Abstract
Background
Clostridium difficile infection (CDI) is the most common infectious cause of nosocomial diarrhea, comprising 10%–20% of all cases. CDI is a significant complication in patients with inflammatory bowel disease (IBD). New monoclonal antibody therapies have emerged as leading treatment options for recurrent CDI (rCDI). Bezlotoxumab, a novel monoclonal antibody, has shown success in decreasing the recurrence rates of patients with rCDI. However, data extrapolating diminished rCDI in patients with concomitant IBD is limited.
Methods
A single infusion of bezlotoxumab @ 10mg/kg was given with fidaxomicin 200mg for 10 days in a patient with rCDI and ulcerative colitis
Results
The patient’s symptoms improved, inflammatory markers normalized, and she has remained asymptomatic for twelve months
Conclusions
This case supports the findings in the MODIFY I/II trials that Bezlotoxumab is a viable treatment option of rCDI in IBD patients.
Keywords: bezlotoxumab, Clostridium difficile, ulcerative colitis
Graphical Abstract
Graphical Abstract.
Introduction
Clostridium difficile infection (CDI) constitutes the most common infectious cause of nosocomial diarrhea, comprising 10%–20% of all cases.1,2 Inflammatory bowel disease (IBD) patients suffer from significantly higher CDI recurrence than the general population, as well as increased mortality.3 New monoclonal antibody therapies, such as bezlotoxumab, have improved management of recurrent CDI (rCDI). The MODIFY I/II trial included 44 patients with known IBD, 28 of which received bezlotoxumab. Among these 28 patients, there was a 27.2% absolute reduction in the incidence of rCDI.4 Encouraged by these results, we recently prescribed bezlotoxumab in an ulcerative colitis (UC) patient with rCDI and herein report our experience.
Case
A 21-year-old female with a history of UC diagnosed in 2017 presented for treatment of rCDI. Her first episode of CDI occurred in 2019 (see Figure 1) during an active UC flare treated with steroids and infliximab induction. CDI was successfully treated with a 4-week course of 125 mg oral vancomycin twice daily followed by once daily for an additional 4 weeks. CDI recurred 1 year later, testing positive for C. difficile via glutamate dehydrogenase antigen and PCR for toxin B while on vedolizumab for UC treatment. She was treated with 125 mg oral vancomycin, 4 times daily, and tapered down by 1 dose over a 4-month period. Two years after her initial CDI, she developed worsening non-bloody diarrhea, nausea, and anorexia with weight loss. She again tested positive for C. difficile via glutamate dehydrogenase antigen and PCR for toxin B. Fecal calprotectin was 2190 µg g−1. She was treated with 14-day course fidaxomicin 200 mg BID and a single infusion of bezlotoxumab 10 mg kg−1, as per protocol. Treatment for UC was not adjusted. At follow-up 1 month later, her symptoms had resolved, and fecal calprotectin normalized (17 µg g−1). Twelve months later, she remains asymptomatic.
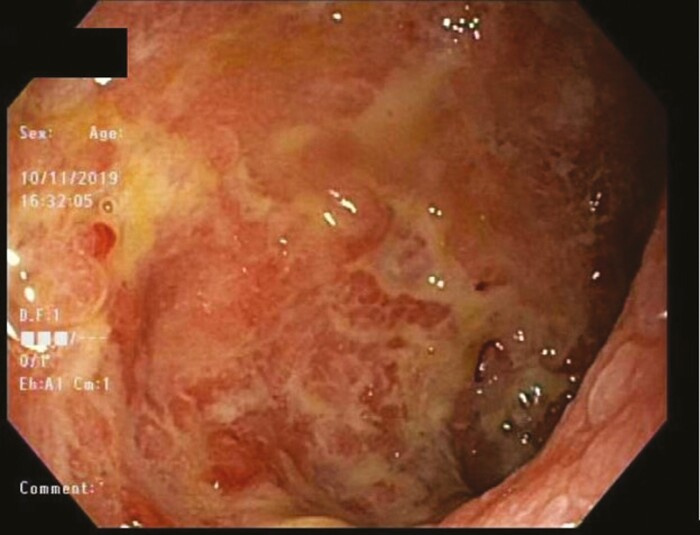
Figure 1. .
Endoscopic findings from sigmoid colon during the patients first episode of Clostridium difficile in 2019 demonstrating friable, congested, ulcerated mucosa (Mayo III).
Discussion
Recurrent CDI is a frequent culprit for inducing IBD flares and is difficult to manage. Fecal transplant (FMT) is an option for rCDI with reported cure rates above 85%5,6; however, FMT can be a logistical challenge to complete at many institutions and risks cross-contamination of endoscopic facilities. Bezlotoxumab is an appealing option for rCDI as a single dose medication with few reported side effects. In this patient, bezlotoxumab quickly resolved both rCDI and UC flare without need for alteration or escalation of biologic therapy with remission lasting >1 year. This case further supports bezlotoxumab use in UC patients with rCDI to improve clinical outcomes. Bezlotoxumab should be considered early in this patient population, particularly in healthcare facilities without timely access to FMT.
Contributor Information
Aaron Fein, Department of Internal Medicine, University of Kentucky Medical Center, Lexington, Kentucky, USA.
Cody Kern, Department of Digestive Disease and Nutrition, University of Kentucky Medical Center, Lexington, Kentucky, USA.
Terrance Barrett, Department of Digestive Disease and Nutrition, University of Kentucky Medical Center, Lexington, Kentucky, USA.
Courtney Perry, Department of Digestive Disease and Nutrition, University of Kentucky Medical Center, Lexington, Kentucky, USA.
Funding
None declared.
Authors’ Contributions
Aaron Fein—lead author. Cody Kern—secondary author and reviewer. Terrance Barrett—attending advisor and reviewer. Courtney Perry—article guarantor, reviewer, and attending advisor.
Conflicts of Interest
None declared.
Data Availability
No new data were created or analyzed.
References
- 1. Polage CR, Solnick JV, Cohen SH.. Nosocomial diarrhea: evaluation and treatment of causes other than Clostridium difficile. Clin Infect Dis. 2012;55(7):982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hung YP, Lee JC, Tsai BY, et al. Risk factors of Clostridium difficile-associated diarrhea in hospitalized adults: vary by hospitalized duration. J Microbiol Immunol Infect. 2021;54(2):276–283. [DOI] [PubMed] [Google Scholar]
- 3. Binion D. Clostridium difficile infection and inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2016;12(5):334–337. [PMC free article] [PubMed] [Google Scholar]
- 4. Kelly C, Wilcox M, Glerup H, et al. Bezlotoxumab for Clostridium difficile infection complicating inflammatory bowel disease. Gastroenterology. 2018;155(4):1270–1271. [DOI] [PubMed] [Google Scholar]
- 5. Rohlke F, Stollman N.. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv Gastroenterol. 2012;5(6):403–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenberg SA, Youngster I, Cohen NA, et al. Five years of fecal microbiota transplantation—an update of the Israeli experience. World J Gastroenterol. 2018;24(47):5403–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed.