Abstract
Background
Patients with inflammatory bowel disease (IBD) have an elevated risk for infection which is further increased by immunosuppressive medications. The aim of this study was to evaluate the safety and immunogenicity of influenza, PVC13, PPSV23, and hepatitis B vaccines in adults with IBD treated with vedolizumab as compared to those treated with anti-tumor necrosis factor (TNF) agents or nonimmunosuppressive therapy.
Methods
In this prospective controlled trial, patients were vaccinated with the influenza, PVC13, PPSV23, and/or hepatitis B vaccines. Participants were grouped based on IBD medication regimen: (1) vedolizumab monotherapy, (2) vedolizumab plus immunomodulator, (3) anti-TNF plus immunomodulator, and (4) no immunosuppressive therapy (control). Vaccine responses were evaluated by comparing pre- and postvaccination titers. Disease activity and adverse events were monitored by the Harvey–Bradshaw Index or Simple Colitis Clinical Activity Index and by standardized phone interviews.
Results
No serious adverse events or significant changes in disease activity were reported. For the influenza vaccine, baseline titers were high in all groups, and no follow-up titers met criteria for adequate response. For the pneumococcal vaccines, all groups showed response to vaccination; there was no statistically significant difference between the groups. For the hepatitis B vaccine, 62.5% of patients receiving vedolizumab and 33.3% receiving anti-TNF therapy achieved a level of response >10 mIU/mL.
Discussion
The inability to observe a response to the influenza vaccine was influenced by high baseline titers. For the hepatitis B vaccine, patients treated with vedolizumab experienced immunogenic response to vaccination that was noninferior to nonimmunosuppressed controls. All studied vaccines were well-tolerated. Vaccination should be encouraged in all adult patients with IBD.
Keywords: inflammatory bowel disease, vedolizumab, vaccination, influenza, pneumococcal vaccine, hepatitis B
INTRODUCTION
Inflammatory bowel disease (IBD) is characterized by immune dysregulation primarily affecting portions of the small and/or large intestine and is generally categorized as either Crohn’s disease (CD) or ulcerative colitis (UC). In addition to this inherent immune dysregulation, patients with IBD are often treated with medications that suppress the immune system, putting them at an increased risk for developing infections.1 Some of these infectious diseases such as influenza, pneumococcal pneumonia, and hepatitis B may be prevented by vaccination. The incidence of influenza and risk of related complications have been shown to be increased in patients with IBD as compared to those without IBD.2 Similarly, it has been demonstrated that patients with CD or UC are at increased risk for pneumonia.3
Current guidelines from the American College of Gastroenterology (ACG) regarding preventative health care for patients with IBD recommend that adult patients with IBD receiving immunosuppressive therapy should receive nonlive vaccines, including the trivalent inactivated influenza vaccine, 13-valent pneumococcal conjugate vaccine, the 23-valent pneumococcal polysaccharide vaccine, and the hepatitis B vaccine series in nonimmune patients, congruent with the guidelines published by the Centers for Disease Control (CDC) and Advisory Committee on Immunization Practices (ACIP).4–7 However, the impact of immunomodulatory medication on the immune response to some vaccines is not fully understood, particularly as newer classes of medications come to market or expand their indications to include patients with IBD.4 For example, it has been previously shown that IBD patients on combination therapy with an anti-tumor necrosis factor (TNF) agent and an immunomodulator (6-mercaptopurine, azathioprine, or methotrexate) had an impaired immune response to vaccination compared to nonimmunosuppressed subjects with IBD and healthy controls.8 A recent study of patients with IBD on anti-TNF monotherapy demonstrated higher postimmunization antibody titers to influenza when they received a high-dose vaccine compared to patients who had received standard dose vaccine, further suggesting that immunosuppressive therapy may adversely affect response rates to vaccines.9
Vedolizumab, a monoclonal antibody which targets the α4β7 integrin, was approved in May 2014 for the treatment of CD and UC. This integrin is important for cellular trafficking to the gastrointestinal tract, and as such it has been hypothesized that the overall effects of vedolizumab are gut specific. Previously, it has been demonstrated that healthy patients given a single infusion of vedolizumab did not have an impaired response to parenteral vaccination with hepatitis B vaccine as compared to a control group of healthy patients on no therapy.10 Also, a recently published study demonstrated immunogenicity to PCV13 by patients with IBD, including those on vedolizumab, though patients treated with vedolizumab were a minority of the study subjects and were aggregated into a study group with patients on other IBD therapies.11 The purpose of this study was to investigate the immune response to the influenza, pneumococcal pneumonia (PCV13 and PPSV23), and the hepatitis B vaccines in patients with IBD treated with vedolizumab as compared to those treated with combination of anti-TNF plus immunomodulator therapy or nonimmunosuppressive therapy. An additional objective of our study was to examine the safety of the influenza, pneumococcal pneumonia (PCV13 and PPSV23), and the hepatitis B vaccines in patients with IBD on various therapies, assessing for adverse events, including any changes in IBD disease activity.
METHODS
Study Population
The study patient population included adult patients aged 18–75 with IBD (diagnosed by standard clinical, radiographic, endoscopic, and histopathologic criteria) who received care at the Center for Digestive Disorders at Boston Medical Center between August 2017 and January 2019. This period covered two influenza seasons. After informed consent, patients were enrolled into one of four study groups based on the treatments they were taking as prescribed by their gastroenterologist: group 1 included patients with IBD receiving vedolizumab monotherapy, group 2 included patients with IBD receiving combination treatment with vedolizumab and concomitant immunomodulator therapy (methotrexate, azathioprine, or 6-mercaptopurine), group 3 included patients with IBD on other biologic therapy (infliximab, adalimumab, certolizumab, and golimumab) in combination with an immunomodulator (methotrexate, azathioprine, or 6-mercaptopurine), and group 4 included patients with IBD not receiving any immunosuppressive therapy. For patients in group 4, treatment with oral or topical 5-aminosalicylates was permissible. Patients in all groups were required to have been on stable doses of their respective treatment for at least 3 months. Inclusion in any group required that subjects did not have a known allergy to any of the vaccine components, had not received immunoglobulin therapy or blood products within a month of enrollment, and had not been receiving corticosteroids either orally or intravenously within 30 days prior to vaccination. Inhaled or topical corticosteroids were permissible. The study was approved by the Institutional Review Board.
Study Design
Enrollment and initiation
Patients with IBD who presented to the Center for Digestive Diseases (CDD) at Boston Medical Center for outpatient office visits or routine medication infusions were invited to participate in the study if they met the inclusion and exclusion criteria (ClinicalTrials.gov Identifier: NCT03056924). At this baseline visit, subjects’ demographic information and medical history, including vaccination history and medications the patient was currently taking and those taken during the preceding 30 days, were reviewed. Either the Harvey–Bradshaw Index (HBI) for CD or Simple Colitis Clinical Activity Index (SCCAI) for UC questionnaire was completed. At both the baseline visit and the follow-up visit, serum was obtained for measurement of antibody titers. For the hepatitis B vaccine, this included both patients with a history of hepatitis B vaccination. Both those with no history of vaccination and those previously vaccinated but with low titers (below 10.00 mIU/mL, per CDC guidelines) were eligible for enrollment.5
Vaccination and follow-up
Influenza vaccination was carried out with the trivalent component vaccine (Afluria, Seqirus USA Inc., King of Prussia, PA) or for patients’ ages 65 years and older (Fluzone, Sanofi Pasteur, Swiftwater, PA). Both of these vaccines were administered in a single dose of 0.5 mL intramuscularly.
Vaccination for pneumococcal pneumonia was carried out with either the PPSV23 (Pneumovax, Merck, Whitehouse Station, NJ) or with the PCV13 (Prevnar 13, Pfizer, Philadelphia, PA). Both vaccines were administered in a single dose of 0.5 mL intramuscularly. The selection of which pneumococcal vaccine was appropriate for the patient to receive was based on ACIP guidelines for these two vaccines.
Vaccination for hepatitis B was carried out with the Energix-B (Glaxo Smith Kline, Research Triangle Park, NC), administered in a three-dose series with 1.0 mL given at 0, 1, and 6 months or for patients receiving a booster, a single intramuscular dose of 1.0 mL was given.
Serum was obtained at the baseline visit prior to vaccination, and subjects returned 2–4 weeks later for follow-up blood collection. At this follow-up visit, subjects again completed the HBI for CD or SCCAI for UC questionnaire and were assessed for adverse events. Patients received a follow-up phone call between the initiation and follow-up visit to assess for adverse events.
Study Drug
The focus of this study was vedolizumab, the α4β7 integrin monoclonal antibody approved for treatment of CD and UC. For patients in this study receiving vedolizumab, either as monotherapy or in combination with an immunomodulator, all patients were treated with the standard vedolizumab maintenance dosing regimen of 300 mg infusions at 8-week intervals. Those receiving vedolizumab included patients with moderate to severe UC or CD who either received vedolizumab as initial therapy or as second-line therapy after failure of anti-TNF or immunomodulator therapy, or dependence on corticosteroids.
Efficacy Endpoints
The primary endpoint was the rate of immune seroconversion after influenza, pneumococcal, or hepatitis B vaccines among patient study groups. This was assessed as a change in antibody titer from baseline at 2–4 weeks postvaccination.
For both the influenza and pneumococcal vaccines arms, serum samples for antibody-specific titers at baseline and postvaccination were evaluated by standard enzyme-linked immunosorbent assay (ELISA).
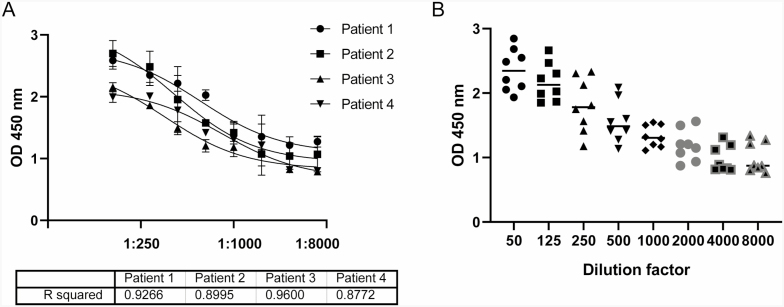
ELISA plates (Fisher Scientific) were coated with the formulation utilized to vaccinate each subject in phosphate-buffered saline (PBS) at 1 µg/mL overnight at 4°C. For the influenza arm of the study, both influenza A and influenza B antigens were tested corresponding to the antigens contained in the vaccine the patient received. Similarly for the pneumococcal vaccines, the antigens contained the corresponding vaccine that the patient received were tested. The plates were then washed and blocked with 1% bovine serum albumin in PBS. The serum was serially diluted up to 1:8000 in duplicate (Fig. 1A) to determine an optimal range from which to measure samples from all patients on one plate. The antibody was detected with anti-IgG horseradish peroxidase (Sigma Aldrich) diluted 1:10,000. The plates for both the influenza and pneumococcal vaccines were developed with tetramethylbenzidine and stopped with 1/20 phosphoric acid and read at 450 nm. Figure 1B demonstrates that the greatest variation between samples is with the 1:500 dilution.
FIGURE 1.
Serum dilution factor to measure differential in individual patient response. (A) Representative titration of serum IgG specific for flu vaccine antigen demonstrating goodness of fit of the developed assay and identifies 1:500. (B) As the concentration to obtain the optimal differential for which to measure all samples on one plate (n = 8).
The primary endpoint was the proportion of subjects with baseline specific antibody concentration that mounted a significant response to the vaccine at follow-up. Based on published literature, the endpoint for the pneumococcal vaccine arm was the proportion of patients demonstrating an approximate ≥2-fold increase in postvaccination titers. For the influenza vaccine arm, a significant response of change in specific antibodies detected in patient serum was defined as a ≥4-fold increase in postvaccination titers.
For the hepatitis B vaccine arm of the study, baseline hepatitis B surface antibody levels were checked as part of the patient’s standard of care. After vaccine administration, repeat levels were drawn from 1 to 6 months postvaccination using the clinical assay available at the institution where the study was conducted (Architect AUSAB; Abbott Laboratories, Abbott Park, IL). Per CDC guidelines, a result level greater than or equal to 10.00 mIU/mL implies immunity.5
Safety Endpoints
Safety was assessed by the incidence of adverse events and serious adverse events as well as by monitoring disease activity using the HBI or the SCCAI questionnaire at the baseline visit and at 2–4 weeks postvaccination. Additionally, subjects were questioned during a follow-up phone call 2 weeks postvaccination and at the follow-up visit about adverse events and serious adverse events, including fevers or chills, rash, and visits to the emergency room or to their primary care physicians.
Statistical Methods
Descriptive statistics were used to assess the sample. Chi-square and Student t test were used to compare outcomes between groups, as appropriate. The incidence of safety outcomes was compared between groups using chi-square. Paired t tests were performed to compare antibody responses to vaccination in the groups for the influenza vaccine, as well as the pneumonia vaccines (PCV13 and PPSV23).
For the hepatitis B vaccine, to compare the response of the groups with patients on IBD medications (vedolizumab or anti-TNF), to the control group of patients not on immunosuppressive therapy, a noninferiority margin of 15% with a lower bound of a one-sided 95% confidence interval (CI) for the per protocol population was chosen. This noninferiority margin was based on the margin in work by Wyant et al who examined similar treatment groups on vedolizumab vs a placebo control, although in a population of healthy patients rather than patients with IBD.10 The Mann–Whitney test was utilized to evaluate differences in group medians.
RESULTS
Demographics
A total of 160 vaccinations were given during the study period. There were 62 influenza vaccines, 57 pneumonia vaccines (36 PCV13 and 21 PSV23), and 41 hepatitis B vaccines administered. Forty-eight of the vaccines given were to patients on vedolizumab therapy (group 1—23 patients, group 2—15 patients). Thirty-six vaccines were given to patients in group 3 on combination anti-TNF and immunosuppressant (6-mercaptopurine or methotrexate). Eighty-six vaccines were given to patients on 5-aminosalicyclic acid (5-ASA) or no therapy (group 4). Seven patients (11%) who received the influenza vaccine did not return for their follow-up serum draw. Six patients (10.5%) who received pneumonia vaccines did not come for their scheduled follow-up blood draw. Patients who received the influenza or pneumococcal vaccines returned for their follow-up serum collection at 11–57 days after their vaccination (average 27 days). Patients who received the hepatitis B vaccine returned for their follow-up serum collection at 1–6 months (average approximately 1.5 months) after their last dose of vaccine; 11 patients (26.8%) did not return for follow-up surface antibody level.
Seventy-five of the vaccines (46.8%) administered were to female patients, and 72 of the vaccines (45%) were administered to patients with CD. Participant demographic, disease characteristics, and vaccine administered to each of the groups are listed in Tables 1 and 2. Statistically significant values (P value) were all calculated to compare the individual study group listed to the control group (group 4). Across all three vaccine arms of the study, the groups in which patients were being treated with vedolizumab (groups 1 and 2) were similar with respect to participant age, gender, and IBD type to the control group. For the influenza and hepatitis B arms, participants receiving anti-TNF therapy (group 3) were overall younger than the control group and had a higher CD prevalence.
TABLE 1.
Demographic and Other Baseline Characteristics: Intention-to-Treat
| Characteristic | Group 1 | Group 2 | Group 3 | Group 4 (Control) |
|---|---|---|---|---|
| Influenza | 12 | 10 | 13 | 27 |
| Age, years (mean ± SD) | 47.8 ± 18 | 41.7 ± 15 | 31.15 ± 6.8 | 42.1 ± 15 |
| (P = 0.308) | (P = 0.941) | (P = 0.017) | ||
| Gender, % female | 33.3% | 30% | 46% | 55.6% |
| (P = 0.210) | (P = 0.177) | (P = 0.588) | ||
| CD | 16.7% | 40% | 84.6% | 37% |
| (P = 0.213) | (P = 0.873) | (P = 0.004) | ||
| UC | 83.3% | 60% | 15.4% | 63% |
| (P = 0.213) | (P = 0.873) | (P = 0.004) | ||
| Pneumonia | 3 | 3 | 12 | 39 |
| PCV13 | 1 | 1 | 6 | 28 |
| PPSV23 | 2 | 2 | 6 | 11 |
| Age, years (mean ± SD) | 39 ± 18 | 36.3 ± 12 | 32 ± 8.4 | 36.38 ± 12.6 |
| (P = 0.738) | (P = 0.267) | (P = 0.248) | ||
| Gender, % female | 33.3% | 33.3% | 50% | 53.8% |
| (P = 0.505) | (P = 0.505) | (P = 0.82) | ||
| CD | 66.7% | 0 | 58.3% | 41% |
| (P = 0.4) | (P = 0.166) | (P = 0.302) | ||
| UC | 1 (33.3%) | 100% | 41.7% | 59% |
| (P = 0.4) | (P = 0.166) | (P = 0.302) | ||
| Hepatitis B | 8 | 2 | 11 | 20 |
| Age, years (mean ± SD) | 43.6 ± 19.2 | 39.5 ± 12 | 41.2 ± 18.4 | 43.1 ± 14.3 |
| (P = 0.937) | (P = 0.736) | (P = 0.739) | ||
| Gender, % female | 25% | 50% | 45.5% | 50% |
| (P = 0.243) | (P = 1) | (P = 0.816) | ||
| CD | 25% | 50% | 81.8% | 42.1%* |
| (P = 0.42) | (P = 0.84) | (P = 0.035) | ||
| UC | 75% | 50% | 18.2% | 57.9%* |
| (P = 0.42) | (P = 0.84) | (P = 0.035) |
Group 1, vedolizumab; group 2, vedolizumab + immunomodulator; group 3, anti-TNF + immunomodulator; group 4, 5ASA or no medication (control).
*One participant’s IBD type was still unclear with respect to CD vs UC at the time of the study and given this was excluded from these percentage calculations.
TABLE 2.
Flow Table of Study Patients and Vaccines Administered
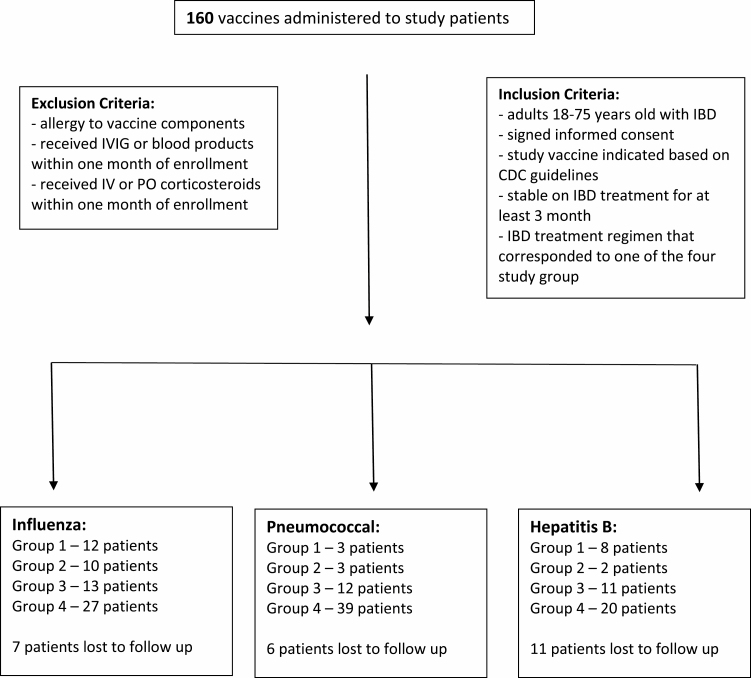
|
A total of 25 patients on vedolizumab therapy (groups 1 and 2) participated in the study across the various vaccine arms. Some of these patients were eligible to receive more than one vaccine. The majority of these patients, 18 out of 25, had exposure to other biologic treatments, in all cases anti-TNF therapy, for IBD prior to their current vedolizumab treatment. The average time on vedolizumab prior to entering the study was approximately 26 months with the shortest amount of time 2 months and the longest 74 months.
Antibody Response
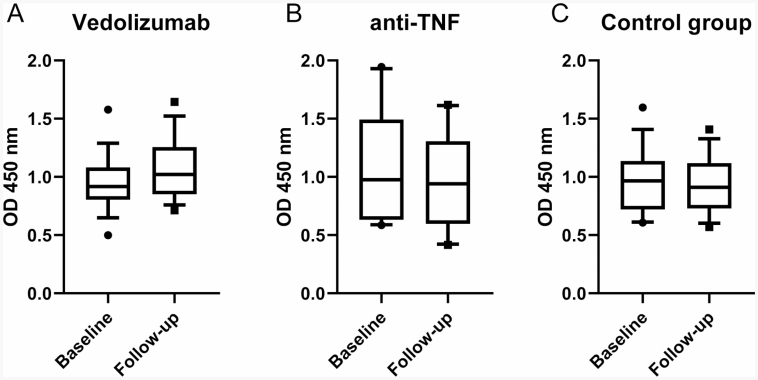
A high baseline influenza titer was noted in all study groups, regardless of medication or age. No follow-up titers met the efficacy criteria for a greater than 4-fold increase over baseline (Fig. 2). In fact, there was no difference noted in response to the influenza vaccine between any of the treatment groups (Figs. 2A–C). There was also no statistical difference in response to the influenza vaccine when specific IBD diagnosis (either CD or UC) was assessed separately (not shown). All subjects included had received an annual influenza vaccine in a year prior to the study; the average amount of time between influenza vaccination in the season prior to the study and the vaccination received as part of the study in the following influenza season was 406 days or approximately 13.4 months. The maximum number of previous influenza vaccines administered was recorded to be eight in our electronic health record.
FIGURE 2.
Responses to flu vaccine. Box and whiskers plot depicting 10%–90% of serum optical densities at 1:500 dilution factor. Nonparametric differences in medians were calculated with the Mann–Whitney test with P values for (A) vedolizumab group, P = 0.20, n = 17; (B) anti-TNF group, P = 0.73, n = 20; (C) control group, P = 0.74, n = 18.
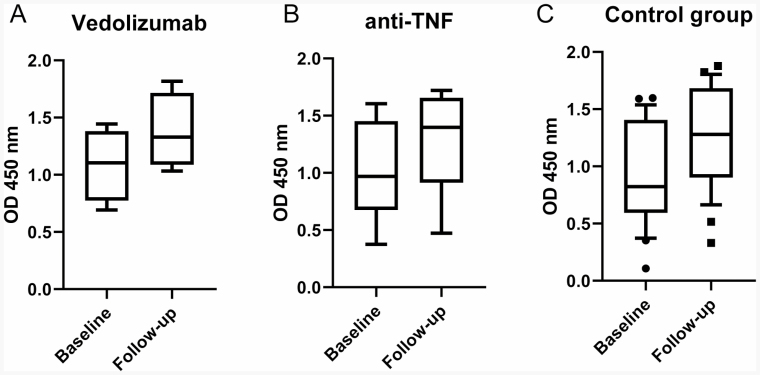
For the pneumonia vaccines, patients in all groups demonstrated an overall increased response to the vaccines (Fig. 3). For participants treated with vedolizumab (groups 1 and 2), the average increase in titer was 33.1% over baseline (Fig. 3A). For participants treated with anti-TNF medications (group 3), the average increase in titer was 44.7% over baseline (Fig. 3B); for the nonimmunosuppressed control (group 4), the average increase was 76.4% (Fig. 3C). While all groups showed response to the vaccine, when compared for significance, there was no statistically significant difference between the groups in terms of titer response rates. When compared to the control group, neither the participants receiving vedolizumab, represented by groups 1 and 2, showed a statistically different response to vaccination, nor the group 3 participants receiving anti-TNF therapy (Fig. 3).
FIGURE 3.
Responses to pneumococcal vaccines (PSV13 and PPSV23). Box and whiskers plot depicting 10%–90% of serum optical densities at 1:500 dilution factor. Nonparametric differences in medians were calculated with the Mann–Whitney test with P values for (A) vedolizumab group, P = 0.34, n = 4; (B) anti-TNF group, P = 0.09, n = 9; (C) control group, P = 0.0098, n = 26.
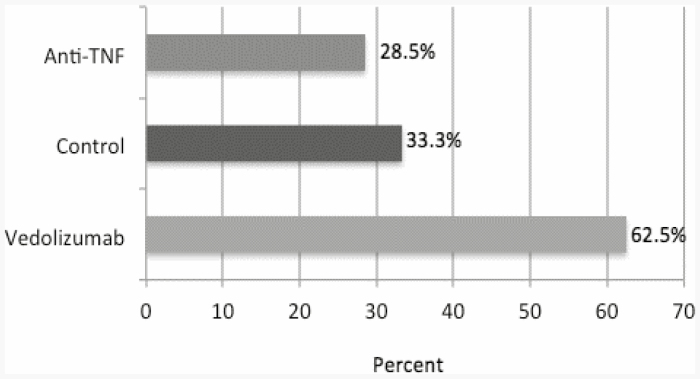
For the patients receiving the hepatitis B vaccine, as with the pneumococcal vaccines, a measurable response to vaccination was achieved by some members of each study group. Patients are considered to be protected from hepatitis B if anti-HBs level is greater than or equal to 10 mIU/mL.5 Of those patients who were treated with vedolizumab (groups 1 and 2 were again aggregated due to small sample size), 62.5% of patients achieved a level of response >10 mIU/mL (5/8 patients) (Fig. 4). For the control group, the response was 33.3% (5/15 patients), and for the group of patients treated with anti-TNF therapy, the response was 28.5% (2/7 patients), as shown in Figure 4. This analysis excludes the 11 enrolled patients who did not return for postvaccination titers.
FIGURE 4.
Response rates of patients receiving hepatitis B vaccines. Bars depict the percent of subjects achieving postvaccination HBsAb titer ≥10.00 mIU/mL by study group.
When comparing patients receiving vedolizumab to the nonimmunosuppressed control group, the response to vaccination was found to be noninferior to those in the control group (95% CI −4.63% to 62.6%), as the lower bound of the 95% CI is contained within the specified margin of −15%.10 Although, this is based on a small sample size. When comparing group 3, patients on combination anti-TNF and immunomodulatory therapy to the control group, the results were inconclusive (95% CI −28.9% to 37.9%). The lower bound of the 95% CI falls outside the −15% margin, and therefore not meeting noninferiority criterion, however inferiority cannot be commented on as the CI additionally includes zero.
Adverse Events
There were no serious adverse events (no events grade 2 or higher)12 reported throughout the study period. There were also no significant changes in SCCAI or HBI scores in the study population in the immediate period after vaccination (P > 0.05) (Table 3). Across all study groups, the majority of disease index scores (73.6% of participants) were unchanged pre- and postvaccination, and 24.8% of participants reported lower disease index score postvaccination as compared to the prevaccination baseline visit. Only two participants, both in the control group, reported higher scores at the postvaccination visit. Importantly, both of these postvaccination visits coincided with planned colonoscopy procedures, so some of this score increase is likely attributable to increased bowel movements as a result of colonoscopy prep.
TABLE 3.
IBD Disease Activity as Measured by HBI or SCCAI
| Treatment Group | Mean Prevaccination Score | Mean Postvaccination Score | Prevaccination Score Range | Postvaccination Score Range |
|---|---|---|---|---|
| Group 1 | 1.44 | 1.11 | 0–5 | 0–5 |
| Group 2 | 0.5 | 0.33 | 0–3 | 0–2 |
| Group 3 | 1.43 | 0.93 | 0–9 | 0–6 |
| Group 4 (control) | 1.45 | 1.01 | 0–18 | 0–10 |
Group 1, vedolizumab; group 2, vedolizumab + immunomodulator; group 3, anti-TNF + immunomodulator; group 4, 5ASA or no medication (control).
DISCUSSION
This study demonstrates that for patients with CD or UC, vaccination with the influenza, pneumococcal, or hepatitis B vaccines does not increase IBD activity and was not associated with serious adverse events. This finding was consistent among all study groups, regardless of medication treatment regimen, including agents with known immunosuppressive effects. As such, gastroenterologists and primary care physicians should strongly recommend participation in vaccination protocols to their patients with IBD. This is particularly important as previous research published in 2011 assessing gastroenterologists’ knowledge in vaccinating IBD patients found that only about two-thirds of gastroenterologists surveyed were correctly recommending inactivated vaccines.13 Additionally, other research has demonstrated that patients with IBD have misconceptions around vaccination, and that a significant percentage of patients with IBD feel that it is their doctor’s responsibility, rather than their own, to keep track of vaccinations.14 Provider recommendation has been cited as one of the top reasons that patients accept vaccines offered.14 These findings have become of increased importance as a resurgence of vaccine-preventable illnesses has occurred in recent years, which has been attributed to increased vaccine hesitancy and refusal among the general population.15
With regard to the immunogenic response to vaccination against influenza, our findings consistently demonstrate no detectable increase in antibody titers across all groups regardless of IBD treatment. This finding was maintained across the 2017–2018 influenza season as well as the 2018–2019 influenza season, despite variation in the influenza virus strains targeted between the two seasons. Additional demographic factors such as age and gender did not alter these results.
It is important to interpret influenza data in the context of patients’ baseline titers. All groups were noted to have high baseline titers to the vaccine antigens administered in this study, and regardless of IBD therapy, a patient’s ability to induce a measurable humoral response to the influenza vaccine is likely impacted by previous annual vaccination and exposure history, evidenced by these high baseline titers. These findings support the idea that repeated vaccination contributes to a consistently seropositive state. As demonstrated by Nuñez et al, this consistent seropositivity occurs across all age groups and results in lower observed seroconversion rates, which are artificially low as individuals enter the study in a seropositive state and maintain that status.16 McLean et al also demonstrated decreased vaccine effectiveness among frequent vaccine recipients.17 This is likely an important factor in the results we observed. Review of the subjects’ previous vaccination history demonstrated that all subjects had a record of receiving at least one prior influenza vaccine, with the maximum number of recorded previous influenza vaccines being eight. These vaccination history data are likely an underestimate, as we were only able to capture what is recorded in the medical center’s electronic medical record and not from outside facilities. Despite these low observed rates, Nuñez et al do note that changes in the annual vaccination formulation do result in broader antibody result, supporting continued annual vaccination.16 Work by Frasca et al demonstrate that even in a population of patients with a lower fold increase in antibody titers after vaccination, the ability to generate memory B cells was maintained, which was thought to be due to amplification as a result of repeated vaccination.18 As such, in our practice, we continue to encourage annual influenza vaccination regardless of the disease subtype and regardless of the IBD medication.
Additionally likely impacting influenza vaccine response is, as described in a growing body of literature, “imprinting” or “original antigenic sin” (OAS). This concept was first used to describe the effect of an individual’s first influenza virus exposure and how it impacts subsequent viral exposures.19 This is now being applied to how the initial viral exposure later affects response to vaccination. Cobey and Hensley describe how the competitive dominance of memory vs naive B cells are at the crux of how OAS impact immunological patterns. Memory B cells targeting epitopes associated with the original influenza strain a patient was exposed to tend to dominate and presumably outcompete naive B cells.20 Following from this, response to components of a vaccine unlike an individual’s original strain or “imprinted” strain is likely to not be robust, thus providing an additional complexity to the low vaccine response seen in our study. It has been shown that OAS strains can be cohorted by age group, for example an H1N1 strain, similar to the pandemic virus of 2009, circulated from 1918 to 1957 likely imprinting individuals who had their first exposure during those years, while those individuals first exposed to influenza between 1957 and 1977 were likely first infected with H3N2 viruses.20 We do not know if stratifying our data by such cohorts would show a significant effect, as the study is underpowered to do so.
We also examined response to the pneumococcal vaccines, PCV13 and PPSV23. In contrast to the influenza arm of the study, receipt of the pneumonia vaccine was typically the subject’s first exposure with these vaccines. Our results demonstrated an immune response to the vaccine in all treatment groups, but trends showed no difference in response between all of the study groups. This supports the findings of Wyant et al, who found that response to parental vaccination remains intact in patients treated with vedolizumab, thus concluding that immunomodulatory effects of vedolizumab are not systemic.10 It is important to note that in the Wyant et al, study only included healthy subjects, none of which who had IBD, and the treatment group only received a single dose of vedolizumab. Our study is the first to test this hypothesis in the population for which the drug is intended, patients with CD or UC; all of whom were on consistent treatment for at least 3 months rather than just a single dose of study drug. Our results support these previous findings, and they provide a much more practical and real-world context. However, given the small number of patients in the study, it is not possible to comment if the response is blunted compared to controls.
In terms of the responses to the hepatitis B vaccine, prior studies have proposed that immunosuppressive medications impair hepatitis B vaccine response.20 A retrospective study at our institution demonstrated that IBD itself may be associated with an impaired response to vaccination.21 In our study, when the group of patients with IBD treated with vedolizumab were compared to those not on any immunosuppressive therapy, the response to vaccination of those on vedolizumab was noninferior to this control group. While the small sample size and therefore a reduced statistical power does limit these conclusions, the fact that patients on vedolizumab are able to mount a response suggests that physicians should continue to encourage vaccination regardless of medication. All patients in our study were vaccinated against hepatitis B using the Energix-B vaccine which was licensed in 1989, however a new vaccine for hepatitis B, Heplisav-B (Dynavax Technologies, Berkeley, CA), has now been on the market since early 2018. Phase 3 trials suggest that this vaccine produces higher seroprotection rates than previous vaccines.22 This vaccine also boasts a possibly more advantageous schedule, a series with just two vaccines given over 4 weeks, as compared to the traditional three vaccines over 24 weeks. With fewer vaccines and shorter schedule, it could be conjectured that this could produce increased series completion rates. While the ACIP cites this new vaccine as an option for adults in prevention of hepatitis B infection, there are no recommendations for specific populations.23 This vaccine has not been specifically studied in patients with IBD, creating a significant opportunity for future research.
Our study had several limitations. We were unable to enroll our target number of patients in each treatment group as many of the patients had already received their pneumococcal and hepatitis B vaccines at the time of the study. Study group sizes were small, in particular for subjects in the pneumococcal pneumonia vaccine arm outside of the control group. This study challenge is likely a natural and welcome consequence of the current vaccination guidelines, which urge providers to vaccinate patients prior to the initiation of biologic medications.4 In addition, the need to return for follow-up serum draw was a major deterrent for patients and many opted out of the study or came back for follow-up serum testing more than the recommended 2–4 weeks postvaccination. Some older literature examining the humoral response to the influenza vaccine even suggests that a shorter window of follow-up, just over a week postvaccination, would best capture antibody response.24 While the cited study was small and used a vaccine that contained different influenza strains than was used in our study, a much earlier follow-up time could improve ability to detect changes in antibody titers. For future research, this short follow-up time could be pursued, however the challenges we faced with timely patient return would likely be amplified with a tighter window of follow-up.
For the influenza arm of the study, as mentioned there are a number of likely factors that contributed to the suboptimal response to vaccination that occurred across all groups. These results may have additionally be limited by serologic evaluation protocol, as Nuñez et al notes, persistent seropositivity result in lower observed seroconversion rates. It is possible that additional serum sample dilutions may have elucidated an observable difference, though diluting serum greater than 1:8000 can lead to less accuracy and increased variability between duplicates.
While these challenges reduced our ability to optimize our study, trends for the pneumococcal and hepatitis B arms of the study do demonstrate that patients on vedolizumab can respond to these vaccines. This taken with the consistent findings that these vaccines are safe for patients with IBD, regardless of medication regimen, support the recommendation that patients with IBD remain current with recommended vaccines.
Funding: This study was funded through an Investigator-Initiated Sponsored Research award from Takeda Pharmaceutical America Inc., study no: IISR-2016-101825.
Conflict of Interest: Dr Farraye has served on advisory boards for Glaxo Smith Kline, Janssen, Pfizer, and Takeda; Mayo Clinic, Department of Gastroenterology and Hepatology, Inflammatory Bowel Disease Center, Jacksonville, Florida. Dr Wasan has received investigator-initiated research funding from Takeda and Pfizer; Section of Gastroenterology, Department of Medicine, Crohn’s and Colitis Center, Boston Medical Center, Boston, Massachusetts. Dr Harrington, Dr Ganley-Leal, and Ms Hamilton have no financial disclosures or conflicts of interest.
DATA AVAILABILITY
Data not publically available.
REFERENCES
- 1. Viget N, Vernier-Massouille G, Salmon-Ceron D, et al. Opportunistic infections in patients with inflammatory bowel disease: prevention and diagnosis. Gut. 2008;57:549–558. [DOI] [PubMed] [Google Scholar]
- 2. Tinsley A, Navabi S, Williams ED, et al. Increased risk of influenza and influenza-related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:369–376. [DOI] [PubMed] [Google Scholar]
- 3. Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241–258. [DOI] [PubMed] [Google Scholar]
- 5. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2012;61:816–819. [PubMed] [Google Scholar]
- 7. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep. 2018;67:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Melmed GY, Agarwal N, Frenck RW, et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148–154. [DOI] [PubMed] [Google Scholar]
- 9. Caldera F, Hillman L, Saha S, et al. Immunogenicity of high dose influenza vaccine for patients with inflammatory bowel disease on anti-TNF monotherapy: a randomized clinical trial. Inflamm Bowel Dis. 2020;26:593–602. [DOI] [PubMed] [Google Scholar]
- 10. Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64:77–83. [DOI] [PubMed] [Google Scholar]
- 11. Pittet LF, Verolet CM, Michetti P, et al. ; Swiss Inflammatory Bowel Disease Cohort Study Group . High immunogenicity of the pneumococcal conjugated vaccine in immunocompromised adults with inflammatory bowel disease. Am J Gastroenterol. 2019;114:1130–1141. [DOI] [PubMed] [Google Scholar]
- 12. Food and Drug Administration (US). Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventative vaccine clinical trials. Fed Regist. 2007;72:54917. [Google Scholar]
- 13. Wasan SK, Coukos JA, Farraye FA. Vaccinating the inflammatory bowel disease patient: deficiencies in gastroenterologists knowledge. Inflamm Bowel Dis. 2011;17:2536–2540. [DOI] [PubMed] [Google Scholar]
- 14. Wasan SK, Calderwood AH, Long MD, et al. Immunization rates and vaccine beliefs among patients with inflammatory bowel disease: an opportunity for improvement. Inflamm Bowel Dis. 2014;20:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phadke VK, Bednarczyk RA, Salmon DA, et al. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016;315:1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nuñez IA, Carlock MA, Allen JD, et al. Impact of age and pre-existing influenza immune responses in humans receiving split inactivated influenza vaccine on the induction of the breadth of antibodies to influenza A strains. PLoS One. 2017;12:e0185666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McLean HQ, Thompson MG, Sundaram ME, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. 2014;59:1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frasca D, Diaz A, Romero M, et al. The generation of memory B cells is maintained, but the antibody response is not, in the elderly after repeated influenza immunizations. Vaccine. 2016;34:2834–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang A, Stacey HD, Mullarkey CE, et al. Original antigenic sin: how first exposure shapes lifelong anti-influenza virus immune responses. J Immunol. 2019;202:335–340. [DOI] [PubMed] [Google Scholar]
- 20. Andrade P, Santos-Antunes J, Rodrigues S, et al. Treatment with infliximab or azathioprine negatively impact the efficacy of hepatitis B vaccine in inflammatory bowel disease patients. J Gastroenterol Hepatol. 2015;30:1591–1595. [DOI] [PubMed] [Google Scholar]
- 21. Pratt PK Jr, David N, Weber HC, et al. Antibody response to hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm Bowel Dis. 2018;24:380–386. [DOI] [PubMed] [Google Scholar]
- 22. Jackson S, Lentino J, Kopp J, et al. ; HBV-23 Study Group . Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. 2018;36:668–674. [DOI] [PubMed] [Google Scholar]
- 23. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cox RJ, Brokstad KA, Zuckerman MA, et al. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12:993–999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not publically available.