Abstract
Background
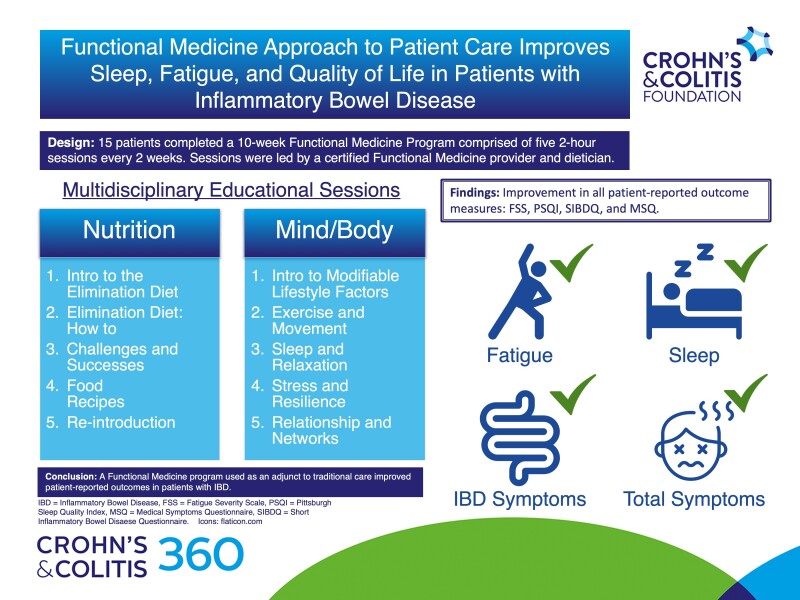
A Functional Medicine program was developed at an inflammatory bowel disease (IBD) center with the goal of integrating strategies to address modifiable lifestyle factors and to complete a 6-week elimination diet under the direction of a trained Functional Medicine dietitian and Functional Medicine providers.
Methods
From January 2019 to November 2019, patients with controlled, but persistent, symptoms from IBD were offered enrollment. Each of the 5 visits incorporated an educational session focused on nutrition followed by a session focusing on modifiable lifestyle factors. The patients were placed on a supervised 6-week elimination diet. At each visit, patients completed the SIBDQ (Short Inflammatory Bowel Disease Questionnaire), FSS (Fatigue Severity Scale), PSQI (The Pittsburgh Sleep Quality Index), and MSQ (Medical Symptoms Questionnaire). Statistical analysis was performed using the Wilcoxon matched pairs signed-rank test.
Results
Nineteen patients enrolled (2 men: 1 ulcerative colitis [UC], 1 Crohn’s disease [CD]; 17 women: 3 UC, 14 CD). 15 patients completed all modules. There was improvement in all patient-reported outcomes (PROs) (FSS, P < .001; PSQI, P < .001; SIBDQ, P < .001; MSQ, P < .001). Every patient who completed the last session demonstrated weight loss.
Conclusions
The psychoemotional roots to immune disease states, particularly IBD, are complicated and often not addressed in traditional care. We are just beginning to understand the impact of nutrition, sleep, stress, movement, and relationships on IBD. In this cohort, utilizing Functional Medicine as an adjunct to traditional care resulted in improvement in all PROs.
Keywords: Functional Medicine, inflammatory bowel disease, Crohn’s disease, ulcerative colitis
Graphical Abstract
Graphical Abstract.
Introduction
Inflammatory bowel disease (IBD) is a chronic, autoimmune, relapsing, and remitting disorder that affects genetically susceptible individuals’ immune response to the world around them. Epidemiologic studies have investigated and implicated risk factors influencing this disease state including genetics, environment, mental health, stress, and diet exposomes as IBD triggers.1–7 By manipulating the impact of these modifiable risk factors, patients with IBD may begin to experience benefits beyond that of conventional disease management.8,9 Since there are many modifiable lifestyle factors that may affect IBD patients, lifestyle interventions in conjunction with conventional care aimed at improving overall health behaviors may have benefits beyond traditional IBD therapies.
The Functional Medicine approach to care expands upon the conventional medicine model by addressing underlying functional imbalance to create balance among all biologic functions.9,10 It is a personalized approach to medical care that focuses on an individual’s lifestyle, environment, relationships, sleep, stress, and nutrition. Functional Medicine care has been shown to be a feasible approach to improve patient outcomes as an adjuvant to conventional care in several studies.9,11,12 Central to the topic of Functional Medicine is nutrition where a focus is placed on anti-inflammatory, low-glycemic index, nonprocessed foods without artificial dye or sugars.13 The Functional Medicine model addresses multiple elements at the same time to combat chronic inflammation and whole-system imbalance.
The aim of this study was to examine the impact of a Functional Medicine program on patient-reported outcomes (PROs) in a cohort of patients with IBD. The authors hypothesized that all measured PROs would improve after implementation of an elimination diet and education on modifiable lifestyle factors (nutrition, sleep, stress, movement, and relationships). This retrospective analysis of a multidisciplinary, shared medical appointment model is grounded on the principles of Functional Medicine and supports the positive impact of a Functional Medicine approach as an adjunct to traditional IBD care.
Materials and Methods
In this single center, retrospective study, we evaluated the change in the SIBDQ (Short Inflammatory Bowel Disease Questionnaire), FSS (Fatigue Severity Scale), PSQI (The Pittsburgh Sleep Quality Index), and MSQ (Medical Symptoms Questionnaire) over 10 weeks. The program consisted of five 2-hour sessions every other week for 10 weeks. The shared medical appointments were led by a certified Functional Medicine provider and a Functional Medicine dietitian.
From January 2019 to November 2019, patients with IBD (Crohn’s disease [CD] or ulcerative colitis [UC]) at an academic tertiary care center with controlled, but persistent, symptoms were offered the opportunity to enter the Functional Medicine program. Prior to the start of each visit, patients completed the SIBDQ, FSS, PSQI, and MSQ. Data were collected by retrospective chart review, approved by our local IRB and stored in a RedCap database. Statistical analysis was performed using R version 4.1.2 (https://cran.r-project.org/, accessed April 4, 2022). Nominal variables are described as count n (% frequency). Continuous variables are reported as mean ± SD or median [Q1, Q3] if distribution was not normal. Normality was tested using the Kolmogorov–Smirnov test. The Wilcoxon matched pairs signed-rank test was used to compare baseline and final scores for all the PROs.
The MSQ is a standardized, self-reported, symptom-based questionnaire.14 The scoring system is organized into 15 symptom-specific domains. Participants assign a score from 0 to 4 for 71 symptoms that have occurred over the last 14 days. A score of 0 correlates with never having symptoms. A score of 1–2 correlates with occasional symptoms, with not severe (“1”) or severe (“2”) effects. Scores of 3 and 4 mean frequent symptoms and not severe (“3”) or severe (“4”) effects. The test is commonly used in Functional Medicine to capture the burden of symptoms in every domain that have occurred in the last 14 days.14 A lower score correlates with lower symptom burden.
The SIBDQ is a standardized, 10-item, self-administered or rater-administered questionnaire that is validated in patients with IBD.15,16 It is an adaptation of a longer questionnaire used in patients with IBD. The questions are organized into 4 domains (bowel, social, emotional, systemic) to capture burdens of disease in the last 14 days. The score ranges from 10 to 70 with higher scores correlating with the least burden.15,16
The PSQI is a standardized, validated, 19-item, self-administered questionnaire with 7 domains: subjective sleep quality, sleep latency, duration, habitual sleep efficiency, sleep disturbances, use of medication, and daytime dysfunction.17 It is designed to capture the participants’ sleep habits during the last month, which are a component of the Functional Medicine approach. The items are grouped into 7 components, each weighted on a 0–3 scale. These components are added together to determine the final score, ranging from 0 to 21. A lower score correlates with better sleep quality.
The FSS is a standardized, validated, 9-item, self-administered questionnaire aimed to quantify an individual’s fatigue during the last week.18 Fatigue is another component of interest in the Functional Medicine model. Participants are asked to grade each of the 9 items from 1 to 7, with 1 indicating strong disagreement and 7 indicating strong agreement. A lower score correlates with less severity.
The first hour of each module focused on nutrition while the second hour addressed modifiable lifestyle factors (Table 1). All patients were placed on an elimination diet for 6 weeks under the close supervision of a dietitian. The elimination diet required avoidance of dairy, eggs, gluten, peanuts, shellfish, beef/red meat (including processed meats), soy, corn, refined sugar, caffeine, and alcohol. The nutrition change was implemented across all cohorts occurred over 6 weeks and was followed by calculated food reintroductions supervised by a dietitian trained in functional nutrition. As part of the group sessions, participants were asked to create SMART goals to optimize their skills training and implement them at home over the following 2 weeks.19
Table 1.
Topics of educational sessions covered in each module of the Functional Medicine program.
| Module | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Nutrition session | Introduction to the elimination diet | Elimination diet: how to | Challenges and successes | Food recipes | Reintroduction |
| Education session | Introduction to modifiable lifestyle factors | Exercise and movement | Sleep and relaxation | Stress and resilience | Relationship and networks |
Ethical Considerations
This study was reviewed and approved by the [Vanderbilt] Institutional Review Board. This manuscript is an original contribution and is not under current consideration for publication nor has it been previously published elsewhere. It was presented as a poster at the Institute of Functional Medicine National Meeting and the American College of Gastroenterology Annual Scientific Meeting.
Results
All IBD patients that were treated at the outpatient IBD center with persistent but stable symptoms were offered enrollment. Through consecutive enrollment, 19 patients enrolled in the Functional Medicine Group Program (2 men: 1 UC, 1 CD; 17 women: 3 UC, 14 CD) (Table 2). Fifteen patients completed the first and last sessions. Dropouts were due to financial burden (5%) and time constraints (16%). Twelve patients (63%) completed all 5 of the sessions.
Table 2.
Baseline characteristics of all participants who completed session 1 of the Functional Medicine program.
| Overall (N = 19) | |
|---|---|
| Age | |
| Mean (SD) | 45.1 (9.55) |
| Gender | |
| Female | 17 (89.5%) |
| Male | 2 (10.5%) |
| Height (cm) | |
| Mean (SD) | 167 (6.70) |
| Weight (kg) | |
| Mean (SD) | 78.8 (15.1) |
| BMI | |
| Mean (SD) | 28.2 (5.78) |
| Type of disease | |
| CD | 16 (84.2%) |
| UC | 3 (15.8%) |
| Years of diagnosis | |
| Mean (SD) | 12.4 (6.13) |
| History of perianal disease | |
| No | 18 (94.7%) |
| Yes | 1 (5.3%) |
| History of penetrating disease | |
| No | 17 (89.5%) |
| Yes | 2 (10.5%) |
| History of stricturing disease | |
| No | 13 (68.4%) |
| Yes | 6 (31.6%) |
| Past surgical history | |
| IC resection | 5 (26.3%) |
| IPAA for UC | 2 (10.5%) |
| Multiple | 1 (5.3%) |
| None | 9 (47.4%) |
| Partial colectomy | 1 (5.3%) |
| Total colectomy | 1 (5.3%) |
| Biologics | |
| No | 7 (36.8%) |
| Yes | 12 (63.2%) |
Abbreviations: BMI, body mass index; CD, Crohn’s disease; IC, ileocecal; IPAA, ileal pouch-anal anastomosis; UC, ulcerative colitis.
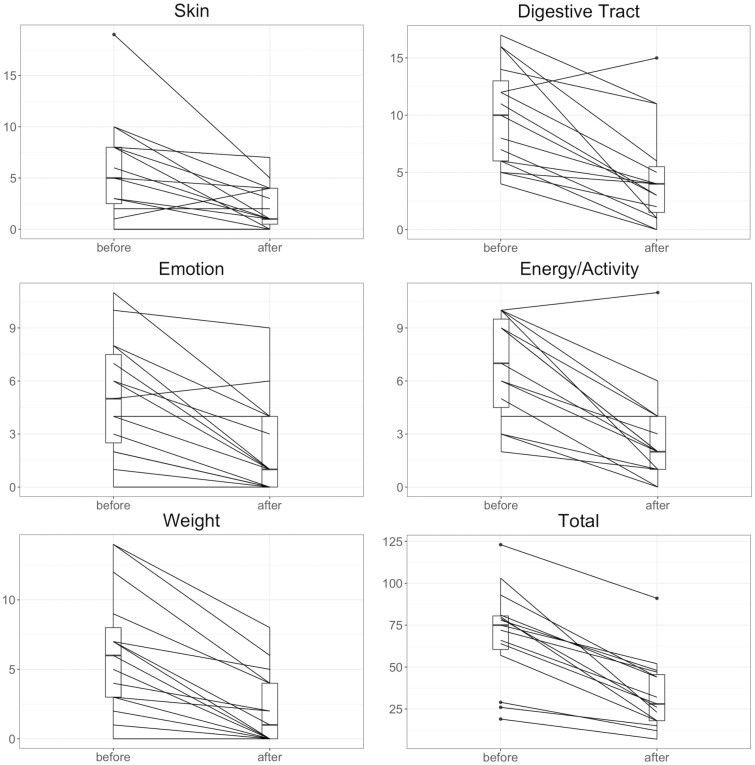
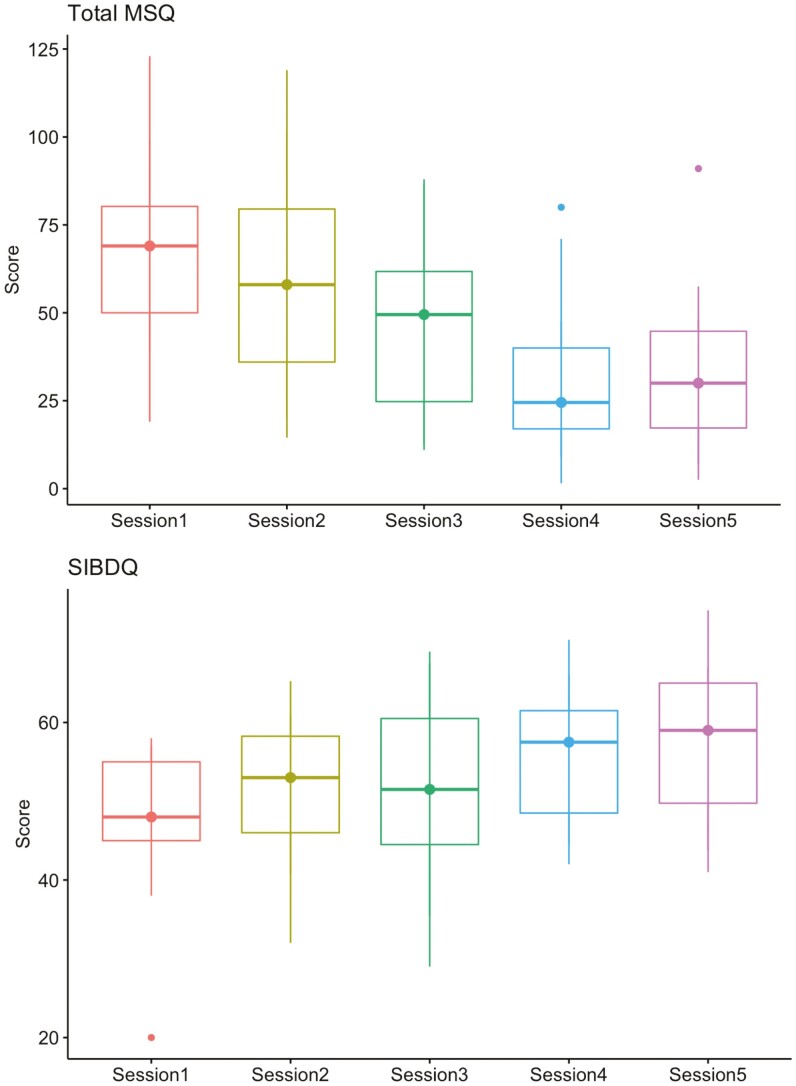
All PROs for the cohort who completed modules 1 and 5 improved with statistical significance (Table 3). Every patient improved their total MSQ scores (Figure 1). When further analyzed by symptom domain, the cohort demonstrated statistically significant improvement in IBD-specific domains including head (P = .0028), skin (P = .005), digestive tract (P < .001), joint and muscle (P < .001), weight (P = .003), energy and activity (P < .001), and emotion (P = .005). There was no significant change in scores for the IBD-related domains of eyes (P = .387) or mouth and throat (P = .924). All but 1 patient (7%) improved their SIBDQ score (Figure 2). The 1 patient who did not improve had UC in histologic remission and reported ongoing social stressors and poor social support throughout the duration of the study period. No patients reported worsening of their PSQI score, however 3 (20%) remained the same. All but 4 (27%) improved their FSS score.
Table 3.
Median scores for participants who completed both the first and last of 5 sessions in the Functional Medicine program with upper and lower quartiles.
| Session 1 (N = 15) | Session 5 (N = 15) | P | |
|---|---|---|---|
| FSS | 43.0 [37.0, 53.5] | 27.0 [18.0, 45.0] | <.001 |
| Global PSQI | 6.00 [5.50, 11.0] | 4.00 [2.00, 6.00] | <.001 |
| SIBDQ | 48.0 [46.5, 55.0] | 58.0 [52.0, 64.5] | <.001 |
| Total MSQ | 75.0 [60.5, 80.5] | 28.0 [18.0, 45.5] | <.001 |
Abbreviations: FSS, Fatigue Severity Scale; MSQ, Medical Symptoms Questionnaire; PSQI, Pittsburgh Sleep Quality Index; SIBDQ, Short Inflammatory Bowel Disease Questionnaire.
Figure 1.
Paired individual scores for select domains in the MSQ. Abbreviation: MSQ, Medical Symptoms Questionnaire. Improvements in each domain were significant with P < .01. Box and whisker plots demonstrate median, interquartile range, and extremes.
Figure 2.
Improvements in MSQ and SIBDQ over 5 educational sessions. Abbreviations: MSQ, Medical Symptoms Questionnaire; SIBDQ, Short Inflammatory Bowel Disease Questionnaire. Box and whisker plot demonstrates median, interquartile range, and extremes.
Those who recorded scores in the first and last sessions were subdivided into groups based on whether they had undergone previous surgical intervention for their IBD. Both subgroups maintained a statistically significant improvement in all PROs from sessions 1 to 5. The 9 (60%) surgical patients improved their FSS from 43.0 [39.0, 55.0] to 27.0 [20.0, 43.0] (P < .001), their global PSQI from 6.0 [6.0, 11.0] to 3.0 [2.00–5.0] (P < .001), their SIBDQ from 48.0 [45.0, 55.0] to 64.0 [51.0, 65.0] (P < .001), and their total MSQ from 72.0 [64.0, 80.0] to 32.0 [18.0, 44.0] (P < .001). The 6 (40%) nonsurgical patients improved their FSS from 41.5 [34.3, 51.0] to 29.0 [17.5, 45.0] (P < .001), their global PSQI from 8.0 [2.3, 10.8] to 5.5 [1.3, 8.3] (P = .018), their SIBDQ from 51.5 [48.0, 55.0] to 57.5 [54.0, 58.0] (P = .002), and their total MSQ from 77.0 [61.5, 97.0] to 24.5 [19.3, 45.5] (P < .001). While both groups had improvement in the SIBDQ, the surgical patients experienced a greater relative improvement in SIBDQ compared to the nonsurgical patients (33% vs 12%). The nonsurgical patients had a greater relative decrease in MSQ (68% vs 56%).
Participants who completed the first and last sessions also recorded a statistically significant change in weight and body mass index (BMI). Weight (kg) decreased from 73.8 [67.8, 91.7] to 73.0 [64.9, 88.7] (P < .001) between sessions 1 and 5. BMI (kg m−2) decreased from 26.6 [24.6, 31.3] to 25.4 [23.5, 30.3] (P < .001) between sessions 1 and 5. This finding is congruent with the MSQ weight domain where patients reported improvement in symptoms of binge eating and drinking, food cravings, compulsive eating, feeling overweight or underweight, and feeling of water retention.
Discussion
Functional Medicine can be defined as a science-based, personalized health care approach aimed at restoring a patient’s health and improving overall function. It considers physiologic systems and function, searches for the causes of imbalance, and focuses on balancing the “whole system.” As part of the patient’s journey, self-discovery plays an important part in creating a personal map moving toward a healthy state of being. In a group setting, focusing on compassion, connection, and partnership creates a space for patients to invoke this.
The foundation of the Functional Medicine model encompasses nutrition and other modifiable lifestyle factors. These were the building blocks of our IBD Functional Medicine group program. By addressing lifestyle factors, we removed dietary triggers and other factors that can negatively affect the environment of the gastrointestinal tract. We equipped these patients with tools they can utilize to manage stress, build resilience, optimize sleep, and increase physical activity. The elimination diet required patients to eliminate common allergic foods and enabled them to discover connections between food and symptoms. Similar studies on patients with IBD undergoing an elimination diet have demonstrated improvement in stool and serologic inflammatory markers, which may explain the improvement in the symptom-based PROs for this cohort.20,21
There is still so much about food and our bodies that we do not understand. To make it more difficult, every person is different. By taking part in a 6-week elimination diet, patients can: identify their own food triggers, reduce inflammation, repair intestinal permeability, introduce healthy phytonutrients, reduce toxic burden, and promote body awareness of foods. In addition to the elimination diet, emphasis was placed on balanced meals with all macronutrients, healthy protein, healthy fats, and healthy carbohydrates. We simultaneously de-emphasized the quantity of calories while preferentially focusing on quality. Fundamental to the educational material was the promotion of “real food.” Success strategies were given as well as positive feedback and support through a group model of care. Reintroduction was a 2-day process for each food and required patients to keep a food log and track symptoms.
SMART goals were implemented into this program. Patients were taught the meaning of this system at the beginning of the program and shared these goals in the group setting to help create accountability and support. The goals were written down and addressed at the beginning and end of each session. SMART is an acronym that is used as a guide for goal setting to make sure personal goals are clear and reachable. To make sure a goal is attainable, each one should be: S—specific, M—measurable, A—achievable, R—relevant, and T—time-bound. For a goal to be specific, it should be clear, well-defined, and sensible. When creating a specific goal, it should answer questions pertaining to “what, why, who, where, and which.” The goal must also be measurable so that tracking is possible, which enables continued motivation. A SMART goal must be achievable by encompassing the parameters of being realistic and attainable. Relevance will ensure the goal matters and aligns with other relevant goals. Lastly, each goal needs a deadline. SMART has been shown to be an effective, easy-to-use tool helps to provide focus and motivation.
PROs were tracked over time and completed at the beginning of every session. PROs have become increasingly important as primary endpoints. The MSQ is a Functional Medicine tool that enables us to score and track symptoms in every organ system. Optimal health has been reported around a score of 25 but no validation measures have been done at this time. Each patient can use this as a personal guide to measure progress. The SIBDQ is a tool to measure health-related quality of life in an IBD patient. The purpose of the SIBDQ is to elicit the burden of disease on not only physical function, but also psychoemotional and social functioning. Our study demonstrated that the Functional Medicine program, which consisted of a disease-specific cohort, yielded improvement in this validated quality of life measure. The FSS measures the severity of fatigue in accordance with a person’s lifestyle and daily activities. Additionally, it can be used to help differentiate between clinical depression and fatigue alone.22 PSQI enables researchers to study sleep quality and efficacy, a focus in our educational program.
We observed that prior to starting the elimination diet, patients were extremely resistant to making nutritional changes. Barriers appeared to be lack of knowledge, perceived lack of time to make the change, financial constraints, and family responsibilities. In this shared medical appointment setting, we observed the power of group support and encouragement of each other in committing to the changes and successful completion of the program. Patients learned to read food labels and navigate the grocery store in a health-conscious way. Feedback after the program was universally positive. Participants did not want to resume old habits of eating and all of them felt a positive shift in their health journey. As they demonstrated improvement in their sleep, fatigue, emotional, and medical symptoms, we also found that every one of them lost weight and improved their BMI.
This study had several limitations. First, this was a retrospective, observational study with no placebo control. Given the nature of the education and model of the program, creating a placebo arm was too logistically challenging at this time. All patients were from an academic IBD center and desired to be in the program, creating vulnerability to sampling bias. The cohort also had a predominance of female participants and those with CD, which may affect the generalizability of our results. Given the open-label and group-oriented nature of our methods, it may be that men were less open to a group-based, alternative approach to their medical conditions. A key tenet of Functional Medicine is personalized care, which is contrary to a one-size-fits-all approach. While not all patients may find this approach appealing, as the body of evidence grows, clinicians and researchers should be more equipped to recruit a more diverse population of patients. Furthermore, time constraints and financial burden contributed to 21% dropout rate. One of our measures was validated in patients with IBD (SIBDQ), 1 measure was developed within the field of Functional Medicine (MSQ), and the other 2 were not disease specific and were validated at large academic centers. This highlights the lack of available disease-specific, patient-centered measures of health quality and quality of life. We acknowledge the extensive time and resources required to create and validate such tools. Lastly, the types of surgeries were collected as a baseline characteristic, but the timing of these procedures were not collected. Future studies may benefit from collecting these data.
While the authors of this study chose to emphasize PRO measures, future studies on the Functional Medicine model would benefit from the addition of non-PROs such as the following: serologic and stool markers of inflammation, micronutrient levels before and after completion of the elimination diet, and endoscopic measures of disease severity. A prospective design with a control arm, such as a sham educational session, would greatly enhance the strength and precision of results.
Conclusion
The foundation of a Functional Medicine model of care encompasses modifiable lifestyle factors—nutrition, sleep, stress, movement, and relationships which were the building blocks of our IBD Functional Medicine group program. This study and program represent the first Functional Medicine-based approach as adjunct care of the IBD patient by promoting health and optimizing function. The findings indicate that a Functional Medicine program positively contributed to high-quality, personalized IBD care. Given the nature of the program, it is difficult to discern which component had the biggest impact on the PROs but it is the first step in learning how a Functional Medicine approach to care may play a part in overall IBD care.
Contributor Information
Thomas M Strobel, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Christine Nguyen, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Taylor Riggs, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Sarah N Horst, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Amy Motley, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Spencer Upadhyaya, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Sarah Campbell, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Emily Spring, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Robin L Dalal, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Elizabeth Scoville, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Baldeep Pabla, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
David A Schwartz, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Dawn B Beaulieu, Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Authors’ Contributions
D.B.B. designed and conducted the study, collected and interpreted the data, and drafted the manuscript. T.M.S. performed a literature search, interpreted the data, performed statistical analysis, and assisted in writing the manuscript. C.N. and T.R. assisted in writing the manuscript. S.H. interpreted and analyzed the data. A.M. interpreted and collected data. S.U., S.C., and E.S. assisted in study design and data interpretation. R.L.D., E.S., B.P., and D.A.S. assisted in data interpretation, writing and editing.
Funding
This study was conducted via REDCap software through the Vanderbilt Institute for Clinical and Translational Research, which is funded by grant number UL1 TR000445 awarded by NCATS/NIH.
Conflicts of Interest
Sarah N. Horst—consultant and advisory board for AbbVie, Jannsen, USB, Takeda, and Gilead. David A. Schwartz—consultant for AbbVie, Genetech, Gilead, Janssen, Pfizer, Takeda, and UCB. Dawn B. Beaulieu—consultant and advisory board for AbbVie, Takeda, consultant for AndHealth. Thomas M. Strobel, Christine Nguyen, Taylor Riggs, Amy Motley, Spencer Upadhyaya, Sarah Campbell, Emily A. Spring, Robin L. Dalal, Elizabeth Scoville, and Baldeep Pabla: None declared.
Data Availability
Data pertaining to this study will be made available to individuals upon request to the corresponding author.
References
- 1. Malik TA. Inflammatory bowel disease: historical perspective, epidemiology, and risk factors. Surg Clin North Am. 2015;95(6):1105–1122, v. [DOI] [PubMed] [Google Scholar]
- 2. Malik TA, Mannon P.. Inflammatory bowel diseases: emerging therapies and promising molecular targets. Front Biosci. 2012;4(3):1172–1189. [DOI] [PubMed] [Google Scholar]
- 3. Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M.. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol. 2010;105(9):1994–2002. [DOI] [PubMed] [Google Scholar]
- 4. Asakura H, Suzuki K, Kitahora T, Morizane T.. Is there a link between food and intestinal microbes and the occurrence of Crohn’s disease and ulcerative colitis? J Gastroenterol Hepatol. 2008;23(12):1794–1801. [DOI] [PubMed] [Google Scholar]
- 5. Martini GA, Brandes JW.. Increased consumption of refined carbohydrates in patients with Crohn’s disease. Klin Wochenschr. 1976;54(8):367–371. [DOI] [PubMed] [Google Scholar]
- 6. Kostic AD, Xavier RJ, Gevers D.. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12(4):205–217. [DOI] [PubMed] [Google Scholar]
- 8. Riordan AM, Hunter JO, Crampton JR, et al. Treatment of active Crohn’s disease by exclusion diet: East Anglian Multicentre Controlled Trial. Lancet. 1993;342(8880):1131–1134. [DOI] [PubMed] [Google Scholar]
- 9. Droz N, Hanaway P, Hyman M, et al. The impact of functional medicine on patient-reported outcomes in inflammatory arthritis: a retrospective study. PLoS One. 2020;15(10):e0240416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanaway P. Form follows function: a functional medicine overview. Perm J. 2016;20(4):16–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beidelschies M, Alejandro-Rodriguez M, Guo N, et al. Patient outcomes and costs associated with functional medicine-based care in a shared versus individual setting for patients with chronic conditions: a retrospective cohort study. BMJ Open. 2021;11(4):e048294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beidelschies M, Alejandro-Rodriguez M, Ji X, et al. Association of the functional medicine model of care with patient-reported health-related quality-of-life outcomes. JAMA Netw Open. 2019;2(10):e1914017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157(2):440–450.e448. [DOI] [PubMed] [Google Scholar]
- 14. Medical Symptoms Questionnaire (MSQ). The Institute for Functional Medicine. Accessed 2021. https://centerforfunctionalmedicine.com/wp-content/uploads/2016/10/MedicalSymptomsQuestionnaire_v2-1.pdf [Google Scholar]
- 15. Jowett S, Seal C, Barton R, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 2001;96(10):2921–2928. [DOI] [PubMed] [Google Scholar]
- 16. Chen X-L, Zhong L-H, Wen Y, et al. Inflammatory bowel disease-specific health-related quality of life instruments: a systematic review of measurement properties. Health Qual Life Outcomes. 2017;15(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 18. Valko PO, Bassetti CL, Bloch KE, et al. Validation of the fatigue severity scale in a Swiss cohort. Sleep. 2008;31(11):1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White ND, Bautista V, Lenz T, et al. Using the SMART-EST goals in lifestyle medicine prescription. Am J Lifestyle Med. 2020;14(3):271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szczubełek M, Pomorska K, Korólczyk-Kowalczyk M, et al. Effectiveness of Crohn’s disease exclusion diet for induction of remission in Crohn’s disease adult patients. Nutrients. 2021;13(11):4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Svolos V, Hansen R, Nichols B, et al. Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology. 2019;156(5):1354–1367.e1356. [DOI] [PubMed] [Google Scholar]
- 22. Krupp LB. The fatigue severity scale. Arch Neurol. 1989;46(10):1121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data pertaining to this study will be made available to individuals upon request to the corresponding author.