Key Points
Kidney biopsy registries in the United States are lacking.
We provide a multicenter, multistate kidney biopsy database in the United States and identified demographic and clinical trends.
Our study catalogs the spectrum of biopsy-proven kidney disease across the Cleveland Clinic enterprise andhighlights the need for a standardized national kidney biopsy registry to bolster glomerular and kidney disease research in the United States.
Keywords: clinical nephrology, database, diabetes, epidemiology, FSGS, glomerular disease, glomerulonephritis, IgA nephropathy, kidney biopsy, lupus nephritis, registry
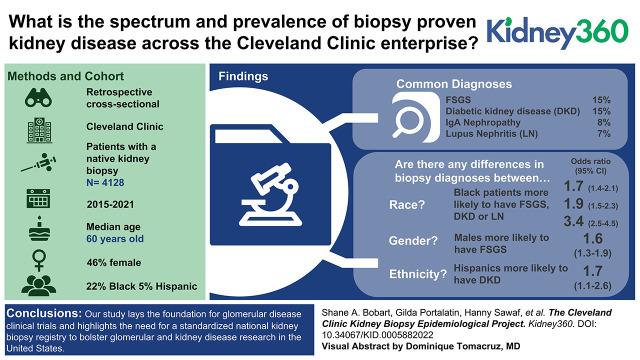
Visual Abstract
Abstract
Background
The kidney biopsy is the gold standard for diagnosing glomerular diseases. Large-scale, epidemiologic studies describing the prevalence of kidney diseases are lacking, especially in the United States. We aimed to determine the spectrum of biopsy-proven kidney disease across the Cleveland Clinic enterprise.
Methods
We identified all patients with a native kidney biopsy performed or reviewed at the Cleveland Clinic from January 2015 to September 2021. Retrospective chart review was performed to obtain clinical and demographic characteristics. Results were stratified by age, sex, race, and location to determine epidemiologic trends.
Results
Of >9600 patients, we excluded transplant and donor biopsies and unavailable records, and included 4128 patients with native kidney biopsy data. The median age was 60 years, with 46% female patients. Self-reported racial demographics included 73% White, 22% Black, 3% multiracial, and 2% Asian background, with 5% Hispanic. Common diagnoses were: FSGS (n=633, 15%), diabetic kidney disease (DKD) (n=602, 15%), IgA nephropathy (n=319, 8%), lupus nephritis (LN) (n=289, 7%), pauci-immune glomerulonephritis (n=275, 7%), membranous nephropathy (n=211, 5%), and amyloidosis (n=110, 3%). There were 3322 patients in Ohio, with 361 patients in Florida. Using multivariate analysis, those aged >70 years were more likely to have FSGS, whereas those <45 years were more likely to have IgA nephropathy or LN. Males were more likely to have FSGS or IgAN, and less likely to have LN. Black patients were more likely to have FSGS, DKD, or LN. Hispanic patients were more likely to have DKD. Finally, patients in Florida were more likely to have LN. There was no change in the disease spectrum before and during the COVID-19 pandemic.
Conclusion
Our study catalogs the spectrum of biopsy-proven kidney disease across the Cleveland Clinic enterprise. This lays the foundation for glomerular disease clinical trials, and highlights the need for a standardized national kidney biopsy registry to bolster glomerular and kidney disease research in the United States.
Introduction
The kidney biopsy is the gold standard for diagnosing kidney diseases, particularly glomerular diseases (1). Glomerular diseases are associated with long-term morbidity and progression to ESKD. Although United States Renal Data System data are helpful, often times, the diagnoses presented are not biopsy proven (2), as such the ability to rely on these data for epidemiologic patterns is limited. Epidemiologic studies help inform clinicians about disease likelihood and trends, provide insights into risk factors or pathogenesis, and help with public health policy and future studies. Despite this, large-scale, epidemiologic studies describing the prevalence of kidney diseases are lacking in the United States. However, efforts to catalog and maintain records of biopsy-proven kidney disease are well established across the world (3).
In one meta-analysis of kidney biopsy registries, 16 major national kidney biopsy catalogs were identified worldwide (Europe, South America, Asia, Canada), of which none are in the United States (3). The United States has a few single-center registries (4,5), but no national registry or multicenter database available. The Cleveland Clinic is unique in that it has several locations across the United States, which allows for greater spectrum and assessment of epidemiologic trends in kidney disease. Furthermore, there are barriers that exist to compiling and maintaining kidney biopsy data, including the ability to have standardized procedures for reporting of kidney biopsy diagnoses and overall lack of time and resources to maintain and carry out data collection (3). Recently, the reporting of kidney biopsies has become more standardized, allowing for easier classification into specific disease diagnoses or patterns (1,6).
The Division of Renal Pathology at Cleveland Clinic Ohio provides renal pathology services to several locations across the United States, including Cleveland Clinic Ohio, Main Campus, and its satellite hospitals in Ohio and Cleveland Clinic Florida, which includes Weston, Florida and locations along the treasure coast of Florida. Given the degree of patient access provided at the Cleveland Clinic, with diverse populations served across several locations within the United States, we aimed to determine the spectrum and prevalence of biopsy-proven kidney disease across the Cleveland Clinic enterprise, with a focus on demographic and geographic trends. Doing so would assist in identifying disease groups that could benefit from clinical trial enrollment and provide a foundation to advocate for a state-wide or national kidney biopsy registry in the United States.
Methods
Patient Population
The study was approved with exempt status by the Cleveland Clinic Institutional Review Board. In this cross-sectional, observational study, we identified all patients who had a kidney biopsy performed or reviewed at the Cleveland Clinic from January 2015 to September 2021. We excluded patients who had a kidney allograft biopsy, those without a Cleveland Clinic medical record number, or those unable to be verified in the electronic medical record.
Kidney Biopsy Review
Across the enterprise, kidney biopsies were performed for common indications, including unexplained kidney failure, nephritic syndrome, nephrotic syndrome, unexplained non-nephrotic range proteinuria, or concern for glomerular hematuria. Biopsies were classified into the following diagnoses on the basis of the report provided by the interpreting nephropathologist, and where descriptive or unclear, subsequently clarified or confirmed by chart review of the treating nephrologists’ notes.
Biopsy diagnoses were: FSGS, global glomerulosclerosis, diabetic kidney disease (DKD), minimal change disease, membranous nephropathy, amyloidosis, monoclonal immunoglobulin deposition disease, C3 glomerulonephritis, dense deposit disease, immunotactoid glomerulonephritis, light chain proximal tubulopathy, proliferative glomerulonephritis with monoclonal immunoglobulin deposits, myeloma cast nephropathy, cryoglobulinemic glomerulonephritis, fibrillary glomerulonephritis, infection related glomerulonephritis, genetic kidney disease (Alport’s, Fabry’s), lupus nephritis (LN), IgA nephropathy (IgAN), pauci-immune glomerulonephritis/ANCA vasculitis, antiGBM nephritis, acute interstitial nephritis (AIN), chronic interstitial nephritis, thrombotic microangiopathy, oxalate nephropathy, or other.
When a diagnosis of an membranoproliferative glomerulonephritis pattern was given, we tried to determine the specific condition associated to better classify the lesion. If the lesion was unable to be classified specifically, then a diagnosis of membranoproliferative glomerulonephritis was maintained. If a patient had multiple biopsies, or the first biopsy was nondiagnostic, then the first diagnostic kidney biopsy was used. If more than one diagnosis was noted on biopsy, the one determined to be the clinical cause of kidney dysfunction or affected management was chosen. Retrospective chart review was performed to obtain clinical and demographic characteristics including age at biopsy, race and ethnicity (self-reported), sex, and state or country of residence. Data were collected and stored in REDCap.
Statistical Analysis
Diagnosis outcomes were summarized using univariate analysis to illustrate their differences among states (Ohio, Florida, and other), among evenly distributed age groups (≤45, >45 to ≤60, >60 to ≤70, and >70 years), among races (White, Black, and other), between females and males, and between Hispanic and non-Hispanic patients.
A separate analysis was conducted comparing Florida and Ohio patients. Demographic factors and diagnosis outcomes were summarized using univariate analysis to illustrate their differences between Ohio and Florida, in which categorical factors were summarized using numbers and percentages, and the differences were tested using chi-squared test or Fisher’s exact test, whereas continuous factors were summarized using median and interquartile range, and the difference was tested using Wilcoxon rank-sum test.
In addition, multivariable logistic regression analysis was conducted to assess if there was any demographic factor related to any diagnosis outcome. A multiplicity adjustment for P value was not applied at the analysis. Finally, an analysis was performed to assess if there were any differences in the number of biopsies or biopsy diagnoses in the 12 months before and the first 12 months of the coronavirus disease 2019 (COVID-19) pandemic. SAS version 9.4 was used to process and analyze the data.
Results
A total of 9608 patients had a kidney biopsy performed or reviewed at the Cleveland Clinic during the defined time period. After excluding transplant or donor kidney biopsies and unavailable records, we identified 4128 patients with native kidney biopsy data. The median age at kidney biopsy was 60 years old, with 46% female and 54% male patients. Self-reported racial demographics were 73% White (of which 5% Hispanic), 22% Black, 3% multiracial, and 2% Asian background.
Within the entire cohort, the most common diagnoses identified were: FSGS (n=633, 15%), DKD (n=602, 15%), IgAN (n=319, 8%), LN (n=289, 7%), ANCA vasculitis/pauci-immune glomerulonephritis (n=275, 7%), acute tubular necrosis (n=256, 6%), membranous nephropathy (n=211, 5%), AIN (n=124, 3%), and amyloidosis (n=110, 3%) (Table 1). Other biopsy diagnoses that were less prevalent are noted in Supplemental Table 1. Across campuses, there were 3322 patients from Ohio, 361 patients from Florida (Table 1), and 445 patients originating from other states or locations (Supplemental Table 2).
Table 1.
Demographics and diagnosis results stratified by state (Ohio, Florida, and other)
| Variable | Overall (n=4128) | Ohio (n=3322) | Florida (n=361) | Other (n=445) | P Value |
|---|---|---|---|---|---|
| Age, median (IQR) | 60.0 (45.0–70.0) | 60.0 (45.0–70.0) | 61.0 (49.0–72.0) | 58.0 (40.0–69.0) | 0.001 |
| Age, n (%) | 0.008 | ||||
| Age ≤45 | 1043 (25) | 835 (25) | 71 (20) | 137 (31) | |
| >45 to ≤60 | 1053 (26) | 836 (25) | 106 (29) | 111 (25) | |
| >60 to ≤70 | 1019 (25) | 838 (25) | 82 (23) | 99 (22) | |
| >70 | 1013 (25) | 813 (25) | 102 (28) | 98 (22) | |
| Race, n (%) | <0.001 | ||||
| White | 2907 (73) | 2321 (72) | 253 (74) | 333 (81) | |
| Black | 867 (22) | 756 (23) | 61 (18) | 50 (12) | |
| Othera | 214 (5) | 159 (5) | 28 (8) | 27 (7) | |
| Ethnicity, n (%) | <0.001 | ||||
| Hispanic | 193 (5) | 113 (4) | 66 (21) | 14 (4) | |
| Non-Hispanic | 3592 (95) | 2952 (96) | 253 (79) | 387 (97) | |
| Sex, n (%) | 0.45 | ||||
| F | 1906 (46) | 1522 (46) | 166 (46) | 218 (49) | |
| M | 2222 (54) | 1800 (54) | 195 (54) | 227 (51) | |
| FSGS, n (%) | 633 (15) | 524 (16) | 66 (18) | 43 (10) | <0.001 |
| DKD, n (%) | 602 (15) | 517 (16) | 56 (16) | 29 (7) | <0.001 |
| Global glomerulosclerosis, n (%) | 341 (8) | 273 (8) | 28 (8) | 40 (9) | 0.80 |
| IgA nephropathy, n (%) | 319 (8) | 249 (8) | 28 (8) | 42 (9) | 0.35 |
| Other, n (%) | 296 (7) | 248 (8) | 12 (3) | 36 (8) | 0.01 |
| Lupus nephropathy, n (%) | 289 (7) | 222 (7) | 39 (11) | 28 (6) | 0.01 |
| ANCA, n (%) | 275 (7) | 196 (6) | 22 (6) | 57 (13) | <0.001 |
| Acute tubular necrosis, n (%) | 256 (6) | 203 (6) | 30 (8) | 23 (5) | 0.16 |
| Membranous nephropathy, n (%) | 211 (5) | 157 (5) | 19 (5) | 35 (8) | 0.02 |
| AIN, n (%) | 124 (3) | 96 (3) | 7 (2) | 21 (5) | 0.05 |
| Amyloidosis, n (%) | 110 (3) | 79 (2) | 13 (4) | 18 (4) | 0.06 |
IQR, interquartile range; F, female; M, male; DKD, diabetic kidney disease; AIN, acute interstitial nephritis.
3.1% multiracial, and 1.6% Asian, 0.7% not reported.
Univariate Analysis, Stratified by Sex, Ethnicity, Race, and Location
Univariate analysis demonstrated that when stratified by sex, FSGS (18% versus 12%, P<0.001), DKD (16% versus 13%, P=0.03) and IgAN (9% versus 6%, P<0.001) were more prevalent among males compared with females (Table 2). Although lupus nephritis (12% versus 3%, P<0.001) and ANCA vasculitis (8% versus 6%, P=0.03) were more prevalent among females compared with males (Table 2). By univariate analysis when stratified by ethnicity, DKD (22% versus 14%, P<0.001), IgAN (14% versus 8%, P=0.002) and lupus nephritis (12% versus 7%, P=0.006) was more prevalent among Hispanic compared with non-Hispanic patients (Table 3). Notably, the prevalence of membranous nephropathy, a disease that primarily affects White patients, was similar between White and Black patients in our cohort (5% versus 5%), (Table 4). Additionally, by univariate analysis, Florida had a higher prevalence of Hispanic patients (21% versus 4%, P<0.001) and those with LN (11% versus 7%, P=0.004) (Table 5).
Table 2.
Diagnosis results stratified by sex
| Diagnosis Result, n (%) | Female (n=1906) | Male (n=2222) | P Valuea |
|---|---|---|---|
| FSGS | 237 (12) | 396 (18) | <0.001 |
| DKD | 254 (13) | 348 (18) | 0.03 |
| Global glomerulosclerosis | 154 (8) | 187 (8) | 0.70 |
| IgA nephropathy | 119 (6) | 200 (9) | <0.001 |
| Other | 153 (8) | 143 (6) | 0.05 |
| Lupus nephropathy | 225 (12) | 64 (3) | <0.001 |
| ANCA | 144 (8) | 131 (6) | 0.03 |
| Acute tubular necrosis | 123 (6) | 133 (6) | 0.53 |
| Membranous nephropathy | 86 (5) | 125 (6) | 0.11 |
| AIN | 57 (3) | 67 (3) | 0.96 |
| Amyloidosis | 44 (2) | 66 (3) | 0.19 |
DKD, diabetic kidney disease; AIN, acute interstitial nephritis.
Each P value was derived from chi-square test or Fisher’s exact test.
Table 3.
Diagnosis results stratified by ethnicity
| Diagnosis Result, n (%) | Non-Hispanic (n=3592) | Hispanic (n=193) | P Valuea |
|---|---|---|---|
| FSGS | 556 (16) | 22 (11) | 0.12 |
| DKD | 492 (14) | 43 (22) | <0.001 |
| Global glomerulosclerosis | 302 (8) | 12 (6) | 0.29 |
| IgA nephropathy | 268 (8) | 26 (14) | 0.002 |
| Other | 270 (8) | 10 (5) | 0.23 |
| Lupus nephropathy | 255 (7) | 24 (12) | 0.006 |
| ANCA | 246 (7) | 12 (6) | 0.73 |
| Acute tubular necrosis | 218 (6) | 6 (3) | 0.09 |
| Membranous nephropathy | 184 (5) | 8 (4) | 0.55 |
| AIN | 108 (3) | 5 (3) | 0.74 |
| Amyloidosis | 100 (3) | 4 (2) | 0.82 |
DKD, diabetic kidney disease; AIN, acute interstitial nephritis.
Each P value was derived from chi-square test or Fisher’s exact test.
Table 4.
Diagnosis results stratified by race
| Diagnosis Result, n (%) | White (n=2907) | Black (n=867) | Other (n=214) | P Valuea |
|---|---|---|---|---|
| FSGS | 398 (14) | 188 (22) | 31 (15) | <0.001 |
| DKD | 367 (13) | 171 (20) | 39 (18) | <0.001 |
| Global glomerulosclerosis | 250 (9) | 76 (9) | 8 (4) | 0.04 |
| IgA nephropathy | 255 (9) | 17 (2) | 33 (15) | <0.001 |
| Other | 237 (8) | 44 (5) | 10 (5) | 0.003 |
| Lupus nephropathy | 123 (4) | 130 (15) | 30 (14) | <0.001 |
| ANCA | 233 (8) | 26 (3) | 7 (3) | <0.001 |
| Acute tubular necrosis | 200 (7) | 41 (5) | 4 (2) | 0.001 |
| Membranous nephropathy | 147 (5) | 45 (5) | 10 (5) | 0.95 |
| AIN | 101 (4) | 15 (2) | 3 (1) | 0.01 |
| Amyloidosis | 90 (3) | 14 (7) | 3 (1) | 0.03 |
DKD, diabetic kidney disease; AIN, acute interstitial nephritis.
Each P value was derived from chi-square test or Fisher’s exact test.
Table 5.
Demographics and diagnosis results stratified by state for subgroup (Ohio and Florida only)
| Variable | Ohio (n=3322) | Florida (n=361) | P Value |
|---|---|---|---|
| Age, median (IQR) | 60.0 (45.0–70.0) | 61.0 (49.0–72.0) | 0.02 |
| Age, n (%) | 0.03 | ||
| Age ≤45 | 835 (25) | 71 (20) | |
| >45 to ≤60 | 836 (25) | 106 (29) | |
| >60 to ≤70 | 838 (25) | 82 (23) | |
| >70 | 813 (25) | 102 (28) | |
| Race, n (%) | <0.001 | ||
| White | 2321 (72) | 253 (74) | |
| Black | 756 (23) | 61 (18) | |
| Othera | 159 (5) | 28 (8) | |
| Ethnicity, n (%) | <0.001 | ||
| Hispanic | 113 (4) | 66 (21) | |
| Non-Hispanic | 2952 (96) | 253 (79) | |
| Sex, n (%) | 0.95 | ||
| F | 1522 (46) | 166 (46) | |
| M | 1800 (54) | 195 (54) | |
| FSGS, n (%) | 524 (16) | 66 (18) | 0.22 |
| DKD, n (%) | 517 (16) | 56 (16) | 0.98 |
| Global glomerulosclerosis, n (%) | 273 (8) | 28 (8) | 0.76 |
| IgA nephropathy, n (%) | 249 (8) | 28 (8) | 0.86 |
| Other, n (%) | 248 (8) | 12 (3) | 0.004 |
| Lupus nephropathy, n (%) | 222 (7) | 39 (11) | 0.004 |
| ANCA, n (%) | 196 (6) | 22 (6) | 0.88 |
| Acute tubular necrosis, n (%) | 203 (6) | 30 (8) | 0.10 |
| Membranous nephropathy, n (%) | 157 (5) | 19 (5) | 0.65 |
| AIN, n (%) | 96 (3) | 7 (2) | 0.30 |
| Amyloidosis, n (%) | 79 (2) | 13 (4) | 0.16 |
IQR, interquartile range; F, female; M, male; DKD, diabetic kidney disease; AIN, acute interstitial nephritis.
Multivariable Logistic Regression Analysis, Stratified by Sex, Ethnicity, Race, and Location
Multivariable logistic regression analysis (Table 6) revealed that patients age >70 years were more likely to have an FSGS lesion on biopsy compared with those <70 years old, odds ratio (OR) 1.32 (95% confidence interval [95% CI], 1.02 to 1.70, P=0.04). Patients with age >45 years were more likely to have DKD (P<0.01), ANCA vasculitis (P<0.01), and amyloidosis (P<0.01). Patients <45 years old were more likely to have IgA nephropathy (P<0.01) and LN (P<0.01).
Table 6.
Multivariable logistic regression analysis for the diagnosis results
| Variable | FSGS | Diabetic Kidney Disease | IgA Nephropathy | Lupus Nephritis | ANCA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | |
| State | ||||||||||
| OH | 1 | 1 | 1 | 1 | 1 | |||||
| FL | 1.30 (0.96 to 1.77) | 0.09 | 1.00 (0.72 to 1.40) | 0.99 | 1.07 (0.69 to 1.67) | 0.77 | 2.06 (1.33 to 3.19) | <0.01 | 0.86 (0.52 to 1.43) | 0.56 |
| Other | 0.62 (0.43 to 0.88) | 0.007 | 0.40 (0.26 to 0.62) | <0.01 | 1.02 (0.70 to 1.49) | 0.91 | 0.93 (0.60 to 1.46) | 0.76 | 2.17 (1.55 to 3.04) | <0.01 |
| Age, yr | ||||||||||
| ≤45 | 1 | 1 | 1 | 1 | 1 | |||||
| >45 to ≤60 | 1.06 (0.82 to 1.37) | 0.66 | 2.70 (2.03 to 3.59) | <0.01 | 0.61 (0.45 to 0.83) | <0.01 | 0.25 (0.18 to 0.35) | <0.01 | 2.31 (1.47 to 3.68) | <0.01 |
| >60 to ≤70 | 0.91 (0.70 to 1.19) | 0.48 | 2.58 (1.93 to 3.44) | <0.01 | 0.41 (0.29 to 0.57) | <0.01 | 0.19 (0.13 to 0.28) | <0.01 | 2.93 (1.87 to 4.58) | <0.01 |
| >70 | 1.32 (1.02 to 1.70) | 0.04 | 1.43 (1.04 to 1.98) | 0.03 | 0.23 (0.15 to 0.34) | <0.01 | 0.09 (0.05 to 0.16) | <0.01 | 3.57 (2.29 to 5.57) | <0.01 |
| Race | ||||||||||
| White | 1 | 1 | 1 | 1 | 1 | |||||
| Black | 1.70 (1.39 to 2.09) | <0.01 | 1.87 (1.51 to 2.32) | <0.01 | 0.18 (0.11 to 0.30) | <0.01 | 3.37 (2.53 to 4.48) | <0.01 | 0.42 (0.28 to 0.64) | <0.01 |
| Other | 1.11 (0.72 to 1.73) | 0.64 | 1.55 (1.03 to 2.35) | 0.04 | 1.35 (0.87 to 2.09) | 0.18 | 2.43 (1.50 to 3.94) | <0.01 | 0.45 (0.20 to 1.00) | 0.05 |
| Ethnicity | ||||||||||
| Hispanic | 0.79 (0.48 to 1.29) | 0.34 | 1.67 (1.09 to 2.55) | 0.02 | 1.19 (0.72 to 1.99) | 0.50 | 1.34 (0.77 to 2.32) | 0.31 | 1.40 (0.72 to 2.72) | 0.32 |
| Non-Hispanic | 1 | 1 | 1 | 1 | 1 | |||||
| Sex | ||||||||||
| F | 1 | 1 | 1 | 1 | 1 | |||||
| M | 1.56 (1.30 to 1.88) | <0.01 | 1.17 (0.97 to 1.41) | 0.11 | 1.53 (1.19 to 1.96) | <0.01 | 0.23 (0.17 to 0.32) | <0.01 | 0.72 (0.56 to 0.93) | 0.01 |
| Acute tubular necrosis | Membranous nephropathy | AIN | Amyloidosis | Global Glomerulosclerosis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value |
| State | ||||||||||
| OH | 1 | 1 | 1 | 1 | 1 | |||||
| FL | 1.23 (0.76 to 1.97) | 0.40 | 1.18 (0.70 to 2.00) | 0.540 | 0.62 (0.26 to 1.47) | 0.281 | 1.42 (0.75 to 2.70) | 0.28 | 0.94 (0.60 to 1.46) | 0.78 |
| Other | 0.83 (0.52 to 1.34) | 0.44 | 1.86 (1.24 to 2.77) | <0.01 | 1.41 (0.83 to 2.41) | 0.205 | 1.60 (0.90 to 2.82) | 0.11 | 1.26 (0.88 to 1.81) | 0.21 |
| Age, yr | ||||||||||
| ≤45 | 1 | 1 | 1 | 1 | 1 | |||||
| >45 to ≤60 | 1.23 (0.81 to 1.87) | 0.33 | 1.23 (0.80 to 1.88) | 0.35 | 1.01 (0.58 to 1.77) | 0.97 | 13.12 (3.1 to 55.4) | <0.01 | 1.29 (0.89 to 1.86) | 0.18 |
| >60 to ≤70 | 1.28 (0.85 to 1.94) | 0.24 | 1.16 (0.75 to 1.79) | 0.52 | 1.16 (0.67 to 1.99) | 0.60 | 18.27 (4.4 to 76.2) | <0.01 | 1.79 (1.26 to 2.54) | <0.01 |
| >70 | 1.74 (1.17 to 2.59) | <0.01 | 1.39 (0.91 to 2.14) | 0.13 | 1.22 (0.70 to 2.10) | 0.49 | 18.88 (4.5 to 78.9) | <0.01 | 1.93 (1.36 to 2.75) | <0.01 |
| Race | ||||||||||
| White | 1 | 1 | 1 | 1 | 1 | |||||
| Black | 0.73 (0.51 to 1.05) | 0.09 | 1.10 (0.77 to 1.57) | 0.62 | 0.53 (0.30 to 0.92) | 0.02 | 0.65 (0.36 to 1.15) | 0.14 | 1.19 (0.90 to 1.58) | 0.21 |
| Other | 0.35 (0.13 to 0.97) | 0.04 | 0.96 (0.46 to 1.98) | 0.90 | 0.43 (0.13 to 1.44) | 0.17 | 0.63 (0.19 to 2.12) | 0.45 | 0.42 (0.19 to 0.93) | 0.03 |
| Ethnicity | ||||||||||
| Hispanic | 0.67 (0.28 to 1.60) | 0.37 | 0.80 (0.35 to 1.83) | 0.60 | 1.05 (0.35 to 3.10) | 0.93 | 0.98 (0.33 to 2.93) | 0.98 | 1.18 (0.62 to 2.25) | 0.62 |
| Non-Hispanic | 1 | 1 | 1 | 1 | 1 | |||||
| Sex | ||||||||||
| F | 1 | 1 | 1 | 1 | 1 | |||||
| M | 0.83 (0.64 to 1.10) | 0.19 | 1.26 (0.93 to 1.70) | 0.13 | 0.95 (0.65 to 1.39) | 0.79 | 1.31 (0.87 to 1.97) | 0.19 | 1.03 (0.82 to 1.31) | 0.79 |
OH, Ohio; FL, Florida; F, female; M, male.
From a sex perspective, males were more likely to have a diagnosis of FSGS (OR, 1.56; 95% CI, 1.30 to 1.88, P<0.01) and IgAN (OR, 1.53, 95% CI, 1.19 to 1.96, P<0.01), whereas males were less likely to have a diagnosis of LN (OR, 0.23; 95% CI, 0.17 to 0.32, P<0.01) and ANCA vasculitis (OR, 0.72; 95% CI, 0.56 to 0.93, P=0.01) (Table 6) with similar results noted on univariate analysis in Table 4.
When comparing diagnoses among those who identify as Black, White, or other race, Black patients were more likely to have a biopsy diagnosis of FSGS (OR, 1.70; 95% CI, 1.39 to 2.09, P<0.01), DKD (OR, 1.87; 95% CI, 1.51 to 2.32, P<0.01), or LN (OR, 3.37; 95% CI, 2.53 to 4.48, P<0.01) and were less likely to have IgA nephropathy (OR, 0.18; 95% CI, 0.11 to 0.30, P<0.01), ANCA vasculitis (OR, 0.42; 95% CI, 0.28 to 0.64, P<0.01), or AIN (OR, 0.53; 95% CI, 0.30 to 0.92, P<0.01). Hispanic patients were more likely to have a diagnosis of DKD, (OR, 1.67; 95% CI, 1.09 to 2.55, P=0.02) compared with non-Hispanic patients.
Geographically, when comparing patients in Ohio to those in Florida or other locations, patients in Florida were more likely to have LN (OR, 2.06; 95% CI, 1.33 to 3.19, P<0.01). However, those collectively in other locations were more likely to have ANCA vasculitis (OR, 2.17; 95 CI, 1.55 to 3.04, P<0.01) and membranous nephropathy (OR, 1.86; 95% CI, 1.24 to 2.77, P<0.01).
Effect of the COVID-19 Pandemic
We compared changes in kidney biopsy numbers and diagnoses before and during the COVID-19 pandemic. The difference in the biopsy amount in the 12 months before COVID-19 (March 2019 to February 2020) and the first 12 months during COVID-19 (March 2020 to February 2021) was significant (696, 53% versus 624, 47%, P=0.05); whereas the difference in the proportion of any disease diagnosed before COVID-19 (March 2019 to February 2020) and during COVID-19 (March 2020 to February 2021) was not significant (Table 7).
Table 7.
Biopsy amount and disease diagnosed 12 months before and after March 2020 (coronavirus disease 2019 pandemic)
| Diagnosis Result, n (%) | 12 Months Before Coronavirus Disease 2019 (3/2019–2/2020) | First 12 Months During Coronavirus Disease 2019 (3/2020–2/2021) | P Value (Chi-Square Test) |
|---|---|---|---|
| Biopsy | 696 (52.73%) | 624 (47.23%) | 0.05 |
| FSGS | 110 (15.8%) | 90 (14.42%) | 0.48 |
| DKD | 108 (15.52%) | 102 (16.35%) | 0.68 |
| Global glomerulosclerosis | 73 (10.49%) | 60 (9.62%) | 0.60 |
| IgA nephropathy | 56 (8.05%) | 41 (6.57%) | 0.31 |
| Other/non-diagnostic | 47 (6.75%) | 39 (6.25%) | 0.71 |
| Lupus nephropathy | 41 (5.89%) | 43 (6.89%) | 0.46 |
| ANCA | 42 (6.03%) | 42 (6.73%) | 0.60 |
| Acute tubular necrosis | 44 (6.32%) | 45 (7.21%) | 0.52 |
| Membranous nephropathy | 33 (4.74%) | 20 (3.21%) | 0.16 |
| AIN | 21 (3.02%) | 16 (2.56%) | 0.62 |
| Amyloidosis | 14 (2.01%) | 14 (2.24%) | 0.77 |
DKD, diabetic kidney disease; AIN, acute interstitial nephritis.
Discussion
This study is an epidemiologic catalog of all patients who had a native kidney biopsy performed or reviewed within the Cleveland Clinic enterprise across a 7-year period from 2015 to 2021. We identify several epidemiologic trends with regards to age, race, sex, and location.
We compared the demographics in our study to that of 2020 US Census data (7). The median age in the United States is younger at 38 years, compared with 60 years old in our study, with 16% of the US population being >65 years old, whereas in our study 25% of patients were >70 years old. Sex distribution was similar in our study compared with the US population (51% female, 50% male). There was a higher percentage of Black patients in our study (22%) compared with 14% across the United States. A possible reason for this is in Ohio, there was a larger percentage of Black patients with biopsy proven kidney disease (23%) compared with 14% of the Ohio population being Black. The higher percentage of Hispanics in the Florida cohort (21%) is similar to the 2020 US Census, which reported in Florida 27% of persons identified as Hispanic or Latino, whereas 19% of the total US population identified as such (7).
In our study, the most common biopsy diagnoses were FSGS and DKD, which goes along with the fact that diabetes and hypertension, alone or in combination, are the top two causes of ESKD in the United States (8). Immune complex kidney disease in the form of IgAN and LN were the second most common diagnoses and more prevalent among those aged <45 years old, consistent with the current epidemiology of these disease processes (9). However, those aged >70 years old were more likely to have FSGS on biopsy, supporting our current understanding of the aging kidney (10).
A few ongoing trends hold true: Black patients were more likely to have FSGS lesions, likely due to the known association with APOL1 risk alleles (11). Females were more likely to have lupus nephritis, a disease that disproportionately affects Black and Hispanic patients (12), thus explaining why Florida had a higher OR for LN. Hispanic and Black patients were more likely to have DKD, similar to the increased prevalence of diabetes in these populations, and consistent with United States Renal Data System data (2). Notably, although membranous nephropathy is a condition that more commonly affects White patients (9,13), in our study, the percentages of membranous nephropathy were equal among White and Black patients, making this a population of interest for further study as data on membranous nephropathy are lacking in non-White patients.
In the United States, a great emphasis has been placed on prospective enrollment of patients into clinical trials, and multidisciplinary approaches to understanding glomerular diseases such as that successfully carried out by the NEPTUNE consortium (14). Despite this, there remains a paucity of organized kidney biopsy data within the United States for ongoing study of glomerular diseases. A review of the literature highlights two main US studies from the University of North Carolina and the Mayo Clinic, which offer sufficient data for comparison with this study.
Firstly, the University of North Carolina provided temporal trends in glomerular disease over a 30-year period. In their cohort of 21,374 patients over a 30-year period, the mean age at diagnosis was lower, at 48 years, similar by sex (51% were male), but with a greater proportion of Black patients (38%), which could be explained by the location of this center in the southern United States. The frequency of biopsy-proven kidney disease was similar, because in their cohort they showed FSGS 25%, DKD 14%, membranous nephropathy 13%, LN 13%, IgAN 10%, and ANCA vasculitis 8% (5). The higher proportion of FSGS in their cohort maybe due to other diagnoses being subsumed into that diagnosis and a higher proportion of Black patients included in that study.
Secondly, in 2006 the Mayo Clinic published the incidence of nondiabetic glomerular diseases in Olmsted County, Rochester, Minnesota from 1974 to 2003. A total of 195 kidney biopsies were included, with a mean age of 44 years, 57% were male, and the population of the county at that time was 96% White. The most common diagnoses were IgAN (22%), FSGS (17%), and membranous nephropathy (10%) (15). These two studies highlight the potential within major academic centers across the United States to compile renal pathology data in a standardized format, with an effort to have larger sample size datasets for further research.
One problem, however, is the heterogeneity that may exist between centers in coding and reporting of renal pathology data. One systematic of review of coding practices in national and regional renal biopsy registries illuminated two main points. Firstly, in the US, there is no major kidney biopsy data source. Secondly, coding among registries is inconsistent. Dendooven et al. found 16 national/regional biopsy registries in the literature (3). Ten were located in Europe, which included the Czech Republic (16), Belgium (17), Italy (18), Denmark (19), Norway (20), Poland (21), Scotland (22), Netherlands (23), Spain (24), and Sweden (25). There were four in Asia, which included Japan (26), Malaysia (3), Philippines (3), and Taiwan (27). There was one in Canada (28) and one in South America (29). There were no multicenter studies noted in the United States and only a few studies shared consistent nomenclature.
Of these studies, the recently published Flemish Collaborative Glomerulonephritis Group registry cataloged >2000 native kidney biopsies from 26 centers across Flanders (Belgium) between 2017 and 2019. The findings were similar to that in our cohort with respect to age at biopsy, (median 61 years), but there were considerably more male (62%) patients, and IgAN (17%), FSGS (9%), and DKD (%) were the most common glomerular diseases biopsied (17). These findings were similar to several other European cohorts but different to ours, likely due to the demographic variations between cohorts.
Our study includes biopsy data before and during the COVID-19 pandemic, which allowed for a unique opportunity to assess changes in biopsy and diagnosis trends. During the 12 months before COVID-19, more biopsies were performed than in the first 12 months of the pandemic. This is an expected finding as during this period throughout the health care system, there were safety protocols, including additional testing and patient prioritization. However, we noted that in our cohort, there was no change in the kidney disease diagnosis spectrum before and during the pandemic.
There are several strengths to our study. We utilized data from 2015 as it was around this time that a more methodical and standardized approach in biopsy reporting was carried out, enterprise wide. The Cleveland Clinic enterprise uses a centralized pathology department at its main campus in Ohio, allowing for the same processing techniques and reporting of biopsies and eliminates inconsistencies that may occur when different reporting agencies are used. Furthermore, the Cleveland Clinic enterprise, being a tertiary and at times quaternary referral center, with several satellite locations in multiple states, allowed for a wider pool and demographic distribution for the data obtained with patients from several US states and countries worldwide being evaluated.
We acknowledge that our study has limitations. Although our study data started from 2015, we maintained this strategy to ensure a standardized approach to biopsy processing and interpretation across campuses. Finally, this study only captures biopsy-proven kidney disease. As such conditions that under certain circumstances are diagnosed serologically such as PLA2R-associated membranous nephropathy (30), or clinically such as DKD (31), may be under-reported.
Our retrospective, observational cohort study essentially catalogs the spectrum of biopsy proven kidney disease across the US Cleveland Clinic enterprise. Key demographic and geographical trends have been identified. Future studies include an in-depth look at clinical outcomes for specific forms of glomerular disease. This study lays the foundation for identifying patient disease groups that would benefit from glomerular disease clinical trials at both our Florida and Ohio campuses and highlights the need for creation of a standardized national kidney biopsy registry to bolster glomerular and kidney disease research in the United States.
Disclosures
S. Bobart reports having honorarium from Travere Therapeutics and other interests or relationships as Faculty for the GlomCon fellowship. L. Herlitz reports having consultancy agreements with ChemoCentryx; and reports having an advisory or leadership role on the ASN Kidney360 editorial board. All remaining authors have nothing to disclose.
Funding
This work was supported by Cleveland Clinic Caregiver Catalyst Grant CCG 0093.
Acknowledgments
The authors would like to acknowledge Dr. Kurt Spindler, Greg Strnad, and Matthew Rerko for systems analysis support, and Bini Jacob and Rachel Burkey of the Medical Specialties Institute for their administrative support to the study staff.
Author Contributions
S. Bobart and S. Gebreselassie conceptualized the study; S. Bobart, A. Carrion Rodriguez, L. Herlitz, G. Portalatin, H. Sawaf, and S. Shettigar were responsible for the data curation; H. Liang was responsible for the formal analysis; S. Bobart and S. Gebreselassie were responsible for the funding acquisition; S. Bobart, S. Gebreselassie, G. Portalatin, and H. Sawaf were responsible for the investigation; S. Bobart, S. Gebreselassie, H. Liang, G. Portalatin, and H. Sawaf responsible for the methodology; S. Bobart and S. Gebreselassie were responsible for the project administration; S. Bobart and S. Gebreselassie were responsible for the resources; S. Gebreselassie was responsible for the software; S. Bobart, S. Gebreselassie and H. Sawaf provided supervision; S. Bobart wrote the original draft; S. Bobart, A. Carrion Rodriguez, S. Gebreselassie, L. Herlitz, H. Liang, G. Portalatin, H. Sawaf, and S. Shettigar reviewed and edited the manuscript. All authors contributed to the writing of the manuscript. All authors have read and approved the manuscript.
Data Sharing Statement
Partial restrictions to the data and/or materials apply: as per the Cleveland Clinic Institutional Review Board, will need data sharing agreement to provide data, but can be arranged upon request.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0005882022/-/DCSupplemental.
Other diagnoses. Download Supplemental Table 1, PDF file, 413 KB (442.2KB, pdf)
Patient location other than Ohio and Florida. Download Supplemental Table 2, PDF file, 413 KB (442.2KB, pdf)
References
- 1.Sethi S, Fervenza FC: Standardized classification and reporting of glomerulonephritis. Nephrol Dial Transplant 34: 193–199, 2019. 10.1093/ndt/gfy220 [DOI] [PubMed] [Google Scholar]
- 2.Johansen KL, Chertow GM, Foley RN, Gilbertson DT, Herzog CA, Ishani A, Israni AK, Ku E, Kurella Tamura M, Li S, Li S, Liu J, Obrador GT, O’Hare AM, Peng Y, Powe NR, Roetker NS, St Peter WL, Abbott KC, Chan KE, Schulman IH, Snyder J, Solid C, Weinhandl ED, Winkelmayer WC, Wetmore JB: US Renal Data System 2020 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 77[Suppl 1]: A7–A8, 2021. 10.1053/j.ajkd.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dendooven A, Peetermans H, Helbert M, Nguyen TQ, Marcussen N, Nagata M, Gesualdo L, Perkowska-Ptasinska A, Capusa C, López-Gómez JM, Geddes C, Abdul-Hamid MA, Segelmark M, Yahya R, Garau M, Villanueva R, Dorman A, Barbour S, Cornet R, Hopfer H, Amann K, Leh S; Kidney Biopsy Codes for Pathologists project (www.kibico.org) : Coding practice in national and regional kidney biopsy registries. BMC Nephrol 22: 193, 2021. 10.1186/s12882-021-02365-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narasimhan B, Chacko B, John GT, Korula A, Kirubakaran MG, Jacob CK: Characterization of kidney lesions in Indian adults: Towards a renal biopsy registry. J Nephrol 19: 205–210, 2006 [PubMed] [Google Scholar]
- 5.O’Shaughnessy MM, Hogan SL, Poulton CJ, Falk RJ, Singh HK, Nickeleit V, Jennette JC: Temporal and demographic trends in glomerular disease epidemiology in the Southeastern United States, 1986-2015. Clin J Am Soc Nephrol 12: 614–623, 2017. 10.2215/CJN.10871016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethi S, Haas M, Markowitz GS, D’Agati VD, Rennke HG, Jennette JC, Bajema IM, Alpers CE, Chang A, Cornell LD, Cosio FG, Fogo AB, Glassock RJ, Hariharan S, Kambham N, Lager DJ, Leung N, Mengel M, Nath KA, Roberts IS, Rovin BH, Seshan SV, Smith RJ, Walker PD, Winearls CG, Appel GB, Alexander MP, Cattran DC, Casado CA, Cook HT, De Vriese AS, Radhakrishnan J, Racusen LC, Ronco P, Fervenza FC: Mayo Clinic/Renal Pathology Society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol 27: 1278–1287, 2016. 10.1681/ASN.2015060612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States : 2020. Census data. Available at: https://www.census.gov/data.html. Accessed August 25, 2022
- 8.Johansen KL, Chertow GM, Gilbertson DT, Herzog CA, Ishani A, Israni AK, Ku E, Li S, Li S, Liu J, Obrador GT, O’Hare AM, Peng Y, Powe NR, Roetker NS, St Peter WL, Saeed F, Snyder J, Solid C, Weinhandl ED, Winkelmayer WC, Wetmore JB: US Renal Data System 2021 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 79[Suppl 1]: A8–A12, 2022. 10.1053/j.ajkd.2022.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, Cook HT, Fervenza FC, Gibson KL, Glassock RJ, Jayne DRW, Jha V, Liew A, Liu ZH, Mejía-Vilet JM, Nester CM, Radhakrishnan J, Rave EM, Reich HN, Ronco P, Sanders JF, Sethi S, Suzuki Y, Tang SCW, Tesar V, Vivarelli M, Wetzels JFM, Lytvyn L, Craig JC, Tunnicliffe DJ, Howell M, Tonelli MA, Cheung M, Earley A, Floege J: Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int 100: 753–779, 2021. 10.1016/j.kint.2021.05.015 [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan ED, Hughes J, Ferenbach DA: Renal aging: Causes and consequences. J Am Soc Nephrol 28: 407–420, 2017. 10.1681/ASN.2015121308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman DJ, Pollak MR: Apolipoprotein L1 and kidney disease in African Americans. Trends Endocrinol Metab 27: 204–215, 2016. 10.1016/j.tem.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portalatin GM, Gebreselassie SK, Bobart SA: Lupus nephritis - An update on disparities affecting African Americans. J Natl Med Assoc 114[3S2]: S34–S42, 2022. 10.1016/j.jnma.2022.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobart SA, Tehranian S, Sethi S, Alexander MP, Nasr SH, Moura Marta C, Vrana JA, Said S, Giesen CD, Lieske JC, Fervenza FC, De Vriese AS: A target antigen-based approach to the classification of membranous nephropathy. Mayo Clin Proc 96: 577–591, 2021. 10.1016/j.mayocp.2020.11.028 [DOI] [PubMed] [Google Scholar]
- 14.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M: Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83: 749–756, 2013. 10.1038/ki.2012.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swaminathan S, Leung N, Lager DJ, Melton 3rd LJ, Bergstralh EJ, Rohlinger A, Fervenza FC: Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol 1: 483–487, 2006. 10.2215/CJN.00710805 [DOI] [PubMed] [Google Scholar]
- 16.Maixnerova D, Jancova E, Skibova J, Rysava R, Rychlik I, Viklicky O, Merta M, Kolsky A, Reiterova J, Neprasova M, Kidorova J, Honsova E, Tesar V: Nationwide biopsy survey of renal diseases in the Czech Republic during the years 1994-2011. J Nephrol 28: 39–49, 2015. 10.1007/s40620-014-0090-z [DOI] [PubMed] [Google Scholar]
- 17.Laurens W, Deleersnijder D, Dendooven A, Lerut E, De Vriese AS, Dejagere T, Helbert M, Hellemans R, Koshy P, Maes B, Pipeleers L, Van Craenenbroeck AH, Van Laecke S, Vande Walle J, Coutteneye MM, De Meester J, Sprangers B; FCGG collaborative group : Epidemiology of native kidney disease in Flanders: Results from the FCGG kidney biopsy registry. Clin Kidney J 15: 1361–1372, 2022. 10.1093/ckj/sfac033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gesualdo L, Di Palma AM, Morrone LF, Strippoli GF, Schena FP; Italian Immunopathology Group, Italian Society of Nephrology : The Italian experience of the national registry of renal biopsies. Kidney Int 66: 890–894, 2004. 10.1111/j.1523-1755.2004.00831.x [DOI] [PubMed] [Google Scholar]
- 19.Heaf J: The Danish renal biopsy register. Kidney Int 66: 895–897, 2004. 10.1111/j.1523-1755.2004.00832.x [DOI] [PubMed] [Google Scholar]
- 20.Tøndel C, Vikse BE, Bostad L, Svarstad E: Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol 7: 1591–1597, 2012. 10.2215/CJN.02150212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkowska-Ptasinska A, Bartczak A, Wagrowska-Danilewicz M, Halon A, Okon K, Wozniak A, Danilewicz M, Karkoszka H, Marszalek A, Kowalewska J, Mroz A, Korolczuk A, Oko A, Debska-Slizien A, Naumnik B, Hruby Z, Klinger M, Ciechanowski K, Myslak M, Sulowicz W, Rydzewski A, Wiecek A, Manitius J, Gregorczyk T, Niemczyk S, Nowicki M, Gellert R, Stompor T, Wieliczko M, Marczewski K, Paczek L, Rostkowska O, Deborska-Materkowska D, Bogdanowicz G, Milkowski A, Durlik M; Polish Society of Nephrology : Clinicopathologic correlations of renal pathology in the adult population of Poland. Nephrol Dial Transplant 32[suppl_2]: ii209–ii218, 2017. 10.1093/ndt/gfw365 [DOI] [PubMed] [Google Scholar]
- 22.McQuarrie EP, Mackinnon B, Young B, Yeoman L, Stewart G, Fleming S, Robertson S, Simpson K, Fox J, Geddes CC; Scottish Renal Biopsy Registry : Centre variation in incidence, indication and diagnosis of adult native renal biopsy in Scotland. Nephrol Dial Transplant 24: 1524–1528, 2009. 10.1093/ndt/gfn677 [DOI] [PubMed] [Google Scholar]
- 23.van Paassen P, van Breda Vriesman PJ, van Rie H, Tervaert JW: Signs and symptoms of thin basement membrane nephropathy: A prospective regional study on primary glomerular disease-The Limburg Renal Registry. Kidney Int 66: 909–913, 2004. 10.1111/j.1523-1755.2004.00835.x [DOI] [PubMed] [Google Scholar]
- 24.Rivera F, López-Gómez JM, Pérez-García R; Spanish Registry of Glomerulonephritis : Frequency of renal pathology in Spain 1994-1999. Nephrol Dial Transplant 17: 1594–1602, 2002. 10.1093/ndt/17.9.1594 [DOI] [PubMed] [Google Scholar]
- 25.Peters B, Nasic S, Segelmark M: Clinical parameters predicting complications in native kidney biopsies. Clin Kidney J 13: 654–659, 2019. 10.1093/ckj/sfz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, Tsuruya K, Kiyomoto H, Iida H, Sasaki T, Higuchi M, Hattori M, Oka K, Kagami S, Kawamura T, Takeda T, Hataya H, Fukasawa Y, Fukatsu A, Morozumi K, Yoshikawa N, Shimizu A, Kitamura H, Yuzawa Y, Matsuo S, Kiyohara Y, Joh K, Nagata M, Taguchi T, Makino H, Committee for Standardization of Renal Pathological Diagnosis, Committee for Kidney Disease Registry, Japanese Society of Nephrology: Japan Renal Biopsy Registry and Japan Kidney Disease Registry: Committee Report for 2009 and 2010. Clin Exp Nephrol 17: 155–173, 2013. 10.1007/s10157-012-0746-8 [DOI] [PubMed] [Google Scholar]
- 27.Chiu HF, Chen HC, Lu KC, Shu KH; Taiwan Society of Nephrology : Distribution of glomerular diseases in Taiwan: Preliminary report of National Renal Biopsy Registry-publication on behalf of Taiwan Society of Nephrology. BMC Nephrol 19: 6, 2018. 10.1186/s12882-017-0810-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbour S, Beaulieu M, Gill J, Djurdjev O, Reich H, Levin A: An overview of the British Columbia Glomerulonephritis network and registry: Integrating knowledge generation and translation within a single framework. BMC Nephrol 14: 236, 2013. 10.1186/1471-2369-14-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garau M, Cabrera J, Ottati G, Caorsi H, Gonzalez Martinez F, Acosta N, Aunchayna MH, Gadola L, Noboa O: Temporal trends in biopsy proven glomerular disease in Uruguay, 1990-2014. PLoS One 13: e0206637, 2018. 10.1371/journal.pone.0206637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bobart SA, Han H, Tehranian S, De Vriese AS, Roman JCL, Sethi S, Zand L, Andrades Gomez C, Giesen CD, Soler MJ, Bomback AS, Fervenza FC: Noninvasive diagnosis of PLA2R-associated membranous nephropathy: A validation study. Clin J Am Soc Nephrol 16: 1833–1839, 2021. 10.2215/CJN.05480421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman NS, Canetta PA, Bomback AS: Glomerular diseases in patients with diabetes mellitus: An underappreciated epidemic. Kidney360 1: 220–222, 2020. 10.34067/KID.0000792019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Other diagnoses. Download Supplemental Table 1, PDF file, 413 KB (442.2KB, pdf)
Patient location other than Ohio and Florida. Download Supplemental Table 2, PDF file, 413 KB (442.2KB, pdf)