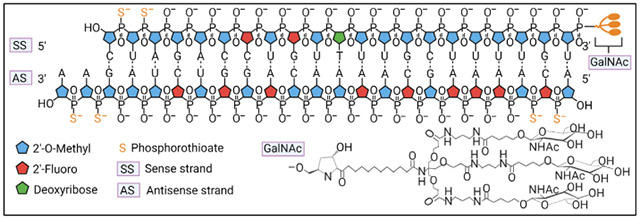
STRUCTURE:
Inclisiran is a synthetic, chemically modified, double-stranded siRNA. The sense strand is conjugated with a triantennary N-acetylgalactosamine (GalNAc) ligand to enable targeting of hepatocytes via the asialoglycoprotein receptor (ASGPR). Its molecular formula is C529H664F12N176Na43O316P43S6 and its molecular weight is 17 284.75 g/mol. There are 32 ribonucleotides chemically modified with 2-O-methyl-ribonucleotide (2′-O-methyl), 11 modified with 2′-fluoro-ribonucleotide (2′-fluoro) and one 2′-deoxy-ribonucleotide. In addition, two phosphorothioate groups are added to the 5′ end of the sense and both the 5′ and 3′ ends of the antisense strands.
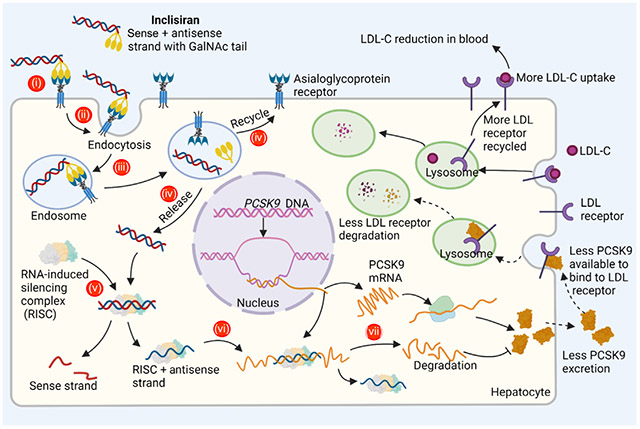
MECHANISM OF ACTION:
After subcutaneous administration into the body, inclisiran is transported across the interstitial space into the bloodstream and, subsequently, to the target organ (liver) via ASGPR-mediated uptake. After reaching the liver, inclisiran goes through a series of events, ultimately silencing the proprotein convertase subtilisin/kexin type 9 (PCSK9), resulting in the reduction of PCSK9 protein in hepatocyte cells. (i) GalNAc ligand of inclisiran binds to the ASGPR expressed on the surface of hepatocyte cell membrane, inducing internalization via (ii) endocytosis. (iii) During endocytosis, inclisiran is trapped in endocytic vesicles, which fuse with endosomes/lysosomes. (iv) The GalNAc ligand of inclisiran is degraded and the double-stranded siRNA is slowly released into the cytoplasm from endosomes/lysosomes, while the ASGPR is recycled to the cell surface. (v) The double-stranded siRNA part of inclisiran is next loaded into the RNA-induced silencing complex (RISC), which leads to removal of the sense strand. (vi) The antisense strand containing active RISC scans for and binds to the complementary sequence in its target mRNA of PCSK9 in the cytoplasm. (vii) The RISC utilizes the catalytic slicer activity that degrades PCSK9 mRNA, resulting in less mRNA available for translation. As a result, less PCSK9 is available to bind low-density lipoprotein cholesterol receptors (LDLR), minimizing their targeting for degradation. Less degradation of LDLR allows for a higher density of LDLR at the cell surface, available to bind and take in LDL-C, with the end result being lower plasma LDL-C.
NAME:
The drug name is inclisiran and the brand name is Leqvio.
DRUG CLASS:
Inclisiran is considered as a large molecule class of drugs known as RNA interference (RNAi) therapeutics. It works by a natural mechanism in cells, by which short interfering RNAs (siRNAs) cause sequence-specific modulation of gene expression.
CLINICAL USE:
Inclisiran is indicated as an adjunct treatment along with statin therapy for patients with heterozygous familial hypercholesteremia and atherosclerotic cardiovascular disease , who require additional lowering of LDL-C. It is particularly useful for patients with uncontrolled cholesterol levels even after maximal stain therapies. Inclisiran is given by a healthcare professional with a subcutaneous injection of 284 mg initially, again at 3 months, and then every 6 months. Inclisiran has shown an over 50% decrease of the LDL-C level with one dose every 6 months.
ADVERSE EFFECTS:
The most common adverse reaction is injection site reactions in 8% of patients, including erythema, pain, and rash. About 5% of patients have an increased arthralgia and development of antidrug antibodies to Leqvio. Lastly, approximately 4% of patients experience bronchitis during treatment. Less than 2% of patients experience myalgia, headache, fatigue, nasopharyngitis, back pain, hypertension, diarrhea, and dizziness. There are no known current contraindications.
DEVELOPED BY:
Inclisiran was developed by Alnylam Pharmaceuticals, a leader in RNAi therapeutics. Novartis obtained global rights to develop, manufacture, and commercialize Leqvio (under a license and collaboration agreement with Alnylam Pharmaceuticals) by purchasing the Medicines Company.
TIMELINE:
2014–2018, Phase I/II trials, NCT02314442, ORION-1 (NCT02597127), ORION-2 (NCT02963311).
2017–2019, Phase III trials, ORION-9 (NCT03397121), ORION-10 (NCT03399370), and ORION-11 (NCT03400800).
December 22, 2021, FDA approval for Leqvio (inclisiran), new drug application: 214012.
Acknowledgments
X-b.Z. is funded by the National Institutes of Health/National Institute of General Medical Sciences (NIGMS) R35GM140862.
Footnotes
Declaration of interests
The authors declare no conflict of interest.
Literature
- 1.Dyrbus K et al. (2020) Inclisiran-new hope in the management of lipid disorders? J. Clin. Lipidol 14, 16–27 [DOI] [PubMed] [Google Scholar]
- 2.Kosmas CE et al. (2018) Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases 6, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang MM et al. (2021) The growth of siRNA-based therapeutics: updated clinical studies. Biochem. Pharmacol 189, 114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raal FJ et al. (2020) Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N. Engl. J. Med 382, 1520–1530 [DOI] [PubMed] [Google Scholar]
- 5.Ray KK et al. (2020) Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N. Engl. J. Med 382, 1507–1519 [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald K et al. (2017) A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med 376, 41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray KK et al. (2017) Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med 376, 1430–1440 [DOI] [PubMed] [Google Scholar]
- 8.Hovingh GK et al. (2020) Inclisiran durably lowers low-density lipoprotein cholesterol and proprotein convertase subtilisin/ kexin type 9 expression in homozygous familial hypercholesterolemia: the ORION-2 pilot study. Circulation 141,1829–1831 [DOI] [PubMed] [Google Scholar]


