Abstract
Background
Bowel urgency is commonly experienced by patients with ulcerative colitis (UC) and is associated with reduced health-related quality of life (QoL). Mirikizumab, a humanized monoclonal antibody directed against the p19 subunit of IL-23, significantly reduced bowel urgency in a double-blind, randomized, placebo-controlled Phase 2 clinical trial in patients with moderate-to-severe UC (NCT02589665).
Methods
All patients (N = 249) reported symptoms including absence or presence of bowel urgency. Absence of urgency was defined as no urgency for the 3 consecutive days prior to each scheduled visit. Missing urgency data were imputed as present. After 12 weeks of induction treatment, patients who achieved clinical response continued maintenance mirikizumab treatment through Week 52. We assessed the relationship of urgency with QoL, clinical outcomes, and inflammatory biomarkers at Weeks 12 and 52.
Results
Patients with absence of urgency demonstrated significantly greater improvement in Inflammatory Bowel Disease Questionnaire (IBDQ) scores even after adjusting for rectal bleeding (RB) and stool frequency (SF), significantly higher rates of all clinical outcomes at Weeks 12 and 52, and a greater decrease in inflammatory biomarkers C-reactive protein and fecal calprotectin compared to those with presence of urgency. Absence of urgency at Week 12 was associated with improved IBDQ scores at Week 52, while Week 12 RB or SF status was not.
Conclusions
Absence of urgency is strongly associated with improvement in QoL as well as clinical measures of UC disease activity. These findings suggest urgency may be a useful surrogate marker of disease activity and an important treatment target for UC.
Keywords: bowel urgency, mirikizumab, IBD
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon and rectum, with mucosal inflammation leading to typical symptoms of diarrhea, rectal bleeding (RB), and bowel urgency.1 While patients often experience all of these concurrently, bowel urgency is a distinct and highly disruptive symptom that is frequently overlooked by healthcare providers2–5 or conflated with stool frequency (SF) or diarrhea. SF refers specifically to the number of trips to the bathroom that involve the passage of stool, mucus, and/or blood over a given period of time6 and diarrhea refers to loose, watery stools.6,7 Bowel urgency, on the other hand, refers to the sudden and immediate need to have a bowel movement.8,9 The conflation of these terms can lead to confusion on the part of both healthcare providers and patients, and mask the impact of bowel urgency on patients with UC.
More than 75%–90% of patients with UC report experiencing bowel urgency.2,9–11 Up to half of patients with UC report at least 1 instance of bowel urgency per day.2,12 Patients report that bowel urgency is an important symptom of UC, distinct from SF and RB.13 In fact, in a recent survey which included both patients and healthcare providers, bowel urgency was ranked most relevant among a list of attributes for inflammatory bowel disease (IBD) treatment decisions,14 and patients with UC consider bowel urgency to be a more relevant and important symptom than RB or SF.14,15 The mechanism(s) underlying bowel urgency have not been fully elucidated; however, bowel urgency has been associated with chronic inflammation, which causes changes in smooth muscle tone, hypersensitivity, and increased contractile responses in the rectum, as well as the development of submucosal fibrosis.16,17 Despite the prevalence of bowel urgency and its importance to patients with UC, there is a dearth of information on the relationship of this symptom to quality of life (QoL) and clinical outcomes, and bowel urgency has not been an outcome typically included in clinical trials.
Mirikizumab (LY3074828) is a humanized immunoglobulin G4 (IgG4)–variant monoclonal antibody that specifically binds to the p19 subunit of IL-23, and thus does not target IL-12, which shares a common p40 subunit with IL-23. Mirikizumab has demonstrated clinical efficacy in psoriasis,18 Crohn’s disease,19 and UC,20,21 and was shown to significantly reduce the presence of bowel urgency in patients with UC.22 We examined the relationship between bowel urgency with QoL, clinical outcomes, and inflammatory biomarkers in a Phase 2 trial of mirikizumab in patients with moderately to severely active UC (NCT02589665).
Methods
Study Design and Participants
Urgency was assessed as part of a multicenter, randomized, double-blind, parallel-arm, placebo-controlled trial which has been reported previously20 (see Supplementary Figure 1 for study design and Supplementary Figure 2 for CONSORT diagram). The trial was conducted at 75 sites in 14 countries. Patients were enrolled from January 2016 to September 2017. A complete list of inclusion and exclusion criteria can be found in the Supplementary Methods.
This study was compliant with the International Conference on Harmonization (ICH) guideline on good clinical practice. All informed consent forms and protocols were approved by appropriate ethical review boards prior to initiation of the study. All patients gave written informed consent prior to receiving study drug.
Procedures
Patients reported daily symptoms in an electronic diary, including patient-assessed bowel urgency status (absence or presence of bowel urgency).
Induction treatment consisted of 50, 200, or 600 mg of mirikizumab or PBO administered IV in a 1:1:1:1 ratio at Weeks 0, 4, and 8. At Week 12, patients receiving mirikizumab who achieved clinical response (a decrease in 9-point modified Mayo score [RB, SF, and endoscopy] inclusive of ≥2 points and ≥35% from baseline with either a decrease of RB subscore of ≥1 or an RB subscore of 0 or 1) were eligible to enter the maintenance period, where they were re-randomized to receive mirikizumab 200 mg subcutaneously (SC) every 4 weeks (Q4W) or every 12 weeks (Q12W). To maintain the blindedness of the study, patients who received PBO in the induction period and achieved clinical response at Week 12 continued to receive PBO SC Q4W in a treat-through study design.
Outcomes
The primary outcome measure for this supplementary study was patient-reported bowel urgency status. Absence of urgency at baseline, Week 12, and Week 52 was defined as reporting no bowel urgency for the 3 consecutive days prior to each scheduled visit as recorded in a daily diary, regardless of bowel urgency status at baseline. If a patient did not report their urgency status for all 3 days prior to a visit date, their urgency status was considered missing. There were 7 patients (2.8%) with missing urgency data at Week 12, and 5 patients (4.7%) with missing urgency data at Week 52. These patients were imputed using nonresponder imputation (NRI), or as having presence of bowel urgency in our analyses.
Other outcome measures examined in this manuscript included clinical remission (Mayo subscores of 0 for RB, 0 or 1 with 1 point decrease from baseline for SF, and 0 or 1 for centrally read endoscopy); clinical response (a decrease in the 9-point modified Mayo subscore [RB, SF, and endoscopy] inclusive of ≥2 points and ≥35% from baseline with either a decrease of RB subscore of ≥1 or an RB subscore of 0 or 1); endoscopic remission (Mayo endoscopic subscore of 0); endoscopic healing (endoscopic subscore of 0 or 1); the Inflammatory Bowel Disease Questionnaire (IBDQ); 36-Item Short Form Health Survey version 2 (SF-36), fecal calprotectin (fCLP), and C-reactive protein (CRP). SF remission and RB remission outcomes were derived from their respective component of the clinical remission definition. Symptomatic response and remission are comprised of the RB and SF components of clinical response and remission, respectively. See Supplement for a brief description of the Mayo score, its components, and the grading scheme.
The IBDQ is a 32-item patient-reported questionnaire that measures 4 aspects of subjects’ lives: symptoms directly related to the primary bowel disturbance, systemic symptoms, emotional function, and social function.23 A total IBDQ score of ≥170 points was defined as the threshold for IBDQ remission in the study, with the Minimal Clinically Important Difference (MCID) defined as an improvement of ≥16 points in the total IBDQ score.24
The SF-36 is a 36-item patient-reported measure designed to be a short, multipurpose assessment of health in the domains of physical functioning, role-physical, role-emotional, bodily pain, vitality, social functioning, mental health, and general health.25,26 The 2 overarching components of mental well-being and physical well-being are captured by the Mental and Physical Component Summary scores (MCS and PCS, respectively). A ≥5-point improvement was defined as the MCID for SF-36 PCS and MCS scores in this study. The SF-36 has demonstrated validity and reliability when used in UC patients.25,27,28
Statistical Analysis
The intention-to-treat (ITT) population, which includes all randomized patients, was used to assess outcomes in the induction period through Week 12 regardless of bowel urgency status at baseline. For maintenance period analyses through Week 52, the analysis population consisted of a subset of the ITT population who demonstrated clinical response at Week 12 and were re-randomized to one of the 2 maintenance mirikizumab arms or continued on to subcutaneous placebo. All analyses were conducted by pooling together patients in the ITT population across treatment groups induction period analyses, and patients in the ITT population that experienced clinical response at Week 12 for the maintenance period analyses.
Multivariable linear regression models were used to assess the association between bowel urgency status and change from baseline on QoL measures (total IBDQ and domain scores, SF-36 PCS and MCS) at Week 12, while adjusting for RB and SF severity. Each model included the baseline value of the QoL measure, bowel urgency status at Week 12, the Mayo SF subscore at Week 12, and the Mayo RB subscore at Week 12. Type III tests for the least squares means were used for statistical comparisons between urgency status groups at Week 12. In addition, the Type III coefficient of partial determination was estimated for each term in the model to compare the relative importance of each factor toward improvement in the QoL variables. A similar model using the Week 52 urgency status, RB score, and SF score was used to assess the association between urgency status and change from baseline on QoL measures at Week 52. Modified baseline observation carried forward (mBOCF) was used to impute missing QoL values and Mayo score components: for patients discontinuing due to an adverse event, the baseline observation was carried forward to the corresponding endpoint for evaluation. For patients discontinuing for any other reason, the last nonmissing postbaseline observation before discontinuation was carried forward to the corresponding endpoint for evaluation.
Separate multivariable linear regression models were used to evaluate whether urgency status, SF, or RB at Week 12 were predictors of longer-term improvement on the IBDQ and SF-36 at Week 52. These models included change from baseline on the Week 52 QoL measures as the dependent variable and included the baseline value of the QoL measure, urgency status at Week 12, Mayo SF subscore at Week 12, and Mayo RB subscore at Week 12 as independent variables.
Analysis of covariance (ANCOVA) models were used to compare the change from baseline on inflammatory biomarkers (CRP and fCLP) between bowel urgency status groups at Weeks 12 and 52. Each model included the baseline value of the inflammatory biomarker, urgency status (at Week 12 or 52), geographic region, prior biologic experience, age, and gender. Type III tests for the least squares means were used for statistical comparisons between urgency status groups. mBOCF was used to impute missing inflammatory biomarker values.
Logistic regression models were used to assess the association between achieving clinical remission, response, endoscopic healing, SF remission, and RB remission with urgency status at Weeks 12 and 52. Each logistic regression model included the clinical outcome as the dependent variable and included urgency status (at Week 12 or 52), geographic region, prior biologic experience, age, and gender as independent variables. NRI was used to impute missing clinical outcome variables.
To further assess the association between achieving absence of urgency and improvement in other symptoms (SF and RB), we compared the time to symptomatic remission and response between patients with and without absence of urgency at baseline and Weeks 4, 8, and 12 using Kaplan–Meier curves and log-rank tests.
This study is registered with ClinicalTrials.gov, number NCT02589665.
Role of Funding Source
The funder of the study was involved in the study design, data collection, data analysis, and data interpretation. The study funder provided funding for writing support and editorial assistance with manuscript preparation.
Data Sharing Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Results
Baseline Demographics and Disease Characteristics
Baseline (BL) demographics and disease characteristics were examined across bowel urgency status at Weeks 12 and 52 and included all patients regardless of urgency status at baseline. Bowel urgency was absent in 27/249 (10.8%) of patients at baseline. In general, baseline characteristics, including Mayo score components of SF, RB, and endoscopy, were similar in patients with both absence and presence of urgency at Weeks 12 and 52. Patients who reported absence of urgency at Week 12 had lower mean disease duration (absence of urgency 6.9 ± 5.7 years, presence of urgency 9.0 ± 9.1 years, P = .04), higher mean baseline SF-36 PCS (absence of urgency 43.9 ± 6.8, presence of urgency 40.8 ± 7.3, P = .001) and total IBDQ scores (absence of urgency 133.6 ± 33.7, presence of urgency 121.5 ± 30.2, P = .004) and were less likely to report urgency at baseline (absence of urgency: 79% urgency at BL, presence of urgency: 95% urgency at BL, P = .0001) compared to those reporting urgency at Week 12. Those who achieved absence of urgency at Week 52 had lower mean weight (absence of urgency 71.3 ± 16.6 kg, presence of urgency 78.7 ± 18.5 kg, P = .04) and higher mean total IBDQ scores (absence of urgency 128.9 ± 30.3, presence of urgency 115.7 ± 28.0, P = .03) at baseline compared to those with urgency at Week 52 (Table 1).
Table 1.
Baseline demographics and disease characteristics by bowel urgency status at Weeks 12 and 52.
| Mean (SD) unless otherwise noted | Week 12 bowel urgency status | Week 52 bowel urgency status | ||
|---|---|---|---|---|
| Bowel urgency absent N = 91 |
Bowel urgency present N = 158 |
Bowel urgency absent N = 66 |
Bowel urgency present N = 40 |
|
| Age, years | 41.1 (13.3) | 43.4 (14.2) | 39.6 (12.8) | 41.6 (15.0) |
| Weight, kg | 73.7 (16.7) | 75.7 (16.7) | 71.3 (16.6)* | 78.7 (18.5) |
| Disease duration, years | 6.9 (5.7)* | 9.0 (9.1) | 8.3 (6.8) | 6.9 (6.6) |
| Prior biologic use, n (%) | 46 (51) | 107 (68) | 32 (48) | 22 (55) |
| Concomitant therapies, n (%) | ||||
| 5-ASA | 66 (73) | 118 (75) | 54 (82) | 31 (78) |
| Corticosteroids | 44 (48) | 78 (49) | 28 (42) | 20 (50) |
| Thiopurines | 23 (25) | 46 (29) | 20 (30) | 9 (23) |
| Bowel urgency at BL, n (%) | 72 (79)* | 150 (95) | 59 (89) | 36 (90) |
| Mayo score, n (%) | ||||
| 6–8 | 45 (50) | 59 (38) | 25 (38) | 20 (50) |
| 9–12 | 45 (50) | 98 (62) | 41 (62) | 20 (50) |
| Mayo SF subscore | 2.2 (0.8) | 2.5 (0.7) | 2.4 (0.8) | 2.3 (0.8) |
| Mayo RB subscore | 1.4 (0.9) | 1.4 (0.8) | 1.6 (0.8) | 1.4 (0.8) |
| Mayo endoscopic subscore | 2.7 (0.5) | 2.8 (0.4) | 2.7 (0.5) | 2.7 (0.5) |
| SF-36 | ||||
| Physical Component Score | 43.9 (6.8)* | 40.8 (7.3) | 42.5 (8.3) | 40.7 (6.9) |
| Mental Component Score | 40.4 (10.1) | 38.7 (10.7) | 39.7 (9.6) | 36.8 (10.1) |
| IBDQ total score | 133.6 (33.7)* | 121.5 (30.2) | 128.9 (30.3)* | 115.7 (28.0) |
| CRP, mg/L, median (range) | 3.3 (0.1–164.0) | 5.1 (0.1–77.1) | 3.3 (0.1–84.9) | 6.7 (0.1–164.0) |
| fCLP, mg/kg, median (range) | 1700.5 (15.0–31 680) | 1442.0 (15.0–22 742) | 1576.0 (15.0–31 680) | 1773.0 (99.0–10 241) |
Baseline demographics and disease characteristics at Week 0, grouped by urgency status at Week 12 or 52. Abbreviations: ASA, aminosalicylic acid; BL, baseline; CRP, C-reactive protein; fCLP, fecal calprotectin; IBDQ, Inflammatory Bowel Disease Questionnaire; RB, rectal bleeding; SF, stool frequency; SF-36, Medical Outcomes Study 36-Item Short Form Health Survey Version 2 Standard.
*P < .05 compared to those with presence of urgency at Week 12 or 52.
Associations Between Urgency Status and Improvement in IBDQ
Improvement on the total IBDQ score was significantly greater in patients who achieved absence of urgency at Week 12 compared to those who still had urgency. A similar pattern was observed for the 4 IBDQ subscores, which corresponded to percent changes from BL (% ΔBL) that were more than 2 times greater in patients who reported absence of urgency compared to patients who reported presence of urgency (Supplementary Figure 3). These differences in IBDQ improvement were highly significant between bowel urgency status groups despite adjusting for RB and SF, which suggests that bowel urgency is an independent symptom significantly associated with IBD-related QoL (Table 2).
Table 2.
Improvement in quality of life measures by bowel urgency status, adjusted for rectal bleeding and stool frequency, at Weeks 12 and 52.
| Week 12 | ||||
|---|---|---|---|---|
| Bowel urgency absent N = 91 |
Bowel urgency present N = 156 |
Difference in LSM ΔBL (95% CI) |
P | |
| LSM ΔBL (SE) | ||||
| IBDQ total score | 25.3 (4.6) | 11.3 (3.9) | 14.0 (5.9, 22.1) | <.001 |
| IBDQ bowel symptoms | 10.0 (1.4) | 5.0 (1.2) | 4.9 (2.6, 7.3) | <.001 |
| IBDQ emotional function | 8.3 (2.1) | 3.7 (1.7) | 4.6 (1.1, 8.2) | .011 |
| IBDQ social function | 3.2 (1.0) | 0.8 (0.8) | 2.4 (0.6, 4.2) | .008 |
| IBDQ systemic symptoms | 3.8 (0.8) | 2.1 (0.7) | 1.7 (0.3, 3.2) | .019 |
| SF-36 MCS | 3.2 (1.6) | 1.1 (1.3) | 2.2 (−0.6, 4.9) | .118 |
| SF-36 PCS | 4.6 (1.0) | 3.2 (0.9) | 1.3 (−0.4, 3.1) | .142 |
| Week 52 | ||||
|---|---|---|---|---|
| Bowel urgency absent N = 66 |
Bowel urgency present N = 40 |
Difference in LSM ΔBL (95% CI) |
P | |
| LSM ΔBL (SE) | ||||
| IBDQ total score | 43.5 (8.3) | 28.0 (7.7) | 15.5 (5.0, 25.9) | .004 |
| IBDQ bowel symptoms | 16.0 (2.4) | 10.9 (2.2) | 5.2 (2.2, 8.1) | <.001 |
| IBDQ emotional function | 12.5 (3.7) | 8.6 (3.4) | 3.9 (−0.7, 8.5) | .096 |
| IBDQ social function | 7.5 (1.8) | 4.8 (1.7) | 2.7 (0.3, 5.0) | .025 |
| IBDQ systemic symptoms | 6.7 (1.6) | 3.3 (1.5) | 3.4 (1.4, 5.4) | .001 |
| SF-36 MCS | 3.7 (3.4) | 2.8 (3.2) | 0.9 (−3.3, 5.2) | .668 |
| SF-36 PCS | 8.3 (2.0) | 5.4 (1.9) | 2.9 (0.3, 5.5) | .026 |
Abbreviations: ΔBL, change from baseline (Week 0); CI, confidence interval; IBDQ, Inflammatory Bowel Disease Questionnaire; LSM, least squares mean; MCS, Mental Component Summary; PCS, Physical Component Summary; SF-36, Medical Outcomes Study 36-Item Short Form Health Survey Version 2 Standard.
Associations Between Urgency Status and Improvement in SF-36
The improvement in the SF-36 PCS and MCS was numerically greater between patients achieving absence of bowel urgency at Weeks 12 and 52 compared to patients still experiencing urgency after adjusting for SF and RB. However, the difference was only statistically significant between urgency status groups for the Week 52 SF-36 PCS (Table 2).
Relative Association Between Urgency, RB, and SF Toward Improvement in QoL at Week 12
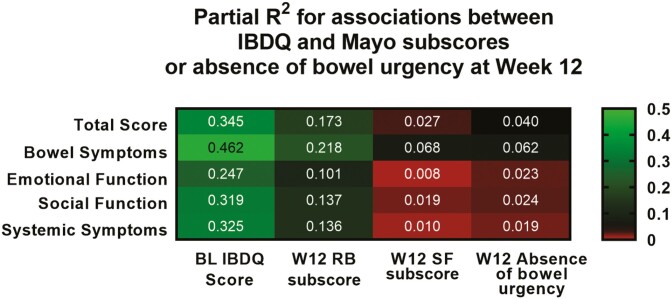
Coefficients of partial determination (partial R2) using Type III sum of squares were calculated from the same ANCOVA model as shown in Table 2 to compare the relative importance of urgency, RB, and SF toward improvement in IBDQ and SF-36 scores at Week 12 (Figure 1). As expected, BL total IBDQ score had the highest relative importance toward explaining improvement on the total IBDQ score at Week 12. Although no symptoms closely correlated with IBDQ at Week 12, the magnitude of correlation was strongest with RB, followed by absence of bowel urgency, and then SF. This suggests that bowel urgency was as important of a symptom, if not more so, in explaining improvements in IBDQ as SF.
Figure 1.
Relative importance of urgency status or Mayo subscores to variation in IBDQ measures at Week 12. Partial R2 values were calculated from an ANCOVA model that includes bowel urgency, rectal bleeding, or stool frequency status, to compare the proportion of variation in QoL gained from the addition of each symptom into the model. Brighter green indicates a stronger contribution while red indicates a weaker contribution. Abbreviations: ANCOVA, analysis of covariance; BL, baseline; IBDQ, Inflammatory Bowel Disease Questionnaire; QoL, quality of life; RB, rectal bleeding; SF, stool frequency; W12, Week 12.
Predictiveness of Urgency, RB, and SF at Week 12 Toward QoL Improvement at Week 52
When comparing whether urgency status, RB, or SF at Week 12 were predictive of improvement in IBDQ at Week 52 in a multiple regression model, Week 12 urgency status was seen to be the only predictor significantly associated with the total IBDQ score (P = .016) as well as every IBDQ domain score except the bowel symptoms domain (P = .055) (Table 3). SF and RB at Week 12 were not seen to be significant predictors of improvement of the total IBDQ score or any domain score at Week 52. After adjusting for the other predictors in the model, all 3 symptom variables at Week 12 were seen to not be significant predictors for improvement on the SF-36 PCS and MCS at Week 52.
Table 3.
Predictiveness of urgency, RB, and SF at Week 12 toward QoL improvement at Week 52.
| Parameter | Estimate (SE) | t-Statistic | P |
|---|---|---|---|
| IBDQ total score | |||
| Baseline value | −0.69 (0.09) | −8.03 | <.001 |
| Week 12 SF score | 2.83 (3.91) | 0.72 | .472 |
| Week 12 absence of urgency | −13.72 (5.57) | −2.46 | .016 |
| Week 12 RB score | 7.65 (9.02) | 0.85 | .398 |
| IBDQ systemic symptoms | |||
| Baseline value | −0.74 (0.09) | −8.04 | <.001 |
| Week 12 SF score | 0.65 (0.71) | 0.92 | .362 |
| Week 12 absence of urgency | −2.55 (1.01) | −2.54 | .013 |
| Week 12 RB score | 2.24 (1.63) | 1.38 | .172 |
| IBDQ social function | |||
| Baseline value | −0.76 (0.08) | −9.73 | <.001 |
| Week 12 SF score | 0.10 (0.83) | 0.12 | .901 |
| Week 12 absence of urgency | −2.93 (1.19) | −2.47 | .015 |
| Week 12 RB score | 1.17 (1.90) | 0.62 | .539 |
| IBDQ emotional function | |||
| Baseline value | −0.50 (0.08) | −6.45 | <.001 |
| Week 12 SF score | 2.99 (1.53) | 1.96 | .053 |
| Week 12 absence of urgency | −4.79 (2.17) | −2.21 | .029 |
| Week 12 RB score | 2.14 (3.52) | 0.61 | .545 |
| IBDQ bowel symptoms | |||
| Baseline value | −0.87 (0.09) | −9.96 | <.001 |
| Week 12 SF score | −1.03 (1.29) | −0.8 | .427 |
| Week 12 absence of urgency | −3.55 (1.83) | −1.95 | .055 |
| Week 12 RB score | 0.24 (3.05) | 0.08 | .939 |
Abbreviations: IBDQ, Inflammatory Bowel Disease Questionnaire; RB, rectal bleeding; SF, stool frequency.
Associations Between Bowel Urgency and Inflammatory Biomarkers
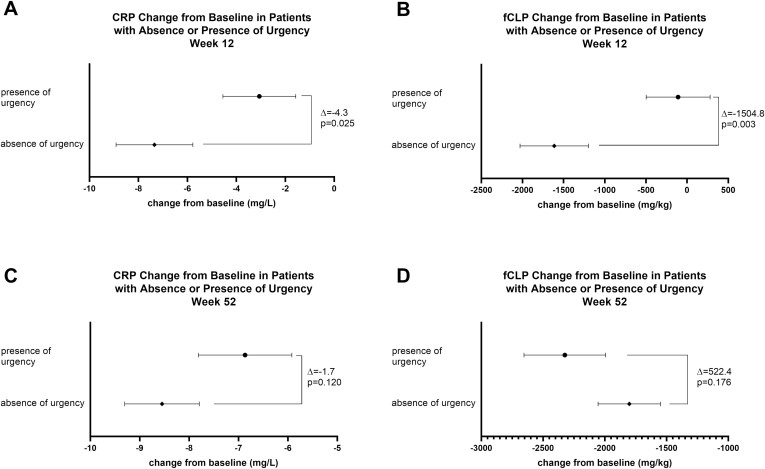
At Week 12, patients who reported absence of urgency had significantly greater ΔBL in both CRP and fCLP compared to patients who reported presence of urgency (CRP: absence of urgency −7.34 ± 1.57; presence of urgency −3.06 ± 1.49, P = .025; fCLP: absence of urgency −1614.07 ± 415.54; presence of urgency −109.24 ± 388.35, P = .003). At Week 52, although there was no statistically significant difference in ΔBL in CRP or fCLP between patients reporting absence or presence of urgency, levels of CRP and fCLP continued to decrease from Weeks 12 to 52 (Figure 2 and Supplementary Table 1).
Figure 2.
Change from baseline CRP or fCLP in patients with or without bowel urgency. Mean change from baseline ± SE of CRP and fCLP at Week 12 (A and B) and Week 52 (C and D) in patients with presence of urgency or absence of urgency at those time points. Abbreviations: CRP, C-reactive protein; fCLP, fecal calprotectin.
Associations Between Bowel Urgency and Clinical Outcomes: Week 12
At Week 12, patients who reported absence of urgency had significantly higher rates of achieving all clinical outcomes examined compared to those reporting presence of urgency (Table 4) (clinical remission: 22/91 [24.2%] of patients with absence of urgency vs 12/158 [7.6%] in those with presence of urgency [P = .0019]; clinical response: 64/91 [70.3%] of patients with absence of urgency vs 42/158 [26.6%] in those with presence of urgency [P < .0001]; SF remission: 62/91 [68.1%] of patients with absence of urgency vs 48/158 [30.4%] in those with presence of urgency [P < .0001]; RB remission: 74/91 [81.3%] of patients with absence of urgency vs 57/158 [36.1%] in those with presence of urgency [P < .0001]; endoscopic healing: 29/91 [31.9%] of patients with absence of urgency vs 17/158 [10.8%] in those with presence of urgency [P = .0004]). Among patients who experienced SF remission or RB remission at Week 12, 44% still experienced urgency. The proportion was lower (35%) among patients who achieved clinical remission, and 37% among patients who achieved endoscopic healing at Week 12. This suggests that while urgency is associated with resolution of symptoms and endoscopic healing, existing clinical assessments do not holistically capture a patient’s experience of bowel urgency.
Table 4.
Clinical outcome measures by bowel urgency status at Weeks 12 and 52.
| All values, n (%) | Week 12 | |||
|---|---|---|---|---|
| Bowel urgency absent N = 91 |
Bowel urgency present N = 158 |
P a | Adjusted odds ratio (95% CI) |
|
| Clinical remissionb | 22 (24.2) | 12 (7.6) | .0019 | 3.6 (1.6, 8.0) |
| Clinical responsec | 64 (70.3) | 42 (26.6) | <.0001 | 6.3 (3.4, 11.6) |
| Mayo stool frequency remissiond | 62 (68.1) | 48 (30.4) | <.0001 | 4.6 (2.6, 8.2) |
| Mayo rectal bleeding remissione | 74 (81.3) | 57 (36.1) | <.0001 | 7.2 (3.8, 13.7) |
| Mayo endoscopic healingf | 29 (31.9) | 17 (10.8) | .0004 | 3.6 (1.8, 7.2) |
| Week 52 | ||||
|---|---|---|---|---|
| Bowel urgency absent | Bowel urgency present | P | Adjusted odds ratio | |
| N = 66 | N = 40 | |||
| Clinical remission | 35 (53.0) | 5 (12.5) | <.0001 | 10.7 (3.3, 35.0) |
| Clinical response | 62 (93.9) | 19 (47.5) | <.0001 | 24.0 (6.2, 92.5) |
| Mayo stool frequency remission | 60 (90.9) | 16 (40.0) | <.0001 | 19.8 (5.7, 68.1) |
| Mayo rectal bleeding remission | 59 (89.4) | 22 (55.0) | .0004 | 6.6 (2.3, 18.6) |
| Mayo endoscopic remission | 40 (60.6) | 11 (27.5) | .0005 | 5.6 (2.1, 14.7) |
Abbreviations: CI, confidence interval; RB, rectal bleeding; SF, stool frequency.
From logistic regression model for remissions or responses, adjusted by urgency status, age, sex, geographic region and prior biologic experience. Missing values for the clinical outcome and absence of urgency were imputed using nonresponder imputation.
Clinical remission: RB Mayo subscore of 0, SF Mayo subscore of 0 or 1 with a ≥1 point decrease from baseline, and Mayo endoscopic subscore of 0 or 1.
Clinical response: a decrease in the 9-point Mayo subscores (comprising the subscores of rectal bleeding, stool frequency and the endoscopic findings) inclusive of ≥2 points and ≥35% from baseline with either a decrease of rectal bleeding subscore of ≥1 or rectal bleeding subscore of 0 or 1.
Stool frequency remission: Mayo SF = 0 or 1 with a ≥1 point decrease from baseline.
Rectal bleeding remission: Mayo RB = 0.
Endoscopic healing: Mayo endoscopy = 0 or 1.
Associations Between Bowel Urgency and Clinical Outcomes: Week 52
Similarly, patients achieving absence of urgency at Week 52 had significantly higher rates of improvement in all clinical outcomes examined at Week 52 (Table 4) (clinical remission: 35/66 [53.0%] of patients with absence of urgency, 5/40 [12.5%] in those with presence of urgency [P < .0001]; clinical response: 62/66 [93.9%] of patients with absence of urgency, 19/40 [47.5%] in those with presence of urgency [P < .0001]; SF remission: 60/66 [90.9%] of patients with absence of urgency, 16/40 [40.0%] in those with presence of urgency [P < .0001]; RB remission: 59/66 [89.4%] of patients with absence of urgency, 22/40 [55.0%] in those with presence of urgency [P = .0004]; endoscopic healing: 40/66 [60.6%] of patients with absence of urgency, 11/40 [27.5%] in those with presence of urgency [P = .0005]). At Week 52, the proportion of patients in clinical remission who still experienced urgency decreased to 5%, while those with endoscopic healing who still experienced urgency decreased to 22%, and those with SF or RB remission who experienced urgency fell to 21% and 27%, respectively.
Association Between Bowel Urgency and Time to Symptomatic Response or Remission
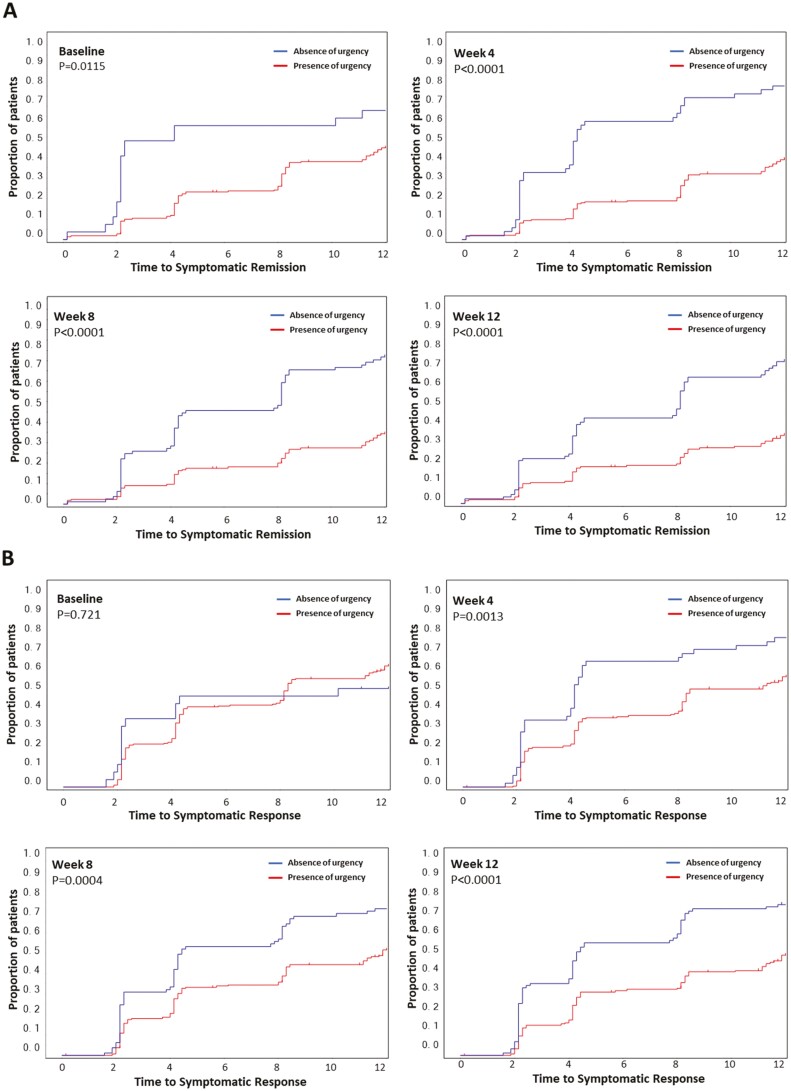
Kaplan–Meier curves for time to symptomatic remission by urgency status at different visit weeks showed that patients who reported absence of urgency at baseline, or achieved absence of urgency at Weeks 4, 8, or 12 had a faster time to symptomatic remission than patients who reported experiencing bowel urgency at those time points (Figure 3A). Likewise, patients who achieved absence of urgency at Weeks 4, 8, or 12 had a faster time to symptomatic response (Figure 3B) than those who reported presence of urgency.
Figure 3.
Time to symptomatic response and remission in patients with or without bowel urgency. Kaplan–Meier analyses demonstrate how time to achieving symptomatic remission (RB = 0, SF = 0 or 1; A) or symptomatic response (≥35% decrease from baseline with either an RB decrease of ≥1 or RB = 0 or 1; B) changes as patients achieve absence of urgency (blue line) at baseline or Weeks 4, 8, and 12. Abbreviations: RB, rectal bleeding; SF, stool frequency.
Discussion
In this analysis of the results of a Phase 2 study of mirikizumab in patients with UC, we show that the absence of bowel urgency is associated with improved QoL measures, decreased levels of inflammatory biomarkers, and improved clinical outcomes. The associations remained strong between urgency status and the IBDQ total and domain scores, even after adjusting for SF and RB, which suggests bowel urgency is independently associated with QoL. Independent association with bowel urgency was not the case for SF-36, however, most likely because SF-36 is a generic health QoL survey25 and thus may not directly address bowel urgency, while IBDQ is specific for IBD. Among the IBDQ subcategories, absence of urgency correlated most strongly with improved bowel symptoms at Week 12, followed by improved social function, while at Week 52 absence of urgency correlated most strongly with improved bowel symptoms followed by improved systemic symptoms. Absence of urgency was strongly associated with achieving all clinical outcomes that were measured at Weeks 12 and 52. At Week 52, of the patients in clinical remission, only 5% reported presence of urgency.
Absence of urgency was strongly associated with reduced levels of both CRP and fCLP after 12 weeks of induction treatment with mirikizumab, suggesting a reduction in colonic inflammation associated with absence of urgency. Among patients who were induction responders, levels of biomarkers continued to decrease in patients with both presence and absence of urgency through Week 52, demonstrating that in the Week 12 responder population, inflammation decreases over time regardless of the presence of urgency, but a greater initial decrease is associated with absence of urgency. This suggests that bowel urgency in the maintenance phase may be associated with factors other than inflammation, such as altered neuromuscular function or psychological factors. The unpredictable and uncontrollable nature of bowel urgency can result in a great deal of anxiety, panic, and stress for patients with UC,3,29–31 and there may be a connection between anxiety and bowel urgency (eg, urgency related “post-traumatic stress disorder”) independent of inflammation.
Patients who reported absence of urgency at Week 12 had significantly shorter disease duration and higher baseline total IBDQ scores compared to those reporting presence of urgency at Week 12, as well as a greater proportion of absence of urgency at baseline. Neither disease duration nor presence of bowel urgency at baseline was significantly different between patients who reported absence of urgency at Week 52 and those who did not. Thus, disease duration and higher QoL as measured by the IBDQ may be an early indicator of rapid treatment-induced resolution of bowel urgency, but not necessarily of long-term response. Interestingly, disease activity, as measured by Mayo score components, was not significantly different at baseline in patients with or without urgency. Given the relatively small sample size of a Phase 2 trial and the post hoc nature of the analyses, the association of these baseline characteristics with resolution of bowel urgency would need to be explored further in a larger Phase 3 trial.
The presence of bowel urgency has previously been reported to have significant impact on patient QoL, limiting participation in physical and social activities, impinging on the ability to travel to and from work, and causing significant stress, anxiety, feelings of social isolation, and stigma.2,3,31–35 Our findings that all IBDQ domain scores were significantly improved in patients with absence of urgency compared to those with presence of urgency, even after adjusting for disease activity, are in agreement with and further support these prior studies, demonstrating the strong impact that bowel urgency has on QoL in patients with UC and suggesting that lack of bowel urgency may be used as a rough surrogate for QoL measures. Furthermore, absence of urgency at Week 12 was significantly associated with and independently predictive of improvements in IBDQ at Week 52 (Figure 1) after adjusting for Week 12 disease activity and baseline IBDQ. Our findings here are in agreement with the recent publication by Ghosh et al, which found that improvements in bowel urgency symptoms were associated with IBDQ score, changes in biomarkers, and most clinical outcomes in a Phase 2 trial of upadacitinib.36 This highlights the importance of urgency in the UC disease state regardless of the mechanism of intervention, as similar results were observed after treatment with both a p19-directed anti-IL-23 antibody and a Janus kinase inhibitor.
The percentage of patients achieving a given clinical outcome was strongly associated with absence of urgency in patients for all clinical outcomes that were assessed. Because clinical outcomes included SF and RB, obtained from the Mayo score, adjustments for these disease activity components were not made when examining the association between urgency status and clinical outcomes derived from the Mayo score. A number of patients with clinical remission or endoscopic healing (and thus no RB and normal SF) still experienced bowel urgency however, suggesting that while urgency is associated with clinical outcomes, it was not completely captured using standard methods to assess disease activity. Our results show that, in agreement with previous studies,3 more than 40% of those patients who have normal SF or a lack of RB still experience urgency after 12 weeks of treatment, affirming the vital importance of including the presence of bowel urgency in assessments of disease activity. Moreover, our analyses show that earlier resolution of bowel urgency is associated with shorter time to symptomatic response and remission (Figure 3). Given the association of urgency improvement with rapid treatment response as well as with changes in inflammatory markers, bowel urgency could potentially be used by clinicians along with RB and SF as surrogate markers to guide communication to patients about expected rapidity of treatment response, as has been suggested previously by Higgins et al.37
Limitations of this study include the binary nature (present/absent) of the measurement of bowel urgency which, similar to the study by Ghosh et al,36 does not allow assessment of changes in severity of bowel urgency if the symptom is still present. Additionally, bowel urgency associations were analyzed post hoc, urgency status was not an inclusion criterion for the trial, and the Phase 2 study had a relatively small sample size, especially during the maintenance period. Bowel urgency has been relatively neglected as a symptom and is not a component of commonly used clinical scoring systems, such as the Mayo Clinic Score. Despite these limitations, the absence of urgency achieved in patients with UC after mirikizumab treatment22 is strongly associated with improved outcomes across the board.
The small proportion of patients without urgency at baseline in this study limited our ability to assess the association between change in urgency status (improvement, worsening, persistent urgency, or persistent lack of urgency) and other endpoints. As such, we were limited to examining associations between bowel urgency status at the end of the induction and maintenance phases with improvement on the other endpoints. These associations for change in bowel urgency remain an area to be explored in future work; the larger population of our ongoing Phase 3 trial will allow us the statistical power to make these types of comparisons, along with the use of the recently developed Urgency Numeric Rating Scale which enables the assessment of bowel urgency severity.13
While there remains a communication gap between healthcare providers and patients regarding bowel urgency, this study, in addition to others, has reinforced the importance of bowel urgency from a clinical perspective.2–5,35 Because of the importance of bowel urgency to patients, recommendations have been made to include bowel urgency in the clinical assessment of IBD patients and considered for inclusion in treatment plans.5,38 Recent American College of Gastroenterology (ACG) Guidelines1 have stated that initial treatment of UC should focus on restoration of normal SF and control of the primary symptoms of bleeding and urgency. The strong associations we observed between bowel urgency status and both QoL and clinical outcomes support the importance of evaluating bowel urgency in a clinical setting, and the inclusion of resolution of bowel urgency as an independent treatment goal for patients with UC.
Supplementary Material
Acknowledgments
The authors would like to thank the following employees of Eli Lilly: Lorena Hernandez Maxwell for providing analyst support, Catherine Milch and Noah Agada for providing medical peer review, Deirdre Hoban for providing quality review, and Meenu Kaur for providing statistical review for this manuscript.
Contributor Information
Marla C Dubinsky, *Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Remo Panaccione, Division of Gastroenterology and Hepatology, University of Calgary, Calgary, Alberta, Canada.
James D Lewis, Division of Gastroenterology and Hepatology, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Bruce E Sands, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Toshifumi Hibi, Department of Gastroenterology, Kitasato Institute Hospital Center for Advanced IBD Research and Treatment, Minato-ku, Tokyo, Japan.
Scott D Lee, Division of Gastroenterology, University of Washington Medical Center, Seattle, Washington, USA.
April N Naegeli, Eli Lilly and Company, Indianapolis, Indiana, USA.
Mingyang Shan, Eli Lilly and Company, Indianapolis, Indiana, USA.
Linden A Green, Eli Lilly and Company, Indianapolis, Indiana, USA.
Nathan Morris, Eli Lilly and Company, Indianapolis, Indiana, USA.
Vipin Arora, Eli Lilly and Company, Indianapolis, Indiana, USA.
Alison Potts Bleakman, Eli Lilly and Company, Indianapolis, Indiana, USA.
Ruth Belin, Eli Lilly and Company, Indianapolis, Indiana, USA.
Simon Travis, Nuffield Department of Medicine, Oxford University Hospital, Oxford, UK.
Authors’ Contributions
M.C.D., R.P., J.D.L., B.E.S., A.N.N., M.S., L.A.G., V.A., A.P.B., and S.T. contributed to study design. M.S., N.M., and V.A. contributed to data analysis. M.C.D., R.P., B.E.S., T.H., M.S., N.M., and V.A. contributed to data collection. M.C.D., R.P., J.D.L., B.E.S., T.H., S.D.L., A.N.N., M.S., L.A.G., N.M., V.A., A.P.B., R.B., and S.T. contributed to data interpretation. M.C.D., R.P., J.D.L., B.E.S., T.H., S.D.L., A.N.N., M.S., L.A.G., N.M., V.A., A.P.B., R.B., and S.T. contributed to manuscript writing and/or critical review/analysis of the manuscript.
Funding
This study was funded by Eli Lilly and Company.
Conflicts of Interest
M.C.D.: consulting fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Eli Lilly, F. Hoffman-LaRoche, Genentech, Gilead, Janssen, Pfizer, Prometheus Biosciences, Takeda, and UCB SA; contracted research with AbbVie, Janssen, Pfizer, and Prometheus Biosciences; ownership interest in Trellus Health; licensing fees from Takeda. R.P.: consulting fees, paid speaker, and/or advisory board member, and/or received educational/research support from Abbott, AbbVie, ActoGeniX, AGI Therapeutics, Alba Therapeutics, Albireo, Alfa Wasserman, Amgen, AM-Pharma BV, Anaphore, Aptalis, Astellas, Athersys, Atlantic Healthcare, AstraZeneca, Baxter, BioBalance, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celek, Cellerix, Cerimon, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cubist, Cytokine Pharmasciences, Eagle, Eisai, Elan, EnGene, Eli Lilly and Company, Enteromedics, Exagen Diagnostics, Ferring, Flexion Therapeutics, Funxional Therapeutics, Genentech, Genzyme, Gilead, Given Imaging, GlaxoSmithKline, Hospira, Human Genome Sciences, Ironwood, Janssen, KaloBios, Lexicon, Lycera, Meda, Merck & Co., Merck Research Laboratories, MerckSerono, Millennium, Nisshin Kyorin, Novartis, Novo Nordisk, NPS Pharmaceuticals, Optimer, Orexigen, PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProtAb, Purgenesis Technologies, Receptos, Relypsa, Salient, Salix, Santarus, Shire Pharmaceuticals, Sigmoid Pharma, Sirtris, S.L.A. Pharma, Takeda, Targacept, Teva, Therakos, Tillotts, TxCell SA, UCB, Vascular Biogenics, Viamet, and Warner Chilcott. J.D.L.: personal fees from Johnson & Johnson Consumer Inc; grants, personal fees, and other from Takeda Pharmaceuticals and Pfizer; personal fees and nonfinancial support from AbbVie; grants and personal fees from Janssen Pharmaceuticals, Nestle Health Science; personal fees from Eli Lilly and Company, Samsung Bioepis, UCB, Bristol-Myers Squibb, Bridge Biotherapeutics, Celgene, Merck, Gilead, Arena Parmaceuticals, and Protagonist Therapeutics. B.E.S.: consultant fees from AbbVie, Abivax, Alimentiv, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Bacainn Therapeutics, Baxalta Bioscience India, Boehringer-Ingelheim, Boston Pharmaceuticals, Calibr, Capella Bioscience, Celgene, Celltrion Healthcare, ClostraBio, F.Hoffmann-La Roche, Galapagos, Gilead, Gossamer Bio, Immunic, Index Pharmaceuticals, Innovation Pharmaceuticals, Ironwood Pharmaceuticals, Janssen, Johnson & Johnson, Lilly, Morphic Therapeutic, Oppilan Pharma, OSE Immunotherapeutics, Otsuka, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Redhill Biopharma, Rheos Medicines, Salix Pharmaceuticals, Seres Therapeutics, Shire, Surrozen, Takeda, Target RWE, Theravance Biopharma R&D, USWM Enterprises, and Vivelix Pharmaceuticals; research grants from Theravance Biopharma R&D and Arena Pharmaceuticals. T.H.: advisory/consultancy fees from AbbVie, Bristol-Myaers Squibb K.K., Celltrion, EA Pharma, Eli Lilly and Company, Gilead Sciences, Janssen, Kyorin, Mitsubishi-Tanabe Pharma, Nichi-Iko Pharmaceutical, Pfizer, Takeda Pharmaceutical, and Zeria Pharmaceutical; research grants from AbbVie, EA Pharma, JIMRO, Otuska Holdings, and Zeria Pharmaceuticals. S.D.L.: grant/research support from AbbVie, AbGenomics, Arena Pharmaceuticals, Celgene, GlaxoSmithKline, Janssen, Salix Pharmaceuticals, Shield Therapeutics, Takeda, Tetherex Pharmaceuticals, and UCB Pharma; consultancy/advisory board fees from Applied Molecular Transport, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Cornerstones Health, Eli Lilly and Company, Janssen, KCRN Research, and UCB Pharma. S.T.: advisory board member for Abacus, AbbVie, Actial, ai4gi, Alcimed, Allergan, Amgen, Aptel, Arena, Asahi, Aspen, Astellas, Atlantic, AstraZeneca, Barco, Biocare, Biogen, BLPharma, Boehringer Ingelheim, BMS, Buhlmann, Calcico, Celgene, Cellerix, Cerimon, ChemoCentryx, Chiesi, CisBio, ComCast, Coronado, Cosmo, Ducentis, Dynavax Elan, Enterome, Falk, Ferring, FPRT Bio, Galapagos, Genentech/Roche, Genzyme, Gilead, Glenmark, Grunenthal, GSK, GW Pharmaceuticals, Immunocore, Immunometabolism, Indigo, Janssen, Lexicon, Lilly, Medarex, Medtrix, Merck, Merrimack, Millenium, Neovacs, Novartis, Novo Nordisk, NPS-Nycomed, Ocera, Optima, Origin, Otsuka, Palau, Pentax, Pfizer, Pharmaventure, Phillips, P&G, Pronota, Proximagen, Resolute, Robarts, Sandoz, Santarus, Satisfai, Sensyne, Shire, SigmoidPharma, Souffinez, Syndermix Synthon, Takeda, Theravance, Tigenix, Tillotts, Topivert, Trino Therapeutics with Wellcome Trust, TxCell, and UCB Pharma; speakers bureau for AbbVie, Amgen, Falk, Ferring, Janssen, Pfizer, Shire, and Takeda; grants/research support from AbbVie, Buhlmann, ECCO, IOIBD, Janssen, Helmsley Trust, Lilly, MediAdd, Norman Collisson Foundation, Pfizer, Takeda, UCB, and Vifor. A.N.N., M.S., L.A.G., N.M., V.A., A.P.B., and R.B.: current employees and shareholders of Eli Lilly and Company.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- 1. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. . ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413. [DOI] [PubMed] [Google Scholar]
- 2. Petryszyn PW, Paradowski L.. Stool patterns and symptoms of disordered anorectal function in patients with inflammatory bowel diseases. Adv Clin Exp Med. 2018;27(6):813–818. [DOI] [PubMed] [Google Scholar]
- 3. Hibi T, Ishibashi T, Ikenoue Y, et al. . Ulcerative colitis: disease burden, impact on daily life, and reluctance to consult medical professionals: results from a Japanese internet survey. Inflamm Intest Dis. 2020;5(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schreiber S, Panes J, Louis E, et al. . Perception gaps between patients with ulcerative colitis and healthcare professionals: an online survey. BMC Gastroenterol. 2012;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell R, Kremer A, Westwood N, et al. . Talking about life and IBD: a paradigm for improving patient-physician communication. J Crohns Colitis. 2009;3(1):1–3. [DOI] [PubMed] [Google Scholar]
- 6. US Food and Drug Administration. Ulcerative Colitis: Clinical Trial Endpoints Guidance for Industry. 2016. www.fda.gov/files/drugs/published/Ulcerative-Colitis-Clinical-Trial-Endpoints-Guidance-for-Industry.pdf. [Google Scholar]
- 7. National Institute of Diabetes and Digestive and Kidney Diseases. Diarrhea: Definition and Facts. 2020. www.niddk.nih.gov/health-information/digestive-diseases/diarrhea. [Google Scholar]
- 8. Buchmann P, Kolb E, Alexander-Williams J.. Pathogenesis of urgency in defaecation in Crohn’s disease. Digestion. 1981;22(6):310–316. [DOI] [PubMed] [Google Scholar]
- 9. Newton L, Randall JA, Hunter T, et al. . A qualitative study exploring the health-related quality of life and symptomatic experiences of adults and adolescents with ulcerative colitis. J Patient Rep Outcomes. 2019;3(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nobrega VG, Silva INN, Brito BS, et al. . The onset of clinical manifestations in inflammatory bowel disease patients. Arq Gastroenterol. 2018;55(3):290–295. [DOI] [PubMed] [Google Scholar]
- 11. Dulai PS, Jairath V, Khanna R, et al. . Development of the symptoms and impacts questionnaire for Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2020;51(11):1047–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ueno F, Nakayama Y, Hagiwara E, et al. . Impact of inflammatory bowel disease on Japanese patients’ quality of life: results of a patient questionnaire survey. J Gastroenterol. 2017;52(5):555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubinsky MC, Irving PM, Panaccione R, et al. . Incorporating patient experience into drug development for ulcerative colitis: development of the Urgency Numeric Rating Scale, a patient-reported outcome measure to assess bowel urgency in adults. J Patient Rep Outcomes. 2022;6(1):31. doi: 10.1186/s41687-022-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Louis E, Ramos-Goni JM, Cuervo J, et al. . A qualitative research for defining meaningful attributes for the treatment of inflammatory bowel disease from the patient perspective. Patient. 2020;13(3):317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Deen WK, Obremskey A, Moore G, et al. . An assessment of symptom burden in inflammatory bowel diseases to develop a patient preference-weighted symptom score. Qual Life Res. 2020;29(12):3387–3396. [DOI] [PubMed] [Google Scholar]
- 16. Limdi JK, Vasant DH.. Anorectal dysfunction in distal ulcerative colitis: challenges and opportunities for topical therapy. J Crohns Colitis. 2016;10(4):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drewes AM, Frokjaer JB, Larsen E, et al. . Pain and mechanical properties of the rectum in patients with active ulcerative colitis. Inflamm Bowel Dis. 2006;12(4):294–303. [DOI] [PubMed] [Google Scholar]
- 18. Reich K, Rich P, Maari C, et al. . Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019;181(1):88–95. [DOI] [PubMed] [Google Scholar]
- 19. Sands BE, Sandborn WJ, Peyrin-Biroulet L, et al. . Efficacy and safety of mirikizumab (LY3074828) in a phase 2 study of patients with Crohn’s disease. In: Digestive Disease Week 2019. 2019.
- 20. Sandborn WJ, Ferrante M, Bhandari BR, et al. . Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology. 2020;158(3):537–549.e10. [DOI] [PubMed] [Google Scholar]
- 21. Sandborn WJ, Ferrante M, Bhandari BR, et al. . Efficacy and safety of continued treatment with mirikizumab in a phase 2 trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;20(1):105–115.e14. [DOI] [PubMed] [Google Scholar]
- 22. Dubinsky M, Lee SD, Panaccione R, et al. . P068 Mirikizumab treatment improves bowel movement urgency in patients with moderately to severely active ulcerative colitis. Gastroenterology. 2020;158(3):S17–S18. [Google Scholar]
- 23. Guyatt G, Mitchell A, Irvine EJ, et al. . A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96(3):804–810. [PubMed] [Google Scholar]
- 24. Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflamm Bowel Dis. 2008;14(4):554–565. [DOI] [PubMed] [Google Scholar]
- 25. Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 26. McHorney CA, Ware JE Jr, Rogers W, et al. . The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care. 1992;30(5 Suppl):MS253–MS265. [DOI] [PubMed] [Google Scholar]
- 27. Yarlas A, Bayliss M, Cappelleri JC, et al. . Psychometric validation of the SF-36((R)) Health Survey in ulcerative colitis: results from a systematic literature review. Qual Life Res. 2018;27(2):273–290. [DOI] [PubMed] [Google Scholar]
- 28. Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976). 2000;25(24):3130–3139. [DOI] [PubMed] [Google Scholar]
- 29. Waljee AK, Joyce JC, Wren PA, et al. . Patient reported symptoms during an ulcerative colitis flare: a Qualitative Focus Group Study. Eur J Gastroenterol Hepatol. 2009;21(5):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larsson K, Loof L, Nordin K.. Stress, coping and support needs of patients with ulcerative colitis or Crohn’s disease: a qualitative descriptive study. J Clin Nurs. 2017;26(5-6):648–657. [DOI] [PubMed] [Google Scholar]
- 31. Dibley L, Norton C.. Experiences of fecal incontinence in people with inflammatory bowel disease: self-reported experiences among a community sample. Inflamm Bowel Dis. 2013;19(7):1450–1462. [DOI] [PubMed] [Google Scholar]
- 32. Ghosh S, Louis E, Loftus EV Jr, et al. . P283 Bowel urgency in patients with moderate to severe ulcerative colitis: prevalence and correlation with clinical outcomes, biomarker levels, and health-related quality of life from U-ACHIEVE, a Phase 2b study of upadacitinib. J Crohns Colitis. 2019;13(Suppl_1):S240–S241. [Google Scholar]
- 33. Dibley LB, Norton C, and Whitehead E. The experience of stigma in inflammatory bowel disease: An interpretive (hermeneutic) phenomenological study. J Adv Nurs. 2018; 74(4):838–851. [DOI] [PubMed] [Google Scholar]
- 34. Rasmussen B, Haastrup P, Wehberg S, et al. . Predictors of health-related quality of life in patients with moderate to severely active ulcerative colitis receiving biological therapy. Scand J Gastroenterol. 2020;55(6):656–663. [DOI] [PubMed] [Google Scholar]
- 35. Rubin DT, Sninsky C, Siegmund B, et al. . International perspectives on management of inflammatory bowel disease: opinion differences and similarities between patients and physicians from the IBD GAPPS survey. Inflamm Bowel Dis. 2021;27(12):1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghosh S, Sanchez Gonzalez Y, Zhou W, et al. . Upadacitinib treatment improves symptoms of bowel urgency and abdominal pain, and correlates with quality of life improvements in patients with moderate to severe ulcerative colitis. J Crohns Colitis. 2021;15(12):2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higgins PD, Schwartz M, Mapili J, et al. . Is endoscopy necessary for the measurement of disease activity in ulcerative colitis? Am J Gastroenterol. 2005;100(2):355–361. [DOI] [PubMed] [Google Scholar]
- 38. Lonnfors S, Vermeire S, Greco M, et al. . IBD and health-related quality of life—discovering the true impact. J Crohns Colitis. 2014;8(10):1281–1286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.