Abstract
Background
Real-world assessments of biosimilars are needed to understand their effectiveness and safety in practice settings that may differ from those seen in clinical trials or healthcare systems in different countries. To assess the effectiveness and safety of a biosimilar (infliximab-dyyb) and its reference product (infliximab) in patients with inflammatory bowel disease (IBD) in the United States.
Methods
We conducted a retrospective cohort study of biologic-naive patients with IBD who started treatment with infliximab-dyyb or infliximab. The study included 3206 patients identified through electronic health records in a US integrated healthcare delivery system. The effectiveness outcome was a composite of IBD-related surgery, IBD-related emergency room visit, and IBD-related hospitalization within 12 months of initiation. Safety outcomes included incidence of any or serious infection, cancer, acute liver dysfunction, and tuberculosis. We used a non-inferiority test with an upper-limit margin of 10% to analyze effectiveness. Doubly robust methods incorporating Cox proportional hazard regression with standardized inverse probability of treatment weighting were used to analyze both effectiveness and safety outcomes.
Results
The composite effectiveness outcome occurred in 107 of 870 patients (12.3%) in the infliximab-dyyb and 379 of 2336 patients (16.2%) in the infliximab groups. Infliximab-dyyb was non-inferior (P < .01) and was not different (hazard ratio [HR] 0.81; confidence interval [CI] 0.65–1.01; P = .06) to infliximab. Safety outcomes were not different between infliximab-dyyb and infliximab for any infections (HR 1.01; CI 0.86–1.17; P = .95), serious infections (HR 0.83; CI 0.54–1.26; P = .38), cancers (HR 0.83; CI 0.44–1.54; P = .55), and tuberculosis (HR 0.59; CI 0.10–3.55; P = .57).
Conclusions
Initiation of infliximab-dyyb was non-inferior to infliximab among biologic-naive patients with IBD in an US integrated healthcare delivery system.
Keywords: biosimilar, infliximb-dyyb, inflammatory bowel disease
Graphical Abstract
Introduction
Biosimilars have the promise of substantially reducing costs of and improving access to biologic therapy. They are biological products that have no clinically meaningful differences in terms of safety, purity, and potency to their reference product.1 The United States’ (US) Food and Drug Administration (FDA) approved Inflectra (infliximab-dyyb), a biosimilar for reference product Remicade (infliximab), in April 2016.2–4 Infliximab-dyyb received approval for ulcerative colitis (UC) and Crohn’s disease (CD), collectively known as inflammatory bowel disease (IBD), through a process called extrapolation. Extrapolation allows a biosimilar to be approved for its reference product’s indications without having clinical trials for every indication, provided it is equivalent to at least one of the reference product’s indications.1 In the case of infliximab-dyyb, approval for IBD was extrapolated based on data from clinical trials in ankylosing spondylitis and rheumatoid arthritis.5,6
Despite the FDA’s oversight of biosimilar introduction, there has been slow uptake of them in the United States. Key concerns for clinicians and patients regarding the use of biosimilars include safety, effectiveness, and persistence, particularly for the indications where extrapolated data have been used to gain FDA approval.7,8 Real-world evidence (ie, data collected during routine clinical practice) of biosimilar use may address these concerns by filling data gaps for extrapolated indications, such as IBD.
The United States has a sizeable population of patients with IBD, but little data on biosimilar use have been published. Kaiser Permanente (KP), a large integrated healthcare delivery system in the United States, has treated patients with IBD with infliximab-dyyb since its approval in 2016; thus, it has extensive data on IBD treatment with infliximab-dyyb and infliximab. The aim of this study is to compare the effectiveness and safety with infliximab-dyyb and infliximab in biologic-naive patients with IBD to provide real-world evidence for patients and clinicians.
Materials and Methods
Study Design and Setting
We conducted a retrospective, multicenter cohort study. Data were obtained from 3 KP regions: Colorado, Northern California, and Southern California. KP owns 39 hospitals and serves over 12.2 million members across 8 states and the District of Columbia.9 All sites use the same electronic health record (EHR) (HealthConnect, Epic Systems Corporation) that provides e-prescribing capabilities and interfaces with the internal pharmacy and laboratory systems. KP operates outpatient infusion centers where patients receive intravenous infusions, where detailed information about each infusion is captured electronically in the EHR. Coded and free-text medical, pharmacy, laboratory, emergency department, hospitalization, and membership information from the EHR, as well as from other contracted and affiliated facilities, are captured in KP’s harmonized administrative and claims databases. The study was approved by the Institutional Review Boards at all participating sites (KP Colorado: CO-17-2472, KP Northern California: CN-17-3003, and KP Southern California: 11385) with a waiver for informed consent due to the retrospective nature of the study.
Study Population
Patients aged ≥18 years with IBD (ie, UC or CD) who were biologic-naive and initiated biologic treatment with either infliximab-dyyb or infliximab between January 1, 2013 and September 30, 2018 were eligible for the study. The first infusion date of either infliximab-dyyb or infliximab was termed as the index date. IBD was defined as an inpatient or outpatient encounter with an International Classification of Disease Clinical Modification (ICD) ninth edition code of 555.x and 556.x or tenth edition code of K50.x and K51.x within 12 months prior to the index date. If a patient had both a CD and UC diagnosis, the patient was categorized based on the first occurring diagnosis. Biologic-naive was defined as no record of adalimumab, certolizumab, golimumab, infliximab, natalizumab, ustekinumab, or vedolizumab administration within 12 months prior to the index date. In addition, patients were required to have had ≥12 months of continuous health plan enrollment prior to the index date. Patients were followed for 12 months, until the termination of health plan membership, or death, whichever occurred first. Patients who switched from infliximab to infliximab-dyyb during follow-up were excluded due to non-medical switching, which was happening across the health system when infliximab-dyyb was available. These switches may not have been due to an intolerance or failure to infliximab.
Study Outcomes
The primary effectiveness outcome was a composite of IBD-related surgery, emergency room (ER) visit, or hospitalization.10 IBD-related surgeries included colectomy, colostomy, hemicolectomy, ileostomy, ileocolectomy, and proctocolectomy. IBD-related ER visits and hospitalizations were identified by a primary diagnosis of UC or CD for the encounter. The safety outcomes included: ≥1 clinical encounter for any infection, ≥1 clinical encounter for serious infection that required hospitalization, new cancer diagnosis, ≥1 acute liver dysfunction diagnosis, and new tuberculosis diagnosis within the 60 days after the last infusion of index drug (diagnosis codes found in Supplementary Table S1).11–14
The secondary outcome was switching, which was defined as starting of tofacitinib, a different biologic (adalimumab, certolizumab, golimumab, natalizumab, ustekinumab, or vedolizumab), or infliximab for patients whose index therapy was infliximab-dyyb.
Data Collection
Integrated electronic medical, pharmacy, and membership administrative records were used to identify patients, treatments, and outcomes for this study. Baseline characteristics of patients were collected using the data prior to the index date, including age at the index date, gender, race, Charlson comorbidity index (CCI), IBD medication history, IBD disease duration, IBD-related surgical history, IBD-related ER visit history, and hospitalization history. The history of IBD medication including aminosalicylates (mesalamine and sulfasalazine), oral budesonides, immunomodulators (azathioprine, mercaptopurine, and methotrexate), and systemic corticosteroids was obtained from pharmacy dispensing records during the 180 days prior to the index date. IBD disease duration was calculated from the date of first IBD diagnose recorded in the KP system to the index date. IBD-related surgical history was ascertained during the 24 months prior to the index date. IBD-related ER visit history and hospitalization history were extracted during the 180 days prior to the index date. The Quan adaptation of CCI15 was applied in the study. Each specific comorbidity used in the calculation of CCI was identified from the diagnosis records during the 1 year prior to the index date. Outcomes were identified from inpatient, emergency, and outpatient administrative and claims databases during the follow-up period.
Data Analysis
Based on our prior study,10 assuming an outcome rate of 10% for the infliximab-dyyb group and 17% for the infliximab group, with an absolute upper-limit non-inferiority margin of 10%, 50 patients would be needed in each group to reach 80% power at a 1-sided alpha level of 0.05. This more stringent non-inferiority margin was selected as a 10% margin is closer to a clinically acceptable difference, as compared to the 15%–20% used in most prospective trials.16–18
Baseline characteristics were reported as means with SD, medians with interquartile range, or frequencies with percentage, and were compared between the 2 groups using chi-squared or Fisher’s exact tests for categorical values and Student’s t-tests or Wilcoxon rank-sum tests for continuous values. Non-inferiority test with an upper-limit margin of 10% was used for the analysis of the effectiveness outcome only, as the FDA did not recommend or provide guidance on safety outcomes using non-inferiority analyses.18
Cox proportional hazards regression with a doubly robust approach were used to analyze all outcomes. To adjust for differences in baseline patient characteristics (Table 1), a propensity score of receiving infliximab-dyyb was calculated for each patient using multivariate logistic regression.19 Stabilized inverse probability of treatment weighting (sIPTW) were generated using propensity scores.20 To evaluate the balance among all baseline covariates between groups after applying sIPTW, standardized differences were calculated and differences less than 10% were considered balanced. Adjusted hazard ratios (HRs) were reported using Cox proportional hazards regression with the sIPTW cohort. All analyses were conducted using SAS statistical software version 9.4 (SAS Institute) and the alpha was set at 0.05.
Table 1.
Baseline characteristics of patients by biosimilar use status and overall
| Characteristic | Infliximab-dyyb (n = 870) | Infliximab (n = 2336) | Overall (n = 3206) | P |
|---|---|---|---|---|
| Age, mean (SD), y | 44.7 (16.6) | 44.1 (17.0) | 44.3 (16.9) | .28 |
| Female, No. (%) | 417 (47.9) | 1199 (51.3) | 1616 (50.4) | .09 |
| Race, No. (%) | .40 | |||
| White | 559 (64.3) | 1514 (64.8) | 2073 (64.7) | |
| Black | 73 (8.4) | 240 (10.3) | 313 (9.8) | |
| Hispanic | 123 (14.1) | 290 (12.4) | 413 (12.9) | |
| Asian | 69 (7.9) | 174 (7.4) | 243 (7.6) | |
| Other | 46 (5.3) | 118 (5.1) | 164 (5.1) | |
| CCI score, No. (%) | <.01 | |||
| 0 | 578 (66.4) | 1344 (57.5) | 1922 (60.0) | |
| 1 | 184 (21.1) | 625 (26.8) | 809 (25.2) | |
| 2 or higher | 108 (12.4) | 367 (15.7) | 475 (14.8) | |
| IBD type, No. (%) | .59 | |||
| Crohn’s disease | 387 (44.5) | 1064 (45.5) | 1451 (45.3) | |
| Ulcerative colitis | 483 (55.5) | 1272 (54.5) | 1755 (54.7) | |
| IBD disease duration, median (IQR), y | 2.1 (0.4–7.3) | 3.1 (0.7–8.6) | 2.8 (0.6–8.3) | <.01 |
| IBD-related surgeries within past 2 years, No. (%) | 156 (17.9) | 264 (11.3) | 420 (13.1) | <.01 |
| IBD-related ER visits within past 6 months, No. (%) | 85 (9.8) | 191 (8.2) | 276 (8.6) | .15 |
| IBD-related hospitalizations within past 6 months, No. (%) | 152 (17.5) | 444 (19.0) | 596 (18.6) | .32 |
| IBD medication history, No. (%) | ||||
| Aminosalicylates | 486 (55.9) | 1215 (52.0) | 1701 (53.1) | .05 |
| Budesonide | 143 (16.4) | 287 (12.3) | 430 (13.4) | <.01 |
| Immunomodulators | 618 (71.0) | 1533 (65.6) | 2151 (67.1) | <.01 |
| Corticosteroids | 499 (57.4) | 1113 (47.6) | 1612 (50.3) | <.01 |
| Dose of infliximab at index date, mean (SD), mg | 428 (138) | 421 (143) | 423 (141) | .01 |
Abbreviations: CCI, Charlson comorbidity index; ER, emergency room; IBD, inflammatory bowel disease; IQR, interquartile range; y, year.
Results
Patient Characteristics
Among 8615 patients who received infliximab-dyyb or reference product infliximab, 3206 biologic-naive patients with IBD met inclusion and exclusion criteria: 870 (27.1%) patients and 2336 (72.9%) patients began treatment with infliximab-dyyb and infliximab between January 2013 and September 2018, respectively. The mean age of the cohort was 44.3 years (SD 16.9 years) and women comprised of half of the cohort. Approximately half (54.7%) of the patients had a diagnosis of UC. The infliximab-dyyb group had a shorter median IBD disease duration (P < .01) and higher burden of chronic disease (P < .01), and was more likely to have had a history of IBD-related surgeries (P < .01), and an IBD-related medication including oral budesonide (P < .01), immunomodulators (P < .01), and corticosteroids (P < .01) (Table 1). After sIPTW weighting, all the standardized mean differences were below 0.1, so the baseline characteristics were considered balanced between the groups (Table 2).
Table 2.
Patient demographics in original and stabilized inverse probability of treatment weighting cohorts
| Demographic | Before sIPTW | After sIPTW | ||||
|---|---|---|---|---|---|---|
| IFX-dyyb (n = 870) | IFX-RP (n = 2336) | Standardized difference, % | IFX-dyyb (n = 860) | IFX-RP (n = 2341) | Standardized difference, % | |
| Age, mean (SD), y | 0.06 | 0.04 | ||||
| ≤34 | 287 (33) | 832 (35.6) | 825 (35.2) | 287 (33.4) | ||
| 35–55 | 301 (34.6) | 808 (34.6) | 812 (34.7) | 300 (34.8) | ||
| ≥56 | 282 (32.4) | 696 (29.8) | 704 (30.1) | 273 (31.8) | ||
| Female, n (%) | 417 (47.9) | 1199 (51.3) | −0.06 | 429 (49.9) | 1181 (50.4) | −0.04 |
| Race, n (%) | 0.08 | 0.03 | ||||
| White | 559 (64.3) | 1514 (64.8) | 556 (64.7) | 1513 (64.6) | ||
| Black | 73 (8.4) | 240 (10.3) | 82 (9.5) | 228 (9.7) | ||
| Hispanic | 123 (14.1) | 290 (12.4) | 116 (13.5) | 304 (13.0) | ||
| Asian | 69 (7.9) | 174 (7.4) | 66 (7.7) | 177 (7.6) | ||
| Other | 46 (5.3) | 118 (5.1) | 40 (4.6) | 118 (5.0) | ||
| Region, n (%) | 0.22 | 0.04 | ||||
| Colorado | 127 (14.6) | 190 (8.1) | 88 (10.3) | 233 (10.0) | ||
| Northern California | 409 (47.0) | 1085 (46.4) | 418 (48.6) | 1096 (46.8) | ||
| Southern California | 334 (38.4) | 1061 (45.4) | 354 (41.1) | 1011 (43.2) | ||
| CCI score ≥1, n (%) | 292 (33.6) | 992 (42.5) | −0.18 | 340 (39.6) | 935 (39.9) | −0.01 |
| IBD disease duration, n (%) | −0.18 | −0.01 | ||||
| <2 years | 431 (49.5) | 953 (40.8) | 379 (44.1) | 1015 (43.4) | ||
| ≥2 years | 439 (50.5) | 1383 (59.2) | 481 (55.9) | 1326 (56.6) | ||
| IBD type, n (%) | 0.01 | 0.04 | ||||
| Crohn’s disease | 387 (44.5) | 1064 (45.5) | 391 (45.5) | 1061 (45.3) | ||
| Ulcerative colitis | 483 (55.5) | 1272 (54.5) | 469 (54.5) | 1280 (54.7) | ||
| IBD-related surgeries within past 2 years, n (%) | 156 (17.9) | 264 (11.3) | 0.19 | 116 (13.4) | 310 (13.2) | 0.01 |
| IBD-related ER visits within past 6 months, n (%) | 85 (9.8) | 191 (8.2) | 0.06 | 76 (8.8) | 203 (8.7) | 0.01 |
| IBD-related hospitalizations within past 6 months, n (%) | 152 (17.5) | 444 (19.0) | −0.04 | 163 (18.9) | 434 (18.5) | 0.01 |
| Medication history, n (%) | ||||||
| Budesonide | 143 (16.4) | 287 (12.3) | 0.12 | 123 (14.3) | 317 (13.6) | 0.02 |
| Corticosteroids | 499 (57.4) | 1113 (47.6) | 0.20 | 442 (51.3) | 1182 (50.5) | 0.02 |
Abbreviations: CCI, Charlson comorbidity index; ER, emergency room; IBD, inflammatory bowel disease; IFX-dyyb, infliximab-dyyb; IFX-RP, infliximab reference product; sIPTW, stabilized inverse probability of treatment weighting; y, year.
Outcomes
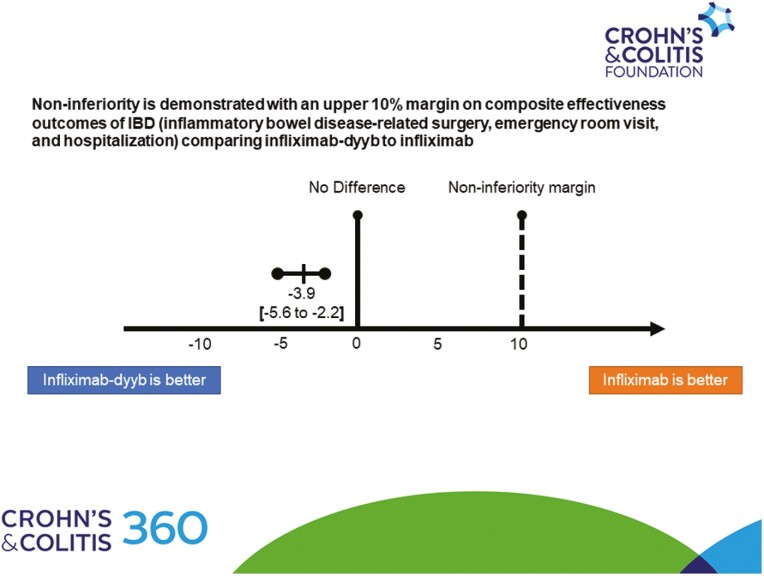
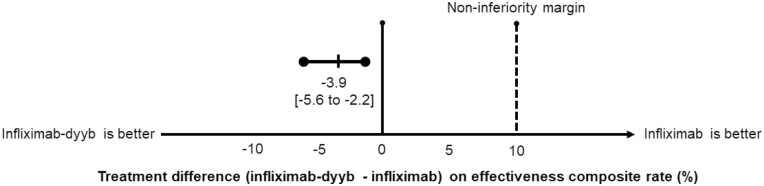
The mean follow-up time for the cohort was 329 days (SD 82 days) and 78.8% of the patients were followed for 12 months. The composite effectiveness rate of IBD-related surgery, ER visit, and hospitalization was non-inferior in the 2 groups; occurring in 107 (12.3%) and 379 (16.2%) patients in the infliximab-dyyb and infliximab groups, respectively (P < .01 for non-inferiority) (Figure 1). There was no statistically significant difference in the risk of the composite outcome between the groups (HR 0.81; 95% confidence interval [CI] 0.65–1.01; P = .06). There were 906 (28.3%) patients who had an encounter for any infection; of which 126 (3.9%) were serious infections (Table 3). In addition, there were 60 (1.9%) cases of cancer, 3 (0.1%) acute liver dysfunctions, and 8 (0.2%) cases of tuberculosis. There were no statistically significant differences in the risks for any infections (HR 1.01; 95% CI 0.86–1.17; P = .95), serious infections (HR 0.83; 95% CI 0.54–1.26; P = .38), cancers (HR 0.83; 95% CI 0.44–1.54; P = .55), or tuberculosis (HR 0.59; 95% CI 0.10–3.55; P = .57). As there were no cases of acute liver dysfunction in the infliximab-dyyb group, adjusted analysis was not performed. There was a higher rate of switching from infliximab therapy to another biologic among patients in the infliximab-dyyb than infliximab group (21.0% vs 15.2%; HR 1.55; CI 1.30–1.85; P < .01).
Figure 1.
Effectiveness composite outcome (composite of inflammatory bowel disease-related surgery, emergency room visit, and hospitalization). The dashed vertical line represents the upper-limit non-inferiority margin of 10%.
Table 3.
Safety outcomes by biosimilar status
| Outcome, n (%) | Infliximab-dyyb (n = 870) | Infliximab (n = 2336) | Adjusted HR (95% CI)a | P |
|---|---|---|---|---|
| Any infection | 219 (25.2) | 687 (29.4) | 1.01 (0.86–1.17) | .95 |
| Serious infectionb | 27 (3.1) | 99 (4.2) | 0.83 (0.54–1.26) | .38 |
| Cancer | 11 (1.3) | 49 (2.1) | 0.83 (0.44–1.54) | .55 |
| Acute liver dysfunction | 0 (0) | 3 (0.1) | —c | —c |
| Tuberculosis | 1 (0.1) | 7 (0.3) | 0.59 (0.10–3.55) | .57 |
Abbreviations: CCI, Charlson comorbidity index; CI, confidence interval; ER, emergency room; HR, hazard ratio; IBD, inflammatory bowel disease.
aModels are adjusted for age, gender, race, CCI, IBD disease duration, IBD type, Kaiser Permanente region, IBD-related surgical history, IBD-related ER visit history, IBD-related hospitalization history, budesonide exposure, and corticosteroid exposure.
bSerious infection was defined as an infection requiring hospitalization.
cAnalysis was not performed due to lack of events in infliximab-dyyb group.
Discussion
This retrospective, longitudinal, multicenter, cohort study of over 3200 patients with IBD who were naive to biologic treatment identified that the biosimilar infliximab-dyyb was non-inferior to the reference product infliximab in the composite effectiveness outcome. In addition, we identified no differences between the groups in safety outcomes using doubly robust statistical analyses. We also report that patients who received infliximab-dyyb had a higher likelihood of switching to another biologic. These findings are important as they provide real-world evidence of infliximab-dyyb’s effectiveness and safety in the United States, a country with slow biosimilar uptake in contrast to more widespread use in Asia and Europe. This further supports the extrapolation review process that the FDA uses to approve biosimilars, and that biosimilars can be effective, safe, and cost-saving biologic therapies in the United States.
While studies have compared biosimilar infliximab to reference product infliximab, these studies are limited and their study populations and assessed outcomes differ from ours.16,21–23 Ye et al conducted a small (N = 214), international RCT comparing the infliximab-dyyb, also known as Remsima (CT-P13) to reference product infliximab in biologic-naive patients with CD.16 They reported infliximab-dyyb’s non-inferior efficacy and safety to infliximab based on results from the Crohn’s Disease Activity Index, Short Inflammatory Bowel Disease Questionnaire, and adverse events. Their study did not include patients with UC or report healthcare encounter effectiveness outcomes and their rigid inclusion/exclusion criteria precluded patients with various comorbidities from the study; thus, limiting the real-world applicability of their results. The PROSIT-BIO observational study of a mix of biologic-naive and non-naive patients with IBD from Italy reported that infliximab-dyyb was effective (based on clinical remission/response) and safe when compared to rates for infliximab previously reported.21 This study did not have a comparison group, had short follow-up (24 weeks), and did not assess healthcare encounter effectiveness outcomes.
Meyer et al assessed infliximab-dyyb and reference product infliximab in infliximab-naive patients with CD in a large, retrospective cohort study conducted in France.22 Based on a composite outcome of death, CD-related surgery, or all-cause hospitalization, infliximab-dyyb was reported to be equivalent for effectiveness; in addition, it reached statistical significance for a lower risk (HR 0.92; 95% CI 0.85–0.98). Safety outcomes were equivalent between the groups. Meyer et al also assessed infliximab-dyyb and reference product infliximab in infliximab-naive patients with UC.23 They reported similar equivalency results; however, the infliximab-dyyb group did not reach statistical significance for the composite outcome (HR 1.04; 95% CI 0.94–1.15) but did for a serious infections safety outcome (HR 0.65; 95% CI 0.48–0.88). These study populations were drawn from patients who received their infusions from hospitals in France and did not use doubly robust statistical methods to balance their groups, leaving safety and efficacy assessed with vigorous methods in a purely US community-based settings unanswered.22,23 Our study narrows the evidence gap of a real-world assessment within the United States to provide data on the effectiveness and safety of infliximab-dyyb in a community practice setting with biologic-naive patients with IBD that differ from those reported in clinical trials or health systems within other countries.
Our rates of any infection and serious infection ranged from 25.2% to 29.4% and 3.1% to 4.2%, respectively. The rates of any infection for patients taking infliximab-dyyb among biologic-naive patients with IBD have not been evaluated in any real-world studies; however, our observations were consistent with the clinical trials for the infliximab in IBD which have shown the any infection rate ranged from 18.5% to 48.3%.24 Similar rates of serious infections were reported in real-world studies of non-biologic-naive patients with IBD ranging from 2.1% to 6.5%.22,23
The rates of biosimilar and reference product infliximab switching to another biologic were higher numerically in our study than those reported in the Meyers’ studies.22,23 However, other studies have reported that approximately 9%–20% of patients initiating infliximab switched to another biologic in the first year, which was broadly similar to our results.25,26 We hypothesize that this may be due, in part, to how switches were defined and other trends in management during the study period. First, we might have inflated the switch rate for the infliximab-dyyb group by including switching from infliximab-dyyb to infliximab, as we cannot distinguish the reasons as treatment failure or nocebo effect, the latter defined as a switch mediated by of a negative belief about a therapy (eg, biosimilar).27,28 A nocebo effect is unlikely to fully explain the observed difference as only 7% of the infliximab-dyyb patients who switched therapy received infliximab as the subsequent therapy. Second, higher switch rates may be related to the growing number of treatment options throughout the study period, thus patients started on infliximab-dyyb (all from after 2016) had other treatment options besides dose optimization or switch to a different TNF-inhibitor.3,29–34 Regardless, our real-world data highlight that higher rates of switches in biologic-naive patients who initiate biosimilar therapy may be observed. Future studies are warranted to understand specific reasoning for switching.
Our study exhibits several strengths. It had a large patient sample and was conducted in an integrated healthcare delivery system, which allowed for the opportunity to reliably ascertain all healthcare services (ie, clinic-administered medications, outpatient medication dispenses, hospitalizations, and surgeries for patients across 3 different geographic regions within the United States). All data were captured using uniform approaches from electronic databases. We limited the study cohort to biologic-naive patients to limit confounding arising from treatment with prior biologics. Lastly, we identified multiple safety outcomes including any and serious infections, cancers, acute liver dysfunction, and tuberculosis to strengthen our findings.
Our study also has limitations. Since the 2 cohorts were drawn from different time periods, residual confounding owing to practice changes may have influenced which patients received infliximab at the outset. To address this, we utilized propensity scores with sITPW to adjust for any imbalances between groups. Second, we identified IBD-related ER visits, hospitalizations, or surgeries as a proxy for disease worsening, which may have variability in the quality and reliability of associated coding. However, both groups were subject to same type of identification and this limitation should not bias 1 group over the other. Since we were unable to conduct chart reviews, we were not able to assess more nuanced markers of disease worsening such as disease activity, radiologic imaging, endoscopic data, nor biomarkers. Third, patients were followed for only 12 months, limiting our ability to identify and compare longer-term outcomes. Additional work is needed to carefully understand longer-term outcomes. Fourth, our results may not be generalizable outside of an insured population within the United States. Finally, we chose to analyze the primary effectiveness outcome on an intent-to-treat basis, not censoring patients at date of infliximab therapy discontinuation; thus, our findings likely represent a conservative estimate of the outcomes.
Conclusions
Our findings suggest that the real-world effectiveness of infliximab-dyyb is non-inferior to infliximab in biologic-naive patients with IBD within 12 months of initiation. Our findings support the extrapolation review process to approve biosimilars and that biosimilars can be effective and safe cost-saving biologic therapies in the United States.
Supplementary Material
Acknowledgments
We thank Gabriel Escobar, MD, Sameer Awsare, MD, Tracy Lieu, MD, and Douglas Corley, MD, PhD for their help in reviewing the final manuscript.
Funding
J.T.S. is supported by funding from The Permanente Medical Group Delivery Science Fellowship Program.
Authors’ Contributions
J.T.S., F.S.V., V.L., and R.L.H. contributed to the study concept and design. All authors contributed to the acquisition, analysis, or interpretation of data. J.T.S., F.S.V., T.D., F.N., and R.L.H. contributed to the drafting of the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content. J.T.S., F.N., and R.L.H. contributed to the statistical analysis. V.L. and K.L. contributed to administrative, technical, or material support. R.L.H. supervised the study. F.N. and R.L.H. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval
This study was reviewed and approved by the Institutional Review Boards of Kaiser Permanente Colorado, Northern California, and Southern California.
Informed Consent
Informed consent was waived for the study.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
None declared.
Data Availability
Data not publicly available.
References
- 1. U.S. Food and Drug Administration. Biosimilar development, review, and approval.2017. Accessed October 12, 2020. https://www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval
- 2. U.S. Food and Drug Administration. FDA approves Inflectra, a biosimilar to Remicade. Accessed October 12, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-inflectra-biosimilar-remicade
- 3. Prescribing information for Inflectra®. Pfizer Labs; 2019. Accessed October 12, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125544s009lbl.pdf [Google Scholar]
- 4. Prescribing information for Remicade®. Janssen Biotech; 2013. Accessed October 12, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf [Google Scholar]
- 5. Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarnola K, Merikoski M, Jyrkkä J, Hämeen-Anttila K. Physicians’ perceptions of the uptake of biosimilars: a systematic review. BMJ Open. 2020;10(5):e034183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehr S. An interesting comparison: the latest data on US and EU biosimilar uptake. Accessed October 12, 2020. https://biosimilarsrr.com/2020/04/23/an-interesting-comparison-the-latest-data-on-us-and-eu-biosimilar-uptake/
- 9. Kaiser Permanente. Fast facts | Kaiser Permanente. Accessed October 12, 2020. https://about.kaiserpermanente.org/who-we-are/fast-facts
- 10. Ho SL, Niu F, Pola S, Velayos FS, Ning X, Hui RL. Effectiveness of switching from reference product infliximab to infliximab-dyyb in patients with inflammatory bowel disease in an integrated healthcare system in the United States: a retrospective, propensity score-matched, non-inferiority cohort study. BioDrugs. 2020;34(3):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore N, Duret S, Grolleau A, et al. Previous drug exposure in patients hospitalised for acute liver injury: a case-population study in the French National Healthcare Data System. Drug Saf. 2019;42(4):559–572. [DOI] [PubMed] [Google Scholar]
- 12. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318(17):1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155(2):337–346.e10. [DOI] [PubMed] [Google Scholar]
- 14. Winthrop KL, Baxter R, Liu L, et al. The reliability of diagnostic coding and laboratory data to identify tuberculosis and nontuberculous mycobacterial disease among rheumatoid arthritis patients using anti-tumor necrosis factor therapy. Pharmacoepidemiol Drug Saf. 2011;20(3):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53(9):e65–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ye BD, Pesegova M, Alexeeva O, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019;393(10182):1699–1707. [DOI] [PubMed] [Google Scholar]
- 17. Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–2316. [DOI] [PubMed] [Google Scholar]
- 18. U.S. Food and Drug Administration. Non-inferiority clinical trials to establish effectiveness.2016. Accessed October 12, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/non-inferiority-clinical-trials
- 19. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983; 70(1):41–55. [Google Scholar]
- 20. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiorino G, Manetti N, Armuzzi A, et al. The PROSIT-BIO cohort: a prospective observational study of patients with inflammatory bowel disease treated with infliximab biosimilar. Inflamm Bowel Dis. 2017;23(2):233–243. [DOI] [PubMed] [Google Scholar]
- 22. Meyer A, Rudant J, Drouin J, et al. Effectiveness and safety of reference infliximab and biosimilar in Crohn disease: a French equivalence study. Ann Intern Med. 2019;170(2):99–107. [DOI] [PubMed] [Google Scholar]
- 23. Meyer A, Rudant J, Drouin J, et al. The effectiveness and safety of infliximab compared with biosimilar CT-P13, in 3112 patients with ulcerative colitis. Aliment Pharmacol Ther. 2019;50(3): 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah ED, Farida JP, Siegel CA, Chong K, Melmed GY. Risk for overall infection with anti-TNF and anti-integrin agents used in IBD: a systematic review and meta-analysis. Inflamm Bowel Dis. 2017;23(4):570–577. [DOI] [PubMed] [Google Scholar]
- 25. Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis. 2019;25(8):1417–1427. [DOI] [PubMed] [Google Scholar]
- 26. Rubin DT, Mody R, Davis KL, Wang CC. Real-world assessment of therapy changes, suboptimal treatment and associated costs in patients with ulcerative colitis or Crohn’s disease. Aliment Pharmacol Ther. 2014;39(10):1143–1155. [DOI] [PubMed] [Google Scholar]
- 27. Fleischmann R, Jairath V, Mysler E, Nicholls D, Declerck P. Nonmedical switching from originators to biosimilars: does the nocebo effect explain treatment failures and adverse events in rheumatology and gastroenterology? Rheumatol Ther. 2020;7(1):35–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Colloca L, Panaccione R, Murphy TK. The clinical implications of nocebo effects for biosimilar therapy. Front Pharmacol. 2019;10(1372):1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prescribing information for Humira®. Abbott Laboratories; 2007. Accessed October 12, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/125057s089Lbl.pdf [Google Scholar]
- 30. Prescribing information for Cimzia®. UBC; 2017. Accessed October 12, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125160s270lbl.pdf [Google Scholar]
- 31. Prescribing information for Simponi®. Janssen Biotech; 2011. Accessed October 12, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf [Google Scholar]
- 32. Prescribing information for Stelara®. Janssen Biotech; 2016. Accessed October 12, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf [Google Scholar]
- 33. Prescribing information for Entyvio®. Takeda Pharmaceuticals America; 2014. Accessed October 12, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125476s000lbl.pdf [Google Scholar]
- 34. Prescribing information for Xeljanz®. Pfizer Labs; 2018. Accessed October 12, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data not publicly available.