Abstract
Oral squamous cell carcinoma (OSCC) is among the most common malignancies and a leading cause of death in developing countries. Late diagnosis and regional and/or distant metastasis worsen the prognosis of this condition. Despite the advances in diagnostic modalities and management strategies, there is little improvement in the 5-year survival rate. A deeper insight into the molecular events of various tumours has enabled the use of minimally invasive methods for monitoring disease progression, prognostication and treatment monitoring. Although studies in OSCC are preliminary, the use of liquid biopsies has opened new frontiers for the development of biomarkers that can serve as alternatives to conventional biopsies and imaging methods. Circulating biomarkers in blood allow for the real-time monitoring of tumour and therapeutic responses. This review aims to outline the promises and challenges of circulating biomarkers in OSCC with special emphasis on circulating tumour cells, circulating tumor DNA, and exosomes.
Keywords: Circulating tumour cells, circulating tumour DNA, exosomes, liquid biopsy, oral cancer
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common neoplasm worldwide.[1,2] Oral squamous cell carcinoma (OSCC) accounts for almost 25% of all cases within this group of tumours. Despite the ease of oral self-examination, this condition is usually diagnosed late, often at advanced stages.[3,4] Regional and distant metastasis is responsible for the poor prognosis of advanced OSCC, with only 50% of the patients remaining disease-free 5 years after treatment.[5] Advances in the multidisciplinary management of advanced OSCC have not greatly improved the survival rate. This may be attributed to a central biological aspect of this pathology, the field cancerization effect,[6] and necessitates the need to gain a deeper insight into the molecular events involved in OSCC in pursuit of new diagnostic biomarkers and therapeutic targets.[7]
At present, tissue biopsy and imaging methods are the mainstays for the diagnosis and prognostication of OSCC. OSCC is a complex disease with immense tumoural heterogeneity and genomic complexity characterised at the molecular and cellular levels. The limitations of tissue biopsy include difficulty in the accessibility of tumours in deeper sites, the possibility of metastatic spread via invasive techniques, requirement of repetitive biopsies to monitor genetic mutations, discomfort and inherent clinical risk to the patient. These potential hindrances have prevented the development of a reliable prognostic marker, scrutinizing treatment response and facilitating personalized treatment decisions.[8,9] Moreover, conventional biopsies are temporally and spatially limited and often provide a brief snapshot of a single region of a heterogeneous tumour.[10] Additionally, both biopsies and imaging methods are unable to discover micro-metastasis and residual lesions at an early stage.[11] This compels the search for biomarkers that can effectively reflect tumour heterogeneity for early diagnosis, prediction of metastases in the tumour genomic landscape and effective treatment monitoring. While 'biopsy' is the mainstay for biomarkers from solid tissue, biomarker sampling via peripheral venepuncture and body fluid analysis are becoming increasingly popular.[12]
Research efforts are now focused on the discovery of new, minimally invasive methods for the diagnosis and comprehension of the tumour genomic architecture to monitor tumour evolution and therapeutic response in real-time.[13,14] In this sense, the field of liquid biopsy has emerged as a revolution in multiple areas of oncology and the development of tumour-precision medicine.[15] Liquid biopsy effectively allows the analysis of genetic variations occurring during the disease process by detection of tumour-related components in several biological fluids such as blood, saliva, cerebrospinal fluid and so on.[16]
With the rising clinical use of liquid biopsies in various tumours, the need for the identification of circulating biomarkers has gained a great deal of relevance. The characterization of specific circulating biomarkers for common neoplasms will facilitate early diagnosis, prognostication, prediction and monitoring of response to therapy.[17] In addition, these analytes are complementary biomarkers that offer great potential for the development of cancer drug discovery platforms.[18] Circulating tumour cells (CTCs) and circulating genetic material such as genomic DNA (gDNA), mitochondrial DNA (mtDNA), circulating tumour DNA (ctDNA), microRNA (miRNA) and exosomes [Figure 1] can be potential sources of tumour material from the primary tumour and from all disease sites.
Figure 1.
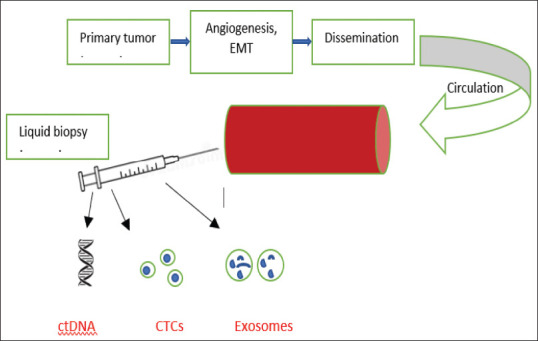
Schematic representation of circulating biomarkers
The relative ease of sampling circulating biomarkers is making it clinically attractive. Knowledge regarding its potential applications, technical limitations and supportive evidence as a screening, diagnostic and prognostic tool in OSCC will allow clinical use.[15] This review will outline the role of circulating biomarkers for early detection, real-time monitoring and prognostic evaluation of OSCC with special emphasis on CTCs, ctDNA and exosomes.
CIRCULATING TUMOUR CELLS (CTCS)
A subpopulation of rare atypical precursor cells released in the circulation by primary tumours are labelled as Circulating tumour cells (CTCs). They are believed to have acquired somatic mutations and genomic rearrangements similar to the primary tumours.[19] They are released from the primary tumour actively or passively like 'seeds'.[20] Various spontaneous or iatrogenic factors are implicated in this process of release into the circulation by the primary tumour. Thus, they act as a mirror to showcase tumour heterogeneity[18] without the need for an invasive tissue biopsy.[21]
The half-life of CTCs varies from 1 to 2.4 h. Harsh conditions in circulation, such as immune attacks, apoptotic processes, shear forces and fluidic turbulences impact it.[22,23] Only a small part of them survive and are able to metastasize. CTCs may circulate in isolation or in groups.[16] CTC clusters are defined as ≥3 tumour cells with strong cell-cell adhesions, CTCs can also cluster with non-malignant cells, such as platelets, immune cells and fibroblasts.[24] Evidence points to the strong metastasis ability of CTC clusters. They traverse narrow capillaries in a single file and retain their cluster properties upon exiting.[25]
CTC analysis is a developing technology in the field of HNSCC in general and in that of OSCC in particular.[16] The identification and characterisation of CTCs require extremely sensitive and specific analytical methods. Enrichment and separation procedures for detection and analysis are often tedious and expensive.[26,27] Antibodies against the epithelial cell adhesion molecule (EpCAM) and subsequent immunocytological detection of CTCs are performed with antibodies to cytokeratins (CKs) for positive selection.[28] Although EpCAM-based enrichment technique has been the most widely used technique, it could not detect CTCs in all metastasized or advanced disease patients of OSCC. This unpredictability could be attributed to either low incidence of CTCs or to exclusion of non-EpCAM and/or non-keratin expressing CTCs as EMT is characterised by loss of epithelial characteristics.[29]
Promise of CTCs in oral cancer
The unique feature of CTCs is their ability to be characterized at the cellular level and even cultured/grown. This sets them apart from other circulating biomarkers renewing continuing interest and promise in their diagnostic and prognostic abilities.[30]
Various recent studies indicate that CTCs may be effective markers to predict disease progression and survival in metastatic and possibly even early-stage cancer patients. High CTC numbers correlate with aggressive disease, increased metastasis and decreased time to relapse.[31]
The detection of CTCs in patients with oral cancer has been found to be associated with a high risk of locoregional recurrence and distant metastasis. Their value as an independent prognostic marker in OSCC is attributed to their ability to predict relapse with more sensitivity than routine staging procedures.[12] Additionally, an increased expression of programmed death-ligand 1 (PD-L1), HOXB9 and ZNF813 in OSCC-derived CTCs on gene expression profiling emphasized their role.[32] This also fuelled the development of anti-PD-L1 therapy for OSCC patients.[16,18]
Apart from its potential as a prognostic biomarker, the role of CTCs in regulating disease behavior has been investigated. The checkpoint inhibitors that block the PD-1/PD-L1 immune checkpoint pathway on CTCs and stimulate the immune system to remove CTCs in circulation may reduce the risk of metastasis and disease recurrence. In patients with OSCC, PD-L1 overexpression in CTCs has been identified and utilized to monitor the treatment response.[32]
CTCs may be shed from different locations within tumours, which are heterogeneous in nature, and even from metastases. There is a clear discrepancy in gene expression between primary tumours and CTCs, as well as heterogeneity within the CTC population. There is a possibility to identify the tissue of origin of CTCs by using expression profiling to detect organ-specific metastatic signatures. This is presumed to localize small metastatic lesions and guide further diagnostic and therapeutic strategies.[31]
CTCs could serve as a real-time marker[31] for recurrent assessments for disease monitoring before, during and after therapy, as sample collection is simple and minimally invasive. Numerous studies[26,27,28,29,30] have demonstrated the potential utility of a cutting-edge technology that may improve the detection and monitoring of cancer using a small number of blood samples.[18]
Thus, CTCs are ideal candidates for the prediction of relapse and local and/or distant spread as they reflect tumour mutational profiles. Additionally, they are non-invasive Adeola and help in determining tailored therapies for better outcomes.[33]
Limitations of CTCs
The potential of CTCs as a biomarker for cancer is tremendous. However, certain challenges that need to be addressed include the technical aspects of isolation and analysis. Although specimen collection is easy, enumeration of CTCs is limited by the detection of sufficient cells for adequate analysis. With frequencies of about one CTC per million blood cells, the detection of CTCs faces the proverbial 'needle in a haystack' problem.[12] They account for a few cells from a solid tumour that are shed in circulation among a sea of haematological ones.[30] The rarity of CTCs in the bloodstream requires differentiation of the normal blood cells (numbering in the billions/ml) from the CTCs (which may be below 10 cells/ml). Hence, even methods achieving a purity of 99.9% must be able to detect one CTC per million background cells.[12]
Detection and analysis of CTCs are technique sensitive and require specialized equipment and expertise. The processing is laborious and challenging, as the sample must be processed quickly after blood collection and the detection process is labour intensive.[12] As the cells have a short life span in circulation,[19] retesting or saving them for further analysis is not possible. In vitro and clinical research in this field are limited greatly due to the high cost of quantification and analysis of CTCs.[12]
Future prospects
As CTCs have been proven to effectively reflect the mutational landscape and genotypic alterations observed in the primary tumour, they have immense potential as non-invasive indicators of disease progression, tumour staging, assessment of treatment response and therapeutic monitoring in oral cancers.
A proposal to include the analysis of CTCs for 'molecular staging' may fill the gap between prognostication based on TNM staging and actual clinical outcomes.[12] There is renewed hope that the analysis of CTCs will enhance understanding of the complex metastatic process. This may unravel new approaches to destroying metastatic cells. However, a factor of crucial importance in any future clinical implementation is the need to improve the sensitivity of the techniques used to quantify and characterise the presence of CTCs.[16]
CIRCULATING DNA (CTDNA)
Fragmented, tumour-derived DNA that is not associated with cells (i.e., cell-free) and found within the circulatory system is referred to as ctDNA. Apoptosis and necrosis under physiologic and pathologic conditions contribute to this tumour-derived circulating cell-free DNA (cfDNA), which accounts for the total DNA shed into the blood and biological fluids.[34]
There is evidence that the proportion of ctDNA detectable within the pool of cell-free plasma DNA is related to tumour burden in cancer patients.[35] The length of ctDNA fragments is less than 167 base pairs, approximately the size of one nucleosome.[36] ctDNA accounts for a small percentage of the total cfDNA in peripheral blood. The quantity is influenced by tumour type, location, vascularization stage and size.[37,38,39]
The first potential application of ctDNA is screening for early diagnosis of cancer to allow early treatment and improved outcomes.[40] The half-life of ctDNA is less than 2 h, which is shorter than the half-life of protein markers. Therefore, ctDNA can reflect the real-time change of the tumour burden during cancer therapy.[41] It is more sensitive than CTCs in early detection and reflects tumour burden in real time.[17]
ctDNA provides a reasonably unbiased sample of the whole tumour burden and, therefore, a means to evade spatial intratumour genetic heterogeneity, which is one of the main causes of the potential non-representative nature of single-tumour biopsies.[17]
Promise of ctDNA in oral cancer
Presently, due to the ease of analysis, cfDNA is being studied extensively in comparison to other biomarkers. Evidence suggests that ctDNA represents the tumour genome from primary tumours and metastasis.[15] The identification of tumour-specific point mutations, promoter hypermethylations and the identification of allelic imbalance using microsatellite markers analysis in ctDNA are helpful tools of assessment in patient management.[42]
The circulating DNA reflects the characteristics of tumour DNA including molecular changes, such as methylation, point mutations and microsatellite instability.[43] DNA methylation is the widely used epigenetic biomarker, which has been the hotspot of ctDNA research in recent years.
In HNSCC patients, SHOX2 and SEPT9 methylation are of diagnostic values and aid in molecular staging, making treatment choices and post-therapeutic monitoring. Apart from DNA methylation, tumour-associated mutations in ctDNA are also explored. The different mutants and epigenetic modification in ctDNA, as well as the detection of ctHPV16DNA, have significance in the early diagnosis of HNSCC.[20]
ctDNA serves as a quantitative marker for longitudinal follow-up and disease monitoring. It also characterizes the genomic landscape of the various stages of cancer and as a means to address the challenge of intratumour genetic heterogeneity.[17]
Limitations of ctDNA
Limitations of ctDNA use as a source of biological material for biomarker analyses include a low detection ratio in the early stages of cancer. Second, multiplexed assays are required to analyze several mutations simultaneously. Finally, there is still a lack of a standardized methods for ctDNA analysis.[15]
The yields of ctDNA may be related to cancer cell death-inducing factors such as hypoxia, proliferation, treatment responses and tumour handling (surgery or biopsy). Assessment of these variables may have an impact on the prognostic and predictive value of ctDNA-derived biomarkers.
It is unlikely that the inter- and intratumour genetic heterogeneity will be reflected by tracking of single-point mutation in ctDNA. Thus, it would be necessary either to have prior knowledge of the tumour's “truncal” driver genetic aberration which is present in all cancer cells, or to test a large panel of the most recurrent cancer-specific alterations or private chromosomal rearrangements.[17]
Future prospects
ctDNA has been reported to be a highly sensitive genetic biomarker in several types of cancer which directly reflect the tumour burden and genetic dynamics. cfDNA may be applied as a screening marker for early detection of precancer and cancer as well as for prognostication of oral cancer.[44] ctDNA analysis in HNSCC should include not only the detection of tumor-specific mutations but also the detection of the human papillomavirus (HPV) DNA for HPV-positive oropharyngeal cancer.[40]
EXOSOMES
Exosomes are nano-scale membrane encapsulated vesicles derived from cells with diameters ranging from 40 to 100 nm, which are exceptionally stable and shuttle through bodily fluids.[45] The component transported in exosomes includes several molecular biomarkers—proteins, a broad range of RNA types (miRNA, lncRNA and mRNA) and various DNA. These biomarkers affect stroma modification and angiogenesis by delivering to recipient cells.[20] The presence of exosomes in the tumour microenvironment suggests their role in tumorigenesis, tumour invasion and metastasis.[46,47] Exosomes can be abundantly released by different types of cells into numerous biological fluids such as urine, semen, saliva, amniotic fluid, cerebrospinal fluid, lymph, bile, ascites, tears, breast milk and blood, both in healthy and diseased conditions.[15]
Promise of exosomes in oral cancer
In OSCC, exosomes have been reported as key elements in the tumour microenvironment that upregulate the transforming growth factor-β (TGF-β) signaling pathway, contributing to the progression and drug resistance of OSCC.[48] Under hypoxic conditions, exosomes secreted by tumour cells are involved in tumour angiogenesis and metastasis.[49,50]
The detection of miRNA biomarkers in both the plasma and tumours of patients with squamous cell carcinoma of the tongue highlights the significance of free and exosomal miRNAs as potential diagnostic biomarkers for tongue cancer. In addition, packaged circulating miRNAs in protein complexes or encapsulated within microvesicles are protected against the activity of blood RNAses, and represent a more dependable approach for the assessment of circulating tumour-miRNA signatures. On miRNA expression profiling, a correlation was found between circulating exosomal miR-21 levels and metastasis in the lymph nodes in OSCC patients.[15]
Exosomal chemokine-like factor (CKLF)-like MARVEL transmembrane domain-containing six (CMTM6) of OSCC cells aid the polarization of alternatively activated macrophages (M2) via activation of the signaling of ERK1/2 in macrophages. M2 macrophages have pro-tumour functions, facilitating initiation and progression of the tumour.[18] Evidence suggests that tumour exosomes play an important role in immune suppression, boosting tumour initiation and progression. This is allowed as tumour exosomes can communicate with immune cells through immunoinhibitory (protumour) and immunostimulatory (antitumour) signals in the tumour microenvironment.[51]
Limited available evidence suggests a potential discriminatory biomarker role of exosomes, between active OSCC disease patients and cured OSCC patients. Oral fluid-derived exosomes have been morphologically characterized in OSCC.[52] The role of some exosomal miRNA (e.g., miR-223, miR-101-3p, miR-338 and miR-34a-5p) as tumour suppressors and the robust potential of exosomes for therapeutic drug delivery to the tumour for effective treatment or to improve prognosis has also been highlighted.[53]
Limitations of exosomes
Various unanswered questions remain regarding the challenges of the clinical use of circulating exosomes as a circulating biomarker. Although in vitro or in vivo xenograft models have investigated the function of tumour exosomes, there is a need for comprehensive studies focusing on the mechanisms of biogenesis, cargo sorting or the physiological relevance of the exosome. Therefore, in vivo studies are required to gain insights into the heterogeneity of tumour exosomes and their functional significance. In addition, a consensus on the isolation strategy, classification and contents of exosomes must be clarified to generate the common and standardized protocols required for clinical use.[15]
CONCLUSION
The disease-free survival rate of OSCC patients remains dismal in spite of the advances in diagnosis and therapeutic modalities. Over half of the oral cancer patients suffer from loco-regional relapses while 1/4th of the patients develop distant organ metastases.[19] The unprecedented advances in the understanding of tumour biology have paved the way for developing minimally invasive methods that facilitate early diagnosis and repeated characterization of tumours.
Circulating biomarkers have been heralded as blood-borne biological surrogates of tissues for molecular analysis of cancers.[28] The detection and analysis of CTCs, ctDNA and circulating exosomes represent a promising opportunity for early cancer detection, molecular profiling analysis, monitoring of treatment response and detection of minimal residual disease and relapse.[15]
Circulating biomarkers provide the advantage of repeated sampling of tumour tissue, and this longitudinal follow-up, combined with the multiplicity of the molecular targets that can be assessed, may provide a wealth of data that can ultimately change the way tumour response to specific therapies is perceived and provide new insights in the complex biology of micrometastasis with important implications for the clinical management of cancer patients.[28]
CTCs have the potential to offer a means of providing living cells directly representative of the primary tumour and may therefore uncover breakthroughs needed in the management of oral cancer. There is a paucity of focused research on their role in OSCC and further prospective longitudinal studies to assess the predictive value of CTC testing for early diagnosis and surveillance are the need of the hour.[12] ctDNA has been employed as a diagnostic tool with higher sensitivity as it carries the tumour-specific gene alteration.[20] Because distinct biological processes give rise to CTCs and ctDNA, it is plausible that the qualitative and quantitative information and potential clinical applications of these modalities of blood-borne biomarkers will not overlap entirely.[17] Exosomes contain various proteins and nucleic acids to mirror tumour cell sources, such as miRNA, genomic DNA and viral RNA, thus, serving as effective biomarkers.[15] Focused research on the utilization of each circulating biomarkers in the context of oral cancer is required to unlock and apply the benefits of these technologies appropriately.
A better knowledge of the biology and origin of circulating biomarkers would be the key to the development of effective therapies for and the management of oral cancer. Although the value of liquid biopsies has been explored in solid tumors of various anatomic locations, studies in oral cancer are limited and preliminary. Longitudinal, multicentric studies are recommended to establish the clinical use of liquid biopsies in oral cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Santiago S, Ramón Y Cajal T, Aguirre E, Alés-Martínez JE, Andrés R, Balmaña J, et al. SEOM Hereditary Cancer Working Group. SEOM clinical guidelines in hereditary breast and ovarian cancer (2019) Clin Transl Oncol. 2020;22:193–200. doi: 10.1007/s12094-019-02262-0. [DOI] [PubMed] [Google Scholar]
- 3.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. European Organization for Research and Treatment of Cancer Trial 22931. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 4.Gan SJ, Dahlstrom KR, Peck BW, Caywood W, Li G, Wei Q, et al. Incidence and pattern of second primary malignancies in patients with index oropharyngeal cancers versus index nonoropharyngeal head and neck cancers. Cancer. 2013;119:2593–601. doi: 10.1002/cncr.28107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong LP, Zhang CP, Ren GX, Guo W, William WN, Jr, Sun J, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744–51. doi: 10.1200/JCO.2012.43.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Tachibana H, Sho R, Takeda Y, Zhang X, Yoshida Y, Narimatsu H, et al. Circulating miR-223 in oral cancer: Its potential as a novel diagnostic biomarker and therapeutic target. PLoS One. 2016;11:e0159693. doi: 10.1371/journal.pone.0159693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potdar PD, Lotey NK. Role of circulating tumour cells in future diagnosis and therapy of cancer. J Cancer Metastasis Treat. 2015;1:44–56. [Google Scholar]
- 9.Wikner J, Gröbe A, Pantel K, Riethdorf S. Squamous cell carcinoma of the oral cavity and circulating tumour cells. World J Clin Oncol. 2014;5:114–24. doi: 10.5306/wjco.v5.i2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellairs JA, Hasina R, Agrawal N. Tumour DNA: An emerging biomarker in head and neck cancer. Cancer Metastasis Rev. 2017;36:515–23. doi: 10.1007/s10555-017-9685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng W, Zhang Y, Guo L, Wang S, Fang M, Mao W, et al. Evaluation of therapeutic efficacy with CytoSorter® circulating tumour cell-capture system in patients with locally advanced head and neck squamous cell carcinoma. Cancer Manag Res. 2019;11:5857–69. doi: 10.2147/CMAR.S208409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtin J, Choi SW, Thomson PJ, Lam AK. Characterization and clinicopathological significance of circulating tumour cells in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2022;51:289–99. doi: 10.1016/j.ijom.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–48. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Chang S, Li G, Sun Y. Application of liquid biopsy in precision medicine: Opportunities and challenges. Front Med. 2017;11:522–7. doi: 10.1007/s11684-017-0526-7. [DOI] [PubMed] [Google Scholar]
- 15.Lousada-Fernandez F, Rapado-Gonzalez O, Lopez-Cedrun JL, Lopez-Lopez R, Muinelo-Romay L, Suarez-Cunqueiro MM. Liquid biopsy in oral cancer. Int J Mol Sci. 2018;19:1704. doi: 10.3390/ijms19061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Ruiz E, Gutiérrez V, Muñoz M, Oliver J, Sánchez M, Gálvez-Carvajal L, et al. Liquid biopsy as a tool for the characterisation and early detection of the field cancerization effect in patients with oral cavity carcinoma. Biomedicines. 2021;9:1478. doi: 10.3390/biomedicines9101478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bidard FC, Weigelt B, Reis-Filho JS. Going with the flow: From circulating tumour cells to DNA. Sci Transl Med. 2013;5:207ps14. doi: 10.1126/scitranslmed.3006305. [DOI] [PubMed] [Google Scholar]
- 18.Adeola HA, Bello IO, Aruleba RT, Francisco NM, Adekiya TA, Adefuye AO, et al. The practicality of the use of liquid biopsy in early diagnosis and treatment monitoring of oral cancer in resource-limited settings. Cancers (Basel) 2022;14:1139. doi: 10.3390/cancers14051139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel S, Shah K, Mirza S, Shah K, Rawal R. Circulating tumour stem like cells in oral squamous cell carcinoma: An unresolved paradox. Oral Oncol. 2016;62:139–46. doi: 10.1016/j.oraloncology.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Yang WY, Feng LF, Meng X, Chen R, Xu WH, Hou J, et al. Liquid biopsy in head and neck squamous cell carcinoma: Circulating tumor cells, circulating tumor DNA, and exosomes. Expert Rev Mol Diagn. 2020;20:1213–27. doi: 10.1080/14737159.2020.1855977. [DOI] [PubMed] [Google Scholar]
- 21.Parikh AR, Corcoran RB. Monitoring resistance through liquid biopsy. Ann Oncol. 2018;29:8–11. doi: 10.1093/annonc/mdx650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehes G, Witt A, Kubista E, Ambros PF. Circulating breast cancer cells are frequently apoptotic. Am J Pathol. 2001;159:17–20. doi: 10.1016/S0002-9440(10)61667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strilic B, Offermanns S. Intravascular survival and extravasation of tumour cells. Cancer Cell. 2017;32:282–93. doi: 10.1016/j.ccell.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, et al. Characterization of circulating tumour cell aggregates identified in patients with epithelial tumours. Phys Biol. 2012;9:016001. doi: 10.1088/1478-3975/9/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Au SH, Storey BD, Moore JC, Tang Q, Chen Y-L, Javaid S, et al. Clusters of circulating tumour cells traverse capillary-sized vessels. Proc Natl Acad Sci U S A. 2016;113:4947–52. doi: 10.1073/pnas.1524448113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Economopoulou P, Kotsantis I, Kyrodimos E, Lianidou ES, Psyrri A. Liquid biopsy: An emerging prognostic and predictive tool in Head and Neck Squamous Cell Carcinoma (HNSCC). Focus on Circulating Tumor Cells (CTCs) Oral Oncol. 2017;74:83–9. doi: 10.1016/j.oraloncology.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Kaldjian EP, Ramirez AB, Sun Y, Campton DE, Werbin JL, Varshavskaya P, et al. The RareCyte® platform for next-generation analysis of circulating tumor cells. Cytometry A. 2018;93:1220–5. doi: 10.1002/cyto.a.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol Med. 2010;16:398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Allen JE, Saroya BS, Kunkel M, Dicker DT, Das A, Peters KL, et al. Apoptotic circulating tumour cells (CTCs) in the peripheral blood of metastatic colorectal cancer patients are associated with liver metastasis but not CTCs. Oncotarget. 2014;5:1753–60. doi: 10.18632/oncotarget.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S. Circulating tumor cells: Advances in basic science and clinical applications. Natl Med J India. 2017;30:46. [Google Scholar]
- 31.Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumour cells. Science. 2013;341:1186–8. doi: 10.1126/science.1235226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira-Costa JP, de Carvalho AF, da Silveira da GG, Amaya P, Wu Y, Park KJ, et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget. 2015;6:20902–20. doi: 10.18632/oncotarget.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anitha N, Jimson S, Masthan KM, Jacobina JJ. Circulating tumour cells in oral squamous cell carcinoma-an enigma or reality? J Pharm Bioallied Sci. 2015;7(Suppl 1):S173–5. doi: 10.4103/0975-7406.155893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronkhorst AJ, Ungerer V, Holdenrieder S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol Detect Quantif. 2019;17:100087. doi: 10.1016/j.bdq.2019.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumour DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 36.Mouliere F, Chandrananda D, Piskorz AM, Moore EK, Morris J, Ahlborn LB, et al. Enhanced detection of circulating tumour DNA by fragment size analysis. Sci Transl Med. 2018;10:eaat4921. doi: 10.1126/scitranslmed.aat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumour DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haber DA, Velculescu VE. Blood-based analyses of cancer: Circulating tumour cells and circulating tumour DNA. Cancer Disc. 2014;4:650–61. doi: 10.1158/2159-8290.CD-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yong E. Cancer biomarkers: Written in blood. Nature. 2014;511:524–6. doi: 10.1038/511524a. [DOI] [PubMed] [Google Scholar]
- 40.Galot R, Machiels JH. Current applications and challenges of circulating tumour DNA (ctDNA) in squamous cell carcinoma of the head and neck (SCCHN) Cancer Treat Rev. 2020;85:101992. doi: 10.1016/j.ctrv.2020.101992. [DOI] [PubMed] [Google Scholar]
- 41.Cheng F, Su L, Qian C. Circulating tumour DNA: A promising biomarker in the liquid biopsy of cancer. Oncotarget. 2016;7:48832–41. doi: 10.18632/oncotarget.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baby NT, Abdullah A, Kannan S. The scope of liquid biopsy in the clinical management of oral cancer. Int J Oral Maxillofac Surg. 2022;51:591–601. doi: 10.1016/j.ijom.2021.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Hamana K, Uzawa K, Ogawara K, Shiiba M, Bukawa H, Yokoe H, et al. Monitoring of circulating tumour-associated DNA as a prognostic tool for oral squamous cell carcinoma. Br J Cancer. 2005;92:2181–4. doi: 10.1038/sj.bjc.6602635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin LH, Chang KW, Kao SY, Cheng HW, Liu CJ. Increased plasma circulating cell-free DNA could be a potential marker for oral cancer. Int J Mol Sci. 2018;19:3303. doi: 10.3390/ijms19113303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu T, Zhang X, Gao S, Jing F, Yang Y, Du L, et al. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551–63. doi: 10.18632/oncotarget.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–15. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Xia W, Lv Z, Ni C, Xin Y, Yang L. Liquid biopsy for cancer: Circulating tumor cells, circulating free DNA or exosomes? Cell Physiol Biochem. 2017;41:755–68. doi: 10.1159/000458736. [DOI] [PubMed] [Google Scholar]
- 48.Languino LR, Singh A, Prisco M, Inman GJ, Luginbuhl A, Curry JM, et al. Exosome-mediated transfer from the tumor microenvironment increases TGFβ signaling in squamous cell carcinoma. Am J Transl Res. 2016;8:2432–7. [PMC free article] [PubMed] [Google Scholar]
- 49.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–99. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016;76:1770–80. doi: 10.1158/0008-5472.CAN-15-1625. [DOI] [PubMed] [Google Scholar]
- 51.Whiteside TL. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017;13:2583–92. doi: 10.2217/fon-2017-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babiuch K, Kuśnierz-Cabala B, Kęsek B, Okoń K, Darczuk D, Chomyszyn-Gajewska M. Evaluation of proinflammatory, NF-kappaB dependent cytokines: IL-1α, IL-6IL-8, and TNF-α in tissue specimens and saliva of patients with oral squamous cell carcinoma and oral potentially malignant disorders. J Clin Med. 2020;9:867. doi: 10.3390/jcm9030867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu Y, Zheng Z, Yuan Y, Pathak JL, Yang X, Wang L, et al. The emerging role of exosomes in oral squamous cell carcinoma. Front Cell Dev Biol. 2021;9:628103. doi: 10.3389/fcell.2021.628103. [DOI] [PMC free article] [PubMed] [Google Scholar]


