Abstract
Background
Over 10 years ago, the step-up/top-down trial demonstrated favorable outcomes of Crohn’s disease (CD) after early initiation of infliximab (IFX) in patients with CD. However, data on long-term effects of this treatment strategy in daily clinical practice are scarce.
Methods
This retrospective study investigated effects of early (<24 months after diagnosis) versus late intervention (>24 months) of IFX in CD on endoscopic remission (ER) rates, surgery rates, and course of CD, long term.
Results
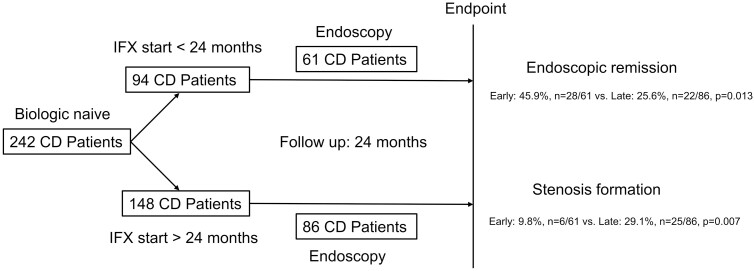
Overall, 242 CD patients (94 early, 148 late intervention) were started on IFX and followed for 24 months. Sixty-one patients with early and 86 with late intervention underwent endoscopy after start of IFX. After IFX induction, 90.3% of patients with early versus 87.8% with late intervention were in clinical remission (P = .676), compared to 89.1% versus 85.8% after 24 months (P = .554). Almost half of patients with early IFX (45.9%, n = 28/61) achieved ER within 24 months compared to only one forth with late IFX intervention (25.6%, n = 22/86, P = .013). In addition, significantly less patients with early IFX intervention (9.8%, n = 6/61) developed intestinal stenosis during 24 months follow-up compared to late IFX start (29.1%, n = 25/86, P = .007). Logistic regression revealed early IFX intervention as only relevant factor achieving ER with an odds ratio of 2.386 (95% confidence interval [1.1180; 4.825], P = .016).
Conclusions
Our data on early IFX therapy in CD support early IFX intervention with more patients achieving ER, and less patients developing stricturing disease behavior. Early IFX intervention could therefore change the course of CD.
Keywords: Crohn’s disease, biological therapy, anti-TNF treatment, infliximab, early intervention, late intervention, mucosal healing, endoscopic remission, need for CD-related surgery, outcome, time to surgery
Graphical Abstract
Introduction
Over 2 decades ago, the first biological therapy and anti-TNF treatment with infliximab (IFX) were approved for the treatment of Crohn’s disease (CD) as a single infusion of IFX, former cA2.1–3 Some years later it become obvious that maintenance treatment with IFX was superior to episodic treatment with IFX infusions on demand.4
In 2008, the step-up/top-down trial for the first time could demonstrate a positive effect in patients with CD when IFX was started early after first diagnosis of inflammatory bowel disease (IBD).5 Patients with CD were randomly assigned to either early combined immunosuppression with IFX or conventional step-up treatment.5 Primary outcome measures were clinical remission without corticosteroids and without bowel resection at weeks 26 and 52. As a result, 60.0% of patients in the early IFX intervention group were in clinical remission without corticosteroids and without surgical resection, compared with only 35.9% controls, for an absolute difference of 24.1% (n = 39/65 vs 23/64 patients, 95% confidence interval [CI] 7.3–40.8, P = .0062). Corresponding rates at week 52 were 61.5% and 42.2% (absolute difference 19.3%, n = 40/65 vs 27/64 patients, 95% CI 2.4–36.3, P = .0278).
In 2010, the SONIC trial compared the efficacy of early (less than median of 2.3 years after first diagnosis of CD) start of IFX monotherapy in CD with azathioprine monotherapy and combination therapy IFX/azathioprine in biological and immunosuppressive naive patients.6
Early start of IFX was most effective, as highest endoscopic remission (ER) rates were seen in IFX treated subgroups 43.9% of patients in the combination group, versus 30.1% in IFX monotreatment, versus only 16.5% in azathioprine monotherapy (P < .001 for the comparison with combination therapy and P = .02 for the comparison with IFX).
Although the positive effect of early initiation of IFX in selected CD patients is known now for several years, data in daily clinical practice are scarce. The conventional step-up approach is still widely used. However, national and international guidelines support a rapid introduction of anti-TNF-alpha agents at least in patients with moderate-to-severe disabling CD.7–9 Some clinical trials could support the results of the reported trials in CD.10–14
The aim of our study was to translate the treatment regimen of these trials into daily clinical practice and to prove if the effects of an early anti-TNF-alpha treatment can also be seen in daily clinical practice, especially in endoscopic findings.
Materials and Methods
Patients
Patients with diagnosis of CD were successively recruited at 1 single center in Munich between 2013 and 2015 at the center of inflammatory bowel disease (IBD center) of the Isarklinikum, Munich, Germany. Inclusion criteria were diagnosis of CD, age of at least 18 years and patients did not receive biological treatment before start of IFX. All eligible CD patients gave written informed consent before being included in this observational trial. A full clinical assessment of all patients was performed by experienced clinicians at start of IFX treatment and during the follow-up (FU) of 24 months when patients received IFX infusions at a maximum range of 8 weeks. CD patients who underwent endoscopy after start of IFX were included in a subanalysis evaluating the effect of early IFX intervention on the chance of achieving ER.
Methods
Clinical data on medical treatment, disease behavior and activity, CD-related complications and endoscopic activity, ER, respectively, were retrospectively obtained from clinical charts and endoscopy reports from patients’ visits at the IBD Centre Munich.
At each patient contact, the CDAI (Crohn’s Disease Activity Index) questionnaire was completed by patients and physicians.15
Early intervention with IFX was defined as start of IFX less than or equal to 24 months after first diagnosis of CD, late intervention with start of IFX more than 24 months after diagnosis of CD.
Primary outcome was defined as the ability of achieving ER at 24 months FU after start of IFX. CD-related complications were defined by the need for CD-related surgery within 24 months of FU, and the de novo development of stenoses and fistulas within 24 months of FU.
ER was defined as macroscopic absence of ulcerations seen in endoscopy.
For assessment of disease location, the Montreal classification was used.16
Information about IFX trough level were not available in our patient cohort. Information about intestinal stenoses and/or fistulas were based on endoscopic findings (luminal narrowing) and/or magnetic resonance enterography (criteria for obstructive disease: narrowing of the intrastenotic luminal diameter, prestenotic dilatation) and pelvic magnetic resonance imaging.17
Statistical Analysis
Statistical analysis was performed with a statistical analysis package from Statistical Analysis System (SAS) version 9.4 for Windows. Results of the analysis of the quantitative data were presented as either mean ± SD and range (Gaussian data) or median and range (non-Gaussian data). Categorical data were summarized as the percentage of the group total. Student’s t-test was used for evaluating differences in distributions of quantitative data (Gaussian data) and the Wilcoxon rank-sum test for non-Gaussian data. For investigation of influencing factors to achieve ER at 24 months FU after start of IFX, logistic regression models were used.
Ethical Considerations
The study was approved by the local ethics committee of the Bayerische Landesärztekammer (Nr. 2020-1130 BLAEK), Munich, Germany.
Results
Patient Cohort and Patients’ Characteristics
A total of 242 patients with CD were included in this retrospective observation trial (Table 1). Overall, 148 CD patients were started with IFX later than 24 months after first diagnosis of CD, defined as late intervention. Ninety-four patients were started early with IFX, defined as start of IFX less than or equal to 24 months after first diagnosis of IBD. Disease progress in all patients was followed for 24 months after start of IFX (Table 1).
Table 1.
Patients’ characteristics of all patients included: a total of 242 patients with Crohn’s disease were included in this retrospective observation trial
| Patients’ characteristics | Early intervention (n = 94) | Late intervention (n = 148) | P |
|---|---|---|---|
| Sex (female (%)/male (%)) | 46 (48.9)/48 (51.1) | 74 (50.0)/74 (50.0) | .896 |
| Median age at diagnosis of CD (median, range) | 25 [15–62] | 21 [6–54] | <.001 |
| Median age at start biological therapy (median, range) | 26 [16–64] | 32 [15–65] | .001 |
| Median interval between diagnosis of CD and start biological therapy (months, median, range) | 12 [0–24] | 98.5 [26–486] | <.001 |
| Montreal classification | |||
| A1 ≤16 years at first diagnosis CD | 2 (2.1) | 32 (21.1) | <.001 |
| A2 17–40 years | 79 (84.0) | 103 (68.4) | |
| A3 >40 years | 13 (13.8) | 13 (10.5) | |
| L1 terminal ileum | 43 (45.7) | 64 (43.4) | .703 |
| L2 colon | 17 (18.1) | 26 (18.4) | .918 |
| L3 ileocolon | 33 (35.1) | 58 (38.2) | .523 |
| L4 only upper GI involvement | 0 | 0 | — |
| L4+ upper GI involvement and distal involvement | 21 (22.3) | 7 (4.6) | <.001 |
| B1 nonstricturing, nonpenetrating | 46 (48.9) | 67 (45.3) | .229 |
| B2 stricturing | 28 (31.8) (n = 88) |
51 (38.9) (n = 131) |
.450 |
| B3 internal penetrating | 0 | 0 | — |
| B3p perianal penetrating | 24 (25.5) | 38 (25.7) | |
| Medical treatment before biological therapy | |||
| 5-ASA (%) | 26 (27.7) | 87 (59.2) (n = 147) |
<.001 |
| Corticosteroids (%) | 80 (85.1) | 138 (93.9) (n = 147) |
.041 |
| Thiopurines (azathioprine, 6-mercaptopurine) (%) | 47 (50.0) | 115 (79.2) (n = 145) |
<.001 |
| Methotrexate (%) | 3 (3.2) (n = 93) |
15 (10.1) (n = 144) |
.046 |
| Biological treatment (%) | 0 | 0 | |
| Disease activity before and after start of biological therapy (CDAI) | |||
| Before (median, range) | 150 [0–403] (n = 93) |
145 [0–400] (n = 148) |
.096 |
| After induction treatment (median, range) | 50 [0–240] (n = 93) |
56 [0–400] (n = 148) |
.575 |
| CDAI at 24 months after start of biological therapy (median, range) | 20.0 [0–300] (n = 92) |
36.5 [0–330] (n = 148) |
.197 |
| Clinical remission after induction treatment (CDAI <150, %) | 84 (90.3) (n = 93) |
130 (87.8) (n = 148) |
.676 |
| Clinical remission at 24 months after start of biological therapy (CDAI <150, %) | 82 (89.1) (n = 92) |
127 (85.8) (n = 148) |
.554 |
| Complications and surgeries | |||
| Surgery because of CD-related complications before start of biological therapy (%) | 7 (7.5) (n = 94) |
65 (43.9) (n = 148) |
<.001 |
| Surgery because of CD-related complications within 24 months after start of biological therapy (%) | 16 (17.0) (n = 94) |
37 (25.0) (n = 148) |
.144 |
| Time to surgery after start of biological therapy within 24 months (months, median, range) | 7.5 [0–22] (n = 17) |
7 [0–24] (n = 40) |
.683 |
Overall, 148 CD patients were started with IFX after 24 months after first diagnosis of CD, defined as late intervention, 94 patients were started early with IFX, defined as start of IFX less than 24 months after first diagnosis of IBD. In the early intervention group, 61 patients and 86 patients in the late intervention group underwent endoscopy after start of IFX treatment. These patients were included in the endoscopy subgroup (Table 2). Bold values were considered significant. Abbreviations: CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; IBD, inflammatory bowel disease; IFX, infliximab.
Regarding sex, no significant difference was observed between early and late intervention subgroups of CD patients (P = .896, Table 1). Patients in the early intervention group were significantly older at first diagnosis of CD but were significantly younger at start of IFX (median of 25 years [range 15–62] vs median of 21 years [range 6–54], P < .001 and median of 26 years [range 16–64] vs median of 32 years [range 15–65], P = .001, Table 1).
According to the definition of early intervention, time to start first biological therapy with IFX was significantly lower in the early intervention group (median of 12 months [range 0–24] vs median of 98.5 months [range 26–486], P < .001, Table 1).
Disease Phenotype
Regarding Montreal classification, significantly more CD patients in the late intervention subgroup were younger than 17 years at first diagnosis of CD (32/148, 21.1%) compared to the early intervention subgroup (2/94, 2.1%, P < .001, Table 1). More patients in the early intervention cohort had additional upper gastrointestinal (GI) involvement (n = 21/94, 22.3% vs n = 7/148, 4.6% in the late intervention cohort, P < .001, Table 1). All other parameters of the Montreal classification were not different between both subgroups. A nonstricturing or nonpenetrating disease type was observed in almost half of both groups, n = 46/94 (48.9%) in the early intervention cohort versus n = 67/148 (45.3%) in the late intervention group (P = .229, Table 1).
Medical Treatment at Start of IFX
Regarding medical treatment at start of IFX, patients in the late intervention subgroup were more intensively treated. Significantly more patients in this subgroup were treated with 5-aminosalicylic acid (5-ASA) and thiopurines (azathioprine, 6-mercaptopurine), when biological treatment was started (for 5-ASA, n = 87/147 (59.2) versus n = 26/94 (27.7), P < .001, for thiopurines: n = 115/145 (79.2) versus n = 47/94 (50.0%), P < .001). Also, more patients with late IFX intervention received corticosteroids (n = 138/147 (93.9%) vs n = 80/94 (85.1%), P = .041) and methotrexate (n = 15/144 (10.1) vs n = 3/93 (3.2), P = .046, Table 1).
Disease Activity Before and After Start of IFX
Before start of biological therapy, disease activity was comparable between patients who were started early with IFX and patients in the late intervention group (CDAI of 150 [range 0–403], n = 93 in early IFX treated patients vs CDAI 145 [range 0–400], n = 148 in patients with late IFX intervention, P = .096).
After start of IFX, CDAI in both subgroups decreased significantly but were comparable between both groups (CDAI of 50 [range 0–240], n = 93 vs CDAI of 56 [range 0–400], n = 148, P = .575). At 24 months FU, CDAI was again comparable between both subgroups (CDAI of 20 [range 0–300], n = 92 vs CDAI of 36.5 [range 0–330], n = 148, P = .197, Table 1).
Most patients in both groups were in clinical remission after IFX induction (IFX infusions at weeks 0, 2, and 6, 5 mg/kg body weight; clinical remission defined as CDAI <150) and comparable between both groups (n = 84/93 (90.3%) versus 130/148 (87.8%), P = .676, Table 2) and stayed in clinical remission until the end of FU at 24 months (n = 82/92 (89.1%) versus n = 127/148 (85.8%), P = .554, Table 1).
Table 2.
Endoscopic subgroup
| Endoscopy subgroup | Early intervention (n = 61) | Late intervention (n = 86) | P |
|---|---|---|---|
| Sex (female (%)/male (%)) | 29 (47.5)/32 (52.5) | 41 (47.7)/45 (52.3) | 1 |
| Median age at diagnosis of CD (median, range) | 25 [15–62] | 20 [6–54] | <.001 |
| Median age at start biological therapy (median, range) | 26 [16–64] | 35 [15–63] | .002 |
| Median interval between diagnosis of CD and start biological therapy (months, median, range) | 12 [0–24] | 125 [28–486] | <.001 |
| Montreal classification | |||
| A1 ≤16 years at first diagnosis CD | 2 (3.2) | 19 (22.1) | .004 |
| A2 >17–40 years | 50 (82.0) | 59 (68.6) | |
| A3 >40 years | 9 (14.8) | 8 (9.3) | |
| L1 terminal ileum | 28 (45.9) | 41 (47.7) | .832 |
| L2 colon | 8 (13.1) | 17 (19.8) | .375 |
| L3 ileocolon | 27 (44.3) | 31 (36.1) | .390 |
| L4 only upper GI involvement | 0 | 0 | — |
| L4+ upper GI involvement and distal involvement | 13 (21.3) | 4 (4.7) | .011 |
| B1 nonstricturing, nonpenetrating | 31 (50.8) | 34 (39.5) | .037 |
| B2 stricturing | 19 (31.2) | 36 (41.9) | .186 |
| B3 internal penetrating | 0 | 0 | — |
| B3p perianal penetrating | 13 (21.3) | 22 (25.6) | .660 |
| Medical treatment before biological therapy | |||
| 5-ASA (%) | 15 (24.6) | 49 (57.7) (n = 85) |
<.001 |
| Corticosteroids (%) | 51 (83.6) | 83 (97.7) (n = 85) |
.004 |
| Thiopurines (azathioprine, 6-mercaptopurine) (%) | 29 (47.5) | 70 (83.3) (n = 84) |
<.001 |
| Methotrexate (%) | 3 (4.9) | 9 (11.0) (n = 82) |
.239 |
| Biological treatment (%) | 0 | 0 | |
| Disease activity before and after start of biological therapy (CDAI) | |||
| Before (median, range) | 150 [10–403] | 163 [40–400] | .204 |
| After induction treatment (median, range) | 50 [0–240] | 63 [0–400] | .244 |
| CDAI at 24 months after start of biological therapy (median, range) | 20 [0–300] | 43 [0–330] | .133 |
| Clinical remission after induction treatment (CDAI <150, %) | 54 (88.5) | 72 (83.7) | .479 |
| Clinical remission at 24 months after start of biological therapy (CDAI <150, %) | 55 (91.7) | 73 (84.9) | .307 |
| Endoscopic remission | |||
| Endoscopy after start biological therapy (months, median, range) | 13 [4–24] | 14 [6–24] | .566 |
| Endoscopic remission within 24 months after start of biological therapy (n, %) | 28 (45.9) | 22 (25.6) | .013 |
| Stenosis within 24 months after start of biological therapy (n, %) | 6 (9.8) | 25 (29.1) | .007 |
| Fistulizing CD within 24 months after start of biological therapy (n, %) | 2 (3.4) | 2 (5.0) (n = 40) |
.647 |
A total of 147 CD patients underwent endoscopy before start of IFX and within of 24 months of FU. In the early intervention group, 61 patients underwent endoscopy after a median of 13 months [range 4–24] whereas 86 patients in the late intervention group underwent endoscopy after a median of 14 months [range 6–24]. Bold values were considered significant. Abbreviations: CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; FU, follow-up; IFX, infliximab.
CD-Related Complications and Surgeries
Almost half of patients in the late intervention group (43.9%, n = 65/148) underwent surgery before starting IFX, compared to a minority of 7.5% (n = 7/94) of patients in the early intervention group (P < .001, Table 1). However, time from first diagnosis of CD until start of IFX was significantly longer (P < .001) in the late intervention cohort (median of 98.5 months [range 26–486] vs median of 12 months [range 0–24] in the early intervention group).
Nevertheless, at start of IFX, proportions of patients with stricturing disease phenotype B2 and perianal penetrating phenotype B3p were now comparable between both cohorts (n = 28/88 (31.8%) with B2 and n = 24/94 (25.5%) with B3p in the early intervention cohort vs n = 51/131 (38.9%) with B2 and n = 38/148 (25.7%) with B3p in the late intervention cohort, P = .450, Table 1).
Within the predefined FU of 24 months, surgery rate due to CD-related complications was numerically but not significantly lower in the early intervention group (Table 1, n = 16/94 (17.0%) vs n = 37/148 (25.0%) in the late intervention group, P = .144). Time to surgery was not different in the subgroups during 24 months of FU (Table 1, median of 7.5 months [range 0–22] early vs median of 7 months [range 0–24] in the late intervention cohort, P = .683).
To investigate the impact of early intervention strategies on ER and on disease behavior we focused then on patients who underwent endoscopy after start of IFX, during the FU of 24 months.
In the early intervention group, 61 patients underwent endoscopy after a median of 13 months [range 4–25] whereas 86 patients in the late intervention group underwent endoscopy after a median of 14 months [range 6–24]. These patients were included in the endoscopy subgroup (Table 2).
Subgroup of Patients Who Underwent Endoscopy Within 24 Months FU After Start of IFX
A total of 147 patients, including 86 with late IFX intervention and 61 with early intervention of IFX underwent endoscopy within 24 months FU (Table 2). Outcome of CD was followed for 24 months with respect to ER. In the late intervention subgroup, 76 of 86 patients (88.4%) underwent endoscopy before start of IFX, and all patients underwent endoscopy within 24 months, after a median of 14 months [range 6–24]. Before start of IFX only 1 patient showed ER, IFX was started in this patient due to steroid dependency. In the early intervention group, 56 of 61 patients (91.8%) underwent endoscopy before starting IFX, and all patients underwent endoscopy after a median of 13 months [range 4–25]. One patient already showed ER before starting IFX, biological treatment was also started in this patient due to steroid dependency. Time to endoscopy did not differ between both groups (P = .566, Table 2). Median age at diagnosis was significantly lower in the late intervention group (median of 20 years [range 6–54] vs median of 25 years [range 15–62] in the early IFX group, P < .001, Table 2), whereas at start of IFX, patients were significantly older in this subgroup (median of 35 years [range 15–63] vs median of 26 years [range 16–64], P = .002, Table 2). According to study design, time to start of IFX was significantly shorter in the early intervention group (median of 12 months [range 0–24] vs median of 125 months [range 28–486], Table 2).
Disease activity did not differ between both subgroups when IFX was started (P = .204, CDAI of 150 points [range 10–403] early vs CDAI of 163 points [range 40–400], Table 2). After induction treatment at weeks 0, 2, and 6 with IFX (5 mg/kg body weight) most patients in the early intervention group were in clinical remission (88.5%, n = 54/61), defined as CDAI <150 and with a median CDAI overall of 50 points [range 0–240] (Table 2). Comparable results were observed in the late intervention cohort, with 83.7% (n = 72/86) of patients being in clinical remission and an overall CDAI of 63 points [range 0–400], P = .479 and P = .244, respectively, Table 2.
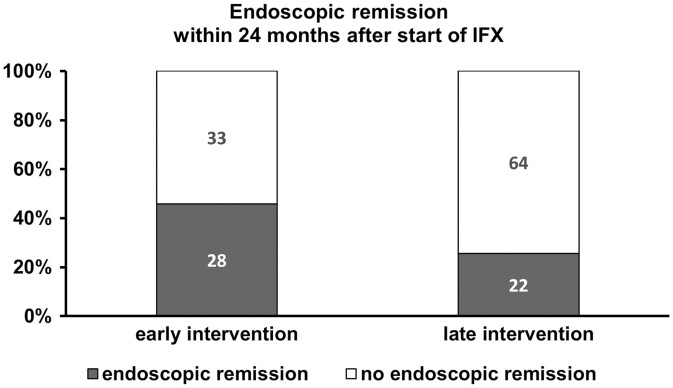
At 24 months FU, rates of patients being in clinical remission did not differ between both subcohorts (91.7% (n = 55/61) vs 84.3% (n = 73/86), early vs late, P = .307, median CDAI of 20 points [range 0–300] vs CDAI of 43 [range 0–330], P = .133, Table 2). However, more patients in the early intervention subgroup achieved ER at 24 months (45.9%, n = 28/61 vs 25.6%, n = 22/86, P = .013, Table 2).
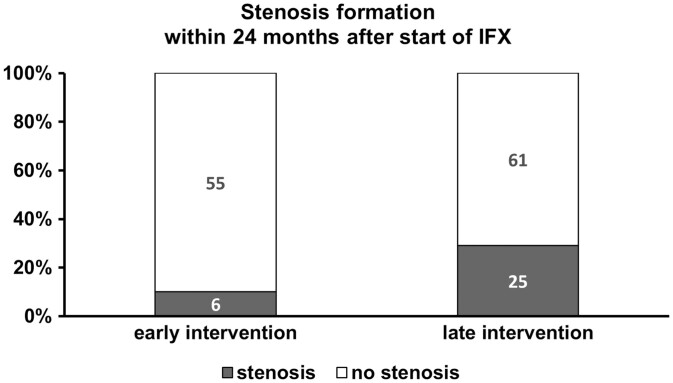
Significantly more patients in the late intervention subgroup (51.2%) had surgery before start of IFX compared to only 6.6% of patients in the early intervention group (6.6%, P < .001). During 24 months after start of IFX, more patients in the late intervention subgroup developed CD-related stenosis (29.1%, n = 25/86 vs 9.8%, n = 6/61, P = .007, Table 2, Figure 2). Stricturing disease behavior was symptomatic in these patients.
Figure 2.
Given are the proportion of patients (%) and the total number of patients who developed intestinal stenosis within 24 months after start of IFX and who underwent endoscopy after start of IFX. Information about intestinal stenoses were based on endoscopic findings (luminal narrowing) and/or magnetic resonance enterography (MRE, criteria for obstructive disease: narrowing of the intrastenotic luminal diameter, prestenotic dilatation). Abbreviation: IFX, infliximab.
Regarding fistulizing disease phenotype, no significant differences were observed between both subgroups (3.4%, n = 2/61 in the early subgroup vs 5.0%, n = 2/40 in the late intervention subgroup, P = .647, Table 2).
Impact of Early Intervention With IFX on Achieving ER and Formation of Intestinal Stenoses
First, logistic regression analysis was performed to prove if the chance of achieving ER was dependent on patients’ characteristics or medical treatment at start of IFX and course of CD before start of IFX. ER within 24 months FU after start of IFX was considered as dependent variable. Following predictor variables were included in the logistic regression model to have an influence on the chance of achieving ER: age at diagnosis, age at start of IFX, surgery before start of IFX, ER before start of IFX, CDAI before start of IFX, disease behavior before start of IFX, sex, concomitant medical treatment at start of IFX, namely 5-ASA treatment, azathioprine or 6-mercaptopurine, methotrexate, and early versus late intervention of IFX, respectively.
After including all variables, regression analysis identified early intervention as the only significant factor influencing the ability of achieving ER with an odds ratio of 3.79 (95% CI [1.233; 11.647], Nagelkerke R2 with 0.125, P = .020, Figure 1).
Figure 1.
Given are the proportion of CD patients (%) and the total number of patients who underwent endoscopy after start of IFX and who achieved endoscopic remission within 24 months after start of IFX. In total, 61 patients in the early intervention group and 86 patients in the late intervention group underwent endoscopy after start of IFX and 91.8%, respectively, 88.4% of these patients also underwent endoscopy before start of IFX. Abbreviations: CD, Crohn’s disease; IFX, infliximab.
Then, logistic regression was performed stepwise. The only variable included in this model was early/late intervention with an odds ratio of 2.386 (95% CI [1.1180; 4.825], Nagelkerke R2 with 0.057, P = .016, Figure 1).
Second, logistic regression was performed to rule out if following baseline characteristics had an influence on the development of intestinal stenoses within 24 months FU after start of IFX: age at diagnosis, age at start of IFX, surgery before start of IFX, ER before start of IFX, CDAI before start of IFX, disease behavior before start of IFX, stenosis formation at start of IFX and within 24 months after start of IFX (including de novo stenosis or restenosis at the anastomosis after bowel resection), sex, concomitant medical treatment at start of IFX, namely 5-ASA treatment, azathioprine or 6-mercaptopurine, methotrexate, and early versus late intervention of IFX, respectively. We especially were interested whether intestinal stenosis formation was dependent on the presence of stenoses at start of IFX and on early or late initiation of IFX therapy.
Late intervention of IFX was associated with a significant higher risk of development of stenoses after start of IFX compared to early IFX treatment (odds ratio of 3.474 (95% CI [1.286; 9.365], P = .014). Regarding the presence of existing strictures at start of IFX (including de novo stenosis or restenosis at the anastomosis after bowel resection), stricturing disease was associated with a significant higher risk of stenosis formation after start of IFX as compared to those without stricturing disease (odds ratio of 3.961 (95% CI [1.682; 9.329], P = .002)).
Discussion
In CD emerging evidence from epidemiological studies demonstrated a link between progressive disease behavior and the development of stenosis and fistulas resulting in a cumulative bowel damage requiring intestinal resection. As a consequence, the treatment target in CD changed from symptom control toward changing the course of disease long term to avoid irreversible bowel damage.18–21 Accordingly, since the introduction of biological therapies in IBD over 2 decades ago with the approval of IFX for CD in 1999, treatment goals were redefined from clinical remission toward ER or healing combined with quiescence of CD and avoiding surgery and hospitalizations.1–3,21–23
However, once the bowel damage has emerged, no therapeutic intervention can reverse the chronic bowel damage in CD and often the only remaining treatment option is surgical resection of the damaged part of the bowel.18
With biological therapies approved to date for the treatment of CD, namely anti-TNFs, eg, IFX, adalimumab, and certolizumab, the anti-integrin (vedolizumab) and the anti-IL 12/23 (ustekinumab), mucosal healing (MH) can be achieved in approximately 30%–50% of CD patients.23–27 The more advanced the damage of the bowel is, the less likely MH can be achieved in CD,18,28 mainly caused by chronic inflammation and significantly being associated with duration of CD.
Here, in our study cohort of 242 CD patients, early start of IFX within 24 months after first diagnosis showed a positive effect on the course of the disease for 24 months FU with a numerically lower need for surgery but a significantly lower risk of stricturing disease, respectively (P = .074 and P = .007, respectively, Table 1, Figure 2), although the proportion of patients with stricturing disease behavior was comparable at start of IFX in both groups (early: 28/88 (31.8%) vs late 51/131 (38.9%), P = .450).
According to logistic regression analysis, the risk for the development of stricturing disease within 24 months of FU depended on the presence of stricturing disease behavior at start of IFX in both subgroups of patients (odds ratio of 3.961, 95% CI [1.682; 9.329], P = .002) but also on the time of intervention: Late intervention of IFX was associated with a significant higher risk of development of stenoses after start as compared to early intervention (odds ratio of 3.474 (95% CI [1.286; 9.365], P = .014).
In a subgroup of CD patients, 61 patients with early intervention and 86 patients with late intervention, data on endoscopy after start of IFX and of the majority of patients before start of IFX were applicable and we focused on ER (Table 2). In concordance to a recently published meta-analysis13 on the efficacy and safety of early biologic treatment in adult and pediatric patients with CD, our data could demonstrate a positive effect of early initiation of IFX in CD patients and ER. Overall, 45.9% in the early IFX subgroup achieved ER after start of IFX versus 25.6% in the late intervention group (P = .013, Table 2). Logistic regression revealed the time point of intervention (early or late) as only significant factor influencing the ability of achieving ER with an odd ratio of 2.386 (95% CI [1.1180; 4.825], P = .016, Figure 1). Hence, patients with early intervention had a more than 2 times higher chance to achieve ER in the following 2 years after start of IFX as compared to patients with a later start.
Other factors like age at diagnosis of CD, age at start of biological therapy, surgery before start of IFX, ER and activity before start of IFX, CDAI at start of IFX, sex, and concomitant medical treatment at start of IFX (eg, 5-ASA, azathioprine or 6-mercaptopurine, corticosteroids, and methotrexate) had no influence on the impact of achieving ER after start of IFX.
However, some limitations of this actual study must be addressed. Firstly, its retrospective study design and secondly, the small numbers of patients in the subgroups, especially in the endoscopic sub cohort. No data on smoking status were available. Furthermore, there was no randomization between the groups, however, it is likely that patients in the early group were more severely affected than those in the late group, because IFX was started earlier in those patients in whom a less favorable disease course is expected. Our definition of early and late was based on start of IFX treatment less than 24 months at start of IFX after first diagnosis of CD versus more than 24 months in the late intervention group, not taking into account previous history of medical therapy. In the overall cohort we could provide endoscopic evaluation in 2/3 of our patients.
ER has been shown to be the most important factor of a favorable long-term outcome of CD and predicts clinical remission long term.28–32 IFX showed excellent efficacy in patients with CD in our cohort of 242 CD patients, no matter if started less than 24 months after first diagnosis of CD or started later, as the great majority of 90.3% and 87.8% of patients were in clinical remission after induction therapy with IFX, respectively.
But its impact of changing the natural course of CD mainly through inducing ER can be exponentiated when started early in CD.
It has become clear and obvious that in CD the pathogenetic factor with the most significant clinical impact is the vicious sequence in which ongoing inflammation in the mucosa and the adjacent wall leads to structural damage that is eventually replaced by fibrotic remodeling. These remodeling forces lead to luminal narrowing, stenosis, abscess formation, bowel shortening, surgical resections, and mutilations.21,33 Delaying the start of a disease modifying therapy in CD increases the risk of irreversible bowel damage in CD patients, consecutively increasing the need for surgical resection of the affected part of the bowel in various CD patients.12,18 Although we know the positive impact of early initiating potent anti-inflammatory drugs like IFX to stop this sequence, conventional and reluctant treatment approaches are still widely used for the treatment of CD.
Recently, the positive effect of early initiation of IFX in pediatric patients was also reported in 70 children with less intestinal surgery and a trend toward decreased hospital admissions than in children when IFX was started after 12 months after first diagnosis of IBD.34
Further large prospective trials should emphasize the impact of early IFX treatment on achieving ER and disease behavior long term, consecutively supporting early initiation of anti-TNF treatment in at least selected CD patients.
Conclusions
In conclusion, our observational trial supports early initiation of IFX in patients with CD and disease duration of less than 24 months until start of IFX. These patients had a more than 2 times higher chance of achieving ER and developed less CD-related stenoses in the first 2 years after initiating IFX.
Funding
This work has been financially supported by HEXAL Aktiengesellschaft.
Conflicts of Interest
None declared.
Data Availability
Data not publicly available. No new data were analyzed.
References
- 1. Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337(15):1029–1035. [DOI] [PubMed] [Google Scholar]
- 2. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350(9):876–885. [DOI] [PubMed] [Google Scholar]
- 3. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. [DOI] [PubMed] [Google Scholar]
- 4. Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126(2):402–413. [DOI] [PubMed] [Google Scholar]
- 5. D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371(9613):660–667. [DOI] [PubMed] [Google Scholar]
- 6. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–1395. [DOI] [PubMed] [Google Scholar]
- 7. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. [DOI] [PubMed] [Google Scholar]
- 8. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(suppl 3):s1–s106. PMID:31562236; PMCID:PMC6872448. doi: 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517. [DOI] [PubMed] [Google Scholar]
- 10. Ma C, Beilman CL, Huang VW, et al. Anti-TNF therapy within 2 years of Crohn’s disease diagnosis improves patient outcomes: a retrospective cohort study. Inflamm Bowel Dis. 2016;22(4):870–879. [DOI] [PubMed] [Google Scholar]
- 11. Rubin DT, Uluscu O, Sederman R. Response to biologic therapy in Crohn’s disease is improved with early treatment: an analysis of health claims data. Inflamm Bowel Dis. 2012;18(12):2225–2231. [DOI] [PubMed] [Google Scholar]
- 12. Safroneeva E, Vavricka SR, Fournier N, et al. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Aliment Pharmacol Ther. 2015;42(8):977–989. [DOI] [PubMed] [Google Scholar]
- 13. Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohns Colitis. 2013;7(3):213–221. [DOI] [PubMed] [Google Scholar]
- 14. Ungaro RC, Aggarwal S, Topaloglu O, Lee WJ, Clark R, Colombel JF. Systematic review and meta-analysis: efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn’s disease. Aliment Pharmacol Ther. 2020;51(9):831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70(3):439–444. [PubMed] [Google Scholar]
- 16. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(suppl A):5A–36A. PMID:16151544. doi: 10.1155/2005/269076 [DOI] [PubMed] [Google Scholar]
- 17. Van Assche G, Herrmann KA, Louis E, et al. Effects of infliximab therapy on transmural lesions as assessed by magnetic resonance enteroclysis in patients with ileal Crohn’s disease. J Crohns Colitis. 2013;7(12):950–957. [DOI] [PubMed] [Google Scholar]
- 18. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8(4):244–250. [DOI] [PubMed] [Google Scholar]
- 19. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389(10080):1741–1755. [DOI] [PubMed] [Google Scholar]
- 20. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–1640. [DOI] [PubMed] [Google Scholar]
- 21. Le Berre C, Peyrin-Biroulet L; group S-Is . Selecting end points for disease-modification trials in inflammatory bowel disease: the SPIRIT Consensus from the IOIBD. Gastroenterology. 2021;160(5):1452–1460.e21. [DOI] [PubMed] [Google Scholar]
- 22. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. [DOI] [PubMed] [Google Scholar]
- 23. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357(3):228–238. [DOI] [PubMed] [Google Scholar]
- 24. Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther. 2018;48(4):394–409. [DOI] [PubMed] [Google Scholar]
- 25. Rutgeerts P, Gasink C, Chan D, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology. 2018;155(4):1045–1058. [DOI] [PubMed] [Google Scholar]
- 26. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–721. [DOI] [PubMed] [Google Scholar]
- 27. Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367(16):1519–1528. [DOI] [PubMed] [Google Scholar]
- 28. Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45(10):1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group . Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133(2):412–422. [DOI] [PubMed] [Google Scholar]
- 30. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138(2):463–468; quiz e10–e11. [DOI] [PubMed] [Google Scholar]
- 31. Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58(4):492–500. [DOI] [PubMed] [Google Scholar]
- 32. Beigel F, Deml M, Schnitzler F, et al. Rate and predictors of mucosal healing in patients with inflammatory bowel disease treated with anti-TNF-alpha antibodies. PLoS One. 2014;9(6):e99293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmoyer CJ, Saidman J, Bohl JL, Bierly CL, Kuemmerle JF, Bickston SJ. The pathogenesis and clinical management of stricturing Crohn disease. Inflamm Bowel Dis. 2021:izab038. PMID:33693860. doi: 10.1093/ibd/izab038 [DOI] [PubMed] [Google Scholar]
- 34. Singh H, Nguyen T, Pho C, Giles E. Early infliximab in Crohn’s is associated with decreased intestinal surgery and similar health care costs. Scand j Gastroenterol. 2021;56(4):397–402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not publicly available. No new data were analyzed.