Abstract
The effect of caspase inhibitors on lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW 267.4 murine macrophage cells was investigated. Pretreatment of RAW cells with a broad caspase inhibitor, benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-FMK), resulted in a striking reduction in LPS-induced NO production. Z-VAD-FMK inhibited LPS-induced NF-κB activation. Furthermore, it blocked phosphorylation of c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) but not that of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinases. Similarly, a caspase 3-specific inhibitor, Z-Asp-Glu-Val-Asp-fluoromethylketone, inhibited NO production, NF-κB activation, and JNK/SAPK phosphorylation in LPS-stimulated RAW cells. The attenuated NO production was due to inhibition of the expression of an inducible-type NO synthase (iNOS). The overexpression of the dominant negative mutant of JNK/SAPK and the addition of a JNK/SAPK inhibitor blocked iNOS expression but did not block LPS-induced caspase 3 activation. It was therefore suggested that the inhibition of caspase 3 might abrogate LPS-induced NO production by preventing the activation of NF-κB and JNK/SAPK. The caspase family, especially caspase 3, is likely to play an important role in the signal transduction for iNOS-mediated NO production in LPS-stimulated mouse macrophages.
Nitric oxide (NO) is an important regulatory and effector molecule with various biological functions (4, 5, 22, 23). NO is synthesized by constitutively expressed NO synthase and an inducible isoform of NO synthase (iNOS) (19, 23, 33). NO production is markedly augmented in several cell types, including macrophages and vascular endothelial cells, by lipopolysaccharide (LPS) (21–23, 31, 32, 35). The augmentation of NO production by LPS is dependent on newly expressed iNOS (20, 30, 33). Once iNOS is induced, it produces large amounts of NO that profoundly influence cell and tissue function and damage (4, 5, 10, 14, 16, 17, 19, 23, 29). Murine macrophages provide the best-studied example of the regulation of NO production (22). The induction of iNOS is mainly triggered and regulated by a series of signaling pathways including NF-κB transcription factor and mitogen-activated protein (MAP) kinases (1, 7, 15, 18, 20, 26, 30). There are several reports on the participation of other signal molecules in LPS-induced iNOS expression in mouse macrophages (33). Recently, LPS has been reported to induce the activation of caspases directly in vitro (2, 13, 35), and their activation has been shown to modulate the activation of MAP kinases (6, 37). Therefore, it was of interest to determine whether the activation of caspases played a role in NO production and iNOS expression in LPS-stimulated macrophages. In this study we examined the effect of caspase inhibitors on LPS-induced NO production in RAW 267.4 murine macrophage cells. Here, we describe the participation of caspase 3 in LPS signaling for NO production and iNOS expression.
MATERIALS AND METHODS
Materials.
LPS from Escherichia coli O55:B5 was obtained from Sigma Chemical Co., St. Louis, MO Benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-FMK), Z-Asp-Glu-Val-Asp-fluoromethylketone (DEVD-FMK), and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) were purchased from Calbiochem-Behring, San Diego, Calif.
Cell culture.
The murine macrophage cell line RAW 267.4 was obtained from the Health Science Resource Bank (Tokyo, Japan) and maintained in RPMI 1640 medium (Sigma) containing 5% heat-inactivated fetal calf serum (GIBCO-BRL, Grand Island, N.Y.) at 37°C under 5% CO2. The cells were washed gently with Hank's balanced salt solution (Sigma) and removed from the flasks. The cells were then suspended in a 12-well plate or a 96-well plate for experiments.
Determination of nitrite concentration.
NO was measured as its end product nitrite, using Griess reagent as described previously (12). Fifty microliters of culture supernatants were mixed with 100 μl of Griess reagent. After 10 min, absorbance at 570 nm was measured in a microplate enzyme-linked immunosorbent assay reader. The concentration of nitrite in the culture supernatant was determined with reference to a sodium nitrite standard curve. Data represent the mean values of triplicate measurements plus or minus the standard deviation (SD).
Immunoblotting.
RAW cells were seeded in 35-mm plastic dishes (4 × 105 cells per dish) and incubated with LPS for either 1 h or 8 h. Cells were lysed in the lysis buffer, which contained 0.5 M Tris-HCl, 4% sodium dodecyl sulfate, and 2 mercaptoethanol, and were boiled for 5 min at 100°C. Aliquots (20 μg per lane) containing equal amounts of protein were electrophoresed under reducing conditions in a 4 to 20% gradient polyacrylamide gel and transferred to a polyvinylidene difluoride membrane filter. The membranes were treated with 5% bovine serum albumin for 1 h to block nonspecific binding, rinsed, and incubated with a panel of rabbit polyclonal antibodies against iNOS (Upstate Biotechnology, Lake Placid, N.Y.), extracellular signal-regulated kinase 1/2 (Erk1/2), phospho-Erk1/2, p38, phospho-p38, phospho-c-Jun N-terminal kinase (JNK/SAPK), and JNK/SAPK (New England Biolabs, Beverly, Mass.) for 1 h. The membranes were further treated with a 1:3000 dilution of horseradish peroxidase-conjugated protein G for 1 h. Immune complexes on the blots were detected with an enhanced chemiluminescence substrate (New England Nuclear, Boston, Mass.) and exposed to Kodak XAR X-ray film.
Luciferase reporter gene assay for NF-κB activation.
RAW cells (3 × 105/ml) were plated in 35-mm plastic dishes. On the following day, the cells were transfected with 0.5 μg of pNF-κB-Luc plasmid, a luciferase reporter gene driven by five tandem repeats of NF-κB binding motif (PathDetect System; Stratagene, La Jolla, Calif.), and 0.5 μg of pCMV-β-gal plasmid (GIBCO-BRL) by the lipofectin method (GIBCO-BRL). The transfected cells were incubated for 48 h and further treated with Z-VAD-FMK or NPPB for 1 h followed by treatment with LPS for 8 h. The cells were lysed using the lysis reagent from Promega (Madison, Wisc.) prior to measurement of luciferase activity. The luciferase activity was determined on cell lysates with a luminometer. β-Galactosidase activity was used to normalize transfection efficiencies, and the transfection rates of RAW cells were approximately 1 to 2%. Bar diagrams in all of the figures below are shown as the mean ± SD for two experiments in which each transfection was performed twice.
Transfection with the dominant negative mutant of JNK/SAPK.
The dominant negative mutant of JNK/SAPK was kindly supplied by J. D. Lee, The Scripps Research Institute, La Jolla, Calif. RAW cells were transfected with various concentrations of this negative mutant of JNK/SAPK, using the lipofectin method (GIBCO-BRL) for 8 h with or without the NF-κB luciferase vector or pCMV-gal. Cells were further incubated with LPS and used for the measurement of NO and NF-κB-dependent luciferase activity.
Determination of caspase 3 activity.
Cells harvested by scraping were suspended in hypotonic cell lysis buffer in the CaspACE assay kit (Promega) and lysed by four cycles of alternate freezing and thawing. Cell lysates were centrifuged at 16,000 × g for 20 min at 4°C, and the supernatant was collected. The activity of caspase 3 in cell extracts was determined with a fluorometric assay using the CaspACE assay kit. The values were expressed as relative fluorescence units. Data represent the mean values of triplicate measurements ± SD.
RESULTS
Activation of caspase 3 in LPS-stimulated RAW cells and its inhibition by caspase inhibitors.
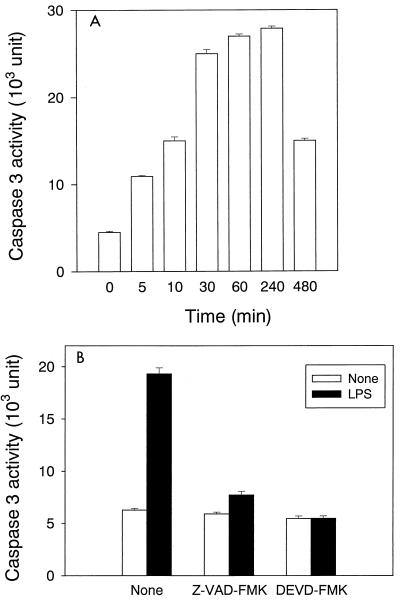
To study the participation of various caspases in LPS-induced NO production, we confirmed the activation of caspases in LPS-stimulated RAW cells and the inhibitory effect of Z-VAD-FMK and DEVD-FMK on their activation. The activity of caspase 3 in cell extracts from LPS-stimulated RAW cells was determined by means of a fluorometric assay (Fig. 1). Caspase 3 activity was markedly augmented in LPS-stimulated RAW cells. Augmented caspase 3 activity was detected immediately after the addition of LPS, and it reached almost maximal activity within 30 min after LPS addition (Fig. 1A). Pretreatment with Z-VAD-FMK or DEVD-FMK abolished the augmentation of the caspase 3 activity by LPS stimulation (Fig. 1B). In addition, there was no significant difference in the expression of caspase 1, 6, 7, 8, and 9 between LPS-stimulated and untreated RAW cells as measured with the immunoblotting analysis (data not shown).
FIG. 1.
The activation of caspase 3 in LPS-stimulated RAW cells and the inhibition by Z-VAD-FMK or DEVD-FMK. (A) RAW cells were treated with LPS (1 μg/ml) for various amounts of time. (B) RAW cells were pretreated with Z-VAD-FMK (100 μM) or DEVD-FMK (10 μM) for 30 min. Both pretreated and untreated cells were exposed to LPS (1 μg/ml) for 1 h.
Inhibition of LPS-induced NO production by caspase inhibitors.
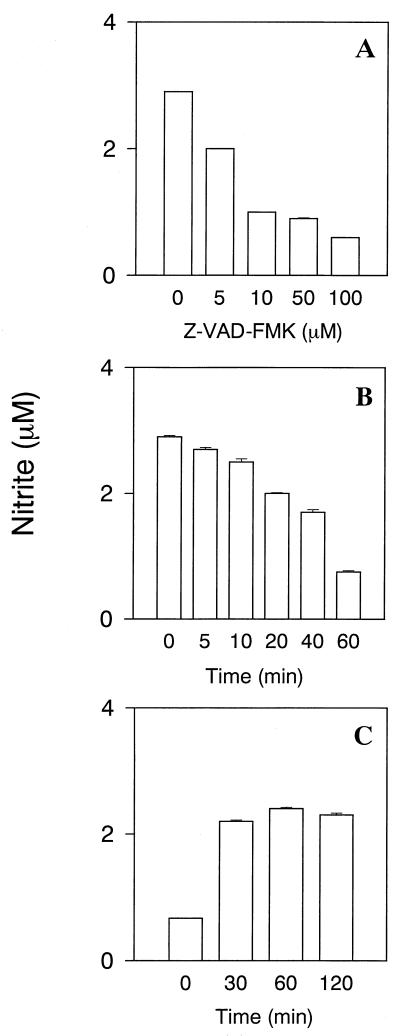
The effect of pretreatment with Z-VAD-FMK on LPS-induced NO production in RAW cells was examined (Fig. 2A). Z-VAD-FMK pretreatment reduced LPS-induced NO production in a dose-dependent manner, although addition of LPS (1 μg/ml) markedly augmented NO production in untreated control cells. Z-VAD-FMK at any of the concentrations tested did not exhibit a cytotoxic effect on RAW cells (data not shown).
FIG. 2.
The inhibitory action of Z-VAD-FMK on NO production in LPS-stimulated RAW cells. (A) The effect of Z-VAD-FMK pretreatment on LPS-induced NO production in RAW cells. RAW cells were pretreated with various concentrations of Z-VAD-FMK for 30 min. Z-VAD-FMK-pretreated RAW cells were incubated with LPS (1 μg/ml) for 24 h. (B) The effect of exposure time of RAW cells to Z-VAD-FMK on LPS-induced NO production. RAW cells were exposed to Z-VAD-FMK (100 μM) for the indicated periods. Z-VAD-FMK-pretreated RAW cells were incubated with LPS (1 μg/ml) for 24 h. (C) The effect of Z-VAD-FMK posttreatment on NO production in LPS-pretreated RAW cells. RAW cells were pretreated with LPS (1 μg/ml) for the indicated periods. After LPS pretreatment, the cells were washed and cultured with Z-VAD-FMK (100 μM) for 30 min, and subsequently incubated with, LPS (1 μg/ml) for 24 h.
The effect of Z-VAD-FMK on LPS-induced NO production was examined when RAW cells were transiently exposed to Z-VAD-FMK (100 μM) for various times. The exposure of RAW cells to Z-VAD-FMK for 60 min resulted in a marked reduction in NO production in response to treatment with LPS (1 μg/ml) (Fig. 2B), suggesting that brief contact with Z-VAD-FMK was insufficient to inhibit LPS-induced NO production in RAW cells.
The posttreatment effect of Z-VAD-FMK on LPS-induced NO production was also examined (Fig. 2C). RAW cells were incubated with LPS (1 μg/ml) for various amounts of time, followed by the addition of Z-VAD-FMK (100 μM) to the cultures. The addition of Z-VAD-FMK into cultures of RAW cells pretreated with LPS for 30 min did not cause the inhibition of LPS-induced NO production (Fig. 2C), suggesting that Z-VAD-FMK pretreatment was required to inhibit LPS-induced NO production.
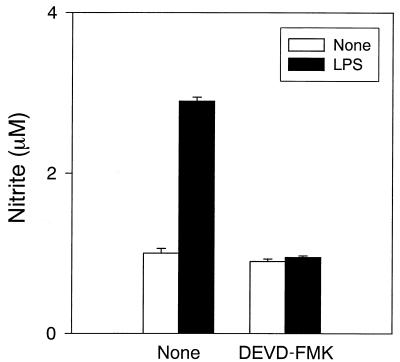
We have shown that a broad caspase inhibitor, Z-VAD-FMK, inhibits NO production in LPS-stimulated RAW cells. The effect of a caspase 3-specific inhibitor, DEVD-FMK, on LPS-induced NO production was investigated (Fig. 3). Pretreatment with DEVD-FMK completely inhibited LPS-induced NO production, suggesting a critical role for caspase 3 in the caspases. The inhibitor for caspase 1, 2, 6, 8, or 9 (Calbiochem-Behring) did not inhibit LPS-induced NO production.
FIG. 3.
The inhibitory action of DEVD-FMK on NO production in LPS-stimulated RAW cells. RAW cells were pretreated with DEVD-FMK (10 μM) for 30 min. DEVD-FMK-pretreated RAW cells were incubated with LPS (1 μ/ml) for 24 h.
Inhibition of LPS-induced iNOS expression by Z-VAD-FMK.
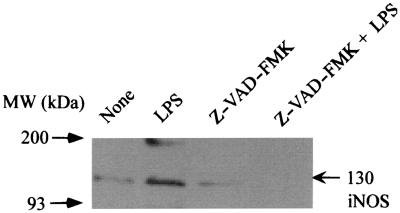
Since Z-VAD-FMK inhibited LPS-induced NO production, the expression of iNOS protein in response to treatment with LPS (1 μg/ml) was studied in Z-VAD-FMK-pretreated RAW cells by immunoblotting using an anti-iNOS antibody (Fig. 4). The iNOS protein was readily detected in LPS-stimulated RAW cells. However, pretreatment of RAW cells with Z-VAD-FMK (100 μM) abrogated the appearance of iNOS protein in response to LPS.
FIG. 4.
The effect of Z-VAD-FMK on the expression of iNOS protein in LPS-stimulated RAW cells. RAW cells were pretreated with Z-VAD-FMK (100 μM) for 30 min. Z-VAD-FMK-pretreated or untreated control cells were incubated with LPS (1 μg/ml) for 8 h. The cells were lysed, and the lysates were analyzed by immunoblotting using an anti-iNOS antibody.
Inhibition of LPS-induced NF-κB activation by Z-VAD-FMK.
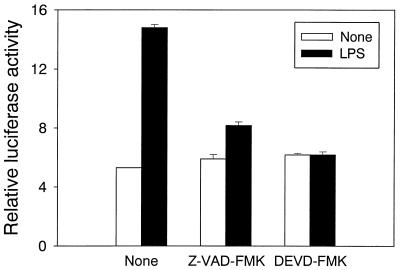
It has been reported that the activation of NF-κB plays an important role in LPS-induced NO production in RAW cells (15, 18, 20, 30). Therefore, we tested the effect of Z-VAD-FMK or DEVD-FMK on LPS-induced NF-κB activation using a luciferase reporter gene assay (Fig. 5). LPS markedly enhanced the reporter gene activity in untreated control RAW cells, indicating NF-κB activation. In contrast, pretreatment with Z-VAD-FMK abrogated the enhancement of the NF-κB-dependent reporter gene activity induced by LPS. LPS-induced NF-κB activation was also completely inhibited in the cells pretreated with DEVD-FMK (10 μM).
FIG. 5.
The effect of Z-VAD-FMK and DEVD-FMK on the activation of NF-κB in LPS-stimulated RAW cells. RAW cells were cotransfected with NF-κB-dependent luciferase reporter gene and β-galactosidase gene. After 48 h the cells were pretreated with Z-VAD-FMK (100 μM) or DEVD-FMK (10 μM) for 30 min. The pretreated cells were incubated with LPS (1 μg/ml) for 8 h.
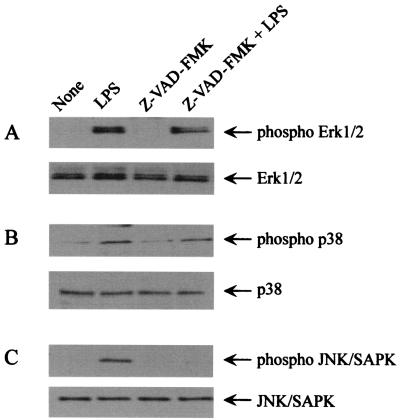
Inhibition of LPS-induced phosphorylation of SAPK/JNK by Z-VAD-FMK.
LPS is known to activate a series of MAP kinases, such as Erk1/2, p38, and JNK/SAPK in macrophages (1, 7, 15). These signal pathways may be involved in LPS-induced NO production in RAW cells. Therefore, the effect of Z-VAD-FMK on the activation of these MAP kinase pathways was examined by immunoblotting using anti-phospho-MAP kinase antibodies (Fig. 6). In Z-VAD-FMK-pretreated cells, LPS did not induce the phosphorylation of JNK/SAPK although it induced the phosphorylation of the MAP kinases p38 and Erk1/2. Z-VAD-FMK inhibited the phosphorylation of JNK/SAPK without affecting the basal level of JNK/SAPK expression. This result suggested that Z-VAD-FMK might inhibit LPS-induced NO production by preventing the phosphorylation of the JNK/SAPK MAP kinase pathway. DEVD-FMK also inhibited phosphorylation of JNK/SAPK (data not shown).
FIG. 6.
The effect of Z-VAD-FMK on the phosphorylation of Erk1/2, p38, and JNK/SAPK MAP kinases in LPS-stimulated RAW cells. RAW cells were pretreated with Z-VAD-FMK (100 μm) for 30 min, followed by treatment with LPS (1 μg/ml) for 30 min. The cells were lysed, and the lysates were analyzed by immunoblotting using antibodies to (A) phospho -Erk1/2, (B) phospho-p38, or (C) phospho-JNK/SAPK. The antibody against Erk1/2, p38, or JNK/SAPK was also used.
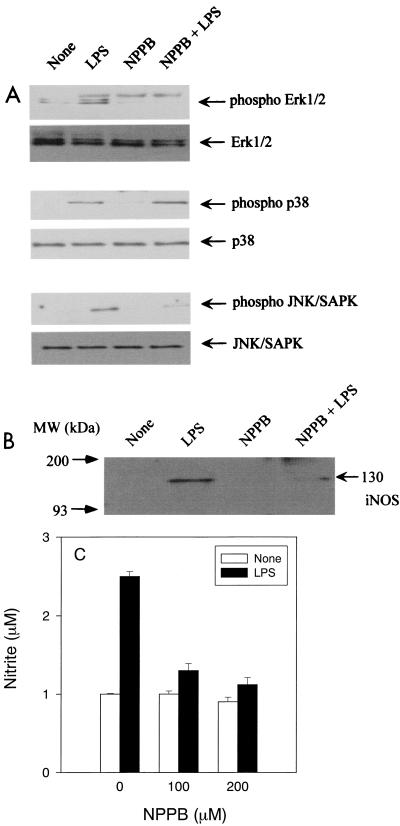
Inhibition of NO production and iNOS expression by a JNK/SAPK inhibitor, NPPB.
It has been reported that NPPB inhibits the activation of Erk1/2 and JNK/SAPK but not p38 (8). Therefore, NPPB was used as a JNK/SAPK inhibitor to confirm the participation of JNK/SAPK activation in LPS-induced iNOS expression. As shown in Fig. 7A, NPPB significantly inhibited phosphorylation of JNK/SAPK but did not inhibit p38 MAP kinase in LPS-stimulated macrophages. NPPB also inhibited phosphorylation of Erk1/2 activation. We examined the effect of NPPB on the expression of iNOS in LPS-stimulated RAW cells (Fig. 7B). LPS-induced iNOS expression was significantly inhibited in NPPB-pretreated RAW cells. The effect of NPPB on LPS-induced NO production was also examined (Fig. 7C). NPPB at 100 or 200 μM inhibited NO production in LPS-stimulated RAW cells. These results supported the premise that SAPK/JNK MAP kinase might be involved in the signal transduction of LPS-induced NO production. However, the experimental result obtained with NPPB could not exclude the participation of Erk1/2 MAP kinase in LPS-induced NO production, since it inhibits both SAPK/JNK and Erk1/2 activation (8).
FIG. 7.
The effect of NPPB on (A) the phosphorylation of Erk1/2, p38, and JNK/SAPK MAP kinases, (B) iNOS protein expression, and (C) NO production in LPS-stimulated RAW cells. RAW cells were pretreated with NPPB (100 μM) for 30 min, followed by treatment with LPS (1 μg/ml) for 30 min. The cells were lysed, and the lysates were analyzed by immunoblotting, using (A) the antibodies to phospho-Erk1/2, phospho-p38, phospho-JNK/SAPK, Erk, p38, or JNK/SAPK or (B) iNOS. (C) RAW cells were pretreated with NPPB (100 μM) for 30 min. NPPB pretreated and untreated RAW cells were incubated with LPS (1 μg/ml) for 24 h.
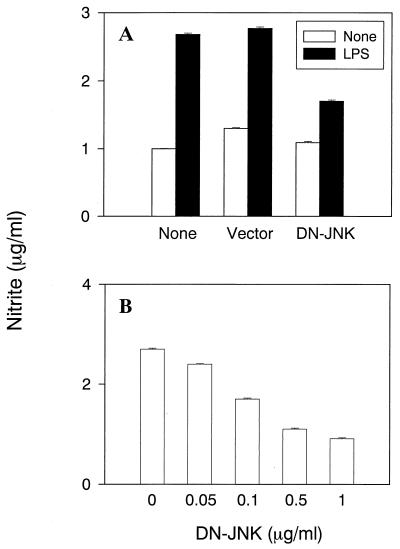
Inhibition of NO production and iNOS expression by the dominant negative JNK/SAPK mutant.
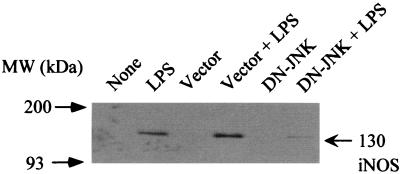
To further elucidate the participation of JNK/SAPK MAP kinase in LPS-induced iNOS expression and NO production, the effect of a dominant negative JNK/SAPK mutant on LPS-induced NO production was examined using transfected RAW cells (Fig. 8). Transfection of RAW cells with the dominant negative JNK mutant resulted in the striking inhibition of LPS-induced NO production (Fig. 8A). However, using the empty vector as a negative control did not affect NO production. Next, the dose dependency of the dominant negative JNK/SAPK mutant on LPS-induced NO production was examined in transfected RAW cells. The inhibition was dependent on the concentration of the dominant negative JNK/SAPK mutant used for transfection (Fig. 8B). LPS-induced iNOS expression was also inhibited by the dominant negative JNK/SAPK mutant (Fig. 9).
FIG. 8.
The effect of DN-JNK on NO production in LPS-stimulated RAW cells. (A) RAW cells were treated with DN-JNK or the empty vector (0.5 μg/ml) for 48 h. The transfected cells were treated with LPS (1 μg/ml) for 24 h. (B) Cells were transfected with various concentrations of DN-JNK for 48 h followed by exposure to LPS (1 μg/ml) for 24 h.
FIG. 9.
The effect of DN-JNK on the expression of iNOS protein in LPS-stimulated RAW cells. Cells transfected with DN-JNK or the empty vector (0.5 μg/ml) were incubated with LPS (1 μg/ml) for 8 h. The cells were lysed, and the lysates were analyzed by immunoblotting using an anti-iNOS antibody.
Inhibition of NF-κB activation by NPPB and the dominant negative JNK/SAPK.
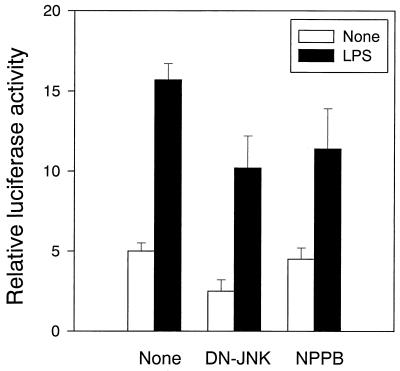
There are several reports on the cross-talk between two NF-κB and JNK/SAPK signal pathways (25, 27, 36). The effect of NPPB and the dominant negative JNK on LPS-induced NF-κB activation was examined by using NF-κB-dependent reporter gene activity. As shown in Fig. 10, NPPB and the dominant negative mutant JNK (DN-JNK) inhibited NF-κB activation to some extent in LPS-stimulated RAW cells (P < 0.05), suggesting some relationship between JNK/SAPK and NF-κB.
FIG. 10.
The effect DN-JNK and NPPB on NF-κB activation in LPS-stimulated RAW cells. RAW cells were transfected with the NF-κB-dependent luciferase reporter gene and β-galactosidase for 48 h and further cotransfected with DN-JNK (0.5 μg/ml) for 48 h or treated with NPPB (100 μM) for 30 min. The cells were incubated with LPS (1 μg/ml) for 8 h.
No inhibition of caspase 3 activity by NPPB and the dominant negative JNK/SAPK.
We have demonstrated that both a broad caspase inhibitor, Z-VAD-FMK, and a caspase 3-specific inhibitor, DEVD-FMK, inhibited JNK/SAPK phosphorylation in LPS-stimulated RAW cells. The effect of NPPB and a dominant negative JNK/SAPK mutant on LPS-induced caspase 3 activation was also studied. Pretreatment with NPPB and the DN-JNK mutant did not affect the augmentation of the caspase 3 activity in LPS-stimulated RAW cells (data not shown), suggesting that caspase 3 might modulate the SAPK/JNK signal pathway as its upstream regulator.
DISCUSSION
In this study, we demonstrate that caspase inhibitors, Z-VAD-FMK and DEVD-FMK, abolished NO production and iNOS expression in LPS-stimulated RAW cells. This suggested that the caspases participate in LPS-induced NO production. Based on the fact that NF-κB and SAPK/JNK are involved in the induction of iNOS protein in mouse macrophages (8), it is likely that the impaired functioning of NF-κB and JNK/SAPK MAP kinase caused by caspase inhibitors may result in the down-regulation of LPS-induced iNOS expression and NO production. Only Z-VAD-FMK and DEVD-FMK, which inhibit caspase 3 activation, blocked JNK/SAPK and NF-κB activation and NO production in LPS stimulation. Moreover, LPS exclusively activated the activity of caspase 3 but not caspase 1, 6, 7, 8, or 9 in RAW cells. Taken together, it is possible that caspase 3 plays an important role in NO production and iNOS induction in LPS-stimulated RAW cells. NO is reported to inhibit caspase 3 activation and apoptosis (11, 24, 28), but this is not the case, since NO production and caspase 3 activation coincide in LPS-stimulated RAW cells and caspase inhibitors block the iNOS expression.
The caspase 3 inhibitors down-regulated both the NF-κB and JNK/SAPK MAP kinase signal pathways. On the other hand, the inhibition of JNK/SAPK MAP kinase by NPPB and the dominant negative mutant of JNK/SAPK did not abolish LPS-induced caspase 3 activation. This finding suggested that caspase 3 might regulate the activation of JNK/SAPK as an upstream regulator. Furthermore, NPPB and the dominant negative mutant of JNK did not affect LPS-induced NF-κB activation very much, suggesting that JNK/SAPK is not much involved in NF-κB activation. Caspase 3 may regulate LPS-induced activation of NF-κB and JNK/SAPK MAP kinase as their upstream regulator and modulate LPS-induced NO production through the iNOS expression. There seemed to be some crosstalk between the two signal pathways based on the experiments using NPPB and DN-JNK (Fig. 10). In fact, there are several reports which suggest that NF-κB activation requires the activation of MAP kinases (9, 26, 37).
It is well known that the caspase family plays a crucial role in apoptotic cell death. Caspase 3 is an especially important executioner molecule for apoptosis induction. It is interesting that our study showed that caspase 3 might act as a regulatory molecule in the signal transduction for LPS-induced iNOS expression and modulate the NF-κB pathway as the upstream regulator. The cleavage of MAP kinase kinase (MEKK1) into 91-kDa fragments by caspase 3 increases the MEKK1 activity which, in turn, enhances SAPK/JNK activation (6, 37). Recently, MEKK1 activation has also been reported to result in NF-κB translocation (3). It is possible that caspase 3 may affect NF-κB activation through the cleavage of MEKK1. Furthermore, there are several reports on the participation of the caspase family in the LPS-signaling pathway (2, 34). Although it is of particular interest that caspases might mediate LPS signaling through NF-κB, the exact mechanism must await further studies.
A series of MAP kinases are involved in the signaling pathway for LPS-induced iNOS expression (1, 7, 8). The Erk1/2 signal pathway is involved in LPS-induced iNOS expression in RAW cells since the Erk1/2 inhibitor reduces the iNOS expression by LPS (1). The participation of MAP kinases p38 and Erk1/2 in LPS-induced iNOS expression has also been demonstrated by several workers (1). This study clearly demonstrated the participation of JNK/SAPK in LPS-induced NO production in RAW cells. On the other hand, the trans-activating factor responsible for LPS-induced iNOS expression has been reported to be NF-κB. The promoter region of iNOS gene contains several binding sites for NF-κB, AP-1, and other transcription factors. AP-1 can be activated by the JNK/SAPK signal pathway. AP-1 and NF-κB are reported to form a synergistic complex in enhancing transcription (33). Recently, it was found in the yeast two-hybrid system that JNK/SAPK may associate with the c-rel subunit of NF-κB and enhance NF-κB activation directly in an overexpression system, although JNK/SAPK does not appear to be a direct IκB kinase (27). Caspase 3 may up-regulate NF-κB and AP-1 through the activation of NF-κB and JNK/SAPK signal pathways and enhance the transcription of the iNOS gene.
In summary, we demonstrate that the inhibition of caspase 3 by caspase inhibitors led to the down-regulation of JNK/SAPK and NF-κB, which are required for LPS-induced iNOS expression and NO production. This result indicated that the activation of caspase 3 by LPS might mediate the activation of SAPK/JNK and NF-κB for iNOS expression and NO production. Hence, the caspase family, especially the caspase 3 pathway, may provide an intracellular signaling mechanism in LPS-stimulated macrophages. The regulation of caspases could provide a protective mechanism in NO-mediated tissue injury in endotoxic shock.
ACKNOWLEDGMENTS
We are grateful to K. Takahashi and A. Morikawa for their excellent technical assistance.
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan and the Daiko Foundation.
REFERENCES
- 1.Ajizian S J, English B K, Meals E A. Specific inhibitors of p38 and Erk pathways block iNOS and TNF-α accumulation in murine macrophages stimulated with LPS and IFN-γ. J Infect Dis. 1999;179:939–944. doi: 10.1086/314659. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman D D, Goldblum S E. Direct effects of endotoxin on the endothelium: barrier function and injury. Lab Investig. 1999;79:1181–1199. [PubMed] [Google Scholar]
- 3.Baumann B, Weber C K, Troppmair J, Whiteside S, Israel A, Rapp U R, Wirth T. Raf induces NF-kappaB by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc Natl Acad Sci USA. 2000;97:4615–4620. doi: 10.1073/pnas.080583397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman J S, Koppenol W H. Nitric oxide, superoxide and peroxynitrite: the good, the bad and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 5.Bredt D S. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 6.Cardone M H, Salvesen G S, Widmann C, Johnson G, Frisch S M. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 7.Carter A B, Mocrick M M, Hunninghake G W. Both Erk and p38 kinases are necessary for cytokine gene transcription. Am J Respir Cell Mol Biol. 1999;20:751–758. doi: 10.1165/ajrcmb.20.4.3420. [DOI] [PubMed] [Google Scholar]
- 8.Chan E D, Riches D W. Potential role of the JNK/SAPK signal transduction pathway in the induction of iNOS by TNF-α. Biochem Biophys Res Commun. 1998;253:790–796. doi: 10.1006/bbrc.1998.9857. [DOI] [PubMed] [Google Scholar]
- 9.Cross J V, Deak J C, Rich E A, Qian Y, Lewis M, Parrott L A, Mochida K, Gustafson D, Van de Pol S, Templeton D J. Quinone reductase inhibitors block SAPK/JNK and NF-kappaB pathways and potentiate apoptosis. J Biol Chem. 1999;274:31150–31154. doi: 10.1074/jbc.274.44.31150. [DOI] [PubMed] [Google Scholar]
- 10.Dimmeler S, Zeiher A M. Nitric oxide and apoptosis: another paradigm for the double-edge role of nitric oxide. Nitric Oxide Biol Chem. 1997;1:275–281. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- 11.Dimmeler S, Haendeler J, Nehls M, Zeiher A M. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green L C, Wagner D D A, Glowgowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite and 15N nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 13.Hamada E, Nishida T, Uchiyama Y, Nakamura J, Isahara K, Kazuo H, Huang T P, Momoi T, Ito T, Matsuda H. Activation of Kupffer cells and caspase-3 involved in rat hepatocyte apoptosis induced by endotoxin. J Hepatol. 1999;30:807–818. doi: 10.1016/s0168-8278(99)80133-0. [DOI] [PubMed] [Google Scholar]
- 14.Hart C M. Nitric oxide in adult lung disease. Chest. 1999;115:1407–1417. doi: 10.1378/chest.115.5.1407. [DOI] [PubMed] [Google Scholar]
- 15.Heitmeier M R, Scarim A L, Corbett J A. Double stranded RNA induced nitric oxide synthase expression and IL-1 release by murine macrophages requires NF-κB activation. J Biol Chem. 1999;273:15301–15307. doi: 10.1074/jbc.273.24.15301. [DOI] [PubMed] [Google Scholar]
- 16.Kilbourn R G, Jubran A, Gross S S, Griffith O W, Levi R, Adams J, Lodato R F. Reversal of endotoxin-mediated shock by NG-methyl-l-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophy Res Commun. 1990;172:1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- 17.Kilbourn R G, Gross S S, Jubran A, Adams J, Griffith O W, Levi R, Lodato R F. NG-methyl-l-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci USA. 1990;87:3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y M, Lee B S, Yi K Y, Paik S G. Upstream NF-kappaB site is required for the maximal expression of mouse inducible nitric oxide synthase gene in interferon-gamma plus lipopolysaccharide-induced RAW 264.7 macrophages. Biochem Biophys Res Commun. 1997;236:655–660. doi: 10.1006/bbrc.1997.7031. [DOI] [PubMed] [Google Scholar]
- 19.Kroncke K D, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection—how, why and where? Nitric Oxide Biol Chem. 1997;1:107–120. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- 20.Liu S F, Ye X, Malik A B. In vivo inhibition of NF-κB activation prevents iNOS synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 1997;159:3976–3983. [PubMed] [Google Scholar]
- 21.Lorsbach R B, Murphy W J, Lowenstein C J, Synder S H, Russell S W. Expression of nitric oxide synthase gene in mouse macrophages activated for tumor cell killing—molecular basis for synergy between IFN-γ and LPS. J Biol Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- 22.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 23.Magazine H I. Detection of endothelial cell derived nitric oxide: current trends and future directions. Adv Neuroimmunol. 1995;5:479–490. doi: 10.1016/0960-5428(95)00030-5. [DOI] [PubMed] [Google Scholar]
- 24.Mannick J B, Miao X Q, Stamler J S. Nitric oxide inhibits Fas-induced apoptosis. J Biol Chem. 1997;272:24125–24128. doi: 10.1074/jbc.272.39.24125. [DOI] [PubMed] [Google Scholar]
- 25.Martel-Pelletier J, Mineau F, Jovanovic D, Di Battista J A, Pelletier J P. Mitogen-activated protein kinase and nuclear factor kappaB together regulate interleukin-17-induced nitric oxide production in human osteoarthritic chondrocytes: possible role of transactivating factor mitogen-activated protein kinase-activated proten kinase (MAPKAPK) Arthritis Rheum. 1999;42:2399–2409. doi: 10.1002/1529-0131(199911)42:11<2399::AID-ANR19>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.May M J, Ghosh S. Signal transduction through NF-κB. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 27.Meyer C F, Wang X, Chang C, Templeton D, Tan T H. Interaction between c-Rel and the mitogen-activated protein kinase kinase kinase 1 signaling cascade in mediating kappaB enhancer activation. J Biol Chem. 1996;271:8971–8976. doi: 10.1074/jbc.271.15.8971. [DOI] [PubMed] [Google Scholar]
- 28.Mohr S, Zech B, Lapetina E G, Brune B. Inhibition of caspase-3 by S-nitrosation and oxidation caused by nitric oxide. Biochem Biophys Res Commun. 1997;238:387–391. doi: 10.1006/bbrc.1997.7304. [DOI] [PubMed] [Google Scholar]
- 29.Morikawa A, Kato Y, Sugiyama T, Koide N, Chakravortty D, Yoshida T, Yokochi T. Role of nitric oxide in lipopolysaccharide-induced hepatic injury in d-galactosamine-sensitized mice as an experimental endotoxic shock model. Infect Immun. 1999;67:1018–1024. doi: 10.1128/iai.67.3.1018-1024.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulsch A, Schray-Utz B, Mordvintcez P I, Hauschild K S, Busse R. Diethyldithiocarbamate inhibits induction of macrophage nitric oxide synthase. FEBS Lett. 1993;321:215–218. doi: 10.1016/0014-5793(93)80111-7. [DOI] [PubMed] [Google Scholar]
- 31.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 32.Paul A, Bryant C, Lawson M F, Chilvers E R, Plevin R. Dissociation of lipopolysaccharide-mediated induction of nitric oxide synthase and inhibition of DNA synthesis in RAW 264.7 macrophages and rat aortic smooth muscle cells. Br J Pharmacol. 1997;120:1439–1444. doi: 10.1038/sj.bjp.0701070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao K M. Molecular mechanisms regulating iNOS expression in various cell types. J Toxicol Environ Health B Crit Rev. 2000;3:27–58. doi: 10.1080/109374000281131. [DOI] [PubMed] [Google Scholar]
- 34.Slomiany B L, Piotrowski J, Slomiany A. Gastric mucosal inflammatory responses to Helicobacter pylori lipopolysaccharide: down-regulation of nitric oxide synthase-2 and caspase-3 by sulglycotide. Biochem Biophys Res Commun. 1999;261:15–20. doi: 10.1006/bbrc.1999.1003. [DOI] [PubMed] [Google Scholar]
- 35.Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta. 1999;1411:437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 36.Tuyt L M, Dokter W H, Birkenkamp K, Koopmans S B, Lummen C, Kruijer W, Vellenga E. Extracellular-regulated kinase 1/2, Jun N-terminal kinase, and c-Jun are involved in NF-κB-dependent IL-6 expression in human monocytes. J Immunol. 1999;162:4893–4902. [PubMed] [Google Scholar]
- 37.Widmann C, Gerwins P, Johnson N L, Jarpe M B, Johnson G L. MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol Cell Biol. 1998;18:2416–2429. doi: 10.1128/mcb.18.4.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]