Abstract
Background
Standardizing care through pathways has the potential to reduce emergency department (ED) utilization. We developed and evaluated inflammatory bowel disease (IBD) care pathways for that purpose.
Methods
Over 2014–2016, IBD patients were retrospectively stratified into those managed and not managed by pathways. Patient data were extracted, and negative binomial regression used to predict the annual number of ED visits.
Results
There was a difference of 30.7 ED visits/100 patients between managed and nonmanaged at 12 months (P < 0.001). The incidence rate ratio of total ED visits occurring annually was 0.750 (P = 0.008).
Conclusions
Management with IBD care pathways reduces ED utilization.
Keywords: inflammatory bowel disease, emergency department, healthcare utilization, clinical care pathway
INTRODUCTION
Inflammatory bowel disease (IBD), including Crohn disease (CD) and ulcerative colitis (UC), is a lifelong inflammatory condition that has significant implications for health, employment, quality of life, and healthcare system burden.1–3 The direct economic cost of IBD-related healthcare services in Canada is extremely high, reaching almost $12,000 per IBD patient per annum and will most likely continue to rise.4 Over the past 2 decades, the prevalence and incidence of IBD have been climbing steadily in the Western world, including Canada and the province of Alberta.5,6
Treatment options for IBD patients have improved dramatically due to the introduction of immunosuppressants and biologics.7,8 Early biologic initiation has subsequently led to significant decreases in complications, hospitalizations, and surgical rates.9–11 One would expect a comparable decrease in unplanned IBD care, such as emergency department (ED) utilization, but surprisingly, ED utilization continues to increase.12–14 Population-based studies from the United States suggest a 165% increase in IBD-related ED visits from 1994 to 2005 and a 52% increase from 2006 to 2014.14,15 Whether a result of unpredictable disease course, unexpected emergence of flares and complications, or high prevalence of extraintestinal manifestations and comorbidities, ED remains one of the most common points of interaction between IBD patients and the healthcare system.14,15
In Canada, less data have been reported, but 1 study reports ED attendance as high as 76% for the incident and 49% for prevalent IBD cases over a period of 3 years.16 Out of IBD patients who visited the ED, only 15.4% were admitted to hospital.16 This implies some degree of preventability of ED visits among the IBD population. Furthermore, 60% of patients in a European cohort felt that an ED visit could have been avoided if there was better management and more information available to them regarding their disease.17 Ultimately, the cause for the high rate of ED utilization in IBD is multifactorial and necessitates further study.
Various interventions have been tested with the attempt to reduce ED use among patients with chronic diseases, including case management, acute disease management and education, primary care linkage, navigation, coordination, increasing specialist access,18 and standardization of care.19 A common approach to standardizing care is the implementation of clinical care pathways.20 Such interventions have been effective in reducing ED utilization among ED users with asthma, anxiety disorders, and alcohol dependence.21–28 Literature on the effect of clinical care pathways for IBD is sparse29–31 and, to our knowledge, they have not been thoroughly investigated for reducing ED utilization by IBD patients.
IBD specialists at the University of Alberta and University of Calgary developed and implemented an innovative model of care, using Inflammatory Bowel Disease Clinical Care Pathways (IBD CCP). The aim of this study was to evaluate the impact of the IBD CCP model on ED use and predictors of ED utilization, for both total and IBD-related visits.
MATERIALS AND METHODS
Setting
The IBD Unit provides specialized, comprehensive, evidence-based, long-term IBD care to approximately 3400 IBD patients from the Edmonton region and surrounding areas, including rural Northern Alberta. The IBD Unit integrates a coordinated network of care providers (IBD specialists, IBD nurses, dietitians, and colorectal surgeons). This multidisciplinary team provides routine and semiurgent coordinated care to IBD patients.
IBD CCP Development, Implementation, and Uptake
To standardize care for IBD patients, IBD care providers at the [redacted institutions] developed the IBD CCP over a series of working group meetings in 2013. These IBD CCP are structured, standardized, evidence-based management algorithms, identifying an appropriate sequence of diagnostic and clinical interventions, and timeframes for IBD patients. They contain recommended diagnostic tests, medication doses, follow-up appointment intervals, admission orders, and discharge planning instructions specific to IBD patients. They are supported by systematic reviews of published evidence and are comprised of protocols, algorithms, and checklists that help to harmonize clinical and administrative IBD care.
The IBD CCP were accepted by the IBD specialists at the IBD Unit and incorporated into their routine clinical practice and office administration. They were initially introduced in a paper-based format, followed by electronic PDF documents. They were also made available in a web-based format on the clinic’s web site http://www.ibdclinic.ca/ibd-ccp, which could be accessed as a shortlisted URL from within electronic medical record (EMR) software used at the institution.
Study Design
This is a retrospective, observational, single-center study, designed to provide a proof-of-principle for the IBD CCP model of care.
Study Population and Inclusion
The inclusion criteria were: patients over 17 years of age with a confirmed diagnosis of IBD who had at least 1 appointment with an IBD specialist at the IBD Unit during April 2014–September 2016 with an ICD-9-CA code(s) indicative of CD (555.x), UC (556.x), or other and unspecified noninfectious gastroenteritis and colitis (558.9, including indeterminate colitis) in the primary diagnosis field. To assess the impact of the IBD CCP on the ED visits rate, patients were stratified into 2 groups. The “managed” group consisted of IBD patients who were under the care of IBD specialists and specialized IBD nurses at the IBD Unit in the 18 months preceding the study period, during which IBD care was provided according to the IBD CCP. The “nonmanaged” control group included IBD patients who were not under the care of the IBD specialists and specialized IBD nurses at the IBD Unit over 18 months preceding the study period.
Data Sources
Patient demographics, clinical and disease information, comorbidities (including malignancy, psychiatric illness, asthma, anemia, diabetes, hypertension, dyslipidemia, gastroesophageal reflux disease, kidney disease, obesity, Barrett esophagus, and celiac disease), vitamin and macronutrient deficiencies (vitamin D, vitamin B12, iron, and calcium), surgical and medication history data were extracted from the EpicCare Ambulatory EMR (Epic Systems Corporation, Verona, WI), and via manual review of patient charts. Data for ED visits were obtained from the National Ambulatory Care Reporting System (NACRS).32
ED Utilization
All ED visits were stratified into IBD-related vs non-IBD-related based on the presence of any presentations associated with IBD (in the form of ICD-10 codes) in the first 5 positions of the NACRS record.32 The clinical definition of a presentation as IBD-related was determined a priori, decided upon by consensus between authors R.N.F. and E.L. If no IBD-related presentations were identified in the first 5 positions of the NACRS record, the ED visit case was classified as non-IBD-related.
All ED visits were captured as cumulative at 12 months before and 3, 6, and 12 months after the IBD specialist’ appointment at the IBD Unit which served as the baseline timepoint. For each IBD patient, the IBD-related and non-IBD-related visits were captured separately, and their sum constituted the total number of ED visits.
ED visits rate was calculated as the number of ED visits throughout the observation study period divided by the patients’ population in the cohort and multiplied by 100. Therefore, the ED visits rate (“ED rate”) was represented as the ED visits rate per 100 patients at each of the study timepoints.
Statistical Analysis
Parametric statistical methods were applied for analysis of baseline patient characteristics and group comparison, as the data were normally distributed. Continuous data were presented as the mean and standard deviation (µ ± SD), whereas categorical data were presented as a percentage and number (% (n)). Means were compared using a 2-tailed independent-samples t test.33–35 Proportions were compared using Fisher exact test. Due to the multiple comparisons problem, the Benjamini–Hochberg procedure was performed with a false discovery rate setup as 5% to strengthen statistical conclusions.36,37
The ED visits data were not normally distributed for the study cohort (Kolmogorov–Smirnov test). Therefore, nonparametric statistical methods were applied to analyze ED visit data. Negative binomial regression was used to predict the annual number of ED visits based on the IBD CCP management status, demographics, phenotypical, clinical, and disease-specific factors. Unadjusted (univariate analysis) and, using purposeful selection methods (cutoff of P ≤ 0.10), adjusted models were constructed for ED visit rates: 1 for all ED visit types and 1 for only IBD-related ED visits.38,39 The primary reference group consisted of patients not managed using IBD CCP. The “main effects” models were explored for interaction and confounding, assumptions of the negative binomial distribution were tested and confirmed.40–42
A combination of software was used: SPSS 23.043 for descriptive and exploratory analyses, R 3.5.1 for regression analyses (MASS, visreg, and stargazer packages44,45), and Tableau 10.546 for data visualization. P-value ≤0.05 established statistical significance, unless otherwise specified (Benjamini–Hochberg procedure).
Ethical Considerations
The study was approved by the University of Alberta Health Research Ethics Board (Pro00069433).
RESULTS
Patient Characteristics
The initial cohort of IBD patients meeting study inclusion criteria was comprised of 2552 patients. Of these, patients with lymphocytic colitis (n = 43), collagenous colitis (n = 20), indeterminate colitis (n = 28), and Behçet disease (n = 3) were excluded from the study as the CCP did not apply to them specifically. As a result, 2458 IBD patients with CD (n = 1504) and UC (n = 954) constituted the study cohort.
Baseline characteristics of the IBD patients stratified by managed vs nonmanaged are presented in Table 1. The mean age of the IBD patient cohort was 41.4 ± 15.9 years, ranging from 17 to 91 years old. The mean age at IBD diagnosis was 29.6 ± 14.6 years and ranged from 2 to 88 years. The mean time from the disease onset was 12.3 ± 10.8; 68.1% of patients had IBD for over 5 years, and almost a half (48.1%) had IBD greater than 10 years. Over a third (34.5%) of patients trialed 2 or more different groups of the IBD medications prior to or at the time of enrollment in the study. Biologic therapy was the most prevalent and nearly the same proportions of patients were on 5-aminosalicylic acid (33.3%) as those on immunosuppressants (32.0%). Of all patients, 12.3% received corticosteroid therapy and 3.3% developed steroid dependence. A larger proportion (61.2%) of patients had CD (n = 1504),47 with a significantly greater prevalence in managed patients (P = 0.003).
TABLE 1.
Comparison of Baseline Patient Characteristics Between IBD Patients Managed Using IBD CCP and Not Managed Using IBD CCP
| Patient Characteristics | Total (n = 2458) | IBD CCP Managed (n = 2072) | IBD CCP Nonmanaged (n = 386) | P (χ 2) |
|---|---|---|---|---|
| Demographical information | ||||
| Age, years, µ ± SD | 41.4 ± 15.9 | 41.4 ± 15.8 | 43.4 ± 16.5 | 0.020 |
| Sex | ||||
| Male, % (n) | 48.9 (1201) | 48.4 (1002) | 51.6 (199) | 0.249 |
| Residing in Edmonton, % (n) | 52.7 (1295) | 54.0 (1118) | 45.9 (177) | 0.004 |
| Residing in Greater Edmonton,* % (n) | 62.4 (1533) | 64.0 (1326) | 53.6 (207) | <0.001 |
| IBD disease characteristics | ||||
| CD, % (n) | 61.2 (1504) | 62.5 (1294) | 54.4 (210) | 0.003 |
| Age at diagnosis, years, µ ± SD | 29.6 ± 14.6 | 29.3 ± 14.4 | 31.5 ± 15.7 | 0.011 |
| Disease duration, years, µ ± SD | 12.3 ± 10.8 | 12.3 ± 10.9 | 12.0 ± 10.3 | 0.656 |
| Medication history, at any time over the course of the disease | ||||
| Two and more IBD medication groups trialed, % (n) | 34.5 (847) | 36.9 (765) | 21.2 (82) | <0.001 |
| 5-ASA, % (n) | 33.3 (818) | 33.3 (689) | 33.4 (129) | 0.953 |
| Oral | 32.2 (790) | 32.2 (667) | 31.9 (123) | 0.953 |
| Rectal | 6.2 (153) | 6.3 (130) | 6.0 (23) | 0.909 |
| Immunosuppressants, % (n) | 32.0 (786) | 33.8 (701) | 22.0 (85) | <0.001 |
| Azathioprine | 25.8 (633) | 27.2 (563) | 18.1 (70) | <0.001 |
| 6-Mercaptopurine | 1.4 (35) | 1.5 (31) | 1.0 (4) | 0.484 |
| Methotrexate | 5.1 (125) | 5.5 (114) | 2.8 (11) | 0.029 |
| Biologic therapy, % (n) | 42.7 (1050) | 46.3 (960) | 23.3 (90) | <0.001 |
| Anti-tumor necrosis factor agents | ||||
| Infliximab | 22.2 (545) | 24.4 (505) | 10.4 (40) | <0.001 |
| Adalimumab | 15.1 (371) | 16.0 (332) | 10.1 (39) | 0.002 |
| Golimumab | 0.8 (19) | 0.9 (18) | 0.3 (1) | 0.341 |
| Anti-integrin agent | ||||
| Vedolizumab | 3.2 (79) | 3.5 (72) | 1.8 (7) | 0.114 |
| Anti-interleukin 12/23 agent | ||||
| Ustekinumab | 3.7 (90) | 3.9 (81) | 2.3 (9) | 0.142 |
| Corticosteroids, % (n) | 12.3 (302) | 12.2 (252) | 13.0 (50) | 0.673 |
| Oral | ||||
| Prednisone | 6.6 (161) | 6.3 (131) | 7.8 (30) | 0.313 |
| Budesonide | 4.9 (121) | 5.0 (104) | 4.4 (17) | 0.701 |
| Rectal | 2.1 (52) | 2.2 (45) | 1.8 (7) | 0.847 |
| Steroid dependence, % (n) | 3.3 (80) | 3.5 (73) | 1.8 (7) | 0.087 |
| Narcotics, % (n) | 9.0 (221) | 8.7 (180) | 10.6 (41) | 0.244 |
| Surgical history, at any time over the course of the disease | ||||
| IBD-related surgical history, % (n) | 30.6 (752) | 31.1 (644) | 28.0 (108) | 0.230 |
| Jejunal resection | 0.2 (6) | 0.3 (6) | 0.0 (0) | 0.290 |
| Ileal resection | 6.8 (167) | 6.7 (139) | 7.0 (27) | 0.837 |
| Ileocecal resection | 10.8 (265) | 10.7 (223) | 10.9 (42) | 0.945 |
| Ileal resection and right hemicolectomy | 3.9 (96) | 3.8 (78) | 4.7 (18) | 0.403 |
| Right hemicolectomy | 1.7 (42) | 1.8 (38) | 1 (4) | 0.267 |
| Subtotal colectomy | 2.3 (57) | 2.4 (49) | 2.1 (8) | 0.726 |
| Total colectomy and end ileostomy | 3.3 (82) | 3.4 (70) | 3.1 (12) | 0.787 |
| Total colectomy and ileal pouch/anal anastomosis | 3.1 (75) | 3.3 (68) | 1.8 (7) | 0.124 |
| No. IBD-related surgeries, µ ± SD (range) | 1.82 ± 2.00 | 1.78 ± 2.06 | 2.00 ± 1.74 | 0.468 |
| Vitamin and micronutrient deficiencies | ||||
| Vitamin and micronutrient deficiencies, % (n) | 55.0 (1352) | 58.9 (1220) | 34.2 (132) | <0.001 |
| Health status | ||||
| Weight status | ||||
| BMI, kg/m2, µ ± SD | 27.2 ± 6.12 | 27.2 ± 6.1 | 27.1 ± 6.2 | 0.849 |
| Underweight | 2.7 (56) | 2.6 (48) | 3.0 (0.682) | 0.682 |
| Overweight | 31.2 (654) | 31.5 (577) | 29.2 (77) | 0.478 |
| Smoking status | ||||
| Current smoker | 15.4 (377) | 15.4 (318) | 15.4 (59) | 1.000 |
| Healthcare utilization | ||||
| Has PCP, % (n) | 91.6 (2249) | 92.6 (1916) | 86.3 (333) | <0.001 |
| Frequent ED visits, ≥3 in 1 year before appointment at the IBD Unit | 15.4 (379) | 14.8 (307) | 18.7 (72) | 0.065 |
| Frequent IBD-related ED visits, ≥3 in 1 year before appointment at the IBD Unit | 6.8 (167) | 6.6 (136) | 8.0 (31) | 0.321 |
Values are expressed as µ ± SD or % (n). Analyses are based on 2-sided t test and Fisher exact test. The Benjamini–Hochberg procedure was applied with a false discovery rate set up as 0.05. P-values for significantly different results are bolded.
*Greater Edmonton (Edmonton census metropolitan area) was defined according to the Electoral of Statistics Canada as a conglomeration of 5 cities (Edmonton, Fort Saskatchewan, Leduc, St. Albert, and Spruce Grove).68
5-ASA, 5-aminosalicylic acid; BMI, body mass index.
IBD CCP Managed Patients’ Covariates
IBD CCP managed and nonmanaged patients were compared across baseline patient characteristics to identify possible covariates which might contribute to the ED visit occurrence (Table 1). Of patients in the study, 84.3% (n = 2072) were managed using IBD CCP. More patients with CD than with UC were managed using IBD CCP throughout the observation period (86.0% vs 81.6%, P = 0.003). The majority of managed patients tended to live in Edmonton (54.0% vs 45.9%, P = 0.004) and Greater Edmonton (64.0% vs 23.6%, P < 0.001). Compared to nonmanaged patients, they were also more likely to be receiving immunosuppressive (33.8% vs 22.0%, P < 0.001) and biologic therapy (46.3% vs 23.3%, P < 0.001). Over one-third of them were on 2 or more medications historically vs only one-fifth among nonmanaged patients (P < 0.001). Managed patients also made up a greater proportion of patients with vitamin and micronutrient deficiencies, namely vitamin D deficiency (58.9% vs 34.2%, P < 0.001; 48.1% vs 19.4%, P < 0.001, respectively). The vast majority of managed and nonmanaged patients had a primary care provider (PCP, 92.6% vs 86.3%, P < 0.001).
It is also important to note that proportions of frequent ED users (stratified by >3, >5, or >10 visits in the preceding year) did not differ significantly between managed and nonmanaged patients in the whole study cohort or within CD and UC groups.
ED Utilization
Cumulatively, there were a total of 937 ED visits at 3 months, 1738 ED visits at 6 months, and 3190 ED visits at 12 months. Out of those, IBD-related ED visits constituted 34.8% at 3 months, 37.1% at 6 months, and 37.9% at 12 months. The 3190 total ED visits consisted of 984 IBD patients, while the 1211 IBD-related visits were had by 510 IBD patients. The CD patients accounted for the majority of IBD-related visits—69.6%, 68.6%, and 67.9% at 3, 6, and 12 months, respectively. The top-10 ED visit reasons/diagnoses for IBD-related and non-IBD-related visits among IBD patients in the study cohort are presented in Table 2.
TABLE 2.
Top-10 ED Visit Reasons/ED Diagnoses Among IBD Patients in the Study Cohort
| IBD-Related ED Visits | Non-IBD-Related ED Visits | ||
|---|---|---|---|
| ICD-10-CA69 Diagnosis Code(s) | Visit Diagnosis | ICD-10-CA84 Diagnosis Code | Visit Diagnosis |
| R104/R520/R1010/R1039/ R1031 | Abdominal pain/acute pain/Right upper quadrant pain/Left lower quadrant pain | J069/J029/J329 | Acute upper respiratory tract infection/acute pharyngitis/ sinusitis |
| K922/T810/K625/K921 | Gastrointestinal hemorrhage/ postprocedural hemorrhage/melena | N390 | Urinary tract infection |
| K566 | Intestinal obstruction | R074/R073 | Chest pain |
| E860 | Dehydration | N200/N23 | Nephrolithiasis/renal colic |
| Z480 | Attention to surgical dressings and sutures | R51/R42 | Headache/dizziness |
| R113/R111 | Nausea with vomiting/nausea | J189 | Pneumonia |
| K590 | Constipation | S9349 | Sprain and strain of ankle |
| D649 | Anemia | Z760 | Issue of repeat prescription |
| A419 | Sepsis | LO311 | Cellulitis |
| R53 | Malaise and fatigue | M545 | Low back pain |
Source: AHSDRRX—NACRS.32
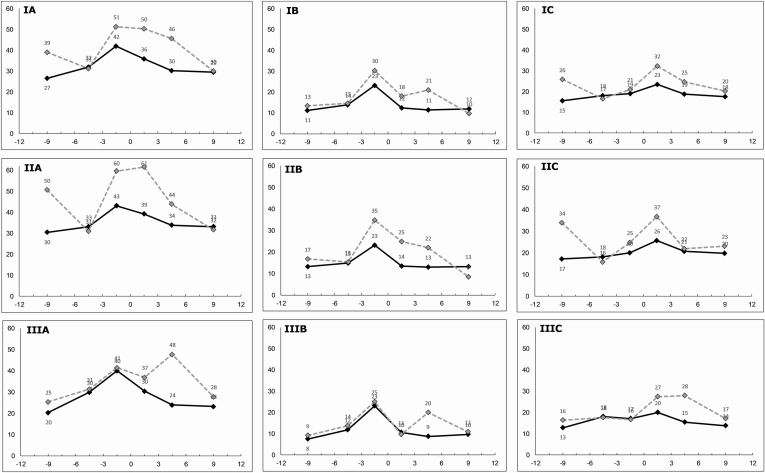
A difference of 30.7 ED visits/100 patients between managed and nonmanaged patients at 12 months corresponded to 636 actual total ED visits avoided annually for all IBD patients in the study who were managed using IBD CCP. The difference in ED visit rates between the 2 cohorts is shown in Figure 1, which visualizes the ED rate trend lines at 3, 6, and 12 months follow-up, and 3, 6, and 12 months prior to baseline appointment with the IBD Unit.
FIGURE 1.
Temporal trends in ED visits per 100 patients per 3 months, before and after baseline appointment at the IBD Unit (month 0) for: (I) All IBD patients; (II) Crohn’s disease; (III) Ulcerative colitis; (A) Total ED visits; (B) IBD-related ED visits; (C) non-IBD-related ED visits. The solid black line represents the ED rate for IBD patients managed using IBD CCP. The dotted grey line represents the ED rate for IBD patients not managed using IBD CCP.
During the first 3 months, there were 28.6% fewer (35.9 vs 50.3, P < 0.001) ED visits among all managed IBD patients compared to nonmanaged (Fig. 1A). The relative difference was similar for both IBD-related and non-IBD-related visits with 30.7% and 27.5% difference between managed and nonmanaged patients, respectively (Figs. 1B, C). The absolute IBD-related ED rate decrease was greater for managed patients compared to those nonmanaged at the 3-month timepoint (0.9 ED visits/100 patients, 9.1%).
At the 6-month timepoint, the absolute difference in ED rate between managed and nonmanaged patients widened, compared to the 3-month timepoint (15.4 vs 14.4), while the overall rate for both decreased (Fig. 1A). For the IBD-related visits, nonmanaged group ED rate increased while the managed group decreased, with an absolute difference of 9.5 ED visits/100 patients (Fig. 1B).
By the 12-month timepoint, the ED visit rate for managed and nonmanaged IBD patients converged, with an absolute difference of only 0.5 ED visits/100 patients (Fig. 1A). This convergence was also seen for IBD-related visits, with an absolute difference of 2.3 ED visits/100 patients (Fig. 1B), and for non-IBD-related visits with an absolute difference of 2.8 ED visits/100 patients (Fig. 1C).
Across both CD and UC and IBD-related and non-IBD related visit types, a trend with significantly lower ED rates for managed IBD patients compared to nonmanaged ones over the course of 12 months was evident (Figs. 1D–I). Of note, there were no significant differences in proportions of frequent ED users (defined by >3, >5, or >10 visits) in the follow-up period, between managed and nonmanaged patients.
Predictors of ED Visits for IBD Patients
The results for univariate (unadjusted) and multivariate (unadjusted) negative binomial regression analyses are presented in Table 3. The possible contributing factors investigated included demographics, disease-specific, clinical, and medication-related covariates. Parameters were considered for model inclusion based on statistically significant differences between managed and nonmanaged patient groups, or significance in univariate prediction of ED visit rates. Purposeful selection was applied to determine the main effects models (Table 3).
TABLE 3.
Results of Univariate (Unadjusted) and Multivariate (Adjusted) Negative Binomial Regression Analysis for the Impact of IBD Patient Management With CCPs, Demographics and Disease Characteristics, on Annual ED Visit Rates
| Univariate (Unadjusted) | Multivariate (Adjusted) | |||||
|---|---|---|---|---|---|---|
| Variable | IRR | 95% CI | P | IRR | 95% CI | P |
| Model for annual rate of total ED visits (IBD and non-IBD-related) | ||||||
| Managed using IBD CCP | 0.803 | 0.641–0.996 | 0.050 | 0.750 | 0.603–0.928 | 0.008 |
| Sex | ||||||
| Female | ref | ref | ||||
| Male | 0.715 | 0.608–0.841 | <0.001 | 0.744 | 0.637–0.868 | <0.001 |
| Residing in Greater Edmonton* | 0.349 | 0.298–0.408 | <0.001 | 0.373 | 0.319–0.435 | <0.001 |
| Disease type | ||||||
| UC | ref | ref | ||||
| CD | 1.323 | 1.118–1.562 | 0.001 | 1.210 | 1.019–1.436 | 0.030 |
| Previous IBD-related surgery | 1.452 | 1.223–1.729 | <0.001 | 1.207 | 1.017–1.433 | 0.033 |
| Medication history | ||||||
| Immunosuppressants | 0.794 | 0.667–0.947 | 0.010 | 0.706 | 0.593–0.840 | <0.001 |
| Biologic therapy | 1.485 | 1.262–1.748 | <0.001 | 1.408 | 1.192–1.664 | <0.001 |
| Corticosteroids | 1.543 | 1.220–1.972 | <0.001 | 1.395 | 1.116–1.756 | 0.004 |
| Vitamin or micronutrient deficiency | 1.531 | 1.300–1.801 | <0.001 | 1.364 | 1.160–1.604 | <0.001 |
| Two or more comorbidities | 1.592 | 1.342–1.894 | <0.001 | 1.323 | 1.123–1.561 | 0.001 |
| Has PCP | 1.648 | 1.205–2.224 | 0.001 | 1.497 | 1.112–2.000 | 0.007 |
| Model for annual rate of IBD-related ED visits only | ||||||
| Managed using IBD CCP | 0.821 | 0.603–1.105 | 0.201 | 0.785 | 0.580–1.056 | 0.114 |
| Sex | ||||||
| Female | ref | ref | ||||
| Male | 0.625 | 0.500–0.782 | <0.001 | 0.651 | 0.525–0.806 | <0.001 |
| Residing in Greater Edmonton* | 0.410 | 0.328–0.511 | <0.001 | 0.442 | 0.355–0.547 | <0.001 |
| Disease type† | ||||||
| UC | ref | ref | ||||
| CD | 1.340 | 1.062–1.688 | 0.013 | 1.106 | 0.869–1.405 | 0.419 |
| Previous IBD-related surgery | 2.024 | 1.607–2.559 | <0.001 | 1.752 | 1.390–2.213 | <0.001 |
| Medication history | ||||||
| Immunosuppressants | 0.745 | 0.585–0.951 | 0.018 | 0.693 | 0.544–0.883 | 0.002 |
| Biologic therapy | 1.667 | 1.334–2.085 | <0.001 | 1.607 | 1.280–2.021 | <0.001 |
| Corticosteroids | 1.811 | 1.321–2.523 | <0.001 | 1.662 | 1.231–2.267 | 0.001 |
| Vitamin or micronutrient deficiency | 2.104 | 1.679–2.637 | <0.001 | 1.805 | 1.444–2.257 | <0.001 |
| Has PCP | 1.697 | 1.093–2.597 | 0.016 | 1.709 | 1.125–2.585 | 0.014 |
Significant P-values are bolded.
*Greater Edmonton (Edmonton census metropolitan area) was defined according to the Electoral of Statistics Canada as a conglomeration of 5 cities (Edmonton, Fort Saskatchewan, Leduc, St. Albert, and Spruce Grove).83
†Retained in model due to clinical relevance.
In the univariate model for total ED visits, management with IBD CCP was associated with a lower rate of ED visits [incidence rate ratio (IRR), 0.803; 95% confidence interval (CI), 0.641–0.996]. This corresponded to 19.7% fewer ED visits among managed patients compared to nonmanaged. In the multivariate model, the association between management with IBD CCP was actually stronger (IRR, 0.750; 95% CI, 0.683–0.823). Covariates associated with lower rates of ED visits included male sex, place of residence within Greater Edmonton and immunosuppressant use as part of a prior or current treatment regimen (Table 3). Covariates which increased likelihood of ED visits included CD, IBD-related surgical history, use of biologic and/or corticosteroid therapy, vitamin and micronutrient deficiencies, having 2 or more comorbidities, and presence of a PCP (Table 3).
In the univariate model for IBD-related ED visits, patients managed with IBD CCP had a lower rate of ED visits, but this was not statistically significant (IRR, 0.821; 95% CI, 0.603–1.105). In the multivariate model, the association between management with IBD CCP vs nonmanaged patients was stronger but still not quite significant (IRR, 0.785; 95% CI, 0.580–1.056). Covariates associated with lower rates of IBD-related ED visits included male sex, place of residence within Greater Edmonton, and immunosuppressant use as part of a prior or current treatment regimen (Table 3). Covariates which increased likelihood of ED visits were CD, IBD-related surgical history, use of biologic and/or corticosteroid therapy, vitamin and micronutrient deficiencies, and presence of a PCP (Table 3). Overall, the covariates for total and IBD-related ED visits were almost exactly the same, except for comorbidity, which was only a significant predictor in the total ED visit model.
Interaction Between IBD CCP Management and PCP
We found little evidence of effect modification when testing for interactions between management with IBD CCP and other covariates in either model. However, there was an interaction with the presence of a PCP in the model for total ED visits. Displayed in Supplementary Table S1, management with CCP was significantly associated with a lower rate of ED visits when patients had a PCP (IRR, 0.693; 95% CI, 0.555–0.865), but this was not significantly associated when patients did not have a PCP. Additionally, having a PCP was associated with a higher ED visit rate for patients who were not managed with CCP (IRR, 3.096; 95% CI, 1.654–5.793), but this was not significant when patients were managed with CCP.
DISCUSSION
Summary and Strengths of the Study
In this study, we analyzed the impact of the innovative IBD CCP model on the unplanned IBD care utilization, namely ED use. We carried out an analysis of potential contributing confounders of ED utilization. We demonstrated a significantly lower occurrence of ED visits among IBD patients managed at the University of Alberta using the IBD CCPs.
This study had a large sample size to compare the IBD CCP intervention to alternative care. The data retrieval process using both the EMR system and NACRS complimented by the manual chart review helped to minimize misclassification bias. Deterministic data linkage assisted in collecting an extensive number of patient characteristics and IBD-associated parameters from different sources, allowing us to compare the 2 groups for preexisting differences and confounders. By having complete data for all patients in the study, we were able to provide a more accurate estimation of ED rate reduction. The results of the study may thus be generalizable to the IBD population across at least Alberta and possibly Canada, since we had patient representation from a wide range of locations both within and outside of the province. However, it may be more difficult to extrapolate the exact effect of implementing CCPs in smaller community or nonacademic settings.
The IBD Population Presenting to the ED
The epidemiology of IBD patients attending the ED has not been well studied and remains limited.14 We provided a detailed snapshot of the main demographic, phenotypic, and clinical characteristics of this IBD subpopulation in Alberta, exploring predictors of ED use. The most common complaints at the time of ED attendance among our IBD patients (abdominal pain and hematochezia) were similar to those reported previously in the literature.16 Also consistent with previous findings, CD patients were overrepresented in the ED.14
Frequent ED users are an important subpopulation. Canadian and European studies consistently identified frequent users as a small proportion (up to 4%) of all ED users.48–51 However, they account for a disproportionately high healthcare cost. Despite the high prevalence of frequent ED users in our study cohort (Supplementary Table S2), there were not any significant differences in proportions between managed and nonmanaged patients, both prior and during the study period. Prospective assessments of disease activity may have helped elucidate the relationship. Further research needs to be conducted to characterize these frequent ED users in the IBD population, which we intend to do. Precise prediction of future ED use has become a frequent target of machine learning techniques such as Random Forests, and creating such models may be useful in the design of targeted interventions.52–55
Explanation of Findings
We did not identify a large number of significant clinical differences between managed and nonmanaged patients who presented to the ED. The fact that managed patients were receiving immunosuppressants and biologics more often than nonmanaged might be a sign of their IBD being under closer and more aggressive clinical management. At the same time, it might be a surrogate of more aggressive disease. Another explanation might be related to the administrative context as patients must be followed by an IBD specialist/gastroenterologist and be entered into special registries in order to be eligible to receive biologic therapy.
A higher proportion of managed patients also had CD, resided in the Greater Edmonton area, and had vitamin deficiencies documented. The CD finding may reflect more severe and complicated disease, and/or confounding by indication. Place of residence within Edmonton likely represents better access to IBD specialist care. This is consistent with recent findings that those with better access to gastroenterologists were both more likely to receive nonemergent specialist care, and had a lower risk of visiting the ED.18 Higher vitamin deficiency in managed patients was an interesting finding. This may also be confounding by indication, where those with more severe disease (contributing directly or through the use of deficiency-inducing medications such as glucocorticoids) may be both more likely to have these deficiencies and to seek specialist care.56 On the other hand, those managed by CCP may be more likely to undergo micronutrient deficiency testing.
Our regression models highlighted some important influences on ED visit rates. Overall, the model for total and IBD-related visits was almost identical, the only difference being the presence of 2 or more comorbidities, having a significant association with total ED visits, less so with IBD-related, which was anticipated. Many of the risk factors in the model were expected and consistent with previous literature, such as Crohn, biologic therapy, corticosteroids use, and vitamin deficiencies, particularly vitamin D.14,16,57 However, age and duration of disease were neither protective nor a risk factor in this population, contradicting the literature.14
Males had an underrepresented use of the ED, compared to females, which has been previously reported in IBD populations and others.12 Thiopurine use was also found to be protective by Nugent et al, although they did not theorize why.16 We could assume that thiopurine use (especially monotherapy) represents less severe disease, and this would be supported modestly by the decrease in IRR when adjusting for biologic therapy, compared to univariate. Further analysis also showed that biologic combination therapy compared to immunomodulators alone was associated with higher ED visit rates (Supplementary Table S3). It is also possible that combination therapy places the patient at increased risk of infections and other complications of immunosuppression, including associated trips to the ED.
Nonintuitively, we found IBD patients who had a PCP were more likely to visit the ED. This paradox was previously published in the literature for other populations, and might be related to patients having unmet needs or being frequent care seekers and having dissatisfaction with their provider.51,58–61 It seems that there are multiple differences in ED users that contribute to increase healthcare utilization, including both primary and emergency care. The interaction of PCP status with IBD CCP management was also an interesting and unexpected finding, which we have not seen previously reported. It could be related to care coordination between specialists and PCP, or an issue of specialist access—since those nonmanaged patients group may not be seeing a gastroenterologist at all. However, because the interaction was found in model fitting techniques and not prespecified or expected, we did not collect data required to fully explain this finding.
Limitations of the Study
Limitations of our study included its retrospective and single-center design. The results are subject to any error in the administrative NACRS database. We did not assess whether ED visits led to patient admission nor did we account for healthcare costs associated with further unplanned events. It is important to note that medications, extra intestinal manifestations, and comorbidities were captured as occurring at any time in the patient’s history prior to, but not exactly at the time of baseline. We also did not have specific data on disease activity which may have contributed to the higher ED utilization. We did not perform an evaluation of the societal and nonhealth benefits of the IBD CCP intervention outside of the actual ED visits, such as time saved on the ED visits, workplace absenteeism avoided, and psychosocial gains from ED avoidance.
Another key limitation is the assumption of the inclusion criteria. We assume that patients in the managed group, by virtue of being seen at the IBD clinic over 18 months prior to the study period (and during which the IBD CCP were implemented), were managed according to the IBD CCP. This assumption leaves a question of whether the “non CCP-managed” group were managed by any gastroenterologist, or no gastroenterologist at all. Unfortunately such data were not available. It would be preferred to isolate the effect of the CCPs through a cluster randomized clinical trial comparing similar practices with and without the CCP.
CONCLUSIONS
Clinical care pathways provide a standardized, patient-centered, comprehensive, and holistic approach to patient care. They have been studied in a variety of settings (surgical intensive care unit, bipolar disorder, asthma, chronic obstructive pulmonary disease, stroke rehabilitation, etc.), but not in IBD, to our knowledge, until now.62–66 This study provides evidence that the IBD CCP model can lead to IBD care optimization in outpatient IBD settings, as well as decreased ED utilization.67 As a “proof-of-principal,” the study suggests utility in allocating healthcare resources to the implementation of IBD CCP at a larger scale as an effective clinical intervention with potential large cost savings to the healthcare system.
The major limitations of this study are the retrospective design and setting at an academic tertiary center with access to more advanced medical therapy. Future studies will be done with prospective, 2 group design to address these limitations. Further work is being done to determine if the IBD CCPs in their current form are cost-effective, and to embed CCPs into the EMR system through automated alerts, call-to-actions, grouped order sets, and instructions.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the faculty and staff of the IBD Unit and Division of Gastroenterology at the [institution] Hospital, who helped with design and implementation of the IBD CCP. We also acknowledge the staff of Alberta Health Services and the Epidemiology and Surveillance with Alberta Health, Government of Alberta, for their assistance with supplying the data. Particularly, we thank Dr Larry Svenson of the Analytics and Performance Reporting Branch, Alberta Health, for his assistance with data acquisition and analysis. This study was supported by the Crohn’s and Colitis Canada (CCC) Promoting Access and Care through Centres of Excellence (PACE) Network. All results and inferences reported in this manuscript are independent of the funding and support sources.
Funding: This work was supported by the Crohn’s and Colitis Canada via the Promoting Access and Care through Centres of Excellence (PACE) initiative.
Conflict of Interest: Nothing to disclose.
Author Contribution: E.L.: literature search, study design, data collection, statistical analysis, manuscript drafting, and revision. R.T.S.: statistical analysis, manuscript drafting, revision, finalization, and preparation for publication. L.A.D.: manuscript revision and approval. F.P.: manuscript revision and approval. K.I.K.: primary coinvestigator, manuscript revision, and approval. R.N.F.: primary investigator, study conception, and manuscript revision.
DATA AVAILABILITY
Data not publicly available.
REFERENCES
- 1. Cohen BL, Zoëga H, Shah SA, et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther. 2014;39:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ananthakrishnan AN, Weber LR, Knox JF, et al. Permanent work disability in Crohn’s disease. Am J Gastroenterol. 2008;103:154–161. [DOI] [PubMed] [Google Scholar]
- 3. Reilly MC, Gerlier L, Brabant Y, et al. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn’s disease. Clin Ther. 2008;30:393–404. [DOI] [PubMed] [Google Scholar]
- 4. Rocchi A, Benchimol EI, Bernstein CN, et al. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alberta Government. Interactive Health Data Application. Alberta Government; 2018. http://www.ahw.gov.ab.ca/IHDA_Retrieval/ (11 April 2020, date last accessed). [Google Scholar]
- 6. IBD in Alberta (June 2018, date last accessed). [Google Scholar]
- 7. Baumgart DC, ed. Crohn’s Disease and Ulcerative Colitis: From Epidemiology and Immunobiology to a Rational Diagnostic and Therapeutic Approach. 2nd ed. Basel, Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 8. Im JP, Ye BD, Kim YS, et al. Changing treatment paradigms for the management of inflammatory bowel disease. Korean J Intern Med. 2018;33:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mao EJ, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45:3–13. [DOI] [PubMed] [Google Scholar]
- 10. Feagan BG, Panaccione R, Sandborn WJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135:1493–1499. [DOI] [PubMed] [Google Scholar]
- 11. Chebli JMF. Effect of azathioprine or mesalazine therapy on incidence of re-hospitalization in sub-occlusive ileocecal Crohn’s disease patients. Med Sci Monit. 2013;19:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gajendran M, Umapathy C, Loganathan P, et al. Analysis of hospital-based emergency department visits for inflammatory bowel disease in the USA. Dig Dis Sci. 2016;61:389–399. [DOI] [PubMed] [Google Scholar]
- 13. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 14. Ananthakrishnan AN, McGinley EL, Saeian K, et al. Trends in ambulatory and emergency room visits for inflammatory bowel diseases in the United States: 1994–2005. Am J Gastroenterol. 2010;105:363–370. [DOI] [PubMed] [Google Scholar]
- 15. Ballou S, Hirsch W, Singh P, et al. Emergency department utilisation for inflammatory bowel disease in the United States from 2006 to 2014. Aliment Pharmacol Ther. 2018;47:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nugent Z, Singh H, Targownik LE, et al. Predictors of emergency department use by persons with inflammatory bowel diseases: a population-based study. Inflamm Bowel Dis. 2016;22:2907–2916. [DOI] [PubMed] [Google Scholar]
- 17. Casellas F, Fontanet G, Borruel N, et al. The opinion of patients with inflammatory bowel disease on healthcare received. Rev Esp Enferm Dig. 2004;96:174–184. [DOI] [PubMed] [Google Scholar]
- 18. Nguyen GC, Bouchard S, Diong C, Promoting Access and Care through Centres of Excellence (PACE) Network. Access to specialists and emergency department visits in inflammatory bowel disease: a population-based study. J Crohns Colitis. 2019;13:330–336. doi:10.1093/ecco-jcc/jjy161. PMID: 30312376. [DOI] [PubMed] [Google Scholar]
- 19. Raven MC, Kushel M, Ko MJ, et al. The effectiveness of emergency department visit reduction programs: a systematic review. Ann Emerg Med. 2016;68:467–483.e15. [DOI] [PubMed] [Google Scholar]
- 20. Lavelle J, Schast A, Keren R. Standardizing care processes and improving quality using pathways and continuous quality improvement. Curr Treat Options Pediatr. 2015;1:347–358. [Google Scholar]
- 21. Shumway M, Boccellari A, O’Brien K, et al. Cost-effectiveness of clinical case management for ED frequent users: results of a randomized trial{star, open}. Am J Emerg Med. 2008;26:155–164. [DOI] [PubMed] [Google Scholar]
- 22. McCormack RP, Hoffman LF, Wall SP, et al. Resource-limited, collaborative pilot intervention for chronically homeless, alcohol-dependent frequent emergency department users. Am J Public Health. 2013;103(suppl 2):S221–S224. doi: 10.2105/AJPH.2013.301373. Epub 2013 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown MD, Reeves MJ, Meyerson K, et al. Randomized trial of a comprehensive asthma education program after an emergency department visit. Ann Allergy Asthma Immunol. 2006;97:44–51. [DOI] [PubMed] [Google Scholar]
- 24. Kolbasovsky A, Reich L, Futterman R, et al. Reducing the number of emergency department visits and costs associated with anxiety: a randomized controlled study. Am J Manag Care. 2007;13:95–102. [PubMed] [Google Scholar]
- 25. Mion LC, Palmer RM, Meldon SW, et al. Case finding and referral model for emergency department elders: a randomized clinical trial. Ann Emerg Med. 2003;41:57–68. [DOI] [PubMed] [Google Scholar]
- 26. Horwitz SM, Busch SH, Balestracci KM, et al. Intensive intervention improves primary care follow-up for uninsured emergency department patients. Acad Emerg Med. 2005;12:647–652. [DOI] [PubMed] [Google Scholar]
- 27. Spiegel TF, Wassermann TB, Neumann N, et al. A clinical pathway for heart failure reduces admissions from the ED without increasing congestion in the ED. Am J Emerg Med. 2018;36:1202–1208. [DOI] [PubMed] [Google Scholar]
- 28. Olajos-Clow J, Szpiro K, Julien B, et al. Emergency department adult asthma care pathway. Adv Emerg Nurs J. 2009;31:44–53. [DOI] [PubMed] [Google Scholar]
- 29. Archer SB, Burnett RJ, Flesch LV, et al. Implementation of a clinical pathway decreases length of stay and hospital charges for patients undergoing total colectomy and ileal pouch/anal anastomosis. Surgery. 1997;122:699–703; discussion 703. [DOI] [PubMed] [Google Scholar]
- 30. Hou JK, Gasche C, Drazin NZ, et al. Assessment of gaps in care and the development of a care pathway for anemia in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2017;23:35–43. [DOI] [PubMed] [Google Scholar]
- 31. Sier MF, Oostenbroek RJ, Dijkgraaf MGW, et al. ; iAID study group . Home visits as part of a new care pathway (iAID) to improve quality of care and quality of life in ostomy patients: a cluster-randomized stepped-wedge trial. Colorectal Dis. 2017;19:739–749. [DOI] [PubMed] [Google Scholar]
- 32. Canadian Institute for Health Information (CIHI). National Ambulatory Care Reporting System (NACRS). Ottawa: CIHI; 2017. [Google Scholar]
- 33. Myers JL, Well AD, Lorch RFJ.. Research Design and Statistical Analysis. Abingdon: Routledge/Taylor & Francis; 2010. [Google Scholar]
- 34. Altman DG, Bland JM. Standard deviations and standard errors. BMJ. 2005;331:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. Vol. 46. United Kingdom: Chapman and Hall/CRC; 2004. [Google Scholar]
- 36. Mcdonald JH. Handbook of Biological Statistics. 3rd ed. Baltimore, Maryland: Sparky House Publishing; 2014. [Google Scholar]
- 37. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser. 1995;57:289–300. [Google Scholar]
- 38. Long JS. Regression Models for Categorical and Limited Dependent Variables. Thousand Oaks, CA: SAGE; 1997. [Google Scholar]
- 39. Cameron AC, Trivedi PK.. Regression Analysis of Count Data. 2nd ed. Econometric Society Monograph No.53, Cambridge University Press; 1998:566. [Google Scholar]
- 40. How to Perform a Poisson Regression Analysis in SPSS Statistics | Laerd Statistics. November 2018. https://statistics.laerd.com/spss-tutorials/poisson-regression-using-spss-statistics.php LB - ac1h (11 April 2020, date last accessed).
- 41. Lavery R. An animated guide: an introduction to Poisson regression. In: NESUG Pap., 2010, 1–17. http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:An+Animated+Guide+:+An+Introduction+To+Poisson+Regression#0 [Google Scholar]
- 42. Breslow NE. Generalized Linear Models: checking assumptions and strengthening conclusions. In: Congr Naz Soc Ital di Biometria, 1996, 19. [Google Scholar]
- 43. IBM Corp. IBM SPSS Statistics for Windows. Armonk, NY; 2010. [Google Scholar]
- 44. Venables WN, Ripley BD.. Modern Applied Statistics with S. 4th ed. New York City: Springer-Verlag New York; 2002. [Google Scholar]
- 45. Hlavac M. Stargazer: Well-Formatted Regression and Summary Statistics Tables. R Package Version 5.2.1. 2018. https://cran.r-project.org/web/packages/stargazer/vignettes/stargazer.pdf (11 April 2020, date last accessed). [Google Scholar]
- 46. Tableau Software, 10.3. Tableau. 2018. https://en.wikipedia.org/wiki/Tableau_Software [Google Scholar]
- 47. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. [DOI] [PubMed] [Google Scholar]
- 48. Moe J, Bailey AL, Oland R, et al. Defining, quantifying, and characterizing adult frequent users of a suburban Canadian emergency department. CJEM. 2013;15:214–226. [DOI] [PubMed] [Google Scholar]
- 49. Chan BT, Ovens HJ. Frequent users of emergency departments. Do they also use family physicians’ services? Can Fam Physician. 2002;48:1654–1660. [PMC free article] [PubMed] [Google Scholar]
- 50. Doupe MB, Palatnick W, Day S, et al. Frequent users of emergency departments: developing standard definitions and defining prominent risk factors. Ann Emerg Med. 2012;60:24–32. [DOI] [PubMed] [Google Scholar]
- 51. Althaus F, Paroz S, Hugli O, et al. Effectiveness of interventions targeting frequent users of emergency departments: a systematic review. Ann Emerg Med. 2011;58:41–52.e42. [DOI] [PubMed] [Google Scholar]
- 52. Qiao Z, Sun N, Li X, et al. Using machine learning approaches for emergency room visit prediction based on electronic health record data. Stud Health Technol Inform. 2018;247:111–115. [PubMed] [Google Scholar]
- 53. Poole S, Grannis S, Shah NH. Predicting emergency department visits. AMIA Jt Summits Transl Sci Proc. 2016;2016:438–445. [PMC free article] [PubMed] [Google Scholar]
- 54. Pereira M, Singh V, Hon CP, et al. Predicting future frequent users of emergency departments in California state. In: Proc 7th ACM Int Conf Bioinformatics, Comput Biol Heal Informatics—BCB’16, 2016, 603–610. [Google Scholar]
- 55. Moe J, Kirkland SW, Rawe E, et al. Effectiveness of interventions to decrease emergency department visits by adult frequent users: a systematic review. Acad Emerg Med. 2017;24:40–52. [DOI] [PubMed] [Google Scholar]
- 56. Kamangar F. Confounding variables in epidemiologic studies: basics and beyond. Arch Iran Med. 2012;15:508–516. [PubMed] [Google Scholar]
- 57. Kabbani TA, Koutroubakis IE, Schoen RE, et al. Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal study. Am J Gastroenterol. 2016;111:712–719. [DOI] [PubMed] [Google Scholar]
- 58. Ullman R, Block JA, Stratmann WC. An emergency room’s patients: their characteristics and utilization of hospital services. Med Care. 1975;13:1011–1020. [DOI] [PubMed] [Google Scholar]
- 59. Moore G, Gerdtz M, Manias E, et al. Socio-demographic and clinical characteristics of re-presentation to an Australian inner-city emergency department: implications for service delivery. BMC Public Health. 2007;7:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hunt KA, Weber EJ, Showstack JA, et al. Characteristics of frequent users of emergency departments. Ann Emerg Med. 2006;48:1–8. [DOI] [PubMed] [Google Scholar]
- 61. West S, King V, Carey TS, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ). 2002:1–11. https://pubmed.ncbi.nlm.nih.gov/11979732/. [PMC free article] [PubMed] [Google Scholar]
- 62. Brattebø G, Hofoss D, Flaatten H, et al. Effect of a scoring system and protocol for sedation on duration of patients’ need for ventilator support in a surgical intensive care unit. BMJ. 2002;324:1386–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bauer MS, McBride L, Williford WO, et al. ; Cooperative Studies Program 430 Study Team . Collaborative care for bipolar disorder: part I. Intervention and implementation in a randomized effectiveness trial. Psychiatr Serv. 2006;57:927–936. [DOI] [PubMed] [Google Scholar]
- 64. Doherty SR, Jones PD, Davis L, et al. Evidence-based implementation of adult asthma guidelines in the emergency department: a controlled trial. Emerg Med Australas. 2007;19:31–38. [DOI] [PubMed] [Google Scholar]
- 65. Smith BJ, Cheok F, Heard AR, et al. Impact on readmission rates and mortality of a chronic obstructive pulmonary disease inpatient management guideline. Chron Respir Dis. 2004;1:17–28. [DOI] [PubMed] [Google Scholar]
- 66. Sulch D, Perez I, Melbourn A, et al. Randomized controlled trial of integrated (managed) care pathway for stroke rehabilitation. Stroke. 2000;31:1929–1934. [DOI] [PubMed] [Google Scholar]
- 67. Metge CJ, Blanchard JF, Peterson S, Bernstein CNet al. Use of pharmaceuticals by inflammatory bowel disease patients: a population-based study. Am J Gastroenterol. 2001;96:3348–55. doi: 10.1111/j.1572-0241.2001.05255.x. PMID: 11774948. [DOI] [PubMed] [Google Scholar]
- 68. Statistics Canada. Focus on Geography Series, 2011 Census. Statistics Canada Catalogue no. 98-310-XWE2011004. Ottawa, Ontario: Statistics Canada; 2011. Analytical products, 2011 Census. Published online 2012. [Google Scholar]
- 69. World Health Organization (WHO). International Classification of Diseases (ICD) 10. http://apps.who.int/classifications/icd10/browse/2016/en#/XVI [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data not publicly available.