatively high, with over 50% reporting an annual income greater than $100,000.
4.2. Associations Between SES and Demographic and Treatment-Related Variables
The two measures of SES, educational attainment and annual household income, were significantly positively associated, (χ2(4, N = 191) = 31.98, p < 0.0001). SES variables were also associated with demographic, treatment-related, and behavioral characteristics. As shown in Table 2, women in the high education or income group generally had lower BMI and were less likely to smoke than those in the low SES groups. Women in the middle education group also had lower BMI than women in the low education group. Women in the high annual household income group were also more likely to be married, though no differences in marital status were noted across education groups. Women in the high annual household income group reported fewer depressive symptoms and better sleep quality than women in the low annual household income group. However, there were no differences in depressive symptoms or sleep across education groups. Age, surgery type, breast cancer stage at diagnosis, comorbidities, and perceived stress did not differ across education or annual household income groups.
Table 2.
Distribution of Demographic, Behavioral, and Cancer-Related Variables by Education and Income Categories
| Education (n=194) | Income (n=191)a | |||||||
|---|---|---|---|---|---|---|---|---|
| <College | College | Post-Grad | p-value b,c | <$60K | $60–100K | >$100K | p-value b,c | |
| Mean Age | 58.2 | 53.7 | 54.6 | 0.06 | 58.1 | 55.5 | 53.7 | 0.07 |
| Mean BMI | 27.9 | 24.7 | 24.0 | < 0.0001 | 27.3 | 25.8 | 24.5 | 0.02 |
| Mean PSS | 16.1 | 15.5 | 15.2 | 0.76 | 16.8 | 16.2 | 13.7 | 0.16 |
| Mean CES-D | 14.9 | 13.2 | 11.4 | 0.20 | 16.5 | 14.2 | 11.2 | 0.01 |
| Mean PSQI | 8.2 | 7.8 | 6.6 | 0.10 | 8.5 | 8.4 | 6.7 | 0.02 |
| % Smoker | 40.4 | 36.8 | 18.0 | 0.03 | 47.9 | 32.5 | 24.3 | 0.01 |
| % Married | 61.4 | 68.4 | 63.9 | 0.82 | 33.3 | 45.0 | 86.4 | < 0.0001 |
| % Mastectomy | 36.8 | 30.3 | 24.6 | 0.16 | 31.3 | 30.0 | 30.1 | 0.83 |
| % Stage 2 or 3 | 36.8 | 48.7 | 34.4 | 0.19 | 41.7 | 47.5 | 38.8 | 0.64 |
Income data were unavailable for three participants
P-values for continuous variables were derived from GLMs.
P-values for categorical variables were derived from chi-square tests.
Note. Significant associations between variables are bolded.
Note. < College = did not complete college (bachelor) degree; College = completed college degree (bachelor) degree; Post-grad = completed master’s or doctoral degree.
Analyses also indicated significant associations between covariates and inflammatory biomarkers. Older age was significantly correlated with higher IL-6, (r(192) = 0.25, p = 0.001), CRP, (r(192) = 0.21, p = 0.003), TNF-α, (r(192) = 0.29, p < 0.0001), and sTNF-RII, (r(192) = 0.37, p < 0.0001). Higher BMI was also significantly correlated with higher levels of IL-6, (r(192) = 0.43, p < 0.0001), CRP, (r(192) = 0.52, p < 0.0001), TNF-α, (r(192) = 0.19, p = 0.01), and sTNF-RII, (r(192) =0.29, p < 0.0001). Women who did not have surgery prior to enrollment in the study had significantly lower levels IL-6, TNF-α, and sTNF-RII than women who had a mastectomy or lumpectomy (all ps < 0.05). Women with Stage 0 or I breast cancer had significantly higher levels of TNF-α and sTNF-RII than women who were diagnosed with Stage II or III breast cancer (ps <.05), but there was no association with IL-6 or CRP (ps > 0.07). Non-Hispanic, White women had significantly higher levels of sTNF-RII than women of other races/ethnicities, (b = 0.12, p = 0.02), but there was no association with IL-6, CRP, or TNF-α (ps > 0.15). Inflammatory biomarkers were not significantly associated with marital status, smoking status, comorbidities, perceived stress, depressive symptoms, or sleep quality (ps > 0.13).
4.3. Associations Between SES and Inflammatory Biomarkers
4.3.1. Education
GLMs were fit to test hypotheses about the associations between educational attainment and IL-6, CRP, TNF-α, and sTNF-RII. In unadjusted models, there were significant differences between education groups in levels of IL-6, (F(2, 191) = 3.16, p = 0.04), CRP, (F(2, 191) = 5.88, p = 0.003), and sTNF-RII, (F(2, 191) = 7.41, p = 0.001), and a marginally significant difference in TNF-α, (F(2, 191) = 2.91, p = 0.06). Pairwise comparisons indicated that participants in the high education group (post-graduate degree) had significantly lower levels of IL-6, CRP, TNF-α, and sTNF-RII than participants in the low education group (less than college degree). In addition, participants in the middle education group (college degree) had lower levels of TNF-α and sTNF-RII (ps <0.05) than participants in the low education group. The middle and high education groups did not differ significantly in any of the inflammatory biomarkers. However, the high education group had lower levels of CRP than the middle education group, and this difference was marginally significant (p = 0.05).
In adjusted models controlling for potential confounds (race/ethnicity, age, cancer stage, surgery, marital status, smoking status), similar findings were observed with significant differences for sTNF-RII, (F(2, 185) = 4.19, p = 0.02), and CRP, (F(2, 185) = 4.72, p = 0.01), but not IL-6 or TNF-α (Figure 1). Pairwise comparisons indicated that women in the middle education group (college degree) had significantly lower levels of sTNF-RII than women in the low education group (less than college degree) (p = 0.004). In addition, women in the high education group (post-graduate degree) had significantly lower levels of CRP than women in the low education group (less than college degree) (p = 0.003). Models controlling for demographic, disease-related, and behavioral confounds (race/ethnicity, age, cancer stage, surgery, marital status, smoking status, comorbidities, perceived stress, depressive symptoms, sleep quality) did not alter associations between educational attainment and inflammatory biomarkers. Specifically, there were significant differences between education groups in levels of sTNF-RII and CRP, but not IL-6 or TNF-α. Further, pairwise comparisons indicated similar significant differences between women in the middle and low education groups for sTNF-RII and between women in the high and low education groups for CRP.
Figure 1.
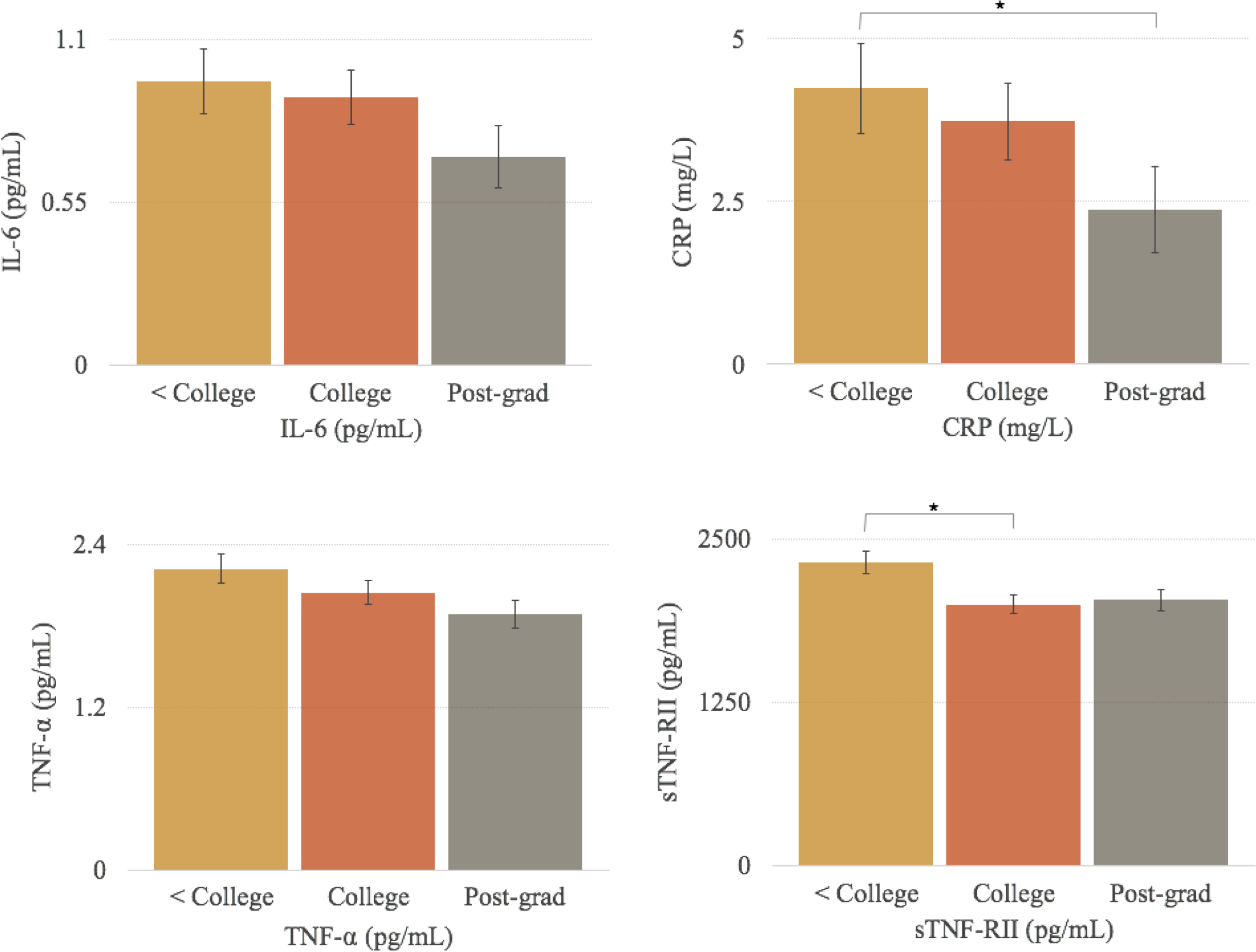
Adjusted mean levels (± SE) of inflammatory biomarkers for women in each education group. *Significant difference in levels of inflammatory biomarkers p < 0.05. Note. Non-natural log transformed values of inflammatory biomarkers are shown; natural log values of IL-6, CRP, TNF-α, and sTNF-RII were used in statistical analyses. GLM tests indicated significant differences in sTNF-RII and CRP across education groups. Post-hoc analyses indicated lower levels of CRP for the Post-grad group relative to the < College group and lower levels of sTNF-RII for the College group relative to the < College group. Analyses controlled for race/ethnicity, age, marital status, breast cancer stage at diagnosis, surgery, and smoking status.
4.3.2. Income
In unadjusted models, income groups differed significantly in levels of sTNF-RII, (F(2,188) = 3.19, p = 0.043). Specifically, participants in the high income group (>$100K) had lower levels of sTNF-RII than participants in the low income group (<$60K). This association was no longer significant in adjusted models controlling for demographic and disease-related confounds (Figure 2) or in models controlling for demographic, disease-related, and behavioral confounds. Income was not significantly associated with IL-6, CRP, or TNF-α in adjusted or unadjusted models (ps > 0.21).
Figure 2.
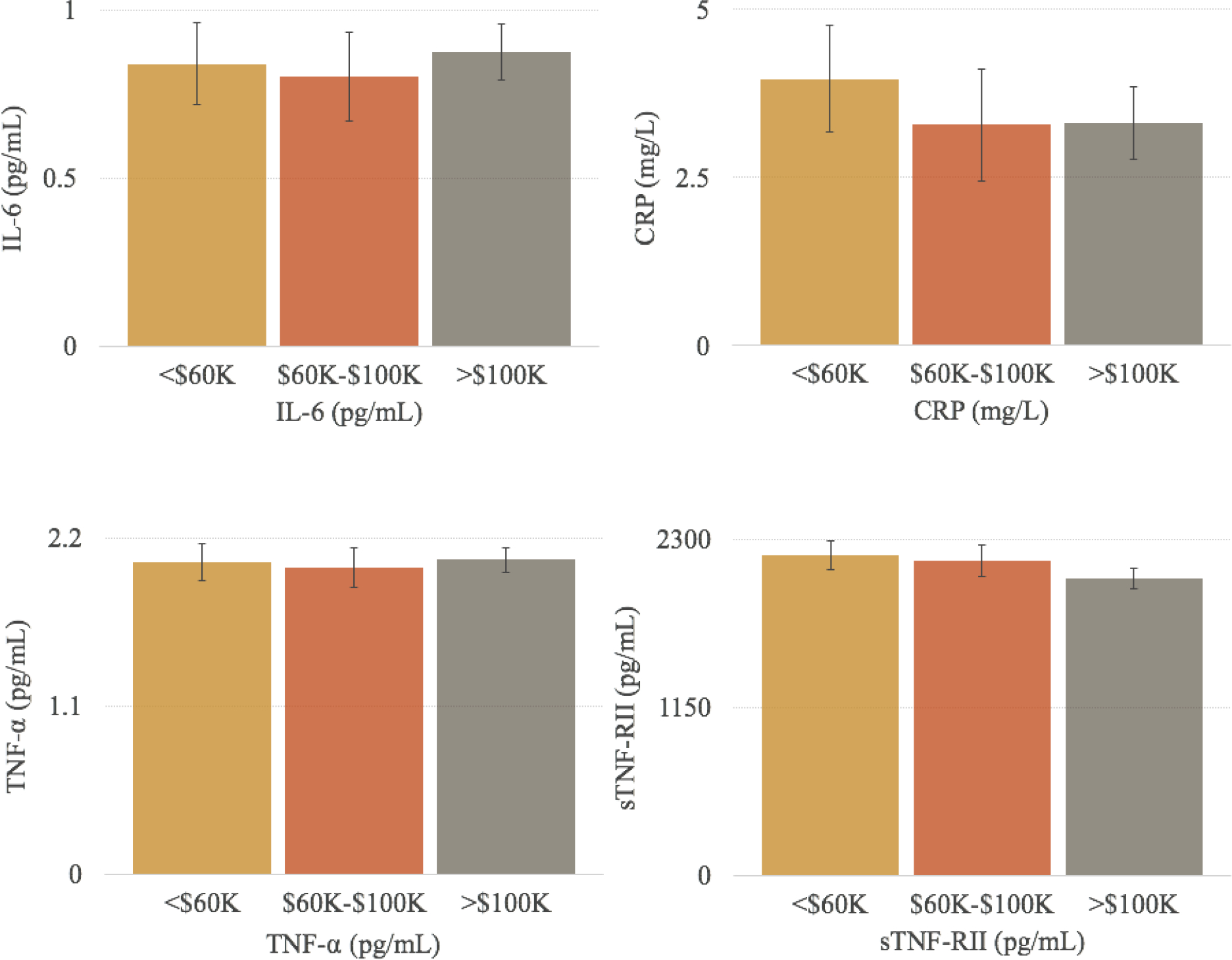
Adjusted mean levels (± SE) of inflammatory biomarkers for women in each annual household income group. Note. Non-natural log transformed values of inflammatory biomarkers are shown; natural log values of IL-6, CRP, TNF-α, and sTNF-RII were used in statistical analyses. GLM tests did not indicate significant differences in inflammatory biomarkers across income groups. Analyses controlled for race/ethnicity, age, marital status, breast cancer stage at diagnosis, surgery, and smoking status.
4.4. BMI as a Mediator of the Relationship Between SES and Inflammatory Biomarkers
4.4.1. Education
PROCESS models were fit to test indirect effects of BMI on the relationships between educational attainment and inflammatory biomarkers, controlling for demographic and disease-related confounds (Table 3). Consistent with bivariate analyses and with previous research, lower educational attainment was significantly associated with higher BMI (a path), and higher BMI was significantly associated with higher levels of IL-6, CRP, TNF-α, and sTNF-RII (b paths).
Table 3.
BMI as a Mediator of the Associations Between Educational Attainment and Plasma Levels of Inflammatory Biomarkers
| College degree (vs. Less than college degree) → BMI → Inflammation | Post-graduate degree (vs. Less than college degree) → BMI → Inflammation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Indirect Effect a1b path (BS SE)a [CI]b | a1 path B (SE) p value |
c’1 path B (SE) p value |
c1 path B (SE) p value |
b path B (SE) p value |
Indirect Effect a2b path (BS SE)a [CI]b | a2 path B (SE) p value |
c’2 path B (SE) p value |
c2 path B (SE) p value |
| IL-6 |
−0.18 (.07)
[−0.32, −0.05] |
−2.98 (0.96) 0.002 |
0.06 (0.13) 0.62 |
−0.11 (0.14) 0.42 |
0.06 (0.01) <0.00001 |
−0.23 (0.07)
[−0.38, −0.09] |
−3.84 (1.02) <0.0002 |
−0.04 (0.14) 0.76 |
−0.27 (0.15) 0.07 |
| CRP |
−0.37 (0.13)
[−0.62, −0.11] |
0.02 (0.22) 0.93 |
−0.35 (0.24) 0.15 |
0.12 (0.02) <0.00001 |
−0.47 (0.14)
[−0.76, −0.21] |
−0.31 (0.23) 0.18 |
−0.78 (0.26) 0.003 |
||
| TNF-α | −0.03 (0.02) [−0.08, 0.004] |
−0.05 (0.07) 0.48 |
−0.08 (0.07) 0.25 |
0.01 (0.01) 0.06 |
−0.04 (0.03) [−0.10, 0.01] |
−0.10 (0.08) 0.23 |
−0.14 (0.08) 0.08 |
||
| sTNF-RII |
−0.04 (0.02)
[−0.09, −0.01] |
−0.12 (0.05) 0.03 |
−0.16 (0.05) 0.004 |
0.01 (0.004) 0.0008 |
−0.05 (0.02)
[−0.10, −0.02] |
−0.05 (0.06) 0.37 |
−0.11 (0.06) 0.07 |
||
Bootstrap estimate of the standard error
95% Confidence Bootstrap interval
Note. All analyses control for race/ethnicity, age, smoking status, breast cancer stage at diagnosis, and type of surgery at all paths.
Note. Significant indirect effects are bolded.
As hypothesized, the mediated effect (ab path) of educational attainment on inflammatory biomarkers through BMI was significant. Participants in the high education group (post-graduate degree) had lower levels of IL-6, CRP, and sTNF-RII through BMI relative to participants in the low education group (less than college degree). In addition, participants in the middle education group (college degree) had lower levels of IL-6, CRP, and sTNF-RII through BMI relative to participants in the low education group (less than college degree). Adjusting for demographic, disease-related, and behavioral confounds did not alter the associations between educational attainment, BMI, and inflammatory biomarkers.
4.4.2. Income
PROCESS models were fit to test indirect effects of BMI on the relationships between annual household income and inflammatory biomarkers, controlling for demographic and disease-related confounds (Table 4). The high annual income group (> $100K) was significantly associated with lower BMI (a path) relative to the low annual income group (<$60K), and higher BMI was significantly associated with higher levels of IL-6, CRP, TNF-α, and sTNF-RII (b paths). However, the mediated effect (ab path) of annual income on inflammatory biomarkers through BMI was not significant. Adjusting for demographic, disease-related, and behavioral confounds did not alter the associations between annual household income, BMI, and inflammatory biomarkers.
Table 4.
BMI as a Mediator of the Associations Between Annual Income and Plasma Levels of Inflammatory Biomarkers
| $60–100K (vs. <$60K) → BMI → Inflammatory Biomarkers | >$100K (vs. <60K) → BMI → Inflammatory Biomarkers | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | a1b path (BS SE)a [CI]b | a1 path B (SE) p value |
c’1 path B (SE) p value |
c1 path B (SE) p value |
b path B (SE) p value |
a2b path (BS SE)a [CI]b | a2 path B (SE) p value |
c’2 path B (SE) p value |
c2 path B (SE) p value |
| IL-6 | −0.08 (0.08) [−0.24, 0.09] |
−1.35 (1.19) 0.26 |
0.14 (0.15) 0.36 |
0.06 (0.17) 0.73 |
0.06 (0.01) <0.00001 |
−0.14 (0.07) [−0.28, 0.01] |
−2.25 (1.07) 0.04 |
0.15 (0.13) 0.27 |
0.01 (0.15) 0.92 |
| CRP | −0.17 (0.18) [−0.51, 0.19] |
0.22 (0.25) 0.39 |
0.05 (0.30) 0.86 |
0.13 (0.02) <0.00001 |
−0.29 (0.15) [−0.58, 0.02] |
0.01 (0.23) 0.95 |
−0.27 (0.26) 0.31 |
||
| TNF-α | −0.02 (0.02) [−0.06, 0.02] |
0.04 (0.09) 0.65 |
0.02 (0.09) 0.79 |
0.01 (0.01) 0.03 |
−0.03 (0.02) [−0.07, 0.003] |
0.03 (0.08) 0.72 |
0.001 (0.08) 0.99 |
||
| sTNF-RII | −0.02 (0.02) [−0.06, 0.02] |
0.01 (0.06) 0.87 | −0.01 (0.07) 0.90 |
0.01 (0.004) <0.0005 |
−0.03 (0.02) [−0.08, 0.002] |
−0.09 (0.06) 0.14 |
−0.09 (0.06) 0.14 |
||
Bootstrap estimate of the standard error
95% Confidence Bootstrap interval
Note. All analyses control for race/ethnicity, age, smoking status, breast cancer stage at diagnosis, and type of surgery at all paths.
Note. Significant indirect effects are bolded.
5. Discussion
The current study examined the association between SES (operationalized as education or income) and inflammation, a known risk factor for poor physical and behavioral health, in women with breast cancer. In addition, we tested BMI as a mediator of this relationship. Consistent with hypotheses, educational attainment was associated with inflammation such that women with college degrees had lower levels of sTNF-RII than women without college degrees, and women with post-graduate degrees had lower levels of CRP than women without college degrees. In contrast, there were no significant differences in inflammatory biomarkers across income groups. The pattern for education is consistent with previous literature on SES and health outcomes (i.e., mortality) in women with breast cancer (Sprague et al., 2011). Similarly, higher education was associated with lower levels of inflammation in healthy samples (Muscatell et al., 2018). Significant associations between education and CRP, relative to IL-6 and TNF-α, are also consistent with results from a study that examined associations between education and multiple inflammatory biomarkers across multiple samples (Maurel et al., 2020). Previous research has also indicated differing results between indicators of SES and inflammation such that education, but not income, was associated with inflammation in a sample of individuals with hepatocellular carcinoma (Cheng et al., 2019). However, to our knowledge, this is the first study to show this association in a breast cancer population. These findings are particularly relevant for this population because elevated levels of inflammation are associated with worse prognosis, higher risk of cancer recurrence, and mortality (Allin et al., 2011; Pierce et al., 2009).
In addition to demonstrating a link between SES and inflammation, we found that BMI mediated the association between education and three of the four inflammatory markers assessed: IL-6, CRP, and sTNF-RII. Similar findings have been reported in non-cancer samples, with BMI mediating the association between indicators of SES and inflammatory biomarkers in older adults (Matthews et al., 2016; Pollitt et al., 2009), adolescents (Pietras and Goodman, 2013), and pregnant women (Finy and Christian, 2018). The present findings extend these results to a breast cancer population and help to elucidate the mechanisms through which SES may contribute to disparities in cancer-related outcomes. The relationship between BMI and inflammation may be particularly meaningful in this population given links between obesity and poor breast cancer-related health outcomes, including prognosis and survival (Jiralerspong & Goodwin, 2016; Picon-Ruiz et al., 2017). Indeed, a meta-analysis showed that obese women with breast cancer had poorer overall and breast cancer-specific survival in comparison to non-obese women with breast cancer (Protani et al., 2010). Our findings suggest that obesity is tied to SES and may influence health in women with breast cancer through links with inflammation. Thus, higher BMI and its socioeconomic determinants may be targets for improving inflammation and, potentially, physical health for women of lower SES.
Examination of two indicators of SES is a strength of this study and revealed their unique relationships with inflammatory biomarkers. In particular, lower education was associated with higher CRP and sTNF-RII whereas household income was not significantly associated with any of the four inflammatory markers. In previous research, education and income, although correlated, show independent effects on health (Schnittker, 2004). In general, education may reflect individuals’ abilities to navigate various resources (Ross and Mirowsky, 1999) whereas income may reflect a lack of material resources to promote health (Braveman et al., 2011). In the current sample, women of higher education may be more effectively mobilizing resources to engage in positive health behaviors and maintain a lower BMI, leading to lower inflammation. Further, low educational attainment is associated with low health literacy which can be a hinderance to seeking preventive care and engaging in health promoting behaviors (Zonderman et al., 2014). Given that previous research shows that lower education is associated with weight gain (Rock et al., 1999), it is possible that individuals with low educational attainment may be missing opportunities to obtain resources and recommendations from physicians to prevent diseases such as obesity. In contrast, nonsignificant effects of income on inflammation suggest that annual household income is a weaker predictor of behaviors that influence BMI in this sample. It is also possible that links between income, BMI, and inflammation may be more apparent in lower income samples, and that our ability to capture links with income was restricted by the upper bound of the item ($>100K annual income). In this study, unique associations between indicators of SES and inflammation underscore that educational attainment and annual household income reflect unique domains of health. Thus, further research is needed to identify how distinct components of SES may uniquely affect women’s risk for worse breast cancer-related health outcomes and to develop resources to address socioeconomic health disparities in this population.
There are several limitations in this study. Given that we examined cross-sectional links between SES, BMI, and inflammation, causality cannot be determined. Another limitation of this study is the use of BMI as a measure of obesity, which lacks specificity in terms of measurement of body fat percentage and visceral fat. There were also limitations in the measurement of SES given that participants reported educational attainment and annual household income using categories on a questionnaire. Further examination of these factors as continuous variables (e.g., years of education, annual household income amount) may provide more accuracy in understanding associations between SES and inflammatory biomarkers. Although there was variability across indices of SES, participants reported relatively high education and income and the majority were non-Hispanic White. Thus, the generalizability of findings requires examination in more diverse groups. Indeed, studies have shown that there are racial/ethnic differences in indicators of pro-inflammatory activity (Martin et al., 2009). Relatedly, race/ethnicity may moderate the association between SES and inflammation (Fuller-Rowell et al., 2015), which we were unable to examine in this sample. The inclusion of diverse groups is particularly important given that breast cancer survival rates are significantly higher in White women relative to Black women (Siegel et al., 2019), highlighting the importance of understanding biobehavioral pathways among individuals of different racial and ethnic backgrounds.
Overall, results from the current study are largely consistent with research showing that lower SES is associated with worse health outcomes for individuals with breast cancer (Byers et al., 2008; Sprague et al., 2011) and identify a biological pathway through which SES may influence outcomes in this context. Findings underscore the need to identify additional malleable pathways through which SES may influence inflammatory biology in breast cancer survivors. BMI may be a useful target to improve health outcomes for women with breast cancer by reducing inflammation. In this study, controlling for behavioral confounds, including sleep, stress, and depressive symptoms, did not have an impact on the relationship between SES and inflammation. This suggests that future research should prioritize examination of factors that more directly contribute to BMI in women with breast cancer such as physical activity and diet. Several randomized and non-randomized trials show that behavioral interventions, ranging two to 18 months in duration, can yield weight loss of five percent or greater in breast cancer survivors (Reeves et al., 2014). For example, the Women’s Intervention Nutrition Study, a randomized trial, showed that women who consumed a low-fat diet had significant weight loss at one and five years and were at a lower risk for breast cancer recurrence than women in the control group (Chlebowski et al., 2006). Relatedly, empirical evidence shows that exercise with weight loss is associated with decreases in pro-inflammatory biomarkers, including TNF-A (Byers and Sedjo, 2011) and IL-6 (Byers and Sedjo, 2011; Dethlefsen et al., 2017; Neilson et al., 2009; Pakiz et al., 2011). Given that physical activity and dietary behaviors are important contributors of BMI, these findings underscore the importance of targeting these factors to improve breast cancer-related health outcomes for women of low SES.
Previous studies have aimed to lower BMI in women with breast cancer through exercise and dietary changes (Ligibel and Strickler, 2013). However, socioeconomic disparities in BMI highlight the need to tailor interventions based on unique socioeconomic-related needs (e.g., health-related knowledge). Qualitative research shows that obese women of low SES identify costs of maintaining weight-loss practices (e.g., expenses to prepare healthy meals, family size) and presentation of health-related information as barriers to weight loss (Coupe et al., 2018; Davis et al., 2005). Thus, in order to promote health equity among women with breast cancer and survivors, interventions should adapt strategies to modify health behaviors, including dietary changes, meal planning, and exercise (Travier et al., 2013), and tailor resources (Demark-Wahnefried et al., 2014) to reduce BMI in individuals of lower SES. In addition, thoughtful consideration of contextual factors that contribute to obesogenic status such as access to healthful food retailers and recreational facilities (Anekwe et al., 2020) is needed to sustain health behavior changes. Developing an understanding of biobehavioral and contextual characteristics that contribute to higher risk for worse breast cancer prognosis and survival may be particularly useful in improving long-term health outcomes for women of low SES with breast cancer.
Financial support:
This work was supported by the National Cancer Institute [R01-CA160427]. Preparation of this article was made possible by the National Institute of Mental Health awarded to YKP [T32MH15750].
Abbreviations:
- SES
socioeconomic status
- BMI
body mass index
- TNF-α
tumor necrosis factor alpha
- IL-6
interleukin-6
- sTNF-RII
soluble TNF receptor type II
- CRP
C-reactive protein
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL, 1994a. Socieoconomic Status and Health: The Challenge of the Gradient. Am. Psychol. 10.1021/jo901279g [DOI] [PubMed] [Google Scholar]
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kann RL, Syme SL, 1994b. Socioeconomic Status and Health: The Challenge of the Gradient. Am. Psychol. 49, 15–24. [DOI] [PubMed] [Google Scholar]
- Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL, 1993. Socioeconomic Inequalities in Health: No Easy Solution. JAMA J. Am. Med. Assoc. 269, 3140–3145. 10.1001/jama.1993.03500240084031 [DOI] [PubMed] [Google Scholar]
- Adler NE, Ostrove JM, 1999. Socioeconomic Status and Health: What We Know and What We Don’t. Ann. NEW YORK Acad. Sci. 896. [DOI] [PubMed] [Google Scholar]
- Allin KH, Nordestgaard BG, Flyger H, Bojesen SE, 2011. Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res. 13, R55. 10.1186/bcr2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anekwe CV, Jarrell AR, Townsend MJ, Gaudier GI, Hiserodt JM, Stanford FC, 2020. Socioeconomics of Obesity. Curr. Obes. Rep. 9, 272–279. 10.1007/s13679-020-00398-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay J-Y, 2003. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br. J. Cancer 88, 1721–6. 10.1038/sj.bjc.6600956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, Kubera M, Köhler CA, Fernandes BS, Stubbs B, Pavlidis N, Carvalho AF, 2017. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 52, 58–70. 10.1016/J.CTRV.2016.11.004 [DOI] [PubMed] [Google Scholar]
- Bower JE, 2019. The Role of Neuro-Immune Interactions in Cancer-Related Fatigue: Biobehavioral Risk Factors and Mechanisms. Cancer 125, 353–364. 10.1002/cncr.31790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Asher A, Garet D, Petersen L, Ganz PA, Irwin MR, Cole SW, Hurvitz SA, Crespi CM, 2019. Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer 125, 633–641. 10.1002/cncr.31827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL, 2002. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 64, 604–611. 10.1097/00006842-200207000-00010 [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW, 2007. Inflammatory responses to psychological stress in fatigued breast cancer survivors: Relationship to glucocorticoids. Brain. Behav. Immun. 21, 251–258. 10.1016/J.BBI.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW, 2011. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol. 29, 3517–22. 10.1200/JCO.2011.36.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N, 2009. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 15, 5534–40. 10.1158/1078-0432.CCR-08-2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P, Egerter S, Williams DR, 2011. The Social Determinants of Health: Coming of Age. Annu. Rev. Public Health 32, 381–398. 10.1146/annurev-publhealth-031210-101218 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ, 1988. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds Iii CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ, 1991. Quantification of Subjective Sleep Quality in Healthy Elderly Men and Women Using the Pittsburgh Sleep Quality Index (PSQI), Sleep. [PubMed] [Google Scholar]
- Byers T, Sedjo RL, 2011. Does intentional weight loss reduce cancer risk? Diabetes, Obes. Metab. 13, 1063–1072. 10.1111/j.1463-1326.2011.01464.x [DOI] [PubMed] [Google Scholar]
- Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, Fulton JP, Schymura MJ, Shen T, Van Heest S, Yin X, 2008. The impact of socioeconomic status on survival after cancer in the United States: Findings from the National Program of Cancer Registries patterns of care study. Cancer 113, 582–591. 10.1002/cncr.23567 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2020. Defining Adult Overweight and Obesity [WWW Document]. URL https://www.cdc.gov/obesity/adult/defining.html
- Chen E, Matthews KA, Thomas Boyce W, Bulletin P, 2002. Socioeconomic differences in children’s health: How and why do these relationships change with age? 128, 295–329. 10.1037/0033-2909.128.2.295 [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, 2013. Socioeconomic Status and Health: Mediating and Moderating Factors. Annu. Rev. Clin. Psychol. 9, 723–749. 10.1146/annurev-clinpsy-050212-185634 [DOI] [PubMed] [Google Scholar]
- Cheng HH, Kamarck TW, Gianaros PJ, Roecklein KA, Vanegas Y, Tsung A, Geller DA, Marsh JW, Ahmed NS, Steel JL, 2019. Socioeconomic disparities of depressive symptoms and cytokines in hepatocellular carcinoma. Psychooncology. 28, 1624–1632. 10.1002/pon.5127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng I, Shariff-Marco S, Koo J, Monroe KR, Yang J, John EM, Kurian AW, Kwan ML, Henderson BE, Bernstein L, Lu Y, Sposto R, Vigen C, Wu AH, Lin Gomez S, Keegan THM, 2015. Contribution of the Neighborhood Environment and Obesity to Breast Cancer Survival: The California Breast Cancer Survivorship Consortium. Cancer Epidemiol Biomarkers Prev 24, 1282–1290. 10.1158/1055-9965.EPI-15-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, Goodman MT, Giuliano AE, Karanja N, McAndrew P, Hudis C, Butler J, Merkel D, Kristal A, Caan B, Michaelson R, Vinciguerra V, Del Prete S, Winkler M, Hall R, Simon M, Winters BL, Elashoff RM, 2006. Dietary Fat Reduction and Breast Cancer Outcome: Interim Efficacy Results From the Women’s Intervention Nutrition Study. JNCI J. Natl. Cancer Inst. 98, 1767–1776. 10.1093/jnci/djj494 [DOI] [PubMed] [Google Scholar]
- Choi J, Joseph L, Pilote L, 2013. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes. Rev. 14, 232–244. 10.1111/obr.12003 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A Global Measure of Perceived Stress, Journal of Health and Social Behavior. [PubMed]
- Coupe N, Cotterill S, Peters S, 2018. Tailoring lifestyle interventions to low socio-economic populations: a qualitative study. BMC Public Health 18, 967. 10.1186/s12889-018-5877-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I, 2020. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell. Oncol. 43, 1–18. 10.1007/s13402-019-00489-1 [DOI] [PubMed] [Google Scholar]
- Davis EM, Clark JM, Carrese JA, Gary TL, Cooper LA, 2005. Racial and Socioeconomic Differences in the Weight-Loss Experiences of Obese Women. Am. J. Public Heal. 95. 10.2105/AJPH [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Jones LW, Snyder DC, Sloane RJ, Kimmick GG, Hughes DC, Badr HJ, Miller PE, Burke LE, Lipkus IM, 2014. Daughters and Mothers Against Breast Cancer (DAMES): Main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer 120, 2522–2534. 10.1002/cncr.28761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA, 2016. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. Mech. Dis. 11, 421–449. 10.1146/annurev-pathol-012615-044359 [DOI] [PubMed] [Google Scholar]
- Department of Health, U. Services, H., 2014. The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General.
- Dethlefsen C, Pedersen KS, Hojman P, 2017. Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Cancer Res Treat 162, 399–408. 10.1007/s10549-017-4129-4 [DOI] [PubMed] [Google Scholar]
- Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D, 2009. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur. J. Haematol. 54, 1–8. 10.1111/j.1600-0609.1995.tb01618.x [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Specter S, 2004. Poverty and obesity: the role of energy density and energy costs. Am. J. Clin. Nutr. 79, 6–16. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, 2005. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115, 911–919. 10.1016/j.jaci.2005.02.023 [DOI] [PubMed] [Google Scholar]
- Finy MS, Christian LM, 2018. Pathways Linking Childhood Abuse History and Current Socioeconomic Status to Inflammation During Pregnancy HHS Public Access. Brain. Behav. Immun. 74, 231–240. 10.1016/j.bbi.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Curtis DS, Doan SN, Coe CL, 2015. Racial Disparities in the Health Benefits of Educational Attainment. Psychosom. Med. 77, 33–40. 10.1097/PSY.0000000000000128 [DOI] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM, 2019. Chronic inflammation in the etiology of disease across the life span. Nat Med 25, 1822–1832. 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Fortmann AL, Monteros KE, de los, Mills PJ, Barrett-Connor E, Roesch SC, Matthews KA, 2012. Individual and Neighborhood Socioeconomic Status and Inflammation in Mexican-American Women: What is the Role of Obesity? Psychosom. Med. 74, 535. 10.1097/PSY.0B013E31824F5F6D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genest J, 2010. C-reactive protein: Risk factor, biomarker and/or therapeutic target?, Canadian Journal of Cardiology. 10.1016/S0828-282X(10)71061-8 [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M, 2010. Immunity, inflammation, and cancer. Cell 140, 883–99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger-Johnson G, Mõttus R, Craig LCA, Starr JM, Deary IJ, 2012. Pathways from childhood intelligence and socioeconomic status to late-life cardiovascular disease risk. Heal. Psychol. 31, 403–412. 10.1037/a0026775 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2011. Hallmarks of Cancer: The Next Generation. Cell 144, 646–674. 10.1016/J.CELL.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hastert TA, Ruterbusch JJ, Beresford SAA, Sheppard L, White E, 2016. Contribution of health behaviors to the association between area-level socioeconomic status and cancer mortality HHS Public Access. Soc Sci Med 148, 52–58. 10.1016/j.socscimed.2015.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, 2017. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Publications. [Google Scholar]
- Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K, 2019. SEER Cancer Statistics Review, 1975–2017.
- James PT, 2004. Obesity: The worldwide epidemic. Clin. Dermatol. 22, 276–280. 10.1016/J.CLINDERMATOL.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Ji P, Gong Y, Jiang CC, Hu X, Di GH, Shao ZM, 2020. Association between socioeconomic factors at diagnosis and survival in breast cancer: A population-based study. Cancer Med. 9, 1922–1936. 10.1002/cam4.2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiralerspong S, Goodwin PJ, 2016. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J. Clin. Oncol. 34, 4203–4216. 10.1200/JCO.2016.68.4480 [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW, 1996. Can Comorbidity Be Measured by Questionnaire Rather than Medical Record Review? [DOI] [PubMed]
- Knupfer H, Preib R, 2007. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res Treat 102, 129–135. 10.1007/s10549-006-9328-3 [DOI] [PubMed] [Google Scholar]
- Kumachev A, Trudeau ME, Chan KKW, 2016. Associations among socioeconomic status, patterns of care and outcomes in breast cancer patients in a universal health care system: Ontario’s experience. Cancer 122, 893–898. 10.1002/cncr.29838 [DOI] [PubMed] [Google Scholar]
- Ligibel JA, Strickler HD, 2013. Obesity and Its Impact on Breast Cancer: Tumor Incidence, Recurrence, Survival, and Possible Interventions. [DOI] [PubMed]
- Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, Sadler GR, Parker BA, Ancoli-Israel S, 2012. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain. Behav. Immun. 26, 706–713. 10.1016/J.BBI.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RS, Aiello AE, Mensah FK, Gasser CE, Rueb K, Cordell B, Juonala M, Wake M, Burgner DP, 2017. Socioeconomic status in childhood and C reactive protein in adulthood: a systematic review and meta-analysis. J. Epidemiol. Community Health 71, 817–826. 10.1136/jech-2016-208646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot MG, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A, Marmot MG, Smith GD, 1991. Health inequalities among British civil servants: the Whitehall II study. Lancet 337, 1387–1393. 10.1016/0140-6736(91)93068-K [DOI] [PubMed] [Google Scholar]
- Martin DN, Boersma BJ, Yi M, Reimers M, Howe TM, Yfantis HG, Tsai YC, Williams EH, Lee DH, Stephens RM, Weissman AM, Ambs S, 2009. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One 4, e4531. 10.1371/journal.pone.0004531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Reza I, Díaz L, García-Becerra R, 2017. Preclinical and clinical aspects of TNF-α and its receptors TNFR1 and TNFR2 in breast cancer. J. Biomed. Sci. 10.1186/s12929-017-0398-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Chang Y, Bromberger JT, Karvonen-Gutierrez CA, Kravitz HM, Thurston RC, Montez JK, 2016. Childhood Socioeconomic Circumstances, Inflammation, and Hemostasis Among Midlife Women: Study of Women’s Health Across the Nation. Psychosom. Med. 78, 311–8. 10.1097/PSY.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC, 2011. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu. Rev. Psychol. 62, 501–530. 10.1146/annurev.psych.031809.130711.Psychological [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel M, Castagné R, Berger E, Bochud M, Chadeau-Hyam M, Fraga S, Gandini M, Hutri-Kähönen N, Jalkanen S, Kivimäki M, Marmot M, McCrory C, Preisig M, Raitakari O, Ricceri F, Salmi M, Steptoe A, Vineis P, Delpierre C, Kelly-Irving M, 2020. Patterning of educational attainment across inflammatory markers: Findings from a multi-cohort study. Brain. Behav. Immun. 90, 303–310. 10.1016/J.BBI.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew NP, Bottalico L, Mesaros C, Blair IA, Tsao PY, Rosado JM, Ganguly T, Song SJ, Gimotty PA, Mao JJ, DeMichele A, 2021. Effects of systemic inflammation on relapse in early breast cancer. npj Breast Cancer 7, 7. 10.1038/s41523-020-00212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, 2018. Socioeconomic influences on brain function: implications for health. New York Acad. od Sci. 1428, 14–32. 10.1111/nyas.13862 [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Brosso SN, Humphreys KL, 2018. Socioeconomic status and inflammation: a meta-analysis. Mol. Psychiatry 1. 10.1038/s41380-018-0259-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson HK, Friedenreich CM, Brockton NT, Millikan RC, 2009. Physical Activity and Postmenopausal Breast Cancer: Proposed Biologic Mechanisms and Areas for Future Research. Cancer Epidemiol Biomarkers Prev 18, 11–27. 10.1158/1055-9965.EPI-08-0756 [DOI] [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR, 2009. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain. Behav. Immun. 23, 887–897. 10.1016/J.BBI.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD, 2020. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain. Behav. Immun. 87, 901–909. 10.1016/J.BBI.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakiz B, Flatt SW, Bardwell WA, Rock CL, Mills PJ, 2011. Effects of a Weight Loss Intervention on Body Mass, Fitness, and Inflammatory Biomarkers in Overweight or Obese Breast Cancer Survivors. Int. J. Behav. Med. 18, 333–341. 10.1007/s12529-010-9079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O’Dwyer A-M, Harkin A, Kennedy MJ, Connor TJ, 2013. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain. Behav. Immun. 34, 108–119. 10.1016/J.BBI.2013.07.177 [DOI] [PubMed] [Google Scholar]
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM, 2017. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA. Cancer J. Clin. 67, 378–397. 10.3322/caac.21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM, 2009. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 27, 3437–44. 10.1200/JCO.2008.18.9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras SA, Goodman E, 2013. Socioeconomic Status Gradients in Inflammation in Adolescence. Psychosom Med 75, 442–448. 10.1097/PSY.0b013e31828b871a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G, 2009. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. Eur. J. Epidemiol. 55–66. 10.1007/s10654-006-9082-1 [DOI] [PubMed] [Google Scholar]
- Protani M, Coory M, Martin JH, 2010. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res. Treat. 123, 627–635. 10.1007/s10549-010-0990-0 [DOI] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population.
- Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W, 2014. Weight loss intervention trials in women with breast cancer: a systematic review. Obes. Rev. 15, 749–768. 10.1111/obr.12190 [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Newman V, Caan BJ, Haan MN, Stefanick ML, Faerber S, Pierce JP, 1999. Factors Associated With Weight Gain in Women After Diagnosis of Breast Cancer. J. Am. Diet. Assoc. 99, 1212–1221. 10.1016/S0002-8223(99)00298-9 [DOI] [PubMed] [Google Scholar]
- Rogers LQ, Courneya KS, Carter SJ, Anton PM, Verhulst S, Vicari SK, Robbs RS, McAuley E, 2016. Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res. Treat. 159, 283. 10.1007/S10549-016-3945-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J, 1999. Refining the association between education and health: the effects of quantity, credential, and selectivity. Demography 36, 445–460. [PubMed] [Google Scholar]
- Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY, 2003. CIRCULATING INTERLEUKIN-6 PREDICTS SURVIVAL IN PATIENTS WITH METASTATIC BREAST CANCER. Int. J. Cancer 103, 642–646. 10.1002/ijc.10833 [DOI] [PubMed] [Google Scholar]
- Schnittker J, 2004. Education and the Changing Shape of the Income Gradient in Health, Journal of Health and Social Behavior. [DOI] [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE, 2007. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain. Behav. Immun. 21, 413–427. 10.1016/J.BBI.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS, 2010. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann. N. Y. Acad. Sci. 1186, 223–239. 10.1111/j.1749-6632.2009.05341.x [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2019. Cancer statistics, 2019. CA. Cancer J. Clin. 69, 7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- Sprague BL, Trentham-Dietz A, Gangnon RE, Ramchandani R, Hampton JM, Robert SA, Remington PL, Newcomb PA, 2011. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer 117, 1542–51. 10.1002/cncr.25589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travier N, Fonseca-Nunes A, Javierre C, Guillamo E, Arribas L, Peiró I, Buckland G, Moreno F, Urruticoechea A, Oviedo GR, Roca A, Hurtós L, Ortega V, Muñoz M, Garrigós L, Cirauqui B, del Barco S, Arcusa A, Seguí MA, Borràs JM, Gonzalez CA, Agudo A, 2013. Effect of a diet and physical activity intervention on body weight and nutritional patterns in overweight and obese breast cancer survivors. Med. Oncol. 31, 783. 10.1007/s12032-013-0783-5 [DOI] [PubMed] [Google Scholar]
- Villasenor A, Flatt SW, Marinac C, Natarajan L, Pierce JP, Patterson RE, 2014. Postdiagnosis C-reactive protein and Breast Cancer Survivorship: findings from the WHEL Study. Cancer Epidemiol. Biomarkers Prev. 23, 189. 10.1158/1055-9965.EPI-13-0852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vona-Davis L, Rose DP, 2009. The Influence of Socioeconomic Disparities on Breast Cancer Tumor Biology and Prognosis: A Review. J. Women’s Heal. 18, 883–893. [DOI] [PubMed] [Google Scholar]
- Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres MA, 2017. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol. Med. 47, 1733–1743. 10.1017/S0033291717000150 [DOI] [PubMed] [Google Scholar]
- Zonderman AB, Ejiogu N, Norbeck J, Evans MK, 2014. The Influence of Health Disparities on Targeting Cancer Prevention Efforts. Am. J. Prev. Med. 46, S87–S97. 10.1016/J.AMEPRE.2013.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]


