Abstract
Accumulating evidence underscores the large role played by the environment in the health of communities and individuals. We review the currently known contribution of environmental exposures and pollutants on kidney disease and its associated morbidity. We review air pollutants, such as particulate matter; water pollutants, such as trace elements, per- and polyfluoroalkyl substances, and pesticides; and extreme weather events and natural disasters. We also discuss gaps in the evidence that presently relies heavily on observational studies and animal models, and propose using recently developed analytic methods to help bridge the gaps. With the expected increase in the intensity and frequency of many environmental exposures in the decades to come, an improved understanding of their potential effect on kidney disease is crucial to mitigate potential morbidity and mortality.
Keywords: clinical nephrology, air pollution, extreme weather events, kidney disease, water pollution
Introduction
There is growing understanding of how the environment affects the health of individuals and communities. Exposure to human-made and naturally occurring toxins in the air, water, and soil can lead to accumulation in organs, and contribute to morbidity and mortality. Even transient events, such as extreme heat and natural disasters, may contribute to adverse health outcomes.
Patients with kidney disease may be especially susceptible to the effects of environmental exposures, given their innate frailty and high comorbidity burden. In this review, we discuss the potential contribution of environmental exposures to kidney disease and associated morbidity (Figure 1). We also discuss the current limitations in understanding and propose the use of recently developed analytic methods that may help to bridge some of these gaps. The intensity and frequency of many environmental exposures are expected to increase due to climate change while, at the same time, the global burden of chronic kidney disease (CKD) is rising for all countries, including those with limited resources (1).
Figure 1.
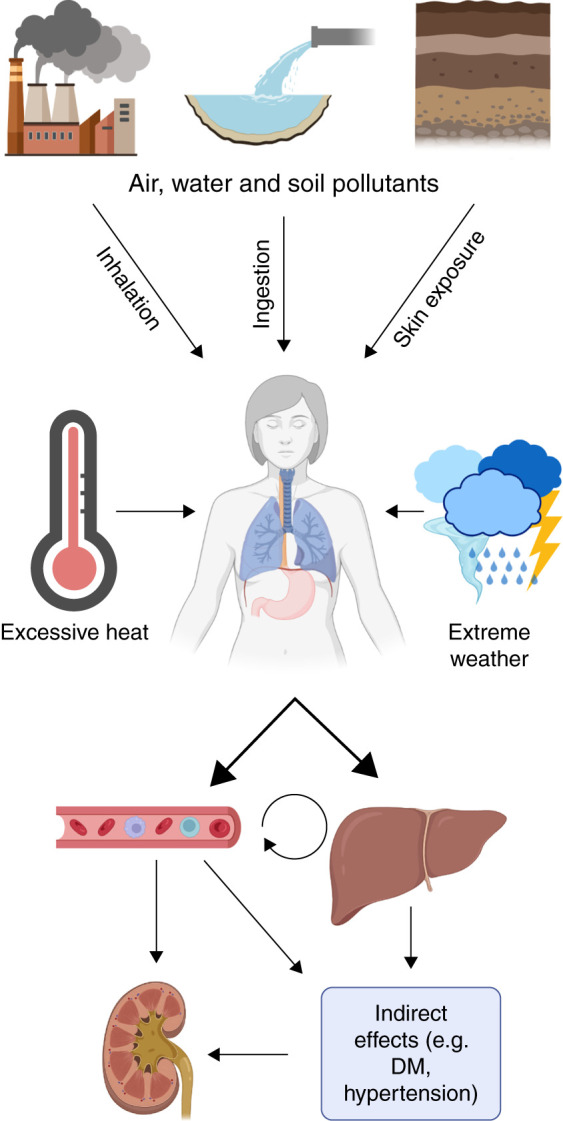
Multiple routes of environmental exposures and their potential end-organ effects. Environmental exposures occur through a variety of routes that can directly and indirectly influence kidney health. Pollutants in the air, water, and soil may be ingested, inhaled, or absorbed through the skin. To varying degrees, these pollutants cross into the bloodstream, where they may travel to, and directly injure, the kidneys. Some compounds will first be absorbed via the gastrointestinal tract, undergoing first-pass metabolism in the liver before returning to the bloodstream. Some exposures may also indirectly contribute to kidney disease by causing conditions such as diabetes and hypertension—well-established risk factors for incident or progressive CKD. DM, diabetes mellitus.
Air
Particulate matter, an air pollutant that is a complex mixture of small particles and liquid droplets arising from the combustion of fossil fuels and biomass, has come into focus for its adverse effects (2,3). Particulate matter with an aero-diameter <2.5 µm (PM2.5) can travel through the respiratory tract and enter the bloodstream after inhalation; its components include sulfates, nitrates, ammonium, hydrogen ions, carbon, volatile organic compounds, and trace metals. PM2.5 is one of the six criteria pollutants regulated by the US Environmental Protection Agency (4).
Cell culture and animal studies demonstrated that both short-term (days) and long-term (months to years) exposure to PM2.5 induces oxidative stress, inflammation, cell autophagy, and cell apoptosis (5–10), whereas studies in humans demonstrated acute thrombus formation and vascular dysfunction (11,12), which is postulated to eventually lead to clinical cardiovascular events and mortality. Mechanisms of injury specific to kidney disease are less clear. A recent study (13) of healthy volunteers demonstrated that inhaled inert gold nanoparticles, a model for PM2.5, entering the bloodstream, are detected in the urine within minutes after exposure. The nanoparticle model suggests PM2.5 can be filtered by the glomerulus and may thus lead to indirect and direct kidney tissue injury.
Epidemiologic data about PM2.5 and kidney disease can be divided into two categories: (1) PM2.5 as a risk factor for kidney disease and progression of CKD to end stage kidney disease (ESKD), and (2) PM2.5 contributing to the morbidity and mortality of individuals with CKD, including ESKD.
A recent systematic review (14) identified 40 epidemiologic studies examining the association of PM2.5 and adverse kidney function. Most of the studies (36 of 40) observed that PM2.5 exposure was associated with adverse kidney function. The assessment of kidney function was clinically diverse, and included outcomes such as glomerular filtration rate (GFR), albuminuria, and glomerulonephritis. We point out some of the included studies to highlight the heterogeneity: (1) long-term PM2.5 exposure was associated with the rise of a specific type of glomerular disease, membranous nephropathy, in an 11-year series of >71,000 native kidney biopsy specimens across China (15); (2) among nearly 1 million US veterans, PM2.5 was associated with incident CKD and the progression of CKD to ESKD (16); (3) among a community-dwelling cohort of middle-aged individuals, PM2.5 was associated with both increased urine albumin and a decline in GFR (17); (4) modeling estimated 7 million incident cases of CKD annually are attributable to PM2.5 worldwide (18); and (5) among a US national cohort of kidney transplant recipients, PM2.5 was associated with increased risk of 1-year kidney rejection post-transplant, graft failure, and all-cause death (19).
Short-term PM2.5 exposure during wildfires (20), and short- and long-term ambient PM2.5, is associated with a 5% increased risk of all-cause and cardiovascular mortality among patients receiving maintenance hemodialysis (21–23). Short-term PM2.5 exposure is also associated with an increased risk of hospital admissions and 30-day readmissions among these patients (24).
In addition, limited evidence suggests an association between tropospheric or ground-level ozone, which is formed by photochemical reactions between volatile organic compounds and nitrogen oxides in the atmosphere, and kidney disease (25,26).
Water
A range of heavy metals, perfluorinated compounds, pesticides, industrial hydrocarbons, and pathogens are common water contaminants. Human exposure to these agents occurs through drinking the water, consumption of animals (especially fish and mollusks) living in the water, or dermal/mucosal contact with the water.
Metals, including arsenic, cadmium, lead, mercury, and uranium, are among the most extensively studied waterborne nephrotoxins. Arsenic is a naturally occurring metalloid found in many parts of the world, especially in groundwater. Arsenic can also be introduced into water via mining and metal smelting (27). Worldwide, >200 million people are estimated to be chronically exposed to arsenic in drinking water at concentrations above the World Health Organization provisional guideline value of 10 μg/L (27,28). Epidemiologic studies linked high drinking water arsenic levels to increased CKD/ESKD incidence (29,30), progression (31), and mortality (32).
Cadmium is released into water, soil, and air via (1) mining and metal refining; (2) production and application of phosphate fertilizers; (3) burning of fossil fuels; and (4) waste incineration, disposal, and recycling (33). Although drinking water contributes only a small proportion of total cadmium exposure in the general population, it can be an important source of exposure for water in the vicinity of cadmium-emitting industries (33,34). Historically, a major outbreak of cadmium toxicity occurred in the Toyama Prefecture (Japan) after contamination of the Jinzu River basin from a zinc mine in 1912. Local inhabitants termed the resulting disease “itai-itai” or “ouch-ouch” disease because of severe, diffuse bone pain from vitamin D–resistant rickets with osteomalacia; other manifestations included proximal tubular dysfunction and hyperphosphaturia (35). Most studies of renal toxicity associated with cadmium have measured exposure via blood or urine levels and have linked exposure with several molecular markers of kidney injury and CKD (33,36,37).
The most common source of lead in drinking water is from leaching of plumbing materials, including lead service lines and residential pipes, lead solder, and certain fixtures (38). However, it can also result from runoff or dumping from lead smelters, lead battery production or recycling operations, and mining (38,39). In most countries, blood lead levels are decreasing, but continue to be of concern (40). Most studies investigating kidney effects related to lead exposure assessed exposure via blood lead levels; they identified associations with CKD incidence (41) and prevalence (42,43), and increased serum creatinine (44) or decreased eGFR (45,46). A recent study demonstrated that high lead levels were associated with a higher prevalence of anemia among patients with ESKD (47). Overall, lead-induced kidney disease may be underdiagnosed or misdiagnosed as hypertensive kidney disease without accurate assessment of lead exposure from patient histories.
Globally, artisanal and small-scale gold mining and coal combustion are the primary sources of anthropogenic mercury emissions (48). General population exposure to mercury, primarily in the form of methylmercury, occurs mostly through consumption of fish, shellfish, and marine mammals from contaminated fresh- or seawater (48). Although chronic exposure to mercury has been shown to induce renal dysfunction, there is limited epidemiologic evidence of an association specifically between methylmercury and CKD (49). Methylmercury toxicity manifests primarily in neurologic changes (Minamata disease) (50) and less commonly in markers of kidney disease, such as proteinuria (51).
For the general population, drinking water is an important source of uranium exposure (52). Contamination of ground- and surface water arises largely from redistribution of uranium and uranium progeny through the natural erosion of rock and soil, although elevated levels can be found near mining operations. Epidemiologic studies have reported associations between uranium levels in drinking water and molecular markers of renal dysfunction, with stronger evidence from animal studies (52).
Per- and polyfluoroalkyl substances, known as PFAS, are a large family of manmade, persistent chemicals widely used in everyday products and widespread drinking water contaminants. PFAS are used in firefighting foam, food packaging, personal care products, nonstick cookware, carpet, upholstery, and many other applications. Two of these, perfluorooctanoic acid and perfluorooctane sulfonate, were manufactured and released into the environment for decades, but are no longer produced or used in the United States or most other industrialized countries (53). However, they and the PFAS that replaced them continue to be found in surface and groundwater sources. These legacy PFAS have been associated directly with increased risk of CKD in some (54–57), but not all (58–60), studies, and inversely with GFR in several studies (56,61,62). The exact nature of the association of PFAS and CKD is ambiguous because serum concentrations of PFAS may increase with decreased kidney function (63). PFAS exposure has also been linked to obesity, diabetes mellitus, hyperlipidemia, and cardiovascular conditions that are direct and indirect risk factors for kidney disease.
For the general population, the primary routes of exposure to trichloroethylene and tetrachloroethylene, used as industrial degreasers and in dry cleaning, are inhalation from ambient or indoor air and ingestion of contaminated drinking water (64,65). These chemicals have been shown to have nephrotoxic effects in epidemiologic and animal studies (64,65).
In addition to chemical pollutants often found in drinking water, biologic contaminants, including the bacteria Leptospira (66) and parasitic worms from the genus Schistosoma (67,68), have been implicated in the pathogenesis of CKD. Aristolochic acids, potent nephrotoxins produced by the Aristolochia plant, were first identified in relation to Balkan endemic nephropathy among individuals using Aristolochia-based herbal remedies (69). A recent study demonstrated the widespread presence and stability of aristolochic acids in groundwater in Serbia (69); however, the prevalence and levels of such groundwater contamination worldwide are unknown.
Exposure to pesticides occurs through several routes, including consumption of contaminated surface or well water. Most studies examining pesticides and CKD have been conducted among farmers or agricultural workers. They have assessed exposure via self-report in terms of applications, i.e., either direct from handling of the pesticides or indirect from being in the vicinity of applications. Pesticides that have been linked to CKD/ESKD in one or more epidemiologic studies include the herbicides alachlor, atrazine, butylate, glyphosate, metolachlor, paraquat, and pendimethalin, and the insecticides methyl parathion and permethrin (70–75). The organochlorine insecticides hexachlorocyclohexane and endosulfan have also been associated with CKD (76,77). The herbicide dicamba has been associated with reduced eGFR (76), whereas the organochlorine insecticide dichlorodiphenyltrichloroethane (DDT) and its primary metabolite have been linked to insulin resistance and increased diabetes risk, which may indirectly impair renal function (78).
Extreme Weather Events and Natural Disasters
An extreme weather event is defined as “time and place in which weather, climate, or environmental conditions—such as temperature, precipitation, drought, or flooding—rank above a threshold value near the upper or lower ends of the range of historical measurements” (79). Natural disasters are weather conditions “…that have the potential to pose a significant threat to human health and safety, property, [and] critical infrastructure…” (80).
To date, the primary epidemiologic focus has been extreme heat, especially in the context of CKD of unknown etiology (CKDu) or CKD of nontraditional origin. CKDu/CKD of nontraditional origin occurs among individuals engaged in intense manual labor in hot environments. The described kidney injury is tubulointerstitial, associated with increased levels of urinary neutrophil gelatinase-associated lipocalin (NGAL) (81–83) and urinary IGF binding protein 7 in some studies (82), and renal biopsy specimens demonstrate acute tubular cell injury and chronic tubulointerstitial nephritis (84). An increase in urinary markers of kidney injury after physical work in the heat has been shown to be exacerbated by longer work durations (85) and the magnitude of hyperthermia and/or dehydration (82). The kidney injury may be exacerbated by the occurrence of muscle-damaging exercise (83) and/or the intake of sugar-sweetened beverages high in fructose (81) that are common in these workplaces (86) and associated with increased urinary neutrophil gelatinase-associated lipocalin. The National Institutes of Health has started a multicenter study of CKDu focused on hot-spot regions in Central America and India, with the intent of characterizing CKDu clinical features and identifying biomarkers for early disease, environmental exposures, and other risk factors.
A recent study demonstrated an association of extreme heat events, defined as temperature >95th percentile for the day and location over 30 years, and hospitalizations and mortality for patients receiving in-center hemodialysis in the northeastern United States (87).
A systematic review evaluated the effects of natural disasters on dialysis populations in the Americas, assessing 15 original research articles published in the English language from 2009 to 2019. They found that disasters have immediate, direct effects related to the ability to receive maintenance dialysis from loss of electricity and other infrastructure, such as water. Additionally, the disasters exacerbate depression and post-traumatic stress disorder in the long term (88). The effect of such extreme heat events and natural disasters (e.g., hurricanes), is expected to increase in the coming years because of climate change.
Mind the Gap
The current literature on the potential role of environmental exposures in kidney disease has important limitations. First, national and international agencies have different accepted levels for pollutants, preventing the establishment of standard toxicity thresholds. Second, we rely either on cell and animal models or on epidemiologic studies—each with their respective challenges. Although in vitro and animal experiments can test highly specific exposures, quantify outcomes, and control conditions, they may not replicate the effects of exposures profiles (dose, duration) seen in the real world. For example, an individual’s PM2.5 exposure is influenced by both environmental and behavioral factors (e.g., air filtration and duration of time outdoors) (89). Furthermore, an individual’s potential effective exposure dose is governed by particle deposition, clearance, and retention within the respiratory tract and extrapulmonary tissues. In addition, interspecies differences in toxicant uptake, metabolism, and response may limit the utility of some animal models. Observational studies often generate and test hypotheses, but they cannot alone establish causation. Furthermore, existing banked specimens from established cohort studies have varying longitudinal follow-up, storage quality, and assay repeatability.
Advances in the “omic” technologies and new study designs may help address some of the limitations. Multiple omic approaches may offer a better picture of the different aspects related to the “exposome”—the measure of all exposures across a person’s lifespan related to health (90). Genomics encompass the study of the DNA structure and its epigenetic regulation, and proteomics include the evaluation of gene products and protein post-translational modifications (91). Metabolomics measure intermediate metabolic chemical processes in biologic tissues and fluids (92). These omic assays have been applied in studies of patients with kidney disease and in healthy individuals (93) and, taken together, they can analyze the flow of biologic information from exposure to gene, protein, and function (and back).
New study designs include meeting-in-the-middle (MITM) (94) and environment-wide association studies. As an example, exposure to PM2.5 is associated with epigenetic changes in DNA methylation (95,96), and exposure to PFAS is associated with plasma metabolites related to kidney injury (97). Alone, these findings are suggestive of associations between exposures and outcomes. MITM studies use advanced regression techniques and mediation analysis to find overlap between proteomic or metabolomic profiles resulting from exposure and those that are predictive of disease (94). This model is particularly useful in prospective cohorts with longitudinal sample collection, such that biologic samples are available before disease onset. One such study examined the relationship between exposure to PFAS during pregnancy and fetal growth restriction (98), combining a metabolome-wide association study of PFAS exposure with a metabolome-wide association study of fetal growth to identify metabolites that were associated with both exposure and outcome. This study identified altered amino acid and lipid metabolism, linking exposure and outcome. Thus, incorporating the MITM approach can strengthen causal inference from these data.
Studies examining multiple exposures present an opportunity to expand the scope of MITM studies. These studies aim to identify environmental factors associated with the disease of interest that are examined individually or as combined exposures (99). Although few such studies are related to kidney disease, recent work in the National Health and Nutritional Examination Survey (NHANES) identified that blood cadmium, lead, and volatile organic compound exposures are associated with CKD (100). This study used biomarkers and additional studies, using survey or geospatial data, are needed to complement these findings.
By integrating multiple environmental exposures, MITM designs could help make connections from environmental exposure to intermediate markers, then to biologic effects, and finally to clinical outcomes in a stepwise fashion. Metabolome- and proteome-wide association studies of environ mental exposures can target metabolic pathways and identify biomarkers of exposure. These “exposure/early effect markers” can then be evaluated for their association with the outcome of interest (Figure 2). Although this type of study is ambitious, the infrastructure to perform it now exists. Multiple large cohorts (including participants with and without kidney disease) would lend themselves to such investigations, including NHANES, the Chronic Renal Insufficiency Study, the Atherosclerosis Risk in Communities study, and the Cure Glomerulopathy Network. Targets identified through these analyses could then be investigated in existing preclinical models to confirm their role in the pathogenesis of kidney disease. Such preclinical models already exist for exposures such as PM2.5 and have helped to elucidate the mechanisms by which particulate matter may directly affect kidney function (101), illustrating how hypotheses generated by examining exposures in observational epidemiologic studies may be tested in vivo.
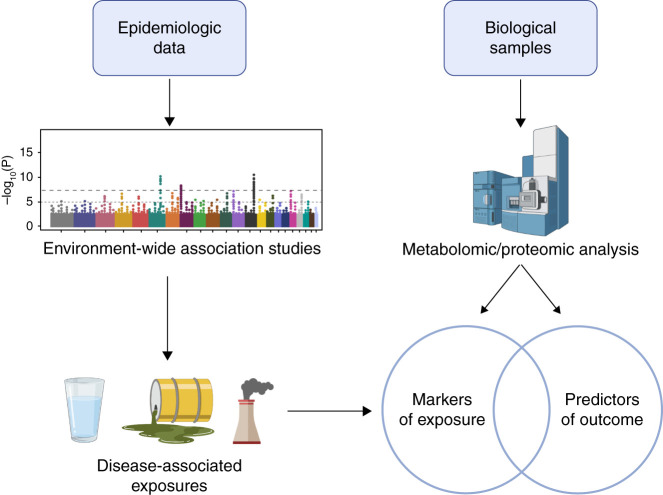
Figure 2.
Integration of epidemiologic data with metabolomic/proteomic analyses to bridge the information gap. Integrating large datasets from previously disparate fields, such as epidemiology and metabolomics, may be key to connecting environmental exposures to biologic outcomes. In the proposed framework, epidemiologic data contribute to epigenome-wide association studies (EWAS), which identify putative disease-associated exposures. Concurrently, metabolomic and proteomic studies can identify signals in biologic samples that are associated with exposure to pollutants, are predictors of outcome, or both. Exposures identified via EWAS can then be compared with these signals, and candidate compounds tested in preclinical models to confirm causality.
Finally, we acknowledge that the health effects from pollution and climate change are differentially distributed among communities in the United States and the world according to wealth/race/ethnicity (102), subjecting analyses to potential confounding and bias, and often limiting the ability to investigate associations in some subgroups. For example, distance to major roads, a proxy indicator for exposure to traffic-related air pollution and community wealth, are inversely associated with estimated GFR (103). Adequate attention to these issues and increased focus on vulnerable communities are needed to better understand the synergy of inequality and environmental exposures in the morbidity of kidney disease.
Summary and Future Directions
Current evidence suggests that environmental exposures may be important contributors to kidney disease morbidity and mortality (Table 1). Some concrete steps may help us bridge existing gaps: (1) regulatory agencies should adopt international consensus data on thresholds of toxicity for pollutants; (2) governments should consider funding an international repository of in vitro, animal, and human data on known environmental pollutants to facilitate pooling and mining of the data; and (3) researchers should integrate novel epidemiologic study designs with large biologic datasets with omics biomarkers. The ultimate goal is to inform individual-level action and public policies to potentially mitigate the risks from these environmental pollutants and reduce the burden of disease.
Table 1.
Summary of environmental pollutants and their potential effect on kidney disease
| Pollutants | Source | Kidney Effect |
|---|---|---|
| Particulate matter <2.5 µm | Air | Associated with kidney function decline, and with CKD and ESKD morbidity/mortality |
| Ozone | Air | Limited association with CKD |
| Heavy metals | ||
| Arsenic | Water, soil, diet | Associated with incident CKD and ESKD, and with CKD progression and mortality |
| Cadmium | Water, soil, air | Bone disease (itai-itai), proximal tubular dysfunction, AKI |
| Lead | Water, soil, air | Associated with incident and prevalent CKD, and with anemia of ESKD |
| Mercury | Seafood, air | Limited association with CKD |
| Uranium | Water | Associated with markers of kidney injury |
| Per- and polyfluoralkyl substances | ||
| Perfluorooctanoic acid | Water, diet | Limited association with kidney disease |
| Perfluorooctane sulfonate | Water, diet | Limited association with kidney disease |
| Industrial degreasers | ||
| Trichloroethylene | Air/water | Both cause nephrotoxicity in animal studies, limited association with CKD |
| Tetrachloroethylene | ||
| Organisms and plants | ||
| Leptospira | Water | Associated with CKD |
| Schistosoma | Water | Associated with CKD |
| Aristolochia | Water, diet | Chronic tubulointerstitial nephritis |
| Insecticides | ||
| Methyl parathion, permethrin, hexachlorocyclohexane, endosulfan, dichlorodiphenyltrichloroethane | Water, diet, dermal contact | All associated with CKD, dichlorodiphenyltrichloroethane specifically associated with insulin resistance and increase risk for diabetes |
| Herbicides | ||
| Alachlor, atrazine, butylate, glycophosate, metolachlor, paraquat, pendimethalin, dicamba | Water, diet, dermal contact | All associated with CKD |
| Heat | N/A | Associated with CKD of unknown cause, and with morbidity for patients with ESKD receiving dialysis |
| Natural disasters | N/A | Associated with increased morbidity for patients with ESKD receiving dialysis |
N/A, not applicable.
Disclosures
N. Franceschini reports serving on the editorial boards of American Journal of Physiology–Renal Physiology and Contemporary Clinical Trials; and serving in an advisory or leadership role as a convener for the National Heart, Lung, and Blood Institute TOPMed kidney working group, as vice-chair of the Women’s Health Initiative Ancillary Committee, and on the Women’s Health Initiative Publication and Presentation Committee. A.V. Kshirsagar reports having consultancy agreements with Alkahest, Rockwell, and Target RWE; serving on the editorial boards of American Journal of Kidney Disease and Kidney Medicine; and having royalties with UpToDate (as contributor). E.M. Zeitler reports receiving research funding, via spouse, from Dexcom, Novo Nordisk, Rhythm Pharmaceuticals, and VTV Therapeutics. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The views expressed in the manuscript do not necessarily reflect the views or policies of the US Environmental Protection Agency.
Author Contributions
A.V. Kshirsagar and E.M. Zeitler conceptualized the manuscript; A.V. Kshirsagar provided supervision and wrote the original draft; and all authors reviewed and edited the manuscript.
References
- 1.GBD Chronic Kidney Disease Collaboration : Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395: 709–733, 2020. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD, Dominici F: Association of short-term exposure to air pollution with mortality in older adults. JAMA 318: 2446–2456, 2017. 10.1001/jama.2017.17923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, Coelho MSZS, Saldiva PHN, Lavigne E, Matus P, Valdes Ortega N, Osorio Garcia S, Pascal M, Stafoggia M, Scortichini M, Hashizume M, Honda Y, Hurtado-Díaz M, Cruz J, Nunes B, Teixeira JP, Kim H, Tobias A, Íñiguez C, Forsberg B, Åström C, Ragettli MS, Guo YL, Chen BY, Bell ML, Wright CY, Scovronick N, Garland RM, Milojevic A, Kyselý J, Urban A, Orru H, Indermitte E, Jaakkola JJK, Ryti NRI, Katsouyanni K, Analitis A, Zanobetti A, Schwartz J, Chen J, Wu T, Cohen A, Gasparrini A, Kan H: ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 381: 705–715, 2019. 10.1056/NEJMoa1817364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Environmental Protection Agency : Integrated science assessment (ISA) for particulate matter (final report Dec 2009), Washington, DC, United States Environmental Protection Agency, 2009. Available at: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=216546. Accessed July 1, 2022 [PubMed] [Google Scholar]
- 5.Feng S, Gao D, Liao F, Zhou F, Wang X: The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol Environ Saf 128: 67–74, 2016. 10.1016/j.ecoenv.2016.01.030 [DOI] [PubMed] [Google Scholar]
- 6.Kouassi KS, Billet S, Garçon G, Verdin A, Diouf A, Cazier F, Djaman J, Courcot D, Shirali P: Oxidative damage induced in A549 cells by physically and chemically characterized air particulate matter (PM2.5) collected in Abidjan, Côte d’Ivoire. J Appl Toxicol 30: 310–320, 2010. 10.1002/jat.1496 [DOI] [PubMed] [Google Scholar]
- 7.Longhin E, Holme JA, Gutzkow KB, Arlt VM, Kucab JE, Camatini M, Gualtieri M: Cell cycle alterations induced by urban PM2.5 in bronchial epithelial cells: Characterization of the process and possible mechanisms involved. Part Fibre Toxicol 10: 63, 2013. 10.1186/1743-8977-10-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelin TD, Joseph AM, Gorr MW, Wold LE: Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol Lett 208: 293–299, 2012. 10.1016/j.toxlet.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Kloog I, Coull BA, Kosheleva A, Zanobetti A, Schwartz JD: Estimating causal effects of long-term PM2.5 exposure on mortality in New Jersey. Environ Health Perspect 124: 1182–1188, 2016. 10.1289/ehp.1409671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing YF, Xu YH, Shi MH, Lian YX: The impact of PM2.5 on the human respiratory system. J Thorac Dis 8: 69–74, 2016. 10.3978/j.issn.2072-1439.2016.01.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucking AJ, Lundback M, Mills NL, Faratian D, Barath SL, Pourazar J, Cassee FR, Donaldson K, Boon NA, Badimon JJ, Sandstrom T, Blomberg A, Newby DE: Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J 29: 3043–3051, 2008. 10.1093/eurheartj/ehn464 [DOI] [PubMed] [Google Scholar]
- 12.Mills NL, Törnqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE: Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112: 3930–3936, 2005. 10.1161/CIRCULATIONAHA.105.588962 [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens PHB, Boere AJF, Krystek P, Campbell CJ, Hadoke PWF, Donaldson K, Cassee FR, Newby DE, Duffin R, Mills NL: Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano 11: 4542–4552, 2017. 10.1021/acsnano.6b08551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasking L, Vanbrabant K, Bové H, Plusquin M, De Vusser K, Roels HA, Nawrot TS: Adverse Effects of fine particulate matter on human kidney functioning: A systematic review. Environ Health 21: 24, 2022. 10.1186/s12940-021-00827-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, Zhang P, Luo Y, Wang Y, Wang X, Schwartz J, Geng J, Hou FF: Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol 27: 3739–3746, 2016. 10.1681/ASN.2016010093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z: Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 29: 218–230, 2018. 10.1681/ASN.2017030253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum MF, Surapaneni A, Stewart JD, Liao D, Yanosky JD, Whitsel EA, Power MC, Grams ME: Particulate matter and albuminuria, glomerular filtration rate, and incident CKD. Clin J Am Soc Nephrol 15: 311–319, 2020. 10.2215/CJN.08350719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z: Estimates of the 2016 global burden of kidney disease attributable to ambient fine particulate matter air pollution. BMJ Open 9: 022450, 2019. 10.1136/bmjopen-2018-022450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang SH, Merzkani M, Murad H, Wang M, Bowe B, Lentine KL, Al-Aly Z, Alhamad T: Association of ambient fine particulate matter air pollution with kidney transplant outcomes. JAMA Netw Open 4: 2128190, 2021. 10.1001/jamanetworkopen.2021.28190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xi Y, Kshirsagar AV, Wade TJ, Richardson DB, Brookhart MA, Wyatt L, Rappold AG: Mortality in US hemodialysis patients following exposure to wildfire smoke. J Am Soc Nephrol 31: 1824–1835, 2020. 10.1681/ASN.2019101066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xi Y, Richardson DB, Kshirsagar AV, Wade TJ, Flythe JE, Whitsel EA, Peterson GC, Wyatt LH, Rappold AG: Effects of short-term ambient PM2.5 exposure on cardiovascular disease incidence and mortality among U.S. hemodialysis patients: A retrospective cohort study. Environ Health 21: 33, 2022. 10.1186/s12940-022-00836-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xi Y, Richardson DB, Kshirsagar AV, Wade TJ, Flythe JE, Whitsel EA, Rappold AG: Association between long-term ambient PM2.5 exposure and cardiovascular outcomes among US hemodialysis patients. Am J Kidney Dis 80: 648–657.e1, 2022. 10.1053/j.ajkd.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 23.Feng Y, Jones MR, Chu NM, Segev DL, McAdams-DeMarco M: Ambient air pollution and mortality among older patients initiating maintenance dialysis. Am J Nephrol 52: 217–227, 2021. 10.1159/000514233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyatt LH, Xi Y, Kshirsagar A, Di Q, Ward-Caviness C, Wade TJ, Cascio WE, Rappold AG: Association of short-term exposure to ambient PM2.5 with hospital admissions and 30-day readmissions in end-stage renal disease patients: Population-based retrospective cohort study. BMJ Open 10: 041177, 2020. 10.1136/bmjopen-2020-041177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C, Wang W, Wang Y, Liang Z, Zhang F, Chen R, Liang C, Wang F, Li P, Ma L, Li S, Deng F, Zhang L: Ambient ozone pollution and prevalence of chronic kidney disease: A nationwide study based on the China National survey of chronic kidney disease. Chemosphere 306: 135603, 2022. 10.1016/j.chemosphere.2022.135603 [DOI] [PubMed] [Google Scholar]
- 26.Weaver AM, Wang Y, Wellenius GA, Young B, Boyle LD, Hickson DA, Diamantidis CJ: Long-term exposure to ambient air pollution and renal function in African Americans: The Jackson Heart Study. J Expo Sci Environ Epidemiol 29: 548–556, 2019. 10.1038/s41370-018-0092-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agency for Toxic Substances and Disease Registry : Toxicological profile for Arsenic, Atlanta, GA, U.S. Department of Health and Human Services Public Health Service, 2007. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf. Accessed July 1, 2022 [Google Scholar]
- 28.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA: The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ Health Perspect 121: 295–302, 2013. 10.1289/ehp.1205875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu LI, Hsieh FI, Wang YH, Lai TS, Wu MM, Chen CJ, Chiou HY, Hsu KH: Arsenic exposure from drinking water and the incidence of CKD in low to moderate exposed areas of Taiwan: A 14-year prospective study. Am J Kidney Dis 70: 787–797, 2017. 10.1053/j.ajkd.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 30.Cheng YY, Chang YT, Cheng HL, Shen KH, Sung JM, Guo HR: Associations between arsenic in drinking water and occurrence of end-stage renal disease with modifications by comorbidities: A nationwide population-based study in Taiwan. Sci Total Environ 626: 581–591, 2018. 10.1016/j.scitotenv.2018.01.043 [DOI] [PubMed] [Google Scholar]
- 31.Cheng YY, Huang NC, Chang YT, Sung JM, Shen KH, Tsai CC, Guo HR: Associations between arsenic in drinking water and the progression of chronic kidney disease: A nationwide study in Taiwan. J Hazard Mater 321: 432–439, 2017. 10.1016/j.jhazmat.2016.09.032 [DOI] [PubMed] [Google Scholar]
- 32.Meliker JR, Wahl RL, Cameron LL, Nriagu JO: Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: A standardized mortality ratio analysis. Environ Health 6: 4, 2007. 10.1186/1476-069X-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agency for Toxic Substances and Disease Registry : Toxicological profile for cadmium, Atlanta, GA, U.S. Department of Health and Human Services Public Health Service, 2012. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf. Accessed July 1, 2022 [Google Scholar]
- 34.Järup L, Akesson A: Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238: 201–208, 2009. 10.1016/j.taap.2009.04.020 [DOI] [PubMed] [Google Scholar]
- 35.Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A: The effects of cadmium toxicity. Int J Environ Res Public Health 17: 3782, 2020. 10.3390/ijerph17113782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Chen X, Wang X, Wang M, Liang Y, Zhu G, Jin T: The association between estimated glomerular filtration rate and cadmium exposure: An 8-year follow-up study. Int J Hyg Environ Health 235: 113774, 2021. 10.1016/j.ijheh.2021.113774 [DOI] [PubMed] [Google Scholar]
- 37.Jalili C, Kazemi M, Cheng H, Mohammadi H, Babaei A, Taheri E, Moradi S: Associations between exposure to heavy metals and the risk of chronic kidney disease: A systematic review and meta-analysis. Crit Rev Toxicol 51: 165–182, 2021. 10.1080/10408444.2021.1891196 [DOI] [PubMed] [Google Scholar]
- 38.Agency for Toxic Substances and Disease Registry : Toxicological profile for lead. Atlanta, GA, U.S. Department of Health and Human Services Public Health Service, 2020. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf. Accessed July 1, 2022 [Google Scholar]
- 39.UNICEF and Pure Earth : The toxic truth: Children’s exposure to lead pollution undermines a generation of future potential, 2020. Available at: https://www.unicef.org/media/109361/file/The%20toxic%20truth.pdf. Accessed July 1, 2022
- 40.World Health Organization : Guidelines for drinking-water quality, 4th edition, incorporating the first addendum, 2017. Available at: https://www.who.int/publications/i/item/9789241549950. Accessed July 1, 2022 [PubMed]
- 41.Harari F, Sallsten G, Christensson A, Petkovic M, Hedblad B, Forsgard N, Melander O, Nilsson PM, Borné Y, Engström G, Barregard L: Blood lead levels and decreased kidney function in a population-based cohort. Am J Kidney Dis 72: 381–389, 2018. 10.1053/j.ajkd.2018.02.358 [DOI] [PubMed] [Google Scholar]
- 42.Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V: Blood cadmium and lead and chronic kidney disease in US adults: A joint analysis. Am J Epidemiol 170: 1156–1164, 2009. 10.1093/aje/kwp248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muntner P, He J, Vupputuri S, Coresh J, Batuman V: Blood lead and chronic kidney disease in the general United States population: Results from NHANES III. Kidney Int 63: 1044–1050, 2003. 10.1046/j.1523-1755.2003.00812.x [DOI] [PubMed] [Google Scholar]
- 44.Kim R, Rotnitsky A, Sparrow D, Weiss S, Wager C, Hu H: A longitudinal study of low-level lead exposure and impairment of renal function. The Normative Aging Study. JAMA 275: 1177–1181, 1996. 10.1001/jama.1996.03530390043032 [DOI] [PubMed] [Google Scholar]
- 45.Pollack AZ, Mumford SL, Mendola P, Perkins NJ, Rotman Y, Wactawski-Wende J, Schisterman EF: Kidney biomarkers associated with blood lead, mercury, and cadmium in premenopausal women: A prospective cohort study. J Toxicol Environ Health A 78: 119–131, 2015. 10.1080/15287394.2014.944680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu CC, Lin JL, Lin-Tan DT: Environmental exposure to lead and progression of chronic renal diseases: A four-year prospective longitudinal study. J Am Soc Nephrol 15: 1016–1022, 2004. 10.1097/01.ASN.0000118529.01681.4F [DOI] [PubMed] [Google Scholar]
- 47.Danziger J, Mukamal KJ, Weinhandl E: Associations of community water lead concentrations with hemoglobin concentrations and erythropoietin-stimulating agent use among patients with advanced CKD. J Am Soc Nephrol 32: 2425–2434, 2021. 10.1681/ASN.2020091281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.United Nations Program and Global Mercury Partnership : Global mercury assessment, Geneva, Switzerland, 2018, pp 1–62. Available at: https://www.unep.org/globalmercurypartnership/resources/report/global-mercury-assessment-2018. Accessed July 1, 2022 [Google Scholar]
- 49.Agency for Toxic Substances and Disease Registry : Toxicological profile for mercury, Atlanta, GA, U.S. Department of Health and Human Services Public Health Service, 2022. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf. Accessed July 1, 2022 [Google Scholar]
- 50.Eto K: Minamata disease. Neuropathology 20: 14–19, 2000. 10.1046/j.1440-1789.2000.00295.x [DOI] [PubMed] [Google Scholar]
- 51.Miller S, Pallan S, Gangji AS, Lukic D, Clase CM: Mercury-associated nephrotic syndrome: A case report and systematic review of the literature. Am J Kidney Dis 62: 135–138, 2013. 10.1053/j.ajkd.2013.02.372 [DOI] [PubMed] [Google Scholar]
- 52.Agency for Toxic Substances and Disease Registry : Toxicological profile for uranium. Atlanta, GA, U.S. Department of Health and Human Services Public Health Service, 2013. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp150.pdf. Accessed July 1, 2022 [Google Scholar]
- 53.Agency for Toxic Substances and Disease Registry : Toxicological profile for perfluoroalkyls. Atlanta, GA, U.S. Department of Health and Human Services Public Health Service, 2021. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf. Accessed July 1, 2022 [PubMed] [Google Scholar]
- 54.Anderson-Mahoney P, Kotlerman J, Takhar H, Gray D, Dahlgren J: Self-reported health effects among community residents exposed to perfluorooctanoate. New Solut 18: 129–143, 2008. 10.2190/NS.18.2.d [DOI] [PubMed] [Google Scholar]
- 55.Steenland K, Woskie S: Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol 176: 909–917, 2012. 10.1093/aje/kws171 [DOI] [PubMed] [Google Scholar]
- 56.Shankar A, Xiao J, Ducatman A: Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol 174: 893–900, 2011. 10.1093/aje/kwr171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie LN, Wang XC, Su LQ, Ji SS, Dong XJ, Zhu HJ, Hou SS, Wang C, Li ZH, Dong B, Zhu Y: Serum concentrations of per-/polyfluoroalkyl substances and its association with renal function parameters among teenagers near a Chinese fluorochemical industrial plant: A cross-sectional study. Environ Pollut 302: 119020, 2022. 10.1016/j.envpol.2022.119020 [DOI] [PubMed] [Google Scholar]
- 58.Raleigh KK, Alexander BH, Olsen GW, Ramachandran G, Morey SZ, Church TR, Logan PW, Scott LL, Allen EM: Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occup Environ Med 71: 500–506, 2014. 10.1136/oemed-2014-102109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steenland K, Zhao L, Winquist A: A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup Environ Med 72: 373–380, 2015. 10.1136/oemed-2014-102364 [DOI] [PubMed] [Google Scholar]
- 60.Dhingra R, Lally C, Darrow LA, Klein M, Winquist A, Steenland K: Perfluorooctanoic acid and chronic kidney disease: Longitudinal analysis of a Mid-Ohio Valley community. Environ Res 145: 85–92, 2016. 10.1016/j.envres.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 61.Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L: Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ Health 14: 89, 2015. 10.1186/s12940-015-0077-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, Savitz DA, Fletcher T, Wellenius GA: Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect 121: 625–630, 2013. 10.1289/ehp.1205838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE: Perfluorinated chemicals as emerging environmental threats to kidney health: A scoping review. Clin J Am Soc Nephrol 13: 1479–1492, 2018. 10.2215/CJN.04670418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agency for Toxic Substances and Disease Registry : Toxicological profile for trichloroethylene. Atlanta, GA, U.S. Department of Health and Human Services Public Health Service, 2019. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp19.pdf. Accessed July 1, 2022 [PubMed] [Google Scholar]
- 65.Agency for Toxic Substances and Disease Registry : Toxicological profile for tetrachloroethylene. Atlanta, GA, U.S. Department of Health and Human Services Public Health Service, 2019. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp18.pdf. Accessed July 1, 2022 [PubMed] [Google Scholar]
- 66.Carrillo-Larco RM, Altez-Fernandez C, Acevedo-Rodriguez JG, Ortiz-Acha K, Ugarte-Gil C: Leptospirosis as a risk factor for chronic kidney disease: A systematic review of observational studies. PLoS Negl Trop Dis 13: 0007458, 2019. 10.1371/journal.pntd.0007458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galvão RLF, Meneses GC, Pinheiro MCC, Martins AMC, Daher EF, Bezerra FSM: Kidney injury biomarkers and parasitic loads of Schistosoma mansoni in a highly endemic area in northeastern Brazil. Acta Trop 228: 106311, 2022. 10.1016/j.actatropica.2022.106311 [DOI] [PubMed] [Google Scholar]
- 68.Barsoum RS: Parasitic kidney disease: milestones in the evolution of our knowledge. Am J Kidney Dis 61: 501–513, 2013. 10.1053/j.ajkd.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 69.Tung KK, Chan CK, Zhao Y, Chan KJ, Liu G, Pavlović NM, Chan W: Occurrence and environmental stability of aristolochic acids in groundwater collected from Serbia: Links to human exposure and Balkan endemic nephropathy. Environ Sci Technol 54: 1554–1561, 2020. 10.1021/acs.est.9b05337 [DOI] [PubMed] [Google Scholar]
- 70.Lebov JF, Engel LS, Richardson D, Hogan SL, Hoppin JA, Sandler DP: Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the Agricultural Health Study. Occup Environ Med 73: 3–12, 2016. 10.1136/oemed-2014-102615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lebov JF, Engel LS, Richardson D, Hogan SL, Sandler DP, Hoppin JA: Pesticide exposure and end-stage renal disease risk among wives of pesticide applicators in the Agricultural Health Study. Environ Res 143: 198–210, 2015. 10.1016/j.envres.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jayasumana C, Paranagama P, Agampodi S, Wijewardane C, Gunatilake S, Siribaddana S: Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ Health 14: 6, 2015. 10.1186/1476-069X-14-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orantes CM, Herrera R, Almaguer M, Brizuela EG, Núñez L, Alvarado NP, Fuentes EJ, Bayarre HD, Amaya JC, Calero DJ, Vela XF, Zelaya SM, Granados DV, Orellana P: Epidemiology of chronic kidney disease in adults of Salvadoran agricultural communities [published corrections appear in MEDICC Rev 16: 30 and 54, 2014]. MEDICC Rev 16: 23–30, 2014. 10.37757/MR2014.V16.N2.5 [DOI] [PubMed] [Google Scholar]
- 74.Zhang F, Pan LP, Ding EM, Ge QJ, Zhang ZH, Xu JN, Zhang L, Zhu BL: Study of the effect of occupational exposure to glyphosate on hepatorenal function. Zhonghua Yu Fang Yi Xue Za Zhi 51: 615–620, 2017. 10.3760/cma.j.issn.0253-9624.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 75.Shearer JJ, Sandler DP, Andreotti G, Murata K, Shrestha S, Parks CG, Liu D, Alavanja MC, Landgren O, Beane Freeman LE, Hofmann JN: Pesticide use and kidney function among farmers in the Biomarkers of Exposure and Effect in Agriculture study. Environ Res 199: 111276, 2021. 10.1016/j.envres.2021.111276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghosh R, Siddarth M, Singh N, Tyagi V, Kare PK, Banerjee BD, Kalra OP, Tripathi AK: Organochlorine pesticide level in patients with chronic kidney disease of unknown etiology and its association with renal function. Environ Health Prev Med 22: 49, 2017. 10.1186/s12199-017-0660-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siddarth M, Datta SK, Mustafa M, Ahmed RS, Banerjee BD, Kalra OP, Tripathi AK: Increased level of organochlorine pesticides in chronic kidney disease patients of unknown etiology: Role of GSTM1/GSTT1 polymorphism. Chemosphere 96: 174–179, 2014. 10.1016/j.chemosphere.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 78.Lind PM, Lind L: Endocrine-disrupting chemicals and risk of diabetes: An evidence-based review. Diabetologia 61: 1495–1502, 2018. 10.1007/s00125-018-4621-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herring D: What is an “extreme event”? Is there evidence that global warming has caused or contributed to any particular extreme event? Available at: https://www.climate.gov/news-features/climate-qa/what-extreme-event-there-evidence-global-warming-has-caused-or-contributed. Accessed July 1, 2022
- 80.Department of Homeland Security : Natural disasters. Available at: https://www.dhs.gov/natural-disasters. Accessed July 1, 2022
- 81.Chapman CL, Johnson BD, Sackett JR, Parker MD, Schlader ZJ: Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am J Physiol Regul Integr Comp Physiol 316: 189–198, 2019. 10.1152/ajpregu.00351.2018 [DOI] [PubMed] [Google Scholar]
- 82.Chapman CL, Johnson BD, Vargas NT, Hostler D, Parker MD, Schlader ZJ: Both hyperthermia and dehydration during physical work in the heat contribute to the risk of acute kidney injury. J Appl Physiol (1985) 128: 715–728, 2020. 10.1152/japplphysiol.00787.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Junglee NA, Di Felice U, Dolci A, Fortes MB, Jibani MM, Lemmey AB, Walsh NP, Macdonald JH: Exercising in a hot environment with muscle damage: Effects on acute kidney injury biomarkers and kidney function. Am J Physiol Renal Physiol 305: 813–820, 2013. 10.1152/ajprenal.00091.2013 [DOI] [PubMed] [Google Scholar]
- 84.Fischer RSB, Vangala C, Truong L, Mandayam S, Chavarria D, Granera Llanes OM, Fonseca Laguna MU, Guerra Baez A, Garcia F, García-Trabanino R, Murray KO: Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int 93: 681–690, 2018. 10.1016/j.kint.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 85.Schlader ZJ, Chapman CL, Sarker S, Russo L, Rideout TC, Parker MD, Johnson BD, Hostler D: Firefighter work duration influences the extent of acute kidney injury. Med Sci Sports Exerc 49: 1745–1753, 2017. 10.1249/MSS.0000000000001254 [DOI] [PubMed] [Google Scholar]
- 86.Fleischer NL, Tiesman HM, Sumitani J, Mize T, Amarnath KK, Bayakly AR, Murphy MW: Public health impact of heat-related illness among migrant farmworkers. Am J Prev Med 44: 199–206, 2013. 10.1016/j.amepre.2012.10.020 [DOI] [PubMed] [Google Scholar]
- 87.Remigio RV, Jiang C, Raimann J, Kotanko P, Usvyat L, Maddux FW, Kinney P, Sapkota A: Association of extreme heat events with hospital admission or mortality among patients with end-stage renal disease. JAMA Netw Open 2: 198904, 2019. 10.1001/jamanetworkopen.2019.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith RS, Zucker RJ, Frasso R: Natural disasters in the Americas, dialysis patients, and implications for emergency planning: A systematic review. Prev Chronic Dis 17: 42, 2020. 10.5888/pcd17.190430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koistinen KJ, Hanninen O, Rotko T, Edwards RD, Moschandreas D, Jantunen MJ: Behavioral and environmental determinants of personal exposure to PM2.5 in EXPOLIS – Helsinki, Finland. Atmos Environ 35: 2473–2481, 2001. 10.1016/S1352-2310(00)00446-5 [DOI] [Google Scholar]
- 90.Wild CP: Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev 14: 1847–1850, 2005. 10.1158/1055-9965.EPI-05-0456 [DOI] [PubMed] [Google Scholar]
- 91.Dubin RF, Rhee EP: Proteomics and metabolomics in kidney disease, including insights into etiology, treatment, and prevention. Clin J Am Soc Nephrol 15: 404–411, 2020. 10.2215/CJN.07420619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson CH, Ivanisevic J, Siuzdak G: Metabolomics: Beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 17: 451–459, 2016. 10.1038/nrm.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Titan SM, Venturini G, Padilha K, Goulart AC, Lotufo PA, Bensenor IJ, Krieger JE, Thadhani RI, Rhee EP, Pereira AC: Metabolomics biomarkers and the risk of overall mortality and ESRD in CKD: Results from the Progredir Cohort. PLoS One 14: 0213764, 2019. 10.1371/journal.pone.0213764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Assi N, Fages A, Vineis P, Chadeau-Hyam M, Stepien M, Duarte-Salles T, Byrnes G, Boumaza H, Knüppel S, Kühn T, Palli D, Bamia C, Boshuizen H, Bonet C, Overvad K, Johansson M, Travis R, Gunter MJ, Lund E, Dossus L, Elena-Herrmann B, Riboli E, Jenab M, Viallon V, Ferrari P: A statistical framework to model the meeting-in-the-middle principle using metabolomic data: Application to hepatocellular carcinoma in the EPIC study. Mutagenesis 30: 743–753, 2015. 10.1093/mutage/gev045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu C, Xu J, Chen Y, Guo X, Zheng Y, Wang Q, Chen Y, Ni Y, Zhu Y, Joyce BT, Baccarelli A, Deng F, Zhang W, Hou L: Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature. Environ Health 14: 65, 2015. 10.1186/s12940-015-0052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Z, Li N, Guo C, Li X, Qian Y, Wu J, Yang Y, Wei Y: Genomic DNA methylation signatures in different tissues after ambient air particulate matter exposure. Ecotoxicol Environ Saf 179: 175–181, 2019. 10.1016/j.ecoenv.2019.04.049 [DOI] [PubMed] [Google Scholar]
- 97.Lu Y, Gao K, Li X, Tang Z, Xiang L, Zhao H, Fu J, Wang L, Zhu N, Cai Z, Liang Y, Wang Y, Jiang G: Mass spectrometry-based metabolomics reveals occupational exposure to per- and polyfluoroalkyl substances relates to oxidative stress, fatty acid β-oxidation disorder, and kidney injury in a manufactory in China. Environ Sci Technol 53: 9800–9809, 2019. 10.1021/acs.est.9b01608 [DOI] [PubMed] [Google Scholar]
- 98.Chang CJ, Barr DB, Ryan PB, Panuwet P, Smarr MM, Liu K, Kannan K, Yakimavets V, Tan Y, Ly V, Marsit CJ, Jones DP, Corwin EJ, Dunlop AL, Liang D: Per- and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: A meet-in-the-middle approach. Environ Int 158: 106964, 2022. 10.1016/j.envint.2021.106964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patel CJ, Bhattacharya J, Butte AJ: An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS One 5: 10746, 2010. 10.1371/journal.pone.0010746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J, Oh S, Kang H, Kim S, Lee G, Li L, Kim CT, An JN, Oh YK, Lim CS, Kim DK, Kim YS, Choi K, Lee JP: Environment-wide association study of CKD. Clin J Am Soc Nephrol 15: 766–775, 2020. 10.2215/CJN.06780619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aztatzi-Aguilar OG, Pardo-Osorio GA, Uribe-Ramírez M, Narváez-Morales J, De Vizcaya-Ruiz A, Barbier OC: Acute kidney damage by PM2.5 exposure in a rat model. Environ Toxicol Pharmacol 83: 103587, 2021. 10.1016/j.etap.2021.103587 [DOI] [PubMed] [Google Scholar]
- 102.Al-Aly Z: We must all join the effort to dismantle environmental racism. J Am Soc Nephrol 33: 12–14, 2022. 10.1681/ASN.2021081118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lue SH, Wellenius GA, Wilker EH, Mostofsky E, Mittleman MA: Residential proximity to major roadways and renal function. J Epidemiol Community Health 67: 629–634, 2013. 10.1136/jech-2012-202307 [DOI] [PMC free article] [PubMed] [Google Scholar]