Abstract
Background
Infliximab is a weight-based prescription for multiple autoimmune diseases and is dispensed only in single-use, 100mg vials. We aim to compute the quantity of infliximab waste at our site and in an ideal world where weight-based prescribing practices are followed. We estimate hypothetical waste reduction and cost-savings if a smaller vial is dispensed. We also surveyed gastroenterologists to study prescription rounding practices for infliximab.
Methods
A pre-existing registry of 426 inflammatory bowel disease patients identified 112 individuals who had received a total of 1003 infliximab administrations from December 2013 to May 2019. We calculated infliximab wastage per administration for the real world and an ideal (weight-based) world. Analysis of potential waste reduction and cost-savings was computed with the hypothetical creation of 50 and 25mg vials. Infliximab-prescribing gastroenterologists completed an online survey, determining reasons for rounding of weight-based prescription, rounding practices, and biosimilar use.
Results
At our site, the total value of infliximab wasted was between $112738.08 and $243209.50. Utilizing 50 and 25mg vials would reduce this waste by 92.2% and 99.4%, respectively. If prescriber guidelines were followed precisely, the total value of waste was between $132781.08 and $286448.19. Utilizing 50 and 25mg vials would reduce waste by 50.39% and 75.34%, respectively. The physician survey revealed that 68.1% rounded doses while only 31.9% prescribed exact weight-based doses.
Conclusions
Infliximab-prescribing gastroenterologists considered reducing drug waste as a common reason in their rounding practices. Our analysis demonstrates significant waste reduction and cost-savings are possible with the introduction of 50 and 25mg vials.
Keywords: biologics, inflammatory bowel disease, pharmaceutical waste, infliximab, vial size
Introduction
Overspending and waste due to drugs distributed in single-use vials, generally containing doses larger than necessary, is a known problem.1–5 Medications administered via intravenous or subcutaneous injection are often dosed based on the body weight of the patient and the excess must be discarded for safety reasons. This is specifically cited in the oncology literature where the use of biologic medications is common.2–5 In the United States alone, hospitals discard roughly 3 billion dollars in unused cancer drugs annually.4 Insurance companies and patients often become accountable for this cost burden because use is assessed by the vial rather than the dose used. Furthermore, this expense can lead to additional downstream costs such as increased insurance premiums for patients.
In the field of inflammatory bowel disease (IBD), the use of biologic medications is becoming more prevalent.6 As the global IBD prevalence rises, IBD-related healthcare costs are also increasing.7, 8 One study estimates the total annual direct economic burden of IBD to be at least $6.3 billion.9 The largest constituent of this high cost is related to pharmaceuticals, particularly biologic medications.10–13 Recent advances in biologic therapies have improved health outcomes for IBD patients and become a mainstay of medical therapy for disease management.
With biologic use rapidly increasing, the overall costs of medical care have shifted from hospitalization and surgery to outpatient pharmacy use.12, 14 Though effective and increasingly utilized for IBD, biologics are extremely expensive with cost for the drug ranging on average from $10000 to $30000 per year and exceed $500000 for the most expensive biologics.10, 11, 15 New biologic medications continue to come to market and utilization continues to increase. In one study, evaluating outpatient IBD drug utilization trends, the proportion of biologic use increased from 21.8% to 43.8% for Crohn’s disease (CD) and from 5.1% to 16.2% for ulcerative colitis (UC) from 2007 to 2015.16 The advent of biosimilar drugs was expected to help mitigate costs, however, poor market penetration has not allowed any significant downward pressure on cost of medical management in the United States.10
Infliximab (brand name Remicade) is a monoclonal anti-tumor necrosis factor alpha (TNF-α) which was the first available biologic to treat IBD.6, 11, 16–20 Infliximab typically comes in single-dose vials, meaning what is not used must be discarded for safety reasons to decrease the risk of infection from contamination.21 Infliximab is typically prescribed for IBD beginning at a dose of 5mg/kg of body weight at 0, 2, and 6 weeks, then every 8 weeks thereafter.22, 23 Given the 5mg/kg prescribing recommendation—with the current production of single-use 100mg vials—there is potential for a large quantity of drug to be wasted during infliximab administration.22 For example, an individual that weighs 81kg would receive a prescription of 405mg of infliximab. Since all vials are 100mg and single use, this would lead to a scenario where 5 vials of infliximab must be prescribed and 95mg of drug is discarded. Since a patient’s body size is not likely to match the amount of drug in the vial, there is often a surplus. Reimbursement and the cost to the patient are currently determined by the number of vials utilized rather than the amount of actual drug administered to a patient.
Medical waste is not unavoidable and various strategies to combat this problem have been proposed including vial sharing, increasing vial dosing options, and dose rounding by physicians. Vial sharing has been unsuccessful because of patient safety concerns. Many physicians have attempted to mitigate drug wastage by dose rounding.1, 2
Although there has been some research into medication dosing and drug utilization in the oncology literature, there is very limited data in the IBD patient population.2, 3 The primary purpose of this study was to calculate the quantity and prevalence of infliximab waste—both in the real world and in an ideal world where prescribing guidelines are followed exactly as written. We evaluated our institution’s quantity of infliximab waste and calculating potential cost-savings achievable by adjusting drug packaging to minimize waste. Secondary outcomes of our study examined current practitioner infliximab dose rounding practices and their rationale to support their decisions via survey.
Materials and Methods
This study was approved by the Northwell Health Institutional Review Board. A pre-existing registry of IBD patients treated by the IBD service at an academic, tertiary care, urban hospital was used to retrospectively identify patients for analysis. Patients were enrolled in the registry between April 2014 and June 2019. The institution’s electronic medical record (EMR) was then searched to identify patients from the registry who had been treated with infliximab in an outpatient setting. Only patients who received infliximab at this institution at least one time and who were treated by the IBD service were included for the real-world analysis. Demographic information including sex, insurance status, type of IBD, year of birth, and weight were recorded. The patient weight associated with each infliximab administration was considered to be the most recent weight recorded in the EMR prior to the administration. If there was no recorded weight for a patient in the 2 months prior to an infliximab administration, no associated weight was recorded for that particular administration in our data set.
Each infliximab administration during the pre-specified study interval was included as a data point and the amount of drug waste for an administration was calculated by subtracting the quantity of drug administered from the next highest multiple of 100—given the current production of only 100mg infliximab vials. Infliximab biosimilars were not used during the study period and thus were not included in these calculations. A theoretical calculation of waste that would exist on 50mg and 25mg vials was performed using the same method of subtracting from the next highest multiple of 50 and 25, respectively. For example, if a patient was prescribed a 425mg dose of infliximab. This patient would have 75mg of drug wasted in the current setting of solely 100mg vial production. If a 50mg vial option was offered, there would be 25mg of drug wasted and, if a 25mg vial option was offered, there would be 0mg of drug wasted.
The amount of actual drug waste was translated to calculate the financial waste, in US dollars, by multiplying the quantity of waste by the price of infliximab. Two prices—Medicare Part B Average Sales Price (ASP) and Average Wholesale Price (AWP)—were utilized to calculate financial waste. Financial waste analyses were also performed with the real-world administration data to calculate waste reduction and potential savings if 50mg and 25mg vials were available.
A second theoretical, weight-based analysis was then performed to examine the waste that would be observed if exact, weight-based administrations were performed (absent any real-world physician rounding practices). Since this was a theoretical analysis, all individuals on the IBD patient registry (regardless of having ever received infliximab or not) were included to provide a sufficient sample size of real-world IBD patient weights. The first EMR-recorded weight for each individual was used to calculate the infliximab quantity that would be administered at an initial dosing rate of 5mg/kg of body weight. The same process as the real-world data were performed, where each theoretical drug administration was subtracted from the next highest multiple of 100, 50, and 25mg to examine the quantity of waste that would be observed with different vial sizes. Once again, the amount of drug waste was multiplied by the ASP and AWP of infliximab to calculate the theoretical financial waste in US dollars.24, 25 All financial and waste calculations for both the real-world and ideal, weight-based analysis was performed using Excel (Microsoft).
To gain a better understanding of infliximab-prescribing practices outside of our institution’s setting, we conducted a survey of practicing gastroenterologists. Physicians were asked to report what type of practice they most often work at and answered questions about their preferred method of dose rounding when prescribing infliximab (eg, rounding up to the nearest 100mg dose, rounding down to the nearest 50mg dose) and the reasoning behind their dose rounding methodology. Those that chose a reason behind their dose rounding were then prompted to rank their motivations in hierarchical order, giving each answer choice a ranked choice score. The motivation that physicians chose as their most important was given a score of 7, second most important was given a score of 6, and so on. A higher ranked choice score demonstrated a more important motivating factor in the physician’s decision to round their prescribed dose of infliximab. The survey also asked physicians if they ever prescribe any infliximab biosimilars and if they would be more likely to prescribe biosimilars if the biosimilars were to be manufactured in 50mg vials rather than 100mg vials. The survey utilized skip logic branching and was between 3 and 8 questions in length, depending on physician responses. Surveys were distributed via a pre-existing email list, with physician responses collected through the online survey program, SurveyMonkey. The survey was pilot-tested with physicians at our hospital to check for clarity. These pilot data were not utilized in the final analysis. Descriptive statistical analysis of the survey results was performed on SurveyMonkey and Excel (Microsoft).
Results
The pre-existing IBD registry included 426 IBD patients. For the real-world analysis, 112 patients (26.3%) had received at least one dose of infliximab at our site with the prescription written by our institution’s IBD specialist. These 112 individuals received a total of 1003 infliximab administrations from December 2013 to May 2019. Patient weights were available for 681 of these 1003 administrations, with an average weight across these 681 administrations of 74.70 ± 16.09kg. Within the sample of patients who had received infliximab, 55.4% of the patients were male. Regarding insurance, 76.8% had private insurance, 16.9% had Medicaid, 6.3% had Medicare, and 0 patients did not have any medical insurance. A majority of the patients (70.5%) had CD, 26.8% had UC, and 2.6% had indeterminate colitis. The average age for the 112 individuals included in our real-world analysis of waste at our site was 41.7 ± 14.0 years. The demographic variables for both the real-world and ideal, weight-based analyses are presented in Table 1.
Table 1.
Demographic variables for both real-world analysis and the ideal, weight-based analysis.
| Real-world analysis (n = 112) | Ideal, weight-based analysis (n = 413) | |
|---|---|---|
| Sex—Male | 62 (55.4%) | 210 (50.8%) |
| Insurance | ||
| Medicaid | 19 (16.9%) | 63 (15.3%) |
| Medicare | 7 (6.3%) | 35 (8.5%) |
| Private | 86 (76.8%) | 309 (74.8%) |
| None | 0 (0%) | 6 (1.5%) |
| Diagnosis at time of consent | ||
| Crohn’s disease | 79 (70.5%) | 240 (58.1%) |
| Ulcerative colitis | 30 (26.8%) | 152 (36.8%) |
| Indeterminate colitis | 3 (2.6%) | 21 (5.1%) |
| Age (years) | 41.7 ± 14.0 | 42.6 ± 15.7 |
The average infliximab dose administered was 489.7mg ± 197.1mg with a range of 200–1000mg. Of the 1003 infliximab administration doses, 997 (99.4%) were a multiple of 25mg, 950 (94.7%) were a multiple of 50mg, and 659 (65.7%) were a multiple of 100mg. The total quantity of actual infliximab waste was 17355mg which, across the 1003 administrations, resulted in a mean waste of 17.3mg ± 24.6mg per infliximab administration. Using the Medicare Part B ASP, the total value of the drug wasted was $112738.08, with an average waste of $112.40 ± $159.76 per administration.24 Using the AWP, the total value of drug wasted was $243209.50 with an average waste of $242.48 ± $344.66 per administration.25 The waste reduction calculations demonstrated that if 50mg vials were sold and subsequently used at our site, there would have been a waste of 1.4mg ± 5.8mg per administration, or a waste reduction of 92.2%. If 25mg vials were available and utilized, there would have been a waste of 0.1mg ± 1.4mg per administration, or a waste reduction of 99.4%. The mean US dollar value of waste per administration would have been $8.78 ± $37.59 and $0.68 ± $8.86 for the 50mg and 25mg vials, respectively. The results of this real-world analysis are summarized in Table 2.
Table 2.
Results of waste analysis for both the real-world analysis (N = 1003 administrations of infliximab) and the ideal, weight-based analysis (N = 413 IBD patients’ presenting weights).
| Real-world analysis (N = 1003) | Ideal, weight-based analysis (N = 413) | ||||||
|---|---|---|---|---|---|---|---|
| Infliximab vial size | 100 mga | 50 mgb | 25 mgb | 100 mga | 50 mgb | 25 mgb | |
| Quantity of drug waste per administration, mean ± SD | 17.3mg ± 24.6 | 1.4mg ± 5.8 | 0.1mg ± 1.4 | 49.5mg ± 28.2 | 24.6mg ± 14.3 | 12.2mg ± 6.8 | |
| Financial value of drug waste per administration, mean ± SD | Medicare Part B Average Sales Price25 | $112.40 ± $159.76 | $8.78 ± $37.59 | $0.68 ± $8.86 | $321.50 ± $183.01 | $159.50 ± $92.73 | $79.28 ± $44.46 |
| Average Wholesale Price26 | $242.48 ± $344.66 | $18.93 ± $81.08 | $1.47 ± $19.11 | $693.58 ± $394.81 | $344.08 ± $200.04 | $171.03 ± $95.91 | |
| Potential waste reduction from current 100mg vials (%) | x | 92.2% | 99.4% | x | 50.4% | 75.3% | |
Currently manufactured vial size
Theoretical vial sizes.
For the ideal, weight-based analysis, 413 (96.9%) patients from the registry had weight data available. The mean weight was 71.65 ± 17.15 (range 37.19–143.79kg). The demographics for this analysis can be found in Table 1. The weights of each IBD patient were multiplied by 5mg/kg to obtain an average theoretical dose of 358.26mg ± 85.75mg (range of 186–719mg). The total amount of theoretical waste was 20440mg, worth between $132781.08 and $286448.19 over the 413 theoretical, weight-based administrations. The average amount of waste using the currently manufactured 100mg vials would be 49.5mg ± 28.2mg per administration of infliximab. Using the Medicare Part B ASP and the AWP, the average financial value of the theoretical drug waste would be between $321.50 ± $183.01 and $693.58 ± $394.81 per administration of infliximab.24, 25 If a 50mg vial were available, the average amount of theoretical drug waste would be 24.6mg ± 14.3mg per administration of infliximab, with a financial value of between $159.50 ± $92.73 and $344.08 ± $200.04—a waste reduction of 50.39%. If a 25mg vial were available, the average amount of theoretical drug waste would be 12.2mg ± 6.8mg per administration of infliximab, with a financial value between $79.28 ± $44.46 and $171.03 ± $95.91—a waste reduction of 75.34%. The results of this ideal, weight-based analysis can be found in Table 2.
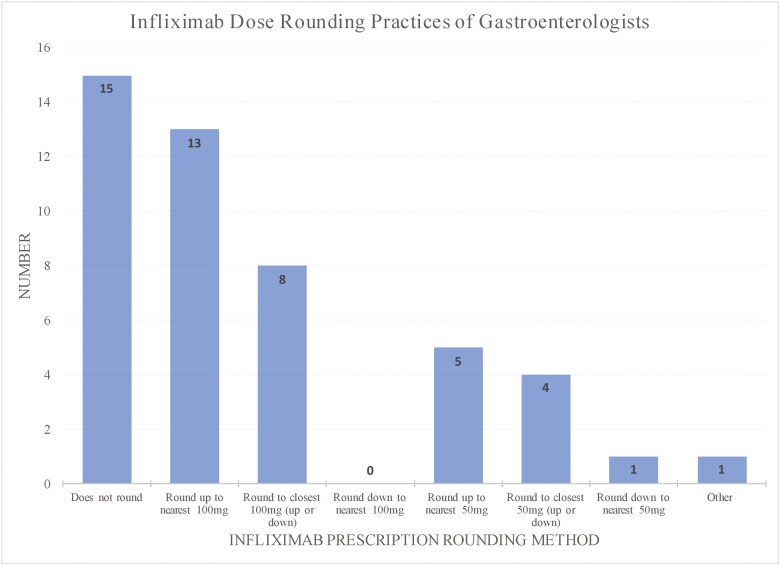
Lastly, we performed a survey of physicians in order to understand the real-world rounding practices being performed to help contextualize the data from the real-world and ideal-world waste calculations. In total, 48 physicians responded to the survey. The average time to complete the survey was 1min and 26s. Four (8.33%) most often worked in a private, solo practice, 14 (29.17%) practiced primarily in a private, group practice, 21 (43.75%) practiced in a University Hospital/Academic Center, and 6 (12.50%) practiced in an employed hospital. Three (6.25%) physicians chose “other” to best describe their current practice, self-reporting the following: Health Maintenance Organization, retired, and locums. All 48 of the physicians reported that they had prescribed infliximab and 31 (64.58%) had prescribed an infliximab biosimilar before. Most (68.1%) respondents rounded the dose when prescribing infliximab or an infliximab biosimilar. One respondent chose to skip that question. Of the 32 physicians that rounded their dose, most typically the dose was rounded up to the nearest 100mg (n = 13, 40.63%) or rounded up or down (whichever is closer) to the nearest 100mg (n = 8, 25.00%). The full results indicating physician methods for rounding infliximab or biosimilars are presented in Figure 1.
Figure 1.
Infliximab dose rounding practices of surveyed gastroenterologists (n = 47).
The physicians that did round their doses were asked to select the motivating factors that determined how they rounded the prescribed drug dose (n = 31). The results of this survey are shown in Table 3. The most commonly chosen motivations for rounding practices were to “Reduce drug left in the vial” and “To achieve therapeutic drug levels.” The highest ranked choice scores, excluding “other,” were 6.29 for “Reduce drug left in the vial” and 6.25 for “It is easier to record and remember rounded numbers.” Write-in respones were revealing for unanticipated factors that influence physician-prescribed infliximab doses. Response to this rounding motivation question can be found in Table 3.
Table 3.
Physician motivations for infliximab dose rounding and ranked choice scores.
| Motivation for rounding | Number that chose as one of their reasons | Ranked choice scorea |
|---|---|---|
| Reduce drug left in the vial | 18 (58.06%) | 6.29 |
| To achieve therapeutic drug levels | 11 (35.48%) | 6.00 |
| It is easier to record and remember rounded numbers | 9 (29.03%) | 6.25 |
| Considering disease activity (moderate vs severe) | 8 (25.81%) | 5.57 |
| Reduce cost of treatment | 4 (12.90%) | 6.00 |
| Decrease risk of infusion reactions | 1 (3.23%) | 3.00 |
| Otherb | 7 (22.58%) | 7.00 |
A higher value in the ranked choice score demonstrates a more important motivating factor in a physician’s decision to round their infliximab prescription.
Three physicians reported that they deferred to pharmacy/infusion center policies and preferences, two physicians were concerned with ease and accuracy of administration, 1 physician focused on alleviating medication waste, and 1 physician named financial waste as a motivating factor.
In the final survey question, the physicians were asked if they would be more likely to prescribe a biosimilar (instead of infliximab) if it was manufactured in a 50mg vial instead of a 100mg vial. 45 physicians responded to this question and 13 (28.89%) indicated that they would be more likely to do so, including 25% (4 of 16) of responding physicians that had never prescribed an infliximab biosimilar before. Three respondents chose to skip this question.
Discussion
Although fewer than 20% of patients with IBD are currently treated with biologic therapies, that population has a 2–3 times higher annual cost compared to those not receiving biologic therapy.11 Prior studies in the pediatric literature reveal that IBD management has evolved to increasingly rely on escalating pharmacotherapies by following levels resulting in increased dosing, specifically for the biologics.16 With this trend, medications, such as infliximab, now outpace hospitalization costs and represent the major cost driver for IBD treatment.12, 14 In fact, the Crohn’s & Colitis Foundation identified the cost of biologics as a key area for improved cost-effectiveness in the treatment of IBD.11
Traditionally, cost control has been managed through the introduction of generic or biosimilar drugs to bring downward pressure on drug costs.10 However, biosimilar adoption has been slower than expected. Infliximab biosimilars including infliximab-dyyb (brand name: Inflectra) in 2016, infliximab-abda (brand name: Renflexis) and infliximab-qbtz (brand name: Ixifi) in 2017, and infliximab-axxq (brand name: Avsola) in 2019 have received US Food and Drug Administration (FDA) approval.26–29 These biosimilars are currently less expensive than Remicade in the United States, with Inflectra wholesaling for $1135.54 per 100mg vial and Renflexis wholesaling for $904.07 per 100mg vial, compared to Remicade’s $1401.38 per 100mg vial.25 Since these biosimilars have hit the market, infliximab (Remicade) sales have significantly dropped in the United States, with a 19% decrease from $4525000 in 2017 to $3664000 in 2018.30 Despite this drop, research in the Medicare population has shown that in the 2 years after launching, biosimilars captured only 10% of the infliximab market with a decrease in reimbursement of 17%.31 Reasons for this slow uptake are unknown, but prescribers may be concerned about biosimilars not being identical, switching back to originators after biosimilar trials, or changing medication regimens in clinically stable patients.31–33
Beyond switching to biosimilars, we have identified another area where cost-savings may be achieved through manufacturing alternative vial sizes for weight-based biologics. In this study, we investigated the cost of infliximab remaining in a vial after an infusion. Recognizing that doses of infliximab were routinely rounded by providers at our site, we further conducted a hypothetical, “ideal” world analysis where exact weight-based doses were prescribed. Physician surveys revealed that this rounding behavior is common, and hence generalizable, though the underlying motivations differ.
The real-world data of infliximab use at our site demonstrate a large amount of infliximab waste from patient infusions, with an average of 17.3mg ± 24.6mg, costing between $112.40 ± $159.76 and $242.48 ± $344.66 per administration, being wasted with each administration. This range of costs spans from using Medicare Part B ASP and AWP. The ASP, effective July 1, 2019 through September 30, 2019, for Infliximab was $649.60 per 100mg.24 The AWP in the United States is $1401.38 per 100mg.25 These two prices were chosen for financial calculations because they represent the extremes of what is reimbursed/negotiated. Thus, by providing an upper and lower bound of potential prices, it is reasonable to assume that the calculation of waste or theoretical potential savings would apply to all institutions involved in the management of IBD patients.
In an ideal world, where exact insert guidelines are followed for prescribing, 49.5mg ± 28.2mg would be wasted, costing between $321.50 ± $183.01 and $693.58 ± $394.81 per administration. On average each patient receives 6–7 infusions in a year, sometimes more if they are initiating or escalating treatment, resulting in potential for a large quantity of waste per patient per year.22 These savings considerations may drive the decision making of payers. Even using the conservative number of annual treatments would mean there could be a waste of over $1900 per year with a high end of over $4800 per patient per year. Since, Inflectra wholesales at $1135.54 per 100mg vial—or 81% the price of infliximab—the maximum waste if only Inflectra was used would be around $3900 per year. Furthermore, Renflexis wholesales at $904.07 per 100mg vial—or 64.5% of infliximab—so the maximum waste if only Renflexis was used would be around $3100 per year. These medications are approved for multiple indications and are generally used long-term, sometimes life-long, and this compounding cost can add up to a significant lifetime financial burden for patients.11, 22
A manufacturing change in infliximab vial sizes to include 50 and 25mg vials could significantly decrease drug waste without compromising safety. We demonstrated this in our real-world analysis as well as ideal, weight-based analysis. With more diversity in vial size options, physicians would be able to dose accurately and with more flexibility, without worrying about drug wastage, which was noted in the physician survey to be a major concern.
The data from our survey build on findings in a prior study focusing on the pediatric IBD population, which found wide variation in infliximab-prescribing practices.1 Interestingly, a majority of the physicians surveyed prefer to round the dose when prescribing. Of those that chose to round, the most popular strategy was to round to the nearest 100mg, mostly to reduce drug left in the vial. Twenty-one out of 47 (44.6%) physicians reported rounding to a multiple of 100mg. Inherently these sites should have no infliximab waste, since they are rounding to the size of the existing vial. Since physicians report reducing the drug left in the vial as the predominant reason for rounding, it is likely that the limited availability of only 100mg vials in large part explains the tendency for physicians to round to 100mg doses. The physician survey also demonstrated that 21.3% of physicians are rounding their dose to a multiple of 50mg. For these physicians, creation of a 50mg vial would lead to a drastic reduction of financial and medication waste—theoretically to a waste of 0mg. In our physician survey, 100% of the physicians had prescribed infliximab before, while only 64.6% had ever prescribed an infliximab biosimilar. Similarly, Chen et al demonstrated that 72.8% of physicians that prescribed infliximab, did not prescribe any biosimilar over a 1-year time frame.31 They further found that only 3.5% of physicians prescribed only a biosimilar.31 Notably, we found that over one quarter of the physicians surveyed indicated they would be more likely to prescribe biosimilars if they were available in 50mg vials, including 25% of physicians that had not previously prescribed a biosimilar.
Based on survey responses, despite a desire to prevent excess cost, some physicians express hesitation about rounding due to clinical concerns regarding therapeutic dosing. Accordingly, 35.48% of our surveyed physicians reported that their rounding practices were based, at least in part, on achieving therapeutic drug levels. Rounding up could hypothetically improve therapeutic levels, but also increase immunosuppression and infection risk, though no surveyed physicians indicated infection risk was a motivating factor.1, 34 Rounding down could potentially result in undertreated disease.1 However, only one physician reported that their rounding was influenced by a desire to decrease the risk of infusion-related reactions. To our knowledge, there have been no studies that have investigated whether rounding up or down with 100mg infliximab vial impacts clinical outcomes.
Despite its strengths, we acknowledge our study contains limitations. For the real-world analysis, the results are biased by the prescription rounding practices of one specialty practice. However, rounding practices are often influenced by clinical, administrative, and personal experiences, so this may be an appropriate reflection of that human differentiation. Furthermore, drug levels may often be adjusted in the real-world based off trough levels - the concentration of drug in the body before the next dose is administered - which may lead to dose changes that are no longer consistently weight-based. However, our real-world data follow patients longitudinally including dose changes which may mean our real-world data are consistent with this reality. Another limitation of this study is a relatively small sample size of 48 physicians for our physician survey, though the sample included a broad practice experience. This survey was dependent on physicians remembering and unbiasedly sharing their prescribing practices. As the survey was anonymous, we are hopeful that physicians felt they could be open about their practices, but we cannot rule out the possibility that there may have been response bias.
Conclusions
In summary, our analysis uniquely explores the cost impact of medication waste from biologics administered at an IBD center in the United States. We determined that this waste can be significantly reduced simply with the creation of a 50 or 25mg infliximab vial, rather than the currently produced 100mg vial. These findings from our study provide one possible pathway to support in the efforts of developing patient-centered, cost-effective pharmacotherapy strategies to treat IBD.
Acknowledgments
None.
Investigation performed at: Lenox Hill Hospital, Northwell Health, 100 E 77th Street, New York, NY, 10075, USA.
Funding
None declared.
Conflicts of Interest
Mr. J.M.S, Ms. L.L.D., Mr. C.R.L., Dr. M.S., and Dr. A.M. have nothing to disclose. Dr. A.S. reports grants from Takeda, grants from Janssen, grants from Pfizer, outside the submitted work.
Data Availability
Data not publicly available.
References
- 1. Adler J, Sandberg KC, Shpeen BH, et al. . Variation in infliximab administration practices in the treatment of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;57(1):35–38. [DOI] [PubMed] [Google Scholar]
- 2. Patel S, Le A.. Rounding rituximab dose to nearest vial size. J Oncol Pharm Pract. 2013;19(3):218–221. doi: 10.1177/1078155212462439. [DOI] [PubMed] [Google Scholar]
- 3. Clark L, Castro AP, Fortes AF, et al. . Ideal vial size for bortezomib: real-world data on waste and cost reduction in treatment of multiple myeloma in Brazil. Value Health. 2011;14(5 Suppl 1):S82–S84. [DOI] [PubMed] [Google Scholar]
- 4. Bach PB, Conti RM, Muller RJ, Schnorr GC, Saltz LB.. Overspending driven by oversized single dose vials of cancer drugs. BMJ. 2016;(8047):352:i788. doi: 10.1136/bmj.i788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hatswell AJ, Porter JK.. Reducing drug wastage in pharmaceuticals dosed by weight or body surface areas by optimising vial sizes. Appl Health Econ Health Policy. 2019;17(3):391–397. doi: 10.1007/s40258-018-0444-0. [DOI] [PubMed] [Google Scholar]
- 6. Loomes DE, Teshima C, Jacobs P, Fedorak RN.. Health care resource use and costs in Crohn’s disease before and after infliximab therapy. Can J Gastroenterol. 2011;25(9):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kappelman MD, Moore KR, Allen JK, Cook SF.. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58(2):519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ng SC, Tang W, Ching JY, et al. . Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145(1):158–165. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 9. Rubin DT, Mody R, Davis KL, Wang CC.. Real-world assessment of therapy changes, suboptimal treatment and associated costs in patients with ulcerative colitis or Crohn’s disease. Aliment Pharmacol Ther. 2014;39(10):1143–1155. [DOI] [PubMed] [Google Scholar]
- 10. Chen BK, Yang YT, Bennett CL.. Why biologics and biosimilars remain so expensive: despite two wins for biosimilars, the supreme court’s recent rulings do not solve fundamental barriers to competition. Drugs. 2018;78(17):1777–1781. [DOI] [PubMed] [Google Scholar]
- 11. Park KT, Ehrlich OG, Allen JI, et al. . The cost of inflammatory bowel disease: an initiative from the Crohn’s & Colitis Foundation. Inflamm Bowel Dis. 2020;26(7):1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park KT, Colletti RB, Rubin DT, Sharma BK, Thompson A, Krueger A.. Health insurance paid costs and drivers of costs for patients with Crohn’s disease in the United States. Am J Gastroenterol. 2016;111(1):15–23. [DOI] [PubMed] [Google Scholar]
- 13. Kostić M, Djakovic L, Šujić R, Godman B, Janković SM.. Inflammatory bowel diseases (Crohn’s disease and ulcerative colitis): cost of treatment in Serbia and the implications. Appl Health Econ Health Policy. 2017;15(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Valk ME, Mangen MJ, Leenders M, et al. . Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63(1):72–79. [DOI] [PubMed] [Google Scholar]
- 15. Gunnarsson C, Chen J, Rizzo JA, Ladapo JA, Naim A, Lofland JH.. The direct healthcare insurer and out-of-pocket expenditures of psoriasis: evidence from a United States national survey. J Dermatolog Treat. 2012;23(4):240–254. [DOI] [PubMed] [Google Scholar]
- 16. Yu H, MacIsaac D, Wong JJ, et al. . Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47(3):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bahler C, Vavricka SR, Schoepfer AM, Brungger B, Reich O.. Trends in prevalence, mortality, health care utilization and health care costs of Swiss IBD patients: a claims data based study of the years 2010, 2012 and 2014. BMC Gastroenterol. 2017;17(1):138. doi: 10.1186/s12876-017-0681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee J, Im JP, Han K, et al. . Changes in direct healthcare costs before and after the diagnosis of inflammatory bowel disease: a nationwide population-based study. Gut Liver. 2019. doi: 10.5009/gnl19023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Valk ME, Mangen MJ, Severs M, et al. . Evolution of costs of inflammatory bowel disease over two years of follow-up. PLoS One. 2016;11(4):e0142481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang DH, Armstrong EP, Lee JK.. Cost-utility analysis of biologic treatments for moderate-to-severe Crohn’s disease. Pharmacotherapy. 2012;32(6):515–526. [DOI] [PubMed] [Google Scholar]
- 21. Protect Patients Against Preventable Harm from Improper Use of Single-Dose/Single-Use Vials. Center for Disease Control and Prevention. 2012. Accessed January 2, 2020. https://www.cdc.gov/injectionsafety/cdcposition-singleusevial.html [Google Scholar]
- 22. Remicade (Infliximab) Package Insert. Janssen Biotech, Inc. 2020. Accessed January 2, 2020. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/REMICADE-pi.pdf [Google Scholar]
- 23. Hanauer SB, Feagan BG, Lichtenstein GR, et al. . Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. doi: 10.1016/s0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 24. ASP Drug Pricing Files. Center for Medicare and Medicaid Services. 2019. Accessed July 22, 2019. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2019ASPFiles.html [Google Scholar]
- 25. Infliximab (Including Biosimilar of Infliximab): Drug Information. UpToDate. 2019. Accessed July 22, 2019. https://www.uptodate.com/contents/infliximab-including-biosimilars-of-infliximab-drug-information [Google Scholar]
- 26. Drugs@FDA: FDA-Approved Drugs. U.S. Food and Drug Administration. 2016. Accessed January 2, 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125544 [Google Scholar]
- 27. Drugs@FDA: FDA-Approved Drugs. U.S. Food and Drug Administration. 2017. Accessed January 2, 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761054 [Google Scholar]
- 28. Drugs@FDA: FDA-Approved Drugs. U.S. Food and Drug Administration. 2017. Accessed January 2, 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761072 [Google Scholar]
- 29. Drugs@FDA: FDA-Approved Drugs. U.S. Food and Drug Administration. 2019. Accessed January 2, 2020. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761086 [Google Scholar]
- 30. Annual Report 2018. Johnson & Johnson. 2018. Accessed January 2, 2020. https://www.investor.jnj.com/annual-meeting-materials/2018-annual-report [Google Scholar]
- 31. Chen AJ, Gascue L, Ribero R, Van Nuys K.. Uptake of infliximab biosimilars among the medicare population. Jama Intern Med. 2020;180(9):1255–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van de Vooren K, Curto A, Garattini L.. Biosimilar versus generic drugs: same but different? Appl Health Econ Health Policy. 2015. doi: 10.1007/s40258-015-0154-9. [DOI] [PubMed] [Google Scholar]
- 33. Husereau D, Feagan B, Selya-Hammer C.. Policy options for Infliximab biosimilars in inflammatory bowel disease given emerging evidence for switching. Appl Health Econ Health Policy. 2018;16(3):279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V.. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not publicly available.