Abstract
Background
This study aims to evaluate sarcopenia defined by skeletal muscle index (SMI) with cutoffs adjusted for sex and body mass index as a predictive marker for postoperative outcomes among individuals with inflammatory bowel disease.
Methods
The SMI was measured using the cross-sectional computed tomography images at the lumbar spine. Multivariate logistic regression was performed to identify independent risk factors of postoperative complications.
Results
Ninety-one patients were included in the study. In multivariate analysis, sarcopenia (odds ratio = 5.37; confidence interval: 1.04–27.6) was predictive of infectious postoperative complications.
Conclusions
Sarcopenia as defined by the SMI is a predictor for 30-day postoperative infection complications in inflammatory bowel disease surgeries.
Keywords: sarcopenia, inflammatory bowel disease, postoperative complications
INTRODUCTION
Medical and surgical management decisions for patients with inflammatory bowel disease (IBD) are based on several clinical factors including risks of adverse events associated with therapy, age, comorbidities, and nutritional status. Up to 80% of patients with Crohn disease (CD) will require surgery in their lifetime and 30% of patients with ulcerative colitis (UC) will ultimately require colectomy.1,2 As a result of a malabsorptive and chronic inflammatory state, individuals with IBD encounter significant challenges in attaining adequate nutrient uptake, and malnutrition is a highly prevalent condition in IBD with up to 70% of individuals with active disease and 38% of patients in remission.3 Given the risks of both malnutrition and need for surgical therapies, individuals with IBD are at high risk for postoperative surgical complications.4 A preoperative nutritional assessment is advised in patients with IBD to adequately identify opportunities to intervene and potentially reduce the risk of postoperative complications.3,5–7
Several reports have demonstrated that altered body composition affects clinical outcomes in IBD.8–10 Holt et al reported on the association between visceral adiposity and endoscopic disease recurrence after surgery in patients with CD.11 Other studies utilizing routine cross-sectional imaging have also demonstrated that sarcopenia, as measured by a reduced muscle area, predicted the need for rescue therapy in active UC and need for intestinal resection in CD.12,13 While body composition analysis has been evaluated for associations with clinical outcomes in IBD, few studies have explored the relationship between reduced skeletal muscle volume and postoperative clinical outcomes in IBD.14–16
Cross-sectional imaging, mainly computed tomography (CT) is frequently used for the purpose of evaluating IBD disease severity.17 It also provides useful information on body composition and muscle volume. The cross-sectional area of skeletal muscle at the level of the third lumbar (L3) vertebra is considered a surrogate marker of total skeletal muscle volume and allows for calculation of the skeletal muscle index (SMI) after standardizing for a subject’s height.18–21 This measure has been developed and validated via regression equations to predict whole body composition adipose tissue and skeletal muscle mass.22 Previous studies have established cut-points for gender-specific SMI values defining sarcopenia and correlated these measures to adverse outcomes in patients with cancer, critical illness, and liver disease.23–28 Martin et al established body mass index (BMI)- and gender-specific set-points to categorize sarcopenia (males ≤43 cm2/m2 if BMI <25 and ≤53 cm2/m2 for all other BMI values; females ≤41 cm2/m2 regardless of BMI) which was predictive of poor survival in patients with gastrointestinal and respiratory tract cancer.29
In order to identify variables in patients with IBD that may benefit from preoperative nutritional support, we performed a retrospective study to evaluate the SMI as a predictor of postoperative outcomes.
METHODS AND MATERIALS
Study Population
Medical records of patients registered in a prospectively collected IBD database were retrospectively reviewed for those individuals that underwent surgery between June 2014 and April 2016. Study patients included adults with UC or CD who had an available abdominal CT scan performed within 3 months of the index surgical operation. Patients were excluded if they had a diagnosis of indeterminate colitis, did not have a CT scan performed within 3 months of surgery, if they did not have 30 days of follow-up after their operation or their operation was performed at an outside hospital.
Covariates and Outcomes
Demographic, clinical, and surgical data were collected via retrospective review of the electronic medical record. Demographic variables of interest included age, gender, weight, height, and BMI. Clinical characteristics included smoking history, IBD diagnosis, and duration of disease. Laboratory values were collected within 6 weeks of index surgery and included total white blood cell count, hemoglobin, platelet count, absolute lymphocytes, prealbumin, albumin, C-reactive protein, total protein, blood urea nitrogen, and creatinine. The prognostic nutritional index (PNI) was calculated using albumin concentration and peripheral blood lymphocyte count and dichotomized into low (<45) and high (≥45) as previously described.30 Similarly, the nutrition risk index (NRI) was calculated using patient weight and albumin as previously described.7 Severe malnutrition was considered for values <83.5.31
IBD surgery included “major surgeries,” which were defined as small bowel resection, colonic resection, ileocecectomy, ileocolectomy, stricturoplasty, proctectomy, total colectomy, and J-pouch creation or resection, and “minor surgeries,” which were defined as an examination under anesthesia, ostomy creation, ileostomy takedown, and hernia repair.
Data were further abstracted for outcomes which included 30-day postoperative complications, need for repeat surgery, length of hospital stay, and readmission to the hospital. The primary endpoint was postoperative complications, which were defined as infectious (wound infection or dehiscence, anastomotic leak, abscess, sepsis, fistula, pulmonary infection, and urinary tract infection) or noninfectious [small bowel obstruction, ileus, deep vein thrombus, pulmonary embolism, bleeding, hematoma, or anemia] occurring within 30 days of the index operation.
Quantification of SMI
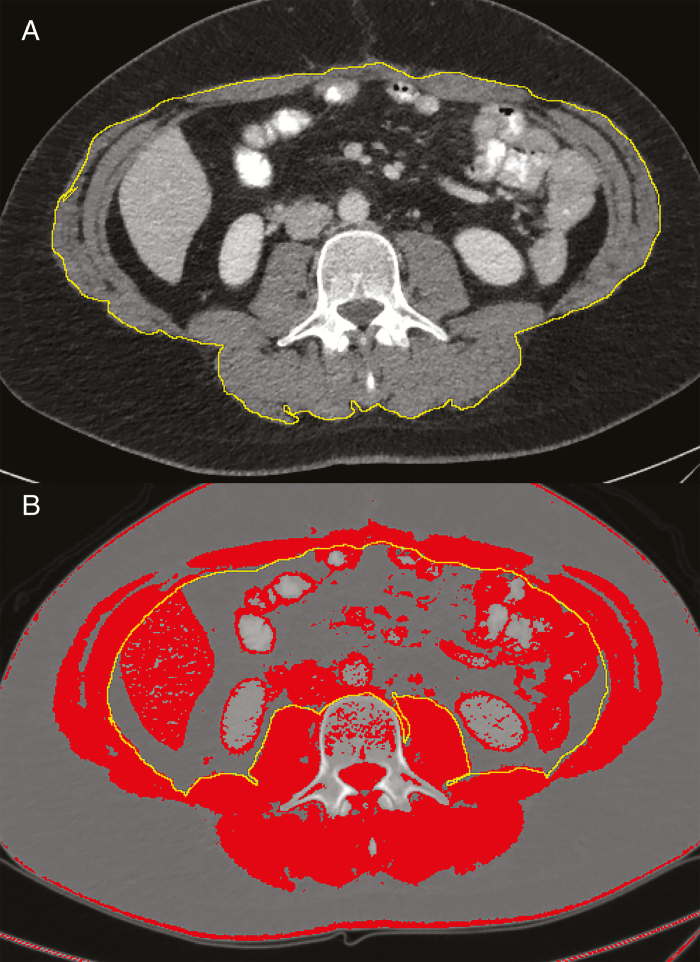
A single cross-sectional image from the mid-L3 vertebral body was taken from the original CT scan and uploaded to ImageJ (National Institutes of Health) as a DICOM file. The cross-sectional area of the abdominal skeletal musculature was determined by a single investigator (MB) and estimated using an electronic drawing pad (WACOM Bamboo, Kazo, Japan), following the methods previously described by Gomez-Perez et al.32 The data for the clinical outcomes were collected separately and therefore MB was blinded to this information while completing the measurements for SMI. The attenuation threshold used for delineating skeletal muscle was between −29 and +150 HU (Fig. 1).33
Figure 1.
Examples of skeletal muscle perimeter measurements of the mid-L3 cross-section on Image. A, Tracing of outer perimeter of skeletal muscle. B, Inner perimeter tracing with threshold set to Hounsfield units (HU) of skeletal muscle (−29 HU to +150 HU).
The SMI was defined as the L3 muscle area (cm2)/individual height (m2). Sarcopenia was defined as previously described by Martin et al (males SMI ≤43 cm2/m2 if BMI <25 and ≤53 cm2/m2 for all other BMI values; females SMI ≤41 cm2/m2 regardless of BMI).24,29
Statistical Analysis
The primary outcome of interest was to evaluate for the presence of sarcopenia as a predictor for postoperative complications in patients with IBD. Secondary outcomes included associations between sarcopenia and hospital readmission, repeat operation within 30 days, hospital length of stay (LOS), and postoperative LOS. Data are presented as mean ± SD or median [interquartile range (IQR)]. Comparison of means was performed using the Mann–Whitney U test and Fisher exact test was used to compare categorical variables. Logistic regression was used to determine associations between the dependent outcome, postoperative complications, and the independent predictive variables. Associations are described as odds ratios (ORs) with 95% confidence intervals (CIs). Variables with an association and P-value <0.1 in univariate analysis in addition to age, sex, disease type, and surgery class were included in backwards stepwise multivariable regression models for the prediction of infectious and noninfectious postoperative complications. A 2-sided P-value ≤0.05 was considered statistically significant in the final model derivation. The discriminative ability of different SMI set-points, the PNI, and NRI was assessed by the receiver operating characteristic curves and the area under the curve. Comparisons between area under the curves were performed by Delong test.34 Statistical analysis was conducted using JMP 13.1.0 (SAS Institute, Inc., Cary, NC).
Ethical Considerations
Patient identifiers were kept in a password protected file. There were no other ethical considerations as data were collected in a retrospective manner. The study was approved by the institutional review board at the University of Chicago (IRB 16-0061).
RESULTS
Baseline Characteristics
Four hundred thirty-nine patients were originally included from the prospectively collected database.35 From this cohort, 91 patients had an available abdominal CT scan performed within 3 months before the index surgical operation. The median patient age was 37 (IQR: 27–54) years and 46 (51%) patients were female. Fifty-nine patients (65%) had a diagnosis of CD and the median BMI was 23.8 (IQR: 20.2–29.2). Twenty-four percent of the study cohort were obese. The median albumin was 3.4 g/dL (IQR: 2.6–3.9) and median C-reactive protein was 23 mg/L (IQR: 6.5–85) (Table 1).
Table 1.
Demographic Features of Included Patients
| Cohort (n = 91) | |
|---|---|
| Demographics | |
| Age, years, median (IQR) | 37 (27–50) |
| Male sex, n (%) | 45 (49.5%) |
| Disease duration, years, median (IQR) | 11.6 (7.5–15.8) |
| CD, n (%) | 59 (64.8) |
| UC, n (%) | 32 (35.2) |
| BMI, median (IQR) | 23.8 (20.2–29.2) |
| Obese, n (%) | 22 (24.2) |
| Past smoking, n (%) | 31 (40.3) |
| SMI, males, median (IQR) | 56.9 (46.2–63.3) |
| SMI, females, median (IQR) | 36.1 (34–43) |
| Major surgery, n (%) | 67 (73.6) |
| Laboratory values | |
| WBC, 109/L, median (IQR) | 10.6 (10–12.6) |
| Hemoglobin, g/dL, median (IQR) | 10.7 (10.2–12) |
| Platelet count, 109/L, median (IQR) | 258.5 (183.8–302.5) |
| Albumin, g/dL, median (IQR) | 3.4 (2.6–3.9) |
| CRP, mg/L, median (IQR) | 23 (6.5–85) |
| Protein, g/dL, median (IQR) | 6.5 (5.5–7.4) |
| BUN, mg/dL, median (IQR) | 9 (8–9) |
| Creatinine, mg/dL, median (IQR) | 0.9 (0.7–1) |
| Medication use | |
| Steroid use, n (%) | 30 (33) |
| Immunomodulator, n (%) | 12 (13.2) |
| Anti-TNF, n (%) | 21 (23.1) |
| Anti-integrin, n (%) | 10 (11) |
BUN, blood urea nitrogen; CRP, C-reactive protein; TNF, tumor necrosis factor; WBC, white blood cell.
Forty-one (45%) patients had sarcopenia. Patients with sarcopenia were more often female (75.6% vs 30%; P < 0.001), and had lower levels of albumin (3.1 ± 0.83 vs 3.5 ± 0.67; P = 0.029), creatinine (0.95 ± 0.77 vs 1.1 ± 0.77; P = 0.047) and tended to more often have a diagnosis of UC (46.3% vs 26%; P = 0.05). Individuals with sarcopenia had a lower NRI (91.4 ± 18.3 vs 102.9 ± 17.9; P = 0.021), while no difference in PNI was demonstrated. The characteristics of the remaining demographic features, concurrent medication use, and laboratory values were similar between the 2 groups (Table 2).
Table 2.
Comparison of Individuals With Sarcopenia With Those Without Sarcopenia
| Sarcopenia (n = 41) | No Sarcopenia (n = 50) | P | |
|---|---|---|---|
| Age, years, mean (95% CI) | 41 (35.9–46.1) | 40 (35.7–44.3) | 0.917 |
| Male sex, n (%) | 10 (24.4) | 35 (70) | <0.001 |
| Disease duration, years, mean (95% CI) | 11.6 (7.5–15.8) | 9.4 (6.8–13.1) | 0.915 |
| CD, n (%) | 22 (53.7) | 37 (74) | 0.05 |
| UC, n (%) | 19 (46.3) | 13 (26) | 0.05 |
| BMI, mean (95% CI) | 24.2 (22.5–26) | 26.2 (24.3–28.1) | 0.186 |
| Past smoking, n (%) | 25 (65.8) | 21 (53.9) | 0.354 |
| Major surgery, n (%) | 29 (70.7) | 38 (76) | 0.636 |
| WBC, 109/L, mean (95% CI) | 11.8 (10.1–13.5) | 11 (10.1–12) | 0.801 |
| Hemoglobin, g/dL, mean (95% CI) | 10.7 (10.2–11.3) | 11.1 (10.6–11.6) | 0.458 |
| Platelet count, 109/L, mean (95% CI) | 267.8 (240.9–294.8) | 249.3 (223.5–275.1) | 0.206 |
| Albumin, g/dL, mean (95% CI) | 3.1 (2.8–3.4) | 3.5 (3.3–3.8) | 0.028 |
| CRP, mg/L, mean (95% CI) | 42.2 (25.9–58.5) | 43.7 (2.6–66.7) | 0.554 |
| Protein, g/dL, mean (95% CI) | 6.2 (5.8–6.6) | 6.7 (6.4–7.1) | 0.093 |
| BUN, mg/dL, mean (95% CI) | 9 (7.9–10.2) | 11.1 (8.4–13.9) | 0.797 |
| Creatinine, mg/dL, mean (95% CI) | 1 (0.7–1.2) | 1.1 (0.8–1.3) | 0.047 |
| PNI, mean (95% CI) | 34.2 (29.4–39.1) | 36.5 (32.1–41) | 0.284 |
| NRI, mean (95% CI) | 91.4 (84.3–98.5) | 102.9 (96.7–109.2) | 0.0211 |
| Steroid use, n (%) | 14 (34.2) | 16 (32) | 1 |
| Immunomodulator, n (%) | 4 (9.8) | 8 (16) | 0.536 |
| Anti-TNF, n (%) | 3 (7.3) | 7 (14) | 0.503 |
| Anti-integrin, n (%) | 9 (22) | 12 (24) | 1 |
Bold: P-value < 0.05.
BUN, blood urea nitrogen; CRP, C-reactive protein; TNF, tumor necrosis factor; WBC, white blood cell.
Risk Factors for Postoperative Complications
There were a total of 30 complications (33%) within 30 days: 13 (14.3%) patients had infectious complications and 17 (18.7%) patients had noninfectious complications. The most common infectious complication was abdominal abscess (n = 8) and the most common noninfectious complications were ileus (n = 3) and bleeding (n = 3). Risk factors associated with 30-day infectious postoperative complications are shown in Table 3. In univariate analysis, male sex (OR: 0.15, 95% CI: 0.03–0.71, P = 0.017) was inversely related to infectious postoperative complications and sarcopenia (OR: 8.8, 95% CI: 1.82–42.48, P = 0.007) was positively correlated to infectious postoperative complications. Risk factors associated with 30-day noninfectious postoperative complications are shown in Table 4. In univariate analysis, BMI (OR: 1.1, 95% CI: 1.02–1.2, P = 0.02) and serum creatinine (OR: 2.2, 95% CI: 1.02–4.7, P = 0.044) were positively associated with noninfectious postoperative complications.
Table 3.
Univariate Analysis for 30-Day Infectious Postoperative Complications
| OR | 95% CI | P | |
|---|---|---|---|
| Age | 0.98 | 0.96–1.04 | 0.894 |
| Male sex | 0.15 | 0.03–0.71 | 0.017 |
| Disease duration | 1.05 | 0.99–1.11 | 0.108 |
| Sarcopenia | 8.8 | 1.82–42.48 | 0.007 |
| CD | 0.85 | 0.25–2.84 | 0.788 |
| BMI | 0.94 | 0.85–1.05 | 0.307 |
| Past smoking | 1.6 | 0.46–5.51 | 0.456 |
| Major surgery | 1.23 | 0.31–4.9 | 0.771 |
| WBC count | 1.13 | 0.99–1.29 | 0.067 |
| Hemoglobin | 1.02 | 0.67–1.55 | 0.922 |
| Platelet count | 1 | 0.99–1.01 | 0.947 |
| Albumin | 0.44 | 0.16–1.22 | 0.115 |
| CRP | 0.99 | 0.97–1.02 | 0.55 |
| Protein | 0.73 | 0.37–1.44 | 0.359 |
| BUN | 0.96 | 0.83–1.12 | 0.618 |
| Creatinine | 0.05 | 0.001–1.9 | 0.105 |
| Steroid use | 0.57 | 0.14–2.23 | 0.417 |
| Immunomodulator | 0.51 | 0.06–4.3 | 0.534 |
| Anti-TNF | 1 | 0.25–4.03 | 1 |
| Anti-integrin | 0.64 | 0.07–5.51 | 0.683 |
Bold: P-value < 0.05.
BUN, blood urea nitrogen; CRP, C-reactive protein; TNF, tumor necrosis factor; WBC, white blood cell.
Table 4.
Univariate Logistic Regression for 30-Day Noninfectious Postoperative Complications
| OR | 95% CI | P | |
|---|---|---|---|
| Age | 1.01 | 0.98–1.05 | 0.385 |
| Male sex | 0.89 | 0.31–2.56 | 0.827 |
| Disease duration | 1.05 | 1–1.11 | 0.071 |
| Sarcopenia | 1.1 | 0.38–3.18 | 0.854 |
| CD | 0.73 | 0.25–2.14 | 0.566 |
| BMI | 1.1 | 1.02–1.2 | 0.02 |
| Past smoking | 0.78 | 0.22–2.82 | 0.705 |
| Major surgery | 1.2 | 0.35–4.13 | 0.768 |
| WBC count | 9.98 | 0.85–1.14 | 0.815 |
| Hemoglobin | 0.76 | 0.53–1.1 | 0.145 |
| Platelet count | 1 | 1–1.01 | 0.174 |
| Albumin | 1.32 | 0.52–3.31 | 0.559 |
| CRP | 1 | 0.98–1.02 | 0.912 |
| Protein | 1.24 | 0.63–2.45 | 0.539 |
| BUN | 1.05 | 0.98–1.13 | 0.157 |
| Creatinine | 2.2 | 1.02–4.7 | 0.044 |
| Steroid use | 0.82 | 0.26–2.58 | 0.73 |
| Immunomodulator | 0.85 | 0.17–4.3 | 0.848 |
| Anti-TNF | 1.03 | 0.3–3.58 | 0.961 |
| Anti-integrin | 0.45 | 0.05–3.83 | 0.466 |
Bold: P-value < 0.05.
BUN, blood urea nitrogen; CRP, C-reactive protein; TNF, tumor necrosis factor; WBC, white blood cell.
With respect to disease type, although individuals with sarcopenia more often had a diagnosis of UC, the presence of sarcopenia was similarly related to infectious postoperative outcomes in both disease types. Among individuals with CD, 6/22 (27.3%) individuals developed a postoperative infectious complication as compared to 2/37 (5.41%) without sarcopenia (P = 0.043). In UC, the outcome trended to significance with 5/19 (26.3%) individuals with sarcopenia developing a postoperative infectious complication as compared to no patients without sarcopenia (P = 0.064).
Multivariate Analysis
After adjusting for age, gender, disease type, and surgery class, sarcopenia (OR: 5.37, 95% CI: 1.04–27.69) remained significantly associated with infectious postoperative complications (Supplementary Table 1). In creation of a model for noninfectious postoperative complications, only disease duration (OR: 1.07, 95% CI: 1–1.15) and BMI (OR: 1.15, 95% CI: 1.02–1.28) were significantly associated with noninfectious postoperative complications (Supplementary Table 2).
Secondary Outcomes
Seventeen patients (18.7%) had a 30-day hospital readmission and 6 (6.6%) patients required a second surgical procedure within 30 days. Sarcopenia was not associated with hospital readmission (sarcopenia: 24.4% vs no sarcopenia: 14%; P = 0.281) but trended to an association with reoperation (sarcopenia: 12.2% vs no sarcopenia: 2%; P = 0.087). No differences were noted for hospital LOS (sarcopenia: 6.2 ± 5.2 vs no sarcopenia: 6.5 ± 4.9 days; P = 0.616) or postoperative LOS (sarcopenia: 5.8 ± 3.5 vs no sarcopenia: 5.5 ± 3.9 days; P = 0.684).
Model Comparisons
A subset of individuals (n = 61) had full data available to include SMI, PNI, and NRI to allow for paired comparison of individual variables for the prediction of infectious postoperative outcomes (Table 5). When compared to the Martin et al criteria used here,29 only the PNI as a dichotomous variable had a significantly lower predictive ability. While the Martin et al criteria which is adjusted for sex and BMI had the highest area under the receiver operating curve, significant difference was not demonstrated among SMI cutoff values.
Table 5.
Discriminative Ability of Different SMI Set-Points, PNI, and NRI Assessed by and the Area Under the Receiver Operating Curve (AUROC)
| Sarcopenia Prevalence (n = 62) | AUROC | P | |
|---|---|---|---|
| Martin et al29 | 28 (45%) | 0.691 | |
| Zhang et al36 | 33 (53%) | 0.644 | 0.052 |
| Galata et al37 | 12 (19%) | 0.517 | 0.059 |
| Prado et al24 | 30 (48%) | 0.607 | 0.198 |
| PNI30 | 47 (76%) | 0.447 | 0.025 |
| NRI7 | 14 (23%) | 0.628 | 0.610 |
Bold: P-value < 0.05.
P-value comparisons to Martin et al criteria.7,29,30,36,37 Sarcopenia criteria for each category: (i) Martin et al: males ≤43 cm2/m2 if BMI <25 and ≤53 cm2/m2 for all other BMI values; females ≤41 cm2/m2 regardless of BMI. (ii) Zhang et al: <55cm2/m2 in males and <39 cm2/m2 in females. (iii) Galata et al: <41.5cm2/m2 in males and <31.8 cm2/m2 in females. (iv) Prado et al: <52.4 for males, <38.5 for females. (v) PNI < 45. (vi) NRI < 83.5.
DISCUSSION
In this retrospective, single-center study including individuals who underwent IBD surgery, we identified several predictors associated with postoperative complications. Namely, we demonstrated that the categorical variable of sarcopenia, as defined by Martin et al, is a significant independent risk factor for 30-day infectious postoperative complications. This supports our understanding on the role of malnutrition associating with immunocompromise and susceptibility to infection.38,39
In comparison to other tools used for nutrition assessment such as the subjective global assessment and the nutritional risk screening 2002 (NRS-2002) which rely on variables requiring subjective interpretation, SMI is an objective marker of malnutrition, thus minimizing interobserver variability.40–42 In addition, the process for calculating SMI is easily reproducible and can be easily transferred to other practices who wish to adopt this model for nutrition assessment.32
With respect to the noninfectious complications, BMI and disease duration demonstrated positive associations. A higher BMI has been previously associated with increased postoperative complications in individuals undergoing liver transplantation and gastrectomy,43,44 and obesity has also been demonstrated to be an independent predictor of anastomotic leak and LOS in colorectal surgery. Increased adipose tissue in the omentum and mesentery limits the exposure to the abdominal compartment and may make it difficult to identify critical structures during surgery.45 Similarly, disease duration has previously been shown to the increase risk of postoperative complications, possibly related to differences in disease behavior or long-term nutritional complications related to active disease.46
In our study, patients with sarcopenia were more often female, had low albumin level and had a diagnosis of UC. Although a previous systematic review demonstrated that sarcopenia was more common in CD, the difference between studies is likely due to the variable inclusion of studies in the systematic review; only one study included individuals with UC that was specific to postoperative complications.47 This also highlights the need for additional studies in IBD to specifically determine the individual risk factors associated with the development of sarcopenia.
Skeletal muscle index has been previously evaluated in 2 studies related to the development of postoperative complications in CD.36,37 Zhang et al retrospectively reviewed 114 CD patients with cross-sectional imaging.36 Similar to our study, total skeletal muscle area at the level of the L3 vertebrae was used to obtain the SMI and sarcopenia was defined by cutoffs proposed by the International Consensus on Sarcopenia with SMI <55 cm2/m2 in men and <39 cm2/m2 in women.48 Utilizing these cutoffs, they demonstrated a higher prevalence of sarcopenia (61.4%) when compared to our study, but a similar risk of major postoperative complications (OR: 9.24, 95% CI: 1.1–77.5).36 Importantly, in the final multivariate analysis, preoperative nutrition support was protective of the development of postoperative complications. Galata et al also used SMI to assess postoperative complications.37 Utilizing a similar criterion for sarcopenia as in our study, Galata et al included 230 patients with CD and demonstrated a higher incidence of sarcopenia (70.4%) and ultimately created sex-specific cutoff values specific to the development of CD postoperative complications. In their study, SMI was an independent predictor of major postoperative outcomes defined as Clavien–Dindo grade ≥III (OR: 0.914, P = 0.002). However, unlike the Martin et al criteria,29 further categorization by BMI or comparison of performance between various definitions was not performed.
An objective marker for defining malnutrition in IBD patients is critical as it can affect preoperative decision making for nutritional status optimization. The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend for patients at risk for malnutrition, abdominal surgery should be postponed for 7–14 days to optimize nutritional status.49 In line with the findings by Zhang et al,36 a meta-analysis of CD patients undergoing abdominal surgery, patients who received preoperative nutritional support, mainly enteral nutrition had a decreased risk of postoperative complications.50 Although nutrition support was not formalized in this study with respect to preoperative nutrition risk, future studies will need to continue to address the role of nutrition support in response to identification of sarcopenia on preoperative imaging.
By using the sex and BMI-adjusted SMI cutoffs, we accounted for overweight and obesity, as obese patients often harbor occult, severe preexisting muscle depletion.29 We also chose the BMI-specific Martin et al criteria as BMI is incorporated in many conventional nutritional assessment tools including the recently published Global Leadership Initiative on Malnutrition (GLIM) criteria, a consensus report by the global clinical nutrition community.29,51 Future collaborative studies will be required to define age, sex, weight, and possible ethnic variations in the presence or absence of sarcopenia and its relationship to both preoperative and postoperative morbidity in IBD.
In our study, sarcopenia did not predict 30-day readmission or postoperative LOS, but numerically these values were greater in the sarcopenic population. Although prior studies have demonstrated longer hospital postoperative stays in the setting of sarcopenia,25 likely due to enhanced recovery programs in place in our hospital for IBD patients, differences in LOS were unable to be detected, in agreement with the study by Zhang et al.36
Our study is not without limitations. Given the retrospective nature of our study, we were not able to evaluate clinical features associated with malnutrition including the subjective global assessment which may have provided a useful model for comparison.40 Secondly, cross-sectional imaging was not widely available within 90 days of the index procedure, thus limiting the power of our study to detect postoperative noninfectious complications related to sarcopenia or additional variables of interest in multivariate modeling. We chose to look at cross-sectional imaging within 3 months of the index surgery because 3–6 months is often used as a standard timeframe for nutrition assessment tools.52,53
CONCLUSIONS
In conclusion, sarcopenia, as defined by SMI and adjusted for sex and BMI, is an independent risk factor for 30-day infectious postoperative complications in IBD. Further large studies or integration of data sets will be required to create age, sex, and BMI defined SMI cutoffs in IBD patients for defining sarcopenia. Creation of such measure will help standardize future studies related to IBD and sarcopenia while allowing for broader nutritional intervention studies to identify patients most likely to benefit from preoperative nutrition support.
Supplementary Material
Funding: There was no grant support or funding for this work.
Conflicts of interest: None declared.
Writing assistance: No support provided.
Authors’ contribution: Berger M: contributed to conception and design of the study, acquisition of data, interpretation of data, and drafting the article. Yamada A, Komaki Y, Komaki F, Sakuraba A: acquisition of the data, analysis and interpretation of data, critical revision of article, and final approval of version to be submitted. Cohen RD, Dalal S, Hurst RD, Hyman N, Pekow J, Shogan BD, Umanskiy K, Rubin DT: contributed to conception and design of the study, critical revision of article, and final approval of version to be submitted. Micic D: contributed to conception and design, acquisition of the data, analysis and interpretation of data, critical revision of article, and final approval of version to be submitted.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy requirements.
REFERENCES
- 1. Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 2. O’Brien S, Kavanagh RG, Carey BW, et al. The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease. Eur Radiol Exp. 2018;2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li S, Ney M, Eslamparast T, et al. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J Gastroenterol. 2019;25:3823–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaplan GG, McCarthy EP, Ayanian JZ, et al. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134:680–687. [DOI] [PubMed] [Google Scholar]
- 5. McKenna NP, Lightner AL. Preoperative considerations in inflammatory bowel disease. Surg Clin North Am. 2019;99:1083–1094. [DOI] [PubMed] [Google Scholar]
- 6. Schuetz P, Fehr R, Baechli V, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393:2312–2321. [DOI] [PubMed] [Google Scholar]
- 7. Buzby G. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525–532. [DOI] [PubMed] [Google Scholar]
- 8. Bryant RV, Trott MJ, Bartholomeusz FD, et al. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:213–225. [DOI] [PubMed] [Google Scholar]
- 9. Valentini L, Schaper L, Buning C, et al. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition. 2008;24:694–702. [DOI] [PubMed] [Google Scholar]
- 10. Schneider SM, Al-Jaouni R, Filippi J, et al. Sarcopenia is prevalent in patients with Crohn’s disease in clinical remission. Inflamm Bowel Dis. 2008;14:1562–1568. [DOI] [PubMed] [Google Scholar]
- 11. Holt DQ, Moore GT, Strauss BJ, et al. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2017;45:1255–1264. [DOI] [PubMed] [Google Scholar]
- 12. Bamba S, Sasaki M, Takaoka A, et al. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS One. 2017;12:e0180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cushing KC, Kordbacheh H, Gee MS, et al. Sarcopenia is a novel predictor of the need for rescue therapy in hospitalized ulcerative colitis patients. J Crohns Colitis. 2018;12:1256. [DOI] [PubMed] [Google Scholar]
- 14. Pedersen M, Cromwell J, Nau P. Sarcopenia is a predictor of surgical morbidity in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1867–1872. [DOI] [PubMed] [Google Scholar]
- 15. Dedhia PH, White Y, Dillman JR, et al. Reduced paraspinous muscle area is associated with post-colectomy complications in children with ulcerative colitis. J Pediatr Surg. 2018;53:477–482. [DOI] [PubMed] [Google Scholar]
- 16. Fujikawa H, Araki T, Okita Y, et al. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surg Today. 2017;47:92–98. [DOI] [PubMed] [Google Scholar]
- 17. Kilcoyne A, Kaplan JL, Gee MS. Inflammatory bowel disease imaging: current practice and future directions. World J Gastroenterol. 2016;22:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanai T, Shiraki M, Nishimura K, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193–199. [DOI] [PubMed] [Google Scholar]
- 19. Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173, 173.e1. [DOI] [PubMed] [Google Scholar]
- 20. Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore). 2016;95:e3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 22. Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 23. Moisey LL, Mourtzakis M, Cotton BA, et al. ; Nutrition and Rehabilitation Investigators Consortium (NUTRIC) . Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17:R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 25. Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan BH, Birdsell LA, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–6979. [DOI] [PubMed] [Google Scholar]
- 27. Montano-Loza AJ, Meza-Junco J, Baracos VE, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640–648. [DOI] [PubMed] [Google Scholar]
- 28. Weijs PJ, Looijaard WG, Dekker IM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 30. Mohri Y, Inoue Y, Tanaka K, et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688–2692. [DOI] [PubMed] [Google Scholar]
- 31. Prasad N, Sinha A, Gupta A, et al. Validity of nutrition risk index as a malnutrition screening tool compared with subjective global assessment in end-stage renal disease patients on peritoneal dialysis. Indian J Nephrol. 2016;26:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomez-Perez SL, Haus JM, Sheean P, et al. Measuring abdominal circumference and skeletal muscle from a single cross-sectional computed tomography image: a step-by-step guide for clinicians using National Institutes of Health ImageJ. JPEN J Parenter Enteral Nutr. 2016;40:308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 35. Yamada A, Komaki Y, Patel N, et al. Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with vedolizumab. Am J Gastroenterol. 2017;112:1423–1429. [DOI] [PubMed] [Google Scholar]
- 36. Zhang T, Cao L, Cao T, et al. Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn’s disease undergoing bowel resection. JPEN J Parenter Enteral Nutr. 2017;41:592–600. [DOI] [PubMed] [Google Scholar]
- 37. Galata C, Reißfelder C, Otto M. Response to skeletal muscle mass index predicts postoperative complications in intestinal surgery for Crohn’s disease. JPEN J Parenter Enteral Nutr. 2020;44:173. [DOI] [PubMed] [Google Scholar]
- 38. Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohns Colitis. 2013;7:107–112. [DOI] [PubMed] [Google Scholar]
- 40. Jeejeebhoy KN, Duerksen DR. Malnutrition in gastrointestinal disorders: detection and nutritional assessment. Gastroenterol Clin North Am. 2018;47:1–22. [DOI] [PubMed] [Google Scholar]
- 41. Kondrup J, Rasmussen HH, Hamberg O, et al. ; Ad Hoc ESPEN Working Group . Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. [DOI] [PubMed] [Google Scholar]
- 42. Baker JP, Detsky AS, Wesson DE, et al. Nutritional assessment: a comparison of clinical judgement and objective measurements. N Engl J Med. 1982;306:969–972. [DOI] [PubMed] [Google Scholar]
- 43. Barone M, Viggiani MT, Losurdo G, et al. Systematic review with meta-analysis: post-operative complications and mortality risk in liver transplant candidates with obesity. Aliment Pharmacol Ther. 2017;46:236–245. [DOI] [PubMed] [Google Scholar]
- 44. Jínek T, Adamčík L, Vrba R, et al. Risk factors and post-operative complications after gastrectomy for cancer. Rozhl Chir. 2018;97:384–393. [PubMed] [Google Scholar]
- 45. Champagne BJ, Nishtala M, Brady JT, et al. Laparoscopic colectomy in the obese, morbidly obese, and super morbidly obese: when does weight matter? Int J Colorectal Dis. 2017;32:1447–1451. [DOI] [PubMed] [Google Scholar]
- 46. Hossne RS, Sassaki LY, Baima JP, et al. Analysis of risk factors and postoperative complications in patients with Crohn’s disease. Arq Gastroenterol. 2018;55:252–257. [DOI] [PubMed] [Google Scholar]
- 47. Ryan E, McNicholas D, Creavin B, et al. Sarcopenia and inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2019;25:67–73. [DOI] [PubMed] [Google Scholar]
- 48. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 49. Forbes A, Escher J, Hébuterne X, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36:321–347. [DOI] [PubMed] [Google Scholar]
- 50. Brennan GT, Ha I, Hogan C, et al. Does preoperative enteral or parenteral nutrition reduce postoperative complications in Crohn’s disease patients: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:997–1002. [DOI] [PubMed] [Google Scholar]
- 51. Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9. [DOI] [PubMed] [Google Scholar]
- 52. Mijac DD, Janković GL, Jorga J, et al. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315–319. [DOI] [PubMed] [Google Scholar]
- 53. Kondrup J, Allison SP, Elia M, et al. ; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN) . ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy requirements.