Abstract
Background
Limited data exist on adherence to fecal calprotectin (FCP) testing in patients with inflammatory bowel disease.
Methods
Completion rates for patients who had at least one FCP test ordered (n = 3082) and a subgroup with C-reactive protein, complete blood count, and Clostridium difficile tests also ordered (n = 1563) were analyzed.
Results
More patients completed blood than stool tests, with FCP having the poorest adherence of all tests analyzed. Older patients had higher FCP completion rates. No differences were noted in completion rates across age, gender, or ethnicity for blood tests.
Conclusions
Further studies are needed to develop strategies that improve the uptake of FCP.
Keywords: biomarkers, calprotectin, Crohn’s disease, ulcerative colitis, inflammatory bowel disease
Introduction
Reliable, noninvasive biomarkers are crucial in the management of inflammatory bowel disease (IBD) to minimize invasive procedures, especially in the era of the coronavirus disease 2019 (COVID-19) pandemic. Conventional blood tests, such as hemoglobin, C-reactive protein (CRP), and erythrocyte sedimentation rate, are widely used in IBD management; however, the sensitivity of these tests is insufficient to reliably identify gut inflammation.1 Furthermore, approximately 15% of patients fail to mount a CRP response, and the disease subtype can further affect the sensitivity of this test.2 These limitations have fostered the development and investigation of alternative tests, specifically stool biomarkers.
Calprotectin is a calcium- and zinc-binding heterodimer of the S100A8/A9 protein that is found primarily in the cytosol of neutrophils and, to a lesser extent, in monocytes and macrophages.3 Intestinal inflammation is characterized by neutrophil migration into the mucosa.4 Because calprotectin is primarily a product of neutrophils, its concentration is directly proportional to the concentration of neutrophils in the gut mucosa, which may be reflected by levels in the stool.5 Furthermore, fecal calprotectin (FCP) is resistant to bacterial degradation and is stable in stool at room temperature for up to 7 days.1
In the past decade, FCP has emerged as a reliable assay that can be used to evaluate the degree of disease activity, monitor response to therapy, and predict potential relapse in IBD patients.6 Moreover, FCP can also aid in distinguishing IBD from functional bowel disorders; FCP has a high negative predictive value in ruling out IBD in undiagnosed, symptomatic patients, and it has a high sensitivity for diagnosing IBD.7 These properties make FCP a useful tool for prioritizing endoscopies, thereby lowering costs for patients and decreasing resource utilization.
Although FCP is a useful marker in the management of IBD patients, adherence to testing could limit its utility. In this retrospective study, we assessed completion rates for FCP as compared to other routine tests done in the care of IBD patients in a tertiary referral center. There are few studies aimed at exploring the adherence to FCP testing within established IBD patients. This study is relevant for identifying factors that may hinder widespread clinical uptake of fecal testing.
Methods
Study Design
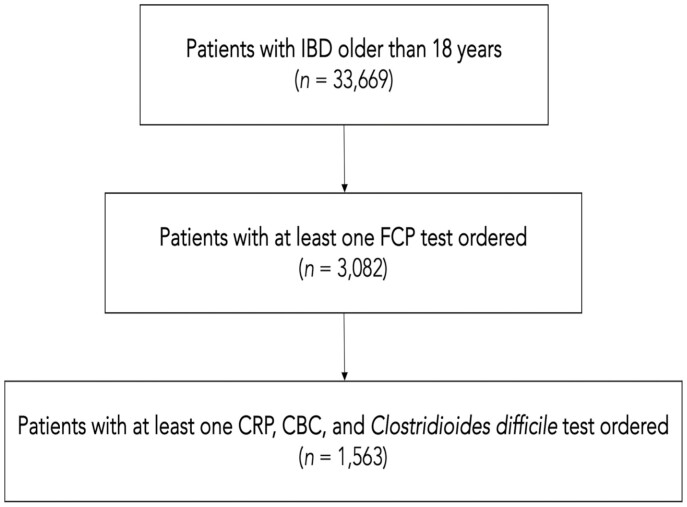
This was a single-center, retrospective cohort study based on patient data retrieved from the electronic medical record (EMR; Epic Systems Corporation) at the University of Miami Miller School of Medicine. We obtained information on patients that fit the diagnosis of IBD from December 2010 to March 2020 using the International Classification of Diseases, 10th revision, Clinical Modification codes (ICD-10-CM); these codes are provided in the supplementary index of the article. Electronic records of 33 699 unique patients older than the age of 18 were reviewed. Of these, 3082 individual patients were found with at least one FCP test ordered. A subset of 1563 patients who also had information on completion rates for CRP, complete blood count (CBC), and Clostridium difficile was also identified. Demographic data, including age and ethnicity, were collected for analysis. The study flow diagram is displayed in Figure 1. The completion rate of the considered tests for each patient was calculated and then the completion rate of the entire cohort was calculated; this was done to ensure that each patient had the same contribution to the group analysis.
Figure 1.
Consort of patient selection. IBD, inflammatory bowel disease; FCP, fecal calprotectin; CRP, C-reactive protein; CBC, complete blood count.
Statistical Analysis
Continuous variables were expressed as mean and standard deviations for normally distributed variables; median and interquartile ranges (IQRs) were used for non-normally distributed variables. Independent T-tests and Mann–Whitney U tests were used to analyze continuous parametric and nonparametric variables, respectively. Categorical variables were expressed as frequencies and proportions, and differences were determined using the Chi-square test. When comparing multiple independent groups, the Kruskal–Wallis test was used. The Bonferroni method was utilized when comparing multiple categorical variables and a P value less than .001 was considered significant. Spearman’s correlation was used to establish a correlation between nonparametric continuous variables. Linear regression was also performed for continuous variables. Statistical significance was accepted for P values less than .05.
Results
Between 2010 and 2020, a total of 3082 patients with IBD and at least one FCP test ordered were identified. In terms of IBD disease type, 1519 (49.3%) patients had Crohn’s disease (CD), 1081 (35.1%) had ulcerative colitis (UC), and 482 (15.6%) had indeterminate colitis (IC). The age of patients ranged from 18 to 96 years with an average age of 43.19 ± 17.2 years. Of this cohort, 1638 patients (53.1%) were female and 1235 patients (40.1%) self-identified as Hispanic. Females were significantly older than males (44.5 ± 17.5 vs. 41.7 ± 16.8 years, P < .001). When comparing differences of age by ethnicity, non-Hispanics were found to be older than Hispanics; however, this difference was not statistically significant (42.8 ± 16.7 vs. 43.5 ± 17.5 years, P = .0234). The demographics of this patient cohort are summarized in Table 1.
Table 1.
Demographics of studied cohort.
| Age | |
| Mean (SD) | 43.2 (17.2) |
| IBD disease type | |
| Crohn’s disease | 1519 (49.3%) |
| Ulcerative colitis | 1081 (35.1%) |
| Indeterminate colitis | 482 (15.6%) |
| Sex | |
| Female | 1638 (53.1%) |
| Male | 1444 (46.9%) |
| Ethnicity | |
| Hispanic | 1235 (40.1%) |
| Non-Hispanic | 1711 (55.5%) |
| Unknown | 136 (4.4%) |
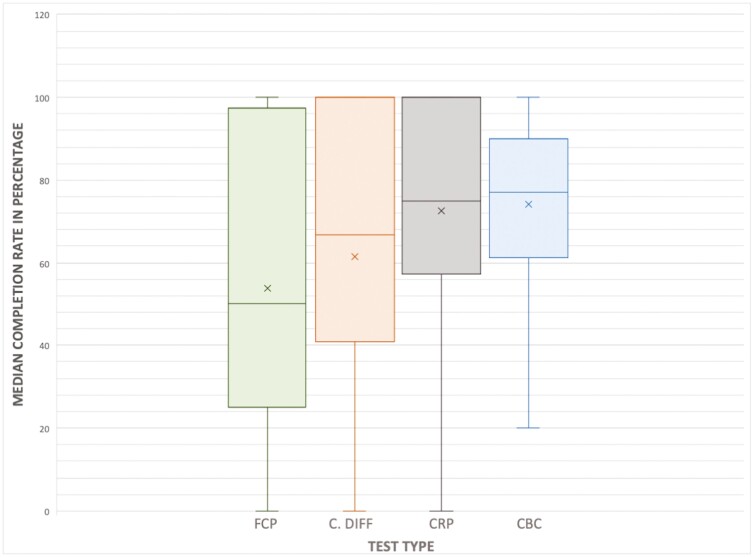
In the 3082 patients initially identified, the median completion rate for FCP for patients who had at least one FCP test ordered was 50% (IQR 0–100). When the subgroup analysis was performed for the 1563 patients for whom complete information was available for FCP, C. difficile, CRP, and CBC testing, the median completion rates (IQR) for each of these tests were noted to be 50.0% (25–100), 66.7% (40–100), 75.0% (57–100), and 77.0% (61–90), respectively. The median completion rates are displayed in Figure 2.
Figure 2.
Box-and-whisker plot displaying median completion rate per test type. FCP, fecal calprotectin; CRP, C-reactive protein; CBC, complete blood count. The median, or 50th percentile, is represented by the solid horizontal line per plot. Mean of the data is represented by an additional “x” symbol per plot.
The patients were then analyzed by IBD disease type. Median FCP completion rates by IBD disease type were 45.5% (0–72.7) for CD, 50% (16.7–100) for UC, and 88.9% (33.3–100) for IC. Median C. difficile completion rates by IBD disease type were 66.6% (33.3–100) for CD, 66.6% (50–100) for UC, and 66.6% for IC (50–100). Median CRP completion rates by IBD disease type were 72.2% (57.1–87.5) for CD, 75% (55.7–90.7) for UC, and 100% (66.7–100) for IC. Finally, median CBC completion rates by IBD disease type were 75% (60.0–88.9) for CD, 76.9% (60.0–89.9) for UC, and 83.3% (66.7–100) for IC. A statistically significant difference was noted in the completion rate for FCP, CRP, and CBC in UC, CD, and IC patients (P < .001), respectively. However, no statistically significant difference was noted in completion rates of C. difficile in UC, CD, or IC patients.
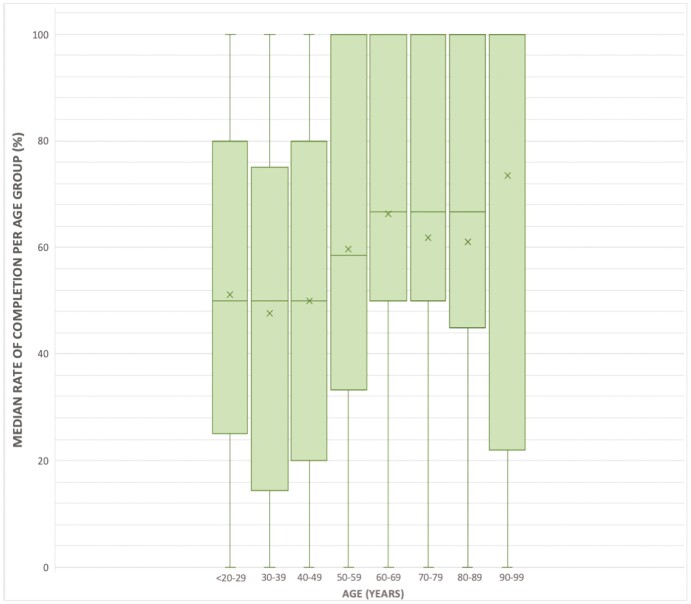
Patients were stratified into 8 separate cohorts according to their age and median rates of completion for each test were compared. Median rates of completion by age group for FCP are displayed in Figure 3. Median FCP completion rate (IQR) for age <20–29 was 50% (25–80), which was significantly lower compared to the completion rate for age 60–69, which was 66.7% (50–100), P < .001. Similarly, the FCP completion rate for age 30–39 was 50% (14.3–75), which again was significantly lower compared to age 60–69 (P < .001). This was further demonstrated when comparing the age range 40–49, which had a completion rate of 50% (20–80), to 60–69 (P < .001). When comparing completion in the age range 30–39 to 50–59 and to 70–79, there were statistically significant differences noted (P < .001 and P < .01, respectively). All other age group comparisons were not statistically significant.
Figure 3.
Box-and-whisker plot displaying age stratification versus median completion rate of fecal calprotectin. The median, or 50th percentile, is represented by the solid horizontal line per plot. Mean of the data is represented by an additional “x” symbol per plot.
The comparison of age stratification versus median completion rates of CBC, CRP, and C. difficile was also calculated. No statistically significant difference was noted among age groups and completion of CBC and CRP. However, for C. difficile, age group 18–29 had a completion rate of 50% (30.2–100), which was significantly lower than the completion rate of 66.7% (50–100) noted among the age group 60–69 (P < .05). Similarly, the age group 50–59 had a significantly higher completion rate of 66.7% (50–100) compared to the age group 18–29 (P < .05). No statistically significant difference was noted based on gender or ethnicity in completion rates of any test. Because we have a large Hispanic population, these data suggest that Hispanics are no less likely than non-Hispanic Whites to complete stool testing.
A Spearman’s rank-order correlation was run to determine the relationship between age and FCP rate of completion which showed no or negligible relationship (r = 0.148, P < .001). In univariate regression analysis, we observed an increase of 0.003% in FCP completion rate for every increase in 1 year of age (P < .001). We also found a positive linear relationship when comparing age and C. difficile completion rate (r = 0.13, P < .001) in which for each increase in 1 year of age, there was a 0.28% increase in test completion rate. No correlation was found between age and CBC (r = 0.018, P = .38) or CRP (r = −0.03, P = .82) rates of completion. Interestingly, a positive correlation between FCP rates of completion and CBC (r = 0.22, P < .001), CRP (r = 0.25, P < .001), and C. difficile (r = 0.37, P < .001) was noted. Linear regression showed that for each increase of 1% of FCP rate of completion, there is an increase of 0.004% of CBC (P < .001), 0.004% of CRP (P < .001), and 0.004% of C. difficile (P < .001) rates of completion.
Discussion and Conclusions
The need for noninvasive tests to aid with diagnosis, to monitor disease activity and response to treatment, and to predict disease relapse is essential. The COVID-19 pandemic has further highlighted this need when the performance of colonoscopies was limited. The sensitivity and specificity of FCP make it a valuable tool in the management of IBD patients. In this study, we assessed completion rates for FCP as compared to other routine tests done in the care of IBD patients at a tertiary referral center caring for a diverse patient population. Despite the utility of FCP as a surrogate marker of disease activity and as a useful alternative to repeated endoscopic evaluations, we observed that adherence to this test compared to standard blood tests among our patient cohort is poor. We observed a similar trend in the completion rate of C. difficile and overall found statistically fewer patients adhering to tests involving the collection of stools compared with blood.
Our analysis shows that age contributes to the differences observed in the completion of stool biomarker tests when compared to blood biomarker tests. In this subset of patients, there was a statistically significant increase in FCP completion rates among older patients compared to younger patients. We found a positive linear relationship when comparing age and FCP completion rate. Furthermore, we found that those patients who completed an FCP test were likely to complete other tests ordered by providers, specifically C. difficile stool tests and blood tests. Patients with colitis, UC, or indeterminate were more likely to complete FCP testing. One possible explanation is that patients perceived a greater value for this test. There was no statistical difference in the completion of FCP tests based on gender or ethnicity. This is important given that 40.1% of our patient cohort are self-identified Hispanics.
We next asked if this finding was also seen in patients who had orders for another common stool test, C. difficile, versus commonly ordered blood tests. A similar pattern was observed for C. difficile stool testing. Whereas we saw that older patients were more likely to perform stool testing, we did not see a difference in completion rates of blood tests based on age, gender, or ethnicity. Not surprisingly, patients willing to have the FCP stool test were also more likely to complete blood tests (CBC, CRP) and other stool tests (C. difficile). This observation indicates that certain patients are generally more likely to adhere to all recommended testing.
Adherence to testing is required in a variety of other common conditions as part of screening guidelines and to aid in the delivery of evidence-based quality care. For example, the Centers for Disease Control and Prevention recommends that diabetic patients undergo a glycated hemoglobin test (HbA1C; hereafter referred to as A1C) every 3 months.8 The national prevalence of diabetic individuals completing an A1C test at least twice in the last year is estimated to be approximately 65.0%.8 The rate of A1C test completion may differ based on race/ethnicity, and one study conducted by Nepal and Banerjee8 demonstrated that the completion among Hispanics is the lowest compared to other races. Additionally, there are several conditions that require close blood drug level monitoring to ensure drug efficacy. For example, patients on warfarin require frequent international normalized ratio (INR) checks for drug level monitoring. In a small study conducted by Pamboukian et al9 in 2008, the authors observed an adherence to INR monitoring in 76.3% of their study cohort. Of interesting note, the authors remarked that younger age and less severe baseline disease were associated with higher rates of nonadherence to blood testing. Stool testing is also used widely as a screening tool for colorectal cancer. In a large-scale, observational study, adherence to fecal immunochemical testing was approximately 40%.10 IBD is distinct as it requires the regular collection of both blood and stool biomarkers to monitor disease activity and guide management.
In conducting this study, our group generated total orders of FCP and other laboratory tests through a search of our EMR, and as such, we do not know the specific indications for which these tests were ordered. Furthermore, it is possible that laboratory tests may be ordered without an accompanying visit. We know from previous studies conducted on our patient cohort that a majority of patients (approximately 70%) are in histologic and endoscopic remission at the time of colonoscopy.11 Our current data demonstrate that approximately half (53%) of the patients are in FCP remission with the laboratory cutoff of 50 μg/g. A systematic review and meta-analysis by Mosli et al2 identified an FCP cutoff point of 50 μg/g as optimal for the detection of endoscopically active disease in symptomatic patients, which is substantially different from the value of 250 μg/g reported by a meta-analysis conducted by Lin et al.12 Given the different cutoffs of FCP, we believe that the indications for ordering an FCP in our cohort are similar to the IBD patients undergoing routine colonoscopies in our center, which is to verify remission and monitor for relapse.
CRP and FCP are among the most commonly used biomarkers in the treatment and monitoring of IBD patients. A multicentered, randomized controlled trial completed in 2017 studying the effect of tight control management on CD (CALM) demonstrated that treatment algorithms based on concentrations of FCP and CRP to monitor inflammatory activity alongside clinical symptoms led to superior outcomes compared with an algorithm based on clinical symptoms alone in patients with CD.13 The use of biomarkers led to faster optimization of therapy and to a higher proportion of patients achieving endoscopic and mucosal healing, biological remission, and steroid-free remission.13 However, CRP is not a specific marker of intestinal inflammation, as it has shown to have a specificity of 0.49 (95% CI: 0.72–0.98) in CD, and 15% of IBD patients do not mount a CRP response during disease flares.2 Furthermore, genetic heterogeneity in CRP generation exists among individual patients.14
Although the use of FCP has emerged as one of the most useful tools for the clinical management of IBD, adherence to FCP testing can be challenging to patients for various reasons. A prospective, observational study conducted in Europe by Maréchal et al15 found that only one-third of the studied patients complied with FCP testing. The main reason for noncompliance was forgetfulness (76%) and a lack of perceived benefit for the test (11%); other reasons included refusal to handle feces and difficulty with collecting the stool sample.15 In a prospective patient survey by Kalla et al16 in 2018, 37% of patients found FCP testing challenging, with the collection of samples being the most difficult aspect (67%). In particular, patients (younger than 49 years; odds ratio: 2.5, 95% CI: 1.6–4.0) had difficulties with the stool test and patients preferred blood testing versus FCP testing (55% vs. 6%, respectively).17 This is similar to our study in which median completion rates were highest for CBC and CRP tests (75% and 77%, respectively) and lowest for FCP (50%), and completion rates were generally higher in older patients. These data suggest that younger patients may benefit from additional support and counseling regarding adherence to tests.
The strengths of this study include that data were obtained in a tertiary referral center with standardized care practices. We included patients of all ages, comprising recently diagnosed IBD and long-standing IBD. A high proportion of patients are self-identified Hispanics and thus these data contribute to understanding whether there are cultural barriers to stool-based tests. Consecutive patients were included to avoid selection bias. The study has limitations, including the retrospective nature of the study over a wide range of dates and that it was performed in a single site. Therefore, it may not reflect the IBD population elsewhere. Furthermore, it is possible that when patients are acutely ill, their likelihood of completing testing may be higher than when they are well. Compared to other stool tests like C. difficile, FCP may be ordered more frequently in stable patients than other stool tests and, as a result, may partially explain our difference in completion rates.
Despite the cited limitations, the data presented here are directly applicable to clinical practice, as it reinforces that patient education for FCP testing is crucial to improve patient outcomes. Further studies are needed to explore the potential improvements that are needed to aid in stool sample collection. At-home testing that minimizes stool handling may help adherence. The development of blood-based biomarkers that reflect intestinal inflammation with greater sensitivity and specificity, as well as ease of adherence, will improve patient care.
Supplementary Material
Author Contributions
Acquisition of data: N.S.K., A.K., A.L., and M.A.Q. Interpretation and statistical analysis: N.S.K. and G.A.R. Drafting of the manuscript: N.S.K., A.L., and A.K. Final approval of the submitted manuscript: N.S.K., A.L., G.A.R., A.K., M.A.Q., A.R.D., D.H.K., O.M.D., and M.T.A.
Funding
This study had no funding sources and did not use any sponsors for study design, collection, analysis, and interpretation and/or the writing of the manuscript. This work is entirely independent and investigator-driven.
Financial Disclosures and Conflicts of Interest
A.R.D. has served as a scientific advisory board member for GI Health Foundation and receives honoraria for serving on a committee on the ABIM. D.H.K. has served as a scientific advisory board member for AbbVie Inc. and as a consultant for Cleveland Clinic. D.H.K. serves as a trainer or lecturer for PRIME Continuing Medical Education and The Academy for Continued Healthcare Learning. M.T.A. has served as a scientific advisory board member for Boehringer Ingelheim Pharmaceuticals, Gilead, AbbVie, Janssen Pharmaceuticals, Shire, Cosmo Pharmaceuticals, and Landos Biopharma. M.T.A. serves as a trainer or lecturer for Imedex, Focus Medical Communications, and Cornerstones Health, Inc. M.T.A. has served as a consultant for Prometheus Biosciences, Eli Lilly Pharmaceuticals, and UCB Biopharma SRL. M.T.A. has a funded project by Prometheus Laboratories. O.M.D. received honoraria from Pfizer and has a funded grant from Pfizer. This does not alter the authors’ adherence to the journal’s policies on sharing data and materials. All other authors declare no conflict of interest.
Data Availability
The authors confirm that the data supporting the key findings of this study are available within the article.
References
- 1. Sipponen T, Kolho KL. Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. Scand J Gastroenterol. 2014;50(1):74–80. doi: 10.3109/00365521.2014.987809 [DOI] [PubMed] [Google Scholar]
- 2. Mosli MH, Zou G, Garg SK, et al. . C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(6):802–19; quiz 820. [DOI] [PubMed] [Google Scholar]
- 3. Alibrahim B, Aljasser MI, Salh B. Fecal calprotectin use in inflammatory bowel disease and beyond: a mini-review. Can J Gastroenterol Hepatol. 2015;29(3):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burri E, Beglinger C. The use of fecal calprotectin as a biomarker in gastrointestinal disease. Expert Rev Gastroenterol Hepatol. 2014;8(2):197–210. [DOI] [PubMed] [Google Scholar]
- 5. Zittan E, Kelly OB, Kirsch R, et al. . Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn’s disease. Inflamm Bowel Dis. 2015;22(3):623–630. doi: 10.1097/MIB.0000000000000652 [DOI] [PubMed] [Google Scholar]
- 6. Molander P, Färkkilä M, Ristimäki A, et al. . Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis. 2015;9(1):33–40. [DOI] [PubMed] [Google Scholar]
- 7. Ayling RM, Kok K.. Fecal Calprotectin. Vol 87. Elsevier Ltd; 2018. doi: 10.1016/bs.acc.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 8. Nepal V, Banerjee D. A1C testing and its sociodemographic predictors: implications for diabetes self-management programs. Health Serv Res Manag Epidemiol. 2014;1:2333392814547129. doi: 10.1177/2333392814547129. PMID: 28462244; PMCID: PMC5278826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pamboukian SV, Nisar I, Patel S, et al. . Factors associated with non-adherence to therapy with warfarin in a population of chronic heart failure patients. Clin Cardiol. 2008;31(1):30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nielson CM, Vollmer WM, Petrik AF, et al. . Factors affecting adherence in a pragmatic trial of annual fecal immunochemical testing for colorectal cancer. J Gen Intern Med. 2019;34(6):978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serigado J, Quintero M, Koru-Sengul T, et al. . Histology and endoscopy discordance in inflammatory bowel disease varies by phenotype and location of disease. ACG 2019;114(p S441). doi: 10.14309/01.ajg.0000592556.97251.70 [DOI] [Google Scholar]
- 12. Lin JF, Chen JM, Zuo JH, et al. . Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20(8):1407–1415. [DOI] [PubMed] [Google Scholar]
- 13. Colombel JF, Panaccione R, Bossuyt P, et al. . Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779–2789. [DOI] [PubMed] [Google Scholar]
- 14. Kathiresan S, Larson MG, Vasan RS, et al. . Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation. 2006;113(11):1415–1423. [DOI] [PubMed] [Google Scholar]
- 15. Maréchal C, Aimone-Gastin I, Baumann C, Dirrenberger B, Guéant JL, Peyrin-Biroulet L. Compliance with the faecal calprotectin test in patients with inflammatory bowel disease. United Eur Gastroenterol J. 2017;5(5):702–707. doi: 10.1177/2050640616686517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalla R, Boyapati R, Vatn S, et al. . Patients’ perceptions of faecal calprotectin testing in inflammatory bowel disease: results from a prospective multicentre patient-based survey. Scand J Gastroenterol. 2018;53(12):1437–1442. [DOI] [PubMed] [Google Scholar]
- 17. Saverymuttu SH, Peters AM, Crofton ME, et al. . 111Indium autologous granulocytes in the detection of inflammatory bowel disease. Gut. 1985;26(9):955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the key findings of this study are available within the article.