Abstract
Hypertension is the leading cause of cardiovascular disease and the primary risk factor for mortality worldwide. For more than half a century, researchers have demonstrated that immunity plays an important role in the development of hypertension; however, the precise mechanisms are still under investigation. The current body of knowledge indicates that proinflammatory cytokines may play an important role in contributing to immune-related pathogenesis of hypertension. Interferon gamma (IFN-γ), in particular, as an important cytokine that modulates immune responses, has been recently identified as a critical regulator of blood pressure by several groups, including us. In this review, we focus on exploring the role of IFN-γ in contributing to the pathogenesis of hypertension, outlining the various immune producers of this cytokine and described signaling mechanisms involved. We demonstrate a key role for IFN-γ in hypertension through global knockout studies and related downstream signaling pathways that IFN-γ production from CD8+ T cell (CD8T) in the kidney promoting CD8T-stimulated salt retention via renal tubule cells, thereby exacerbating hypertension. We discuss potential activators of these T cells described by the current literature and relay a novel hypothesis for activation.
Keywords: hypertension, hypertension, immunity and inflammation, interferon-γ
Hypertension, a major cause of premature death, affects roughly half of the US population and 1.3 billion people worldwide (1,2). This condition has been subjected to intensive study for more than half a century leading to the development of a variety of classes of pharmaceutical options to lower BP; however, fewer than 50% of patients achieve good BP control (3). Further confounding clinical treatment of hypertension is the multifactorial dysfunctional aspect of this disease, wherein an estimated 90%–95% of treated patients present with unclear origin (essential hypertension) (4), and an estimated 20%–30% of cases are resistant to currently available treatment (resistant hypertension) (5). Thus, it is important to investigate unknown mechanisms causing essential hypertension and develop new therapeutic strategies against hypertension (6). Years of intense research have confirmed that the kidney plays a critical role in regulating BP (7), for example transplanting kidneys from hypertensive donors to normotensive recipients transfers hypertension (8). Guyton and others proposed that a physiologic defect in the kidney impairs its salt handling, which contributes to the development of hypertension (9,10). However, the exact identity of this kidney defect is still under investigation. One potential suspect being investigated recently is disorder of immunity, in particular adaptive immune cells, which contribute to hypertension through several possible mechanisms (11), including dysregulation of natriuresis and driving renal injury (12).
Published in the 1970s, Svendsen first demonstrated the role of the immune system in the classic deoxycorticosterone acetate (DOCA)+salt murine model of hypertension (13). In this experiment, nude mice (lacking thymus, immunodeficient) exhibited blunted BP increase compared with haired wild-type (WT) mice, along with fewer round cell infiltrations. When these nude mice received a thymus graft—thereby restoring mature immune cell production—the BP increase to DOCA+salt was restored to normal levels. This study identified a thymus-independent phase of BP elevation followed by a thymus-dependent phase (13). The particular immune cells driving the thymus-dependent phase of BP elevation had not been identified and became of interest to researchers. In 2007, Harrison and colleagues not only corroborated blunted BP elevation due to immunodeficiency through the use of RAG1−/− mice (immunodeficient mice that produce no mature T or B cells) but also found that the adoptive transfer of T cells—but not B cells—would restore the hypertensive response to angiotensin II (AngII) (14). This landmark study was limited, wherein other labs found RAG1−/− mice no longer showed blunted hypertensive response to AngII in later studies (15,16), and a discussion of this change and potential explanations were provided in an excellent editorial from Madhur et al. (17). Many other preclinical studies indicating the role of the immune system have been published in the last 20 years. A few highlights include the use of the immunosuppressant tacrolimus to ameliorate BP elevation in Dahl salt-sensitive rats fed a high salt diet (12) and the use of the immunosuppressants dexamethasone and etanercept to reduce renal fibrosis, albuminuria, T-cell infiltration, and NF-κB activation accompanying AngII-infused double-transgenic rats (18). As researchers began to characterize particular immune players involved, Kamat et al. demonstrated the critical roles of IFN-γ in AngII-mediated hypertension, wherein global knockout (KO) of the cytokine blunted BP elevation to this pressor (19). Known producers of IFN-γ include T cells, natural killer (NK) cells, monocytes/macrophages, neutrophils, and dendritic cells (DCs), and each of these immune cells have been implied as participating in the process of hypertension (11,20–25). Certain T cells (Th17) and macrophages have been specifically implicated to play a role through sodium-driven proinflammatory responses as described here (26); however, the purpose of this review is to highlight current research describing the proposed immune cells contributing to this “thymus-dependent” phase of BP elevation and outline the elucidated mechanisms, and provide insight into the particular role of IFN-γ in the immune cell-mediated stage of hypertensive development. For a comprehensive network of IFN-γ JAK/STAT signaling pathways, we refer the reader to this 2018 review by Bhat et al. (27), and these reviews for a discussion of IFN-γ transcription regulation (28,29).
T Cells
The specific role of the T cell, and not B cell, in contributing to hypertension was first outlined by Guzik et al. in 2007, wherein adoptive transfer of T cells but not B cells to RAG-1−/− immunodeficient mice restored the blunted BP elevation of this model to AngII treatment. In that study, the authors also determined that NADPH oxidase contributed to complete development of hypertension via affecting T-cell infiltration (14). NAPDH oxidases (NOX)—particularly NOX2—mediate release of reactive oxygen species (ROS) alongside cellular mitochondria in a process termed “oxidative burst” to aid in elimination of invading micro-organisms (30,31). The role of NADPH oxidases and T cell–aggravated hypertension has been connected to the central nervous system in an AngII model of hypertension (32,33). Of interest, IFN-γ has been linked to upregulation of NAPDH oxidase in exposed epithelial cells (34,35) to concomitant upregulation of NADPH and IFN-γ in leukocytes in sickle cell disease (36), and to CD8+ enhanced ROS signaling through an NADPH oxidase-dependent CD39 expression mechanism (37,38). From these studies, and others, NAPDH oxidase activity within the immune cell has been tied to IFN-γ production and plays a role in cellular response to IFN-γ.
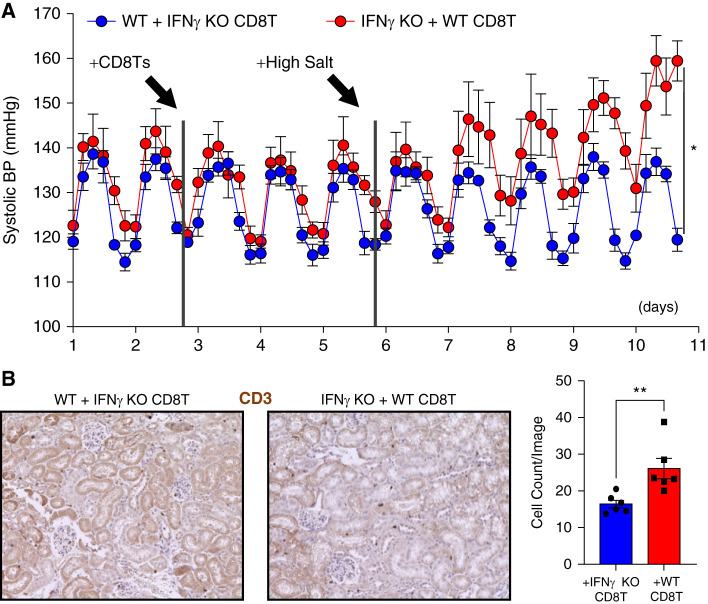
CD4+ Th1 T cells may play a role in the development of kidney damage in hypertension but not necessarily elevated BP itself as indicated by T-bet KO mice showing reduced renal damage but similarly elevated BP to WT mice in the AngII model of hypertension (39,40), Sun et al. further indicated the MR receptor on CD4+ T cells contributes to hypertension through regulation of IFN-γ (41). Our laboratory has found that CD8+ T cells (CD8Ts) play a direct role in the development of hypertension. After induction of hypertension in the DOCA+salt model (Cat. M-121, 50 mg/pellet in 21-day release formula and 1% NaCl drinking water) for 14–18 days, adoptive transfer of 1 × 107 splenocyte-derived CD8Ts from these mice into uninephrectomized C57BL/6J male mice results in salt-sensitive hypertension in the recipient mice that can be alleviated through treatment with a thiazide diuretic (42). We found that these T cells remain within the kidney, directly interacting with the distal tubule and continuing to promote NCC expression and activity despite treatment with the diuretic (42). More recently, we found that this mechanism of hypertensive-derived CD8Ts interacting with the distal tubule thereby generating salt-sensitivity in the recipient mice is via the IFN-γ-programmed cell death ligand 1 (PDL1) pathway (43). IFN-γ KO mice demonstrate blunted BP in either the AngII model of hypertension (19) or DOCA+salt model (43). In performing adoptive transfer of DOCA+salt-induced hypertensive CD8Ts from WT or IFN-γ KO mice to the alternative strain (methods according to previously described laboratory protocols) (42,43), only the WT CD8Ts were able to generate salt-sensitive hypertension in the recipient mouse (Figure 1), indicating that IFN-γ from the CD8T itself is sufficient to drive salt-sensitive hypertension.
Figure 1.
Interferon -γ contributes to CD8T-mediated salt-sensitive hypertension. (A) Radiotelemetry recording of systolic BP in recipient wild-type (C57/B6) mice (blue) and IFN-γ knockout (B6 background) mice (red) after adoptive transfer of 1×107 CD8Ts freshly isolated from deoxycorticosterone acetate+salt-treated IFN-γ knockout or wild-type mice. All mice were purchased from the Jackson Laboratory. Data were recorded every 15 minutes and averaged to six time points a day; n=5–6 mice per group. Statistical analysis was performed by two-way ANOVA. Significance for interaction effect: P<0.001; time effect: P<0.001; and strain recipient: P=0.02. (B) At the end point of BP recording in (A), immunohistochemistry staining (DAB) of kidney sections was performed using CD3 specific monoclonal antibody after the protocol we have published previously (42,43). In the quantification bar graph, each dot shown is the mean of four images taken per sectioned kidney. **P<0.05.
Activation of T cells—characterized by elevated expression of IFN-γ and TNF-α among other cytokines—has been linked to the development of salt-sensitive hypertension (44). In agreement with clinical data finding increased human hypertensive CD8T IFN-γ production compared with normotensive CD8Ts (45), we found that hypertensive murine CD8Ts demonstrated enhanced capacity of producing IFN-γ—but not TNF-α—compared with sham mouse–derived CD8Ts (43). This evidence, the ability of immunosuppressants to reduce BP in animal models of hypertension, and our co-culture model showing activated CD8Ts promoted greater NCC upregulation, sodium retention, and PDL1 expression through IFN-γ and the IFN-γ receptor in mouse distal convoluted tubular cells compared with naïve CD8Ts further supports the hypothesis that activated CD8Ts contribute to the development of hypertension. When we knocked down PDL1 within the renal tubule, BP elevation was blunted in both the DOCA+salt and CD8T adoptive transfer models of hypertension (43). Renal specific targeting of PDL1 may prove a promising clinical target in treating resistant hypertension.
During full activation of naïve CD8Ts, clonal expansion takes place after presentation of antigen, co-stimulatory factor, and a third signal (such as IL-12) wherein T-cell clones that are specific for individual antigens proliferate (46–48). Trott et al. performed a clonotype analysis of kidney CD8+ T-cell receptor sequences and found 3522±1049 unique T-cell receptor sequences in mice treated with AngII with three shared specific clonotypes in Vβ3, 8.1, and 17 families from four of the five mice that were not present in multiple sham mice; however, these clonal subtypes were not observed in other organs (48). The conclusion of this study suggested that rather than a single clonal population, an oligoclonal population of CD8Ts accumulate in the kidney and contribute to hypertension and sodium retention (48). The presence of more than one clonal population of CD8Ts presents at least two potential explanations: either (1) multiple unique antigens are being presented simultaneously leading to CD8T accumulation in the kidney through a IFN-γ-dependent mechanism or (2) activation of these CD8Ts is occurring via nonantigen-specific mechanisms, leading to IFN-γ production.
Some antigen-specific activation evidence includes this study by Rudemiller et al., wherein knocking down CD247 (responsible for coupling antigen recognition to intracellular transduction) (49,50) in Dahl salt-sensitive rats led to reduced T-cell infiltration into the kidneys and reduced BP (51). Blocking antigen presentation by DCs resulted in blunted BP elevation in two murine models of hypertension (52). The study by Hevia et al. found that ablation of CD11c+ antigen presenting cells prevented hypertension in an AngII+ high salt diet murine model (53). A few proposed antigens include isolevuglandin protein adducts (54,55), heat shock protein 70 (56,57), and Toll-like receptor (TLR) 4 or 2 activators such as C-reactive protein (58), uric acid (59), and others (60).
Experimental evidence suggests that such molecules may individually or collectively contribute to the role of T cells in hypertension; however, the presence of more than 3000 unique T-cell receptor sequences in AngII-induced hypertensive mice indicates that clonal expansion may not be occurring due to antigen presentation and recognition. Under certain conditions such as lymphopenia (61) or exposure to both IL-6 or IL-21 and IL-17 or IL-15 (62–65), CD8Ts can be activated without direct antigen presentation (66). IL-7 has also been implicated in the activation of autoimmune CD8Ts (62). In like manner, stimulation of T cells with phorbol myristate acetate (PMA) and the calcium ionophore ionomycin can bypass the T-cell membrane receptor complex and activate the T cell, leading to elevated expression of TNF-α and IFN-γ (67,68). Further research is needed to identify alternative pathways that may contribute to the activation of CD8Ts resulting in their infiltration within the kidney and stimulation of sodium retention.
NK Cells
Group 1 innate lymphocytes, NK cells arise from the same family as T and B cells (69) and have been implicated in both AngII-mediated hypertension and pulmonary hypertension. Depleting NK1.1 cells in WT C57BL/6 mice before AngII treatment resulted in blunted vascular dysfunction. Through a mechanism involving IFN-γ and T-bet, Kossmann et al. described a mutual activation pathway involving IL-12-secreting monocytes stimulating NK cells, leading to increased IFN-γ production and AngII-mediated hypertension (70). This relationship between monocyte and NK cell differentiation has been elucidated to involve TXb21 and IL-15R in a tumor environment (71). IL-12 driving NK production of IFN-γ has been described for some time (72). In a rat model of pulmonary hypertension, NK cells have been proposed to play a protective role rather than pathologic (73); as such, the role of NK cells likely involves environment-specific driven regulation. Such tissue specific regulation has not yet been fully elucidated.
Monocytes and Macrophages
In addition to their role in promoting NK differentiation, monocytes (in particular macrophages) have been described as contributing to the pathogenesis of hypertension. An excellent review was provided by Rucker and Crowley; as such, we refer the reader to this publication for a thorough description of the contribution of macrophages to hypertension (74). Macrophages have been discovered to infiltrate and remain within the renal interstitium in the AngII model of hypertension, correlating with elevated TGF-β and monocyte chemotactic protein (MCP), even after cessation of AngII (75). LysM+ monocytes have been described to contribute to—and be increased by—AngII-driven arterial hypertension through an experiment wherein depletion of LysM+ myelomonocytic cells reduced AngII-induced BP elevation and blunted vascular dysfunction, nitric oxide bioactivity, and vascular oxidative stress. Reconstitution with WT monocytes but not neutrophils restored the AngII-mediated disease phenotype (76).
Due to the ability of immunosuppressants to, at minimum, reduce renal damage elicited in several animal models of hypertension and the correlation between macrophage infiltration and inflammation in models of hypertension (77,78), it would follow that at least a connection can be established between proinflammatory macrophages and tissue inflammation and damage in hypertension. Such a relationship was discussed in the excellent review by Rodriguez-Iturbe et al. (79) in reference to the studies by Bravo et al. (80) and Quiroz et al. (81), wherein macrophage numbers and oxidative stress were correlated in two different animal models of hypertension. The excellent review provided by Rucker and Crowley described the elucidated role of TNF-α from macrophages driving renal inflammation (74); as such, we will focus on the relationship between IFN-γ and macrophages in hypertension.
In addition to aiding in the transition from innate immunity to adaptive immunity, IFN-γ can influence its surroundings directly through actions such as local dilation of blood vessels allowing for immune cells to localize at sites of inflammation (21). However, of particular note is the effect of IFN-γ on macrophages. After release by other immune cells such as NK cells or T cells, IFN-γ stimulates macrophages and primes them for response by inducing a proinflammatory activation and stimulating the release of other cytokines causing the M1 phenotype seen in macrophages (82,83). The M1 phenotype name was derived to match Th1 release of IFN-γ; however, various cytokines and stimuli can affect the activation state of macrophages. Because stimulation with a combination of factors or high salt can shift the activation spectrum of macrophages, the classification of M1 and M2 phenotypes may become insufficient to distinguish the activation status (82,83). Generally, this classic activation by T cells through IFN-γ leads to macrophage production of inflammatory response genes and cytokines such as IL-12, IL-23, and NO generating the proinflammatory macrophage type (82,84). Macrophages also have the ability to secrete IFN-γ to the same extent as T cells via stimulation with certain cytokines such as through simultaneous activation by IL-12 and IL-18 (24), leading to the potential of generating IFN-γ in an autocrine manner (24,85). Accordingly, macrophage activation by IFN-γ is multifaceted in the source and the activation state on the basis of other factors and cytokines present.
Serum IFN-γ levels have been clinically shown to be a predictor of high systolic BP (86). With diastolic BP, there was not only significance with IFN-γ but also MCP-1 levels—after corrections for variables such as age and sex (86). Within models of hypertension with IFN-γ knocked out, such as AngII mini osmotic pump implantation, some labs have demonstrated a reduction in monocyte infiltration within the aorta and a reduction in MCP-1 and other cytokines such as macrophage inflammatory protein 1α, and P-selectin ligand (75,87). Due to MCP-1’s role in recruiting macrophages to tissues, this suggests a potential interaction between hypertension, the renin-angiotensin system, and macrophage activation and recruitment to tissue.
Within the kidney, macrophage infiltration with hypertension has been noted by several research groups (75,88,89). Infiltration not only occurs during high levels of AngII but can also persist after AngII and systolic BP return to the normal range with lingering MCP-1 and TGF-β (75). Macrophages within the kidneys have been suggested to be involved in kidney injury seen with hypertension. For instance, depletion of macrophages with liposome-encapsulated clodronate has been demonstrated to reduce the kidney injury seen in Ang II-induced hypertension (88), and a similar observation was also found in Dahl salt-sensitive rats (88). Continuing to elucidate the ties between hypertension and macrophages remains, including the forms of activation macrophages may experience from IFN-γ release whether by other immune cells or other cytokines. Additionally, further research to expand on the role of infiltrating macrophages in the kidney and the damage induced through inflammation during hypertension is also needed.
Neutrophils
In the innate response to bacterial infection (such as Salmonella-induced colitis), neutrophils—phagocytic cells from the innate immune system (90)—function as a critical source of IFN-γ production, and depletion of these cells results in relief of many IFN-γ-induced disease symptoms (91,92). In contrast, depletion of neutrophils by administration of RB6–8C5 resulted in hypotension in WT C57BL/6 mice but not in IFN-γ or iNOS deficient mice, indicating neutrophils may also maintain physiologic BP via suppression of IFN-γ-dependent iNOS expression (93). Neutrophils play a role in the maintenance of homeostasis (93), and disruption could lead to elevated inflammation and BP dysregulation as evidenced by neutrophil/lymphocyte ratios functioning as predictors of hypertension (94–96); however, the specific relationship between neutrophil-derived IFN-γ and hypertension in an inflammatory situation is still unclear. Additionally, WT monocytes, not neutrophils, restored the AngII-mediated disease phenotype in LysM+-depleted mice (76). This excellent review by Araos et al. further describes the role of neutrophils in hypertension, and we refer the reader here for further information (97).
Myeloid-Derived Suppressor Cells
Immature and heterogeneous myeloid cells have been recently demonstrated to regulate the immune system (77,98). Myeloid-derived suppressor cells (MDSCs) can be identified by expression of CD11B and Gr-1 surface antigens (99). In addition to playing a role in amino acid metabolism and ROS production, it is known that MDSCs are capable of downregulating immune-system facilitated T-cell response in vivo and in vitro (99). This alludes to a possible role of MSDCs in regulating BP. It had been previously observed in tumor models that the T-cell suppression acts by a mechanism that is independent of traditional MHC complex function—meaning there is no classic antigen presentation by these cells (100). In a recent study utilizing AngII-induced hypertension, it was discovered that MSDCs undergo a phenotypic change after the onset of hypertension; MDSCs harvested from hypertensive mice lost the expression of their surface CD80 and MHC-II (77). Concomitantly, these same cells exhibited increased expression of the IFN-γ receptor, IFN-γR1 (77). Hypertension results in an increase of CD8+T-cells that express IFN-γ (43,45). This increase of T cells was attenuated by the presence of MDSCs (77). Further, antibody-mediated depletion of MDSCs resulted in a great increase of IFN-γ-expressing CD4T and CD8T cells (77). Although the complete mechanism of action of MDSCs remains unknown, there is evidence to support that MDSCs might induce development of regulatory T cells. These cells suppress immune response by inhibiting T-cell proliferation and cytokine expression (101). Although MDSCs may have other activities that have still not been discovered, these promising results point to the possibility that MDSCs are capable of inducing regulatory T-cell differentiation to suppress inappropriate T-cell proliferation and attenuate its effects.
DCs
CD11c+ DCs have been demonstrated to play an important role in hypertension, wherein blocking antigen presentation by DCs via antagonizing CD80 and CD86 with CTLA4-Ig or B7 deficient mice blunted BP elevation in both the AngII and DOCA+salt models of hypertension (52). Depleting DCs in the mice prevented the development of hypertension in the AngII model, which could be restored by adoptive transfer of WT CD11c+ antigen presenting cells (53). DCs are found activated in hypertensive animals to stimulate T-cell production of IFN-γ and IL-17A (102–104). However, the mechanisms regulating of DCs in hypertension is still under investigation. For example, sodium has been suggested to play a role in DC activation through amiloride-sensitive channels and the serum/glucocorticoid kinase 1 (105). Other evidence suggest that isoketal-modified proteins (proteins oxidatively modified by highly reactive γ-ketoaldehydes that accumulate in DCs during hypertension) are responsible for DC activation and subsequent T-cell activation in hypertension (102), and more recently, using DC-specific KO of AT1R, Lu et al. demonstrated a protective role of AT1 receptor on DCs against hypertension and T-cell activation (106). Nevertheless, how DCs are regulated in hypertension and what are the antigen(s) they present to activate T cells to exacerbate hypertension are critical questions that need to be further studied.
Limitations
The relationship between IFN-γ and tissue damage and fibrosis appears to be somewhat contextual, wherein blocking IFN-γ signaling in AngII-infused mice can reduce cardiac remodeling and immune cell infiltration (107,108), but alternative models propose a protective role for IFN-γ (109,110). Further exploration is needed for delineation of these effects. In addition, environmental exposure and subsequent gut microbiota alterations play a role directing the development of murine immune systems and host response to various challenges; as such, laboratory mice do not necessarily model clinical situations (111,112). The microbiota, in particular, has become increasingly implicated in regulating the murine response to hypertension in many experimental models (113,114) and may contribute to cardiovascular phenotypes in some immune-deficient mice. To this end, the preclinical established link between the immune cell activation and hypertension may involve gut microbiome alterations that regulate immune responses in such a way that are not inherently translatable to the clinic; however, such gut microbiome dysregulations have been described in clinical studies (115). We refer the reader to this excellent review by Avery et al. for further discussion of the gut microbiome and hypertension (116).
Clinical Translation
Clinical immunosuppression and hypertension have a rather complicated relationship due to adverse drug effects. In renal transplants, immunosuppressant calcineurin inhibitors have been linked to exacerbated BP (117), but BP post renal transplant has been ameliorated through non-nephrotoxic immunosuppression with mycophenolate mofetil with or without rapamycin (118). Immunosuppression (excluding calcineurin inhibitors) has been shown to be favorable in a clinical trial for patients with CKD (119). In agreement, several proinflammatory factors have been found to be upregulated in patients with treatment-resistant hypertension cases of CKD (120). Further clinical studies are needed to verify the efficacy of targeting key immune players in hypertension (121).
In Summary
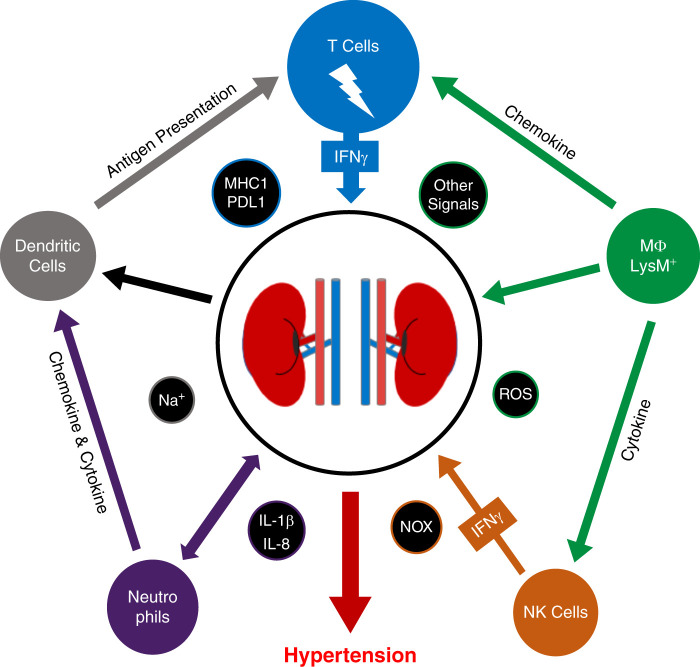
IFN-γ plays a key role in the development and maintenance of hypertension. Global KO of IFN-γ results in blunted BP elevation in both the AngII and DOCA+salt models of hypertension. Potential mechanistic relationships between IFN-γ production and hypertension have been noted with NK cells, neutrophils, macrophages, DCs, and T cells (Figure 2). The source(s) of activation—particularly for CD8Ts—driving elevated IFN-γ production are still being researched; nonetheless, a potential novel mechanism involving activators appears promising in light of the identification of an oligoclonal population of CD8Ts in the kidneys of hypertensive mice.
Figure 2.
Graphical representation of proposed mechanisms by which immune cells contribute to the development of hypertension through enhanced immune cell infiltration, inflammation, and sodium retention.
Disclosures
All authors have nothing to disclose.
Funding
This study was supported by NIH/NHLBI (Grant R01-HL146713) to S. Mu, AHA (Grant 15BGIA25730047) to S. Mu, UAMS Medical Research Endowment Awards to S. Mu, and the Systems Pharmacology and Toxicology Training Program (Grant T32-GM106999) to L. Benson.
Author Contributions
L.N. Benson and Y. Liu were responsible for the investigation; L.N. Benson, K.S. Deck, and C. Mora wrote the original draft of the manuscript; S. Mu, L.N. Benson, and Y. Liu were responsible for the methodology; and S. Mu was responsible for the conceptualization, funding acquisition, supervision, and validation, and reviewed and edited the manuscript.
References
- 1.Centers for Disease Control and Prevention : High Blood Pressure Symptoms and Causes. Available at: https://www.cdc.gov/bloodpressure/about.htm. Accessed January 17, 2022
- 2.World Health Organization : Hypertension. Available at: https://www.who.int/news-room/fact-sheets/detail/hypertension. Accessed January 17, 2022
- 3.Muntner P, Miles MA, Jaeger BC, Hannon Iii L, Hardy ST, Ostchega Y, Wozniak G, Schwartz JE: Blood pressure control among US adults, 2009 to 2012 through 2017 to 2020. Hypertension 79: 1971–1980, 2022. 10.1161/HYPERTENSIONAHA.122.19222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferdinand KC, Nasser SA: Management of essential hypertension. Cardiol Clin 35: 231–246, 2017. 10.1016/j.ccl.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 5.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM; American Heart Association Professional Education Committee : Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 117: e510–e526, 2008. 10.1161/CIRCULATIONAHA.108.189141 [DOI] [PubMed] [Google Scholar]
- 6.Saklayen MG, Deshpande NV: Timeline of history of hypertension treatment. Front Cardiovasc Med 3: 3, 2016. 10.3389/fcvm.2016.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman TM: The inextricable role of the kidney in hypertension. J Clin Invest 124: 2341–2347, 2014. 10.1172/JCI72274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rettig R: Does the kidney play a role in the aetiology of primary hypertension? Evidence from renal transplantation studies in rats and humans. J Hum Hypertens 7: 177–180, 1993 [PubMed] [Google Scholar]
- 9.Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr: Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972. 10.1016/0002-9343(72)90050-2 [DOI] [PubMed] [Google Scholar]
- 10.Guyton AC: Blood pressure control—Special role of the kidneys and body fluids. Science 252: 1813–1816, 1991. 10.1126/science.2063193 [DOI] [PubMed] [Google Scholar]
- 11.Caillon A, Paradis P, Schiffrin EL: Role of immune cells in hypertension. Br J Pharmacol 176: 1818–1828, 2019. 10.1111/bph.14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Miguel C, Guo C, Lund H, Feng D, Mattson DL: Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011. 10.1152/ajprenal.00454.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svendsen UG: Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand A 84: 523–528, 1976. 10.1111/j.1699-0463.1976.tb00150.x [DOI] [PubMed] [Google Scholar]
- 14.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG: Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. 10.1084/jem.20070657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seniuk A, Thiele JL, Stubbe A, Oser P, Rosendahl A, Bode M, Meyer-Schwesinger C, Wenzel UO, Ehmke H: B6.Rag1 knockout mice generated at the Jackson Laboratory in 2009 show a robust wild-type hypertensive phenotype in response to Ang II (angiotensin II). Hypertension 75: 1110–1116, 2020. 10.1161/HYPERTENSIONAHA.119.13773 [DOI] [PubMed] [Google Scholar]
- 16.Ji H, Pai AV, West CA, Wu X, Speth RC, Sandberg K: Loss of resistance to angiotensin II-induced hypertension in the Jackson Laboratory recombination-activating gene null mouse on the C57BL/6J background. Hypertension 69: 1121–1127, 2017. 10.1161/HYPERTENSIONAHA.117.09063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhur MS, Kirabo A, Guzik TJ, Harrison DG: From rags to riches: Moving beyond Rag1 in studies of hypertension. Hypertension 75: 930–934, 2020. 10.1161/HYPERTENSIONAHA.119.14612PMC7067673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC: Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002. 10.1016/S0002-9440(10)64445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA: Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ–/– and interleukin-17A–/– mice. Hypertension 65: 569–576, 2015. 10.1161/HYPERTENSIONAHA.114.04975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukao T, Matsuda S, Koyasu S: Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-gamma production by dendritic cells. J Immunol 164: 64–71, 2000. 10.4049/jimmunol.164.1.64 [DOI] [PubMed] [Google Scholar]
- 21.Schroder K, Hertzog PJ, Ravasi T, Hume DA: Interferon-γ: An overview of signals, mechanisms and functions. J Leukoc Biol 75: 163–189, 2004. 10.1189/jlb.0603252 [DOI] [PubMed] [Google Scholar]
- 22.Frucht DM, Fukao T, Bogdan C, Schindler H, O’Shea JJ, Koyasu S: IFN-gamma production by antigen-presenting cells: Mechanisms emerge. Trends Immunol 22: 556–560, 2001. 10.1016/S1471-4906(01)02005-1 [DOI] [PubMed] [Google Scholar]
- 23.Flaishon L, Hershkoviz R, Lantner F, Lider O, Alon R, Levo Y, Flavell RA, Shachar I: Autocrine secretion of interferon γ negatively regulates homing of immature B cells. J Exp Med 192: 1381–1388, 2000. 10.1084/jem.192.9.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munder M, Mallo M, Eichmann K, Modolell M: Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J Exp Med 187: 2103–2108, 1998. 10.1084/jem.187.12.2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shtrichman R, Samuel CE: The role of gamma interferon in antimicrobial immunity. Curr Opin Microbiol 4: 251–259, 2001. 10.1016/S1369-5274(00)00199-5 [DOI] [PubMed] [Google Scholar]
- 26.Wilck N, Balogh A, Markó L, Bartolomaeus H, Müller DN: The role of sodium in modulating immune cell function. Nat Rev Nephrol 15: 546–558, 2019. 10.1038/s41581-019-0167-y [DOI] [PubMed] [Google Scholar]
- 27.Bhat MY, Solanki HS, Advani J, Khan AA, Keshava Prasad TS, Gowda H, Thiyagarajan S, Chatterjee A: Comprehensive network map of interferon gamma signaling. J Cell Commun Signal 12: 745–751, 2018. 10.1007/s12079-018-0486-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoenborn JR, Wilson CB: Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol 96: 41–101, 2007. 10.1016/S0065-2776(07)96002-2 [DOI] [PubMed] [Google Scholar]
- 29.Jorgovanovic D, Song M, Wang L, Zhang Y: Roles of IFN-γ in tumor progression and regression: A review. Biomark Res 8: 49, 2020. 10.1186/s40364-020-00228-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panday A, Sahoo MK, Osorio D, Batra S: NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell Mol Immunol 12: 5–23, 2015. 10.1038/cmi.2014.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazarewicz RR, Dikalov SI: Mitochondrial ROS in the prohypertensive immune response. Am J Physiol Regul Integr Comp Physiol 305: R98–R100, 2013. 10.1152/ajpregu.00208.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG: Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 107: 263–270, 2010. 10.1161/CIRCRESAHA.110.217299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison DG, Marvar PJ, Titze JM: Vascular inflammatory cells in hypertension. Front Physiol 3: 128, 2012. 10.3389/fphys.2012.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, Rokutan K: Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol 290: C433–C443, 2006. 10.1152/ajpcell.00135.2005 [DOI] [PubMed] [Google Scholar]
- 35.Kamizato M, Nishida K, Masuda K, Takeo K, Yamamoto Y, Kawai T, Teshima-Kondo S, Tanahashi T, Rokutan K: Interleukin 10 inhibits interferon gamma- and tumor necrosis factor alpha-stimulated activation of NADPH oxidase 1 in human colonic epithelial cells and the mouse colon. J Gastroenterol 44: 1172–1184, 2009. 10.1007/s00535-009-0119-6 [DOI] [PubMed] [Google Scholar]
- 36.Marçal LE, Dias-da-Motta PM, Rehder J, Mamoni RL, Blotta MHSL, Whitney CB, Newburger PE, Costa FF, Saad STO, Condino-Neto A: Up-regulation of NADPH oxidase components and increased production of interferon-gamma by leukocytes from sickle cell disease patients. Am J Hematol 83: 41–45, 2008. 10.1002/ajh.20991 [DOI] [PubMed] [Google Scholar]
- 37.Bai A, Moss A, Rothweiler S, Longhi MS, Wu Y, Junger WG, Robson SC: Author correction: NADH oxidase-dependent CD39 expression by CD8+ T cells modulates interferon gamma responses via generation of adenosine. Nat Commun 11: 3036, 2020. 10.1038/s41467-020-16314-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai A, Moss A, Rothweiler S, Serena Longhi M, Wu Y, Junger WG, Robson SC: NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine [published correction appears in Nat Commun 11: 3036, 2020 10.1038/s41467-020-16314-5]. Nat Commun 6: 8819, 2015. 10.1038/ncomms9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanhere A, Hertweck A, Bhatia U, Gökmen MR, Perucha E, Jackson I, Lord GM, Jenner RG: T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun 3: 1268, 2012. 10.1038/ncomms2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Patel MB, Griffths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD: Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 64: 1275–1281, 2014. 10.1161/HYPERTENSIONAHA.114.03863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun XN, Li C, Liu Y, Du LJ, Zeng MR, Zheng XJ, Zhang WC, Liu Y, Zhu M, Kong D, Zhou L, Lu L, Shen ZX, Yi Y, Du L, Qin M, Liu X, Hua Z, Sun S, Yin H, Zhou B, Yu Y, Zhang Z, Duan SZ: T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ Res 120: 1584–1597, 2017. 10.1161/CIRCRESAHA.116.310480 [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Rafferty TM, Rhee SW, Webber JS, Song L, Ko B, Hoover RS, He B, Mu S: CD8+ T cells stimulate Na-Cl co-transporter NCC in distal convoluted tubules leading to salt-sensitive hypertension. Nat Commun 8: 14037, 2017. 10.1038/ncomms14037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benson LN, Liu Y, Wang X, Xiong Y, Rhee SW, Guo Y, Deck KS, Mora CJ, Li LX, Huang L, Andrews JT, Qin Z, Hoover RS, Ko B, Williams RM, Heller DA, Jaimes EA, Mu S: The IFNγ-PDL1 pathway enhances CD8T-DCT interaction to promote hypertension. Circ Res 130: 1550–1564, 2022. 10.1161/CIRCRESAHA.121.320373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren J, Crowley SD: Role of T-cell activation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 316: H1345–H1353, 2019. 10.1152/ajpheart.00096.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ: Activation of human T cells in hypertension: Studies of humanized mice and hypertensive humans. Hypertension 68: 123–132, 2016. 10.1161/HYPERTENSIONAHA.116.07237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J, Yamane H, Paul WE: Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28: 445–489, 2010. 10.1146/annurev-immunol-030409-101212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, Paul WE: CD4 T cells: Fates, functions, and faults. Blood 112: 1557–1569, 2008. 10.1182/blood-2008-05-078154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG: Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64: 1108–1115, 2014. 10.1161/HYPERTENSIONAHA.114.04147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irving BA, Chan AC, Weiss A: Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J Exp Med 177: 1093–1103, 1993. 10.1084/jem.177.4.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sussman JJ, Bonifacino JS, Lippincott-Schwartz J, Weissman AM, Saito T, Klausner RD, Ashwell JD: Failure to synthesize the T cell CD3-zeta chain: Structure and function of a partial T cell receptor complex. Cell 52: 85–95, 1988. 10.1016/0092-8674(88)90533-8 [DOI] [PubMed] [Google Scholar]
- 51.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL: CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63: 559–564, 2014. 10.1161/HYPERTENSIONAHA.113.02191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ: Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 122: 2529–2537, 2010. 10.1161/CIRCULATIONAHA.109.930446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hevia D, Araos P, Prado C, Fuentes Luppichini E, Rojas M, Alzamora R, Cifuentes-Araneda F, Gonzalez AA, Amador CA, Pacheco R, Michea L: Myeloid CD11c+ antigen-presenting cells ablation prevents hypertension in response to angiotensin II plus high-salt diet. Hypertension 71: 709–718, 2018. 10.1161/HYPERTENSIONAHA.117.10145 [DOI] [PubMed] [Google Scholar]
- 54.Xiao L, Patrick DM, Aden LA, Kirabo A: Mechanisms of isolevuglandin-protein adduct formation in inflammation and hypertension. Prostaglandins Other Lipid Mediat 139: 48–53, 2018. 10.1016/j.prostaglandins.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aschner M, Nguyen TT, Sinitskii AI, Santamaría A, Bornhorst J, Ajsuvakova OP, da Rocha JBT, Skalny AV, Tinkov AA: Isolevuglandins (isoLGs) as toxic lipid peroxidation byproducts and their pathogenetic role in human diseases. Free Radic Biol Med 162: 266–273, 2021. 10.1016/j.freeradbiomed.2020.10.024 [DOI] [PubMed] [Google Scholar]
- 56.Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ, Rodriguez-Iturbe B: Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am J Physiol Renal Physiol 304: F289–F299, 2013. 10.1152/ajprenal.00517.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Iturbe B, Lanaspa MA, Johnson RJ: The role of autoimmune reactivity induced by heat shock protein 70 in the pathogenesis of essential hypertension. Br J Pharmacol 176: 1829–1838, 2019. 10.1111/bph.14334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu N, Liu J, Ji Y, Lu P: Toll-like receptor 4 signaling mediates inflammatory activation induced by C-reactive protein in vascular smooth muscle cells. Cell Physiol Biochem 25: 467–476, 2010. 10.1159/000303052 [DOI] [PubMed] [Google Scholar]
- 59.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R: Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 52: 2936–2946, 2005. 10.1002/art.21238 [DOI] [PubMed] [Google Scholar]
- 60.Rodríguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ: The immunological basis of hypertension. Am J Hypertens 27: 1327–1337, 2014. 10.1093/ajh/hpu142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jameson SC: Maintaining the norm: T-cell homeostasis. Nat Rev Immunol 2: 547–556, 2002. 10.1038/nri853 [DOI] [PubMed] [Google Scholar]
- 62.Gagnon J, Ramanathan S, Leblanc C, Cloutier A, McDonald PP, Ilangumaran S: IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8+ T lymphocytes. J Immunol 180: 7958–7968, 2008. 10.4049/jimmunol.180.12.7958 [DOI] [PubMed] [Google Scholar]
- 63.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ: Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med 201: 139–148, 2005. 10.1084/jem.20041057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kishida T, Asada H, Itokawa Y, Cui FD, Shin-Ya M, Gojo S, Yasutomi K, Ueda Y, Yamagishi H, Imanishi J, Mazda O: Interleukin (IL)-21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol Ther 8: 552–558, 2003. 10.1016/S1525-0016(03)00222-3 [DOI] [PubMed] [Google Scholar]
- 65.Davey GM, Starr R, Cornish AL, Burghardt JT, Alexander WS, Carbone FR, Surh CD, Heath WR: SOCS-1 regulates IL-15-driven homeostatic proliferation of antigen-naive CD8 T cells, limiting their autoimmune potential. J Exp Med 202: 1099–1108, 2005. 10.1084/jem.20050003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramanathan S, Gagnon J, Ilangumaran S: Antigen-nonspecific activation of CD8+ T lymphocytes by cytokines: Relevance to immunity, autoimmunity, and cancer. Arch Immunol Ther Exp (Warsz) 56: 311–323, 2008. 10.1007/s00005-008-0033-2 [DOI] [PubMed] [Google Scholar]
- 67.Hou H, Zhou Y, Yu J, Mao L, Bosco MJ, Wang J, Lu Y, Mao L, Wu X, Wang F, Sun Z: Establishment of the reference intervals of lymphocyte function in healthy adults based on IFN-γ secretion assay upon phorbol-12-myristate-13-acetate/ionomycin stimulation. Front Immunol 9: 172, 2018. 10.3389/fimmu.2018.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crawford TQ, Jalbert E, Ndhlovu LC, Barbour JD: Concomitant evaluation of PMA+ionomycin-induced kinase phosphorylation and cytokine production in T cell subsets by flow cytometry. Cytometry A 85: 268–276, 2014. 10.1002/cyto.a.22444 [DOI] [PubMed] [Google Scholar]
- 69.Eissmann P: Natural Killer Cells. Available at: https://www.immunology.org/public-information/bitesized-immunology/cells/natural-killer-cells. Accessed January 17, 2022
- 70.Kossmann S, Schwenk M, Hausding M, Karbach SH, Schmidgen MI, Brandt M, Knorr M, Hu H, Kröller-Schön S, Schönfelder T, Grabbe S, Oelze M, Daiber A, Münzel T, Becker C, Wenzel P: Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol 33: 1313–1319, 2013. 10.1161/ATVBAHA.113.301437 [DOI] [PubMed] [Google Scholar]
- 71.Soderquest K, Powell N, Luci C, van Rooijen N, Hidalgo A, Geissmann F, Walzer T, Lord GM, Martín-Fontecha A: Monocytes control natural killer cell differentiation to effector phenotypes. Blood 117: 4511–4518, 2011. 10.1182/blood-2010-10-312264 [DOI] [PubMed] [Google Scholar]
- 72.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf SF, Trinchieri G: Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med 176: 1387–1398, 1992. 10.1084/jem.176.5.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mickael C, Lee MH, Gu S, Graham BB: Comparing pulmonary hypertension severity between rat strains suggests right ventricle NK cells are protective. Cardiovasc Res 115: 699–700, 2019. 10.1093/cvr/cvy299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Justin Rucker A, Crowley SD: The role of macrophages in hypertension and its complications. Pflugers Arch 469: 419–430, 2017. 10.1007/s00424-017-1950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ozawa Y, Kobori H, Suzaki Y, Navar LG: Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 292: F330–339, 2007. 10.1152/ajprenal.00059.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T: Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124: 1370–1381, 2011. 10.1161/CIRCULATIONAHA.111.034470 [DOI] [PubMed] [Google Scholar]
- 77.Shah KH, Shi P, Giani JF, Janjulia T, Bernstein EA, Li Y, Zhao T, Harrison DG, Bernstein KE, Shen XZ: Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ Res 117: 858–869, 2015. 10.1161/CIRCRESAHA.115.306539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, Schiffrin EL: Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: Evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol 292: H1789–H1795, 2007. 10.1152/ajpheart.01118.2006 [DOI] [PubMed] [Google Scholar]
- 79.Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ: Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: All for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004. 10.1152/ajprenal.00269.2003 [DOI] [PubMed] [Google Scholar]
- 80.Bravo J, Quiroz Y, Pons H, Parra G, Herrera-Acosta J, Johnson RJ, Rodríguez-Iturbe B: Vimentin and heat shock protein expression are induced in the kidney by angiotensin and by nitric oxide inhibition. Kidney Int Suppl 64: S46–S51, 2003. 10.1046/j.1523-1755.64.s86.9.x [DOI] [PubMed] [Google Scholar]
- 81.Quiroz Y, Bravo J, Herrera-Acosta J, Johnson RJ, Rodríguez-Iturbe B: Apoptosis and NFkappaB activation are simultaneously induced in renal tubulointerstitium in experimental hypertension. Kidney Int Suppl 64: S27–S32, 2003. 10.1046/j.1523-1755.64.s86.6.x [DOI] [PubMed] [Google Scholar]
- 82.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL: Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40: 274–288, 2014. 10.1016/j.immuni.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang WC, Zheng XJ, Du LJ, Sun JY, Shen ZX, Shi C, Sun S, Zhang Z, Chen XQ, Qin M, Liu X, Tao J, Jia L, Fan HY, Zhou B, Yu Y, Ying H, Hui L, Liu X, Yi X, Liu X, Zhang L, Duan SZ: High salt primes a specific activation state of macrophages, M(Na). Cell Res 25: 893–910, 2015. 10.1038/cr.2015.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M: The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. 10.1016/j.it.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 85.Gessani S, Belardelli F: IFN-γ expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev 9: 117–123, 1998. 10.1016/S1359-6101(98)00007-0 [DOI] [PubMed] [Google Scholar]
- 86.Mirhafez SR, Mohebati M, Feiz Disfani M, Saberi Karimian M, Ebrahimi M, Avan A, Eslami S, Pasdar A, Rooki H, Esmaeili H, Ferns GA, Ghayour-Mobarhan M: An imbalance in serum concentrations of inflammatory and anti-inflammatory cytokines in hypertension. J Am Soc Hypertens 8: 614–623, 2014. 10.1016/j.jash.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 87.Parissis JT, Korovesis S, Giazitzoglou E, Kalivas P, Katritsis D: Plasma profiles of peripheral monocyte-related inflammatory markers in patients with arterial hypertension. Correlations with plasma endothelin-1. Int J Cardiol 83: 13–21, 2002. 10.1016/S0167-5273(02)00021-9 [DOI] [PubMed] [Google Scholar]
- 88.Huang L, Wang A, Hao Y, Li W, Liu C, Yang Z, Zheng F, Zhou MS: Macrophage depletion lowered blood pressure and attenuated hypertensive renal injury and fibrosis. Front Physiol 9: 473, 2018. 10.3389/fphys.2018.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moore JP, Vinh A, Tuck KL, Sakkal S, Krishnan SM, Chan CT, Lieu M, Samuel CS, Diep H, Kemp-Harper BK, Tare M, Ricardo SD, Guzik TJ, Sobey CG, Drummond GR: M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am J Physiol Heart Circ Physiol 309: H906–H917, 2015. 10.1152/ajpheart.00821.2014 [DOI] [PubMed] [Google Scholar]
- 90.Rosales C: Neutrophil: A cell with many roles in inflammation or several cell types? Front Physiol 9: 113, 2018. 10.3389/fphys.2018.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Netea MG, Fantuzzi G, Kullberg BJ, Stuyt RJL, Pulido EJ, McIntyre RC Jr, Joosten LAB, Van der Meer JWM, Dinarello CA: Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol 164: 2644–2649, 2000. 10.4049/jimmunol.164.5.2644 [DOI] [PubMed] [Google Scholar]
- 92.Kak G, Raza M, Tiwari BK: Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol Concepts 9: 64–79, 2018. 10.1515/bmc-2018-0007 [DOI] [PubMed] [Google Scholar]
- 93.Morton J, Coles B, Wright K, Gallimore A, Morrow JD, Terry ES, Anning PB, Morgan BP, Dioszeghy V, Kühn H, Chaitidis P, Hobbs AJ, Jones SA, O’Donnell VB: Circulating neutrophils maintain physiological blood pressure by suppressing bacteria and IFNgamma-dependent iNOS expression in the vasculature of healthy mice. Blood 111: 5187–5194, 2008. 10.1182/blood-2007-10-117283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aydin M, Yuksel M, Yildiz A, Polat N, Bilik MZ, Akil MA, Acet H, Demir M, Inci U, Toprak N: Association between the neutrophil to lymphocyte ratio and prehypertension. Bratisl Lek Listy 116: 475–479, 2015. 10.4149/BLL_2015_090 [DOI] [PubMed] [Google Scholar]
- 95.Tatsukawa Y, Hsu WL, Yamada M, Cologne JB, Suzuki G, Yamamoto H, Yamane K, Akahoshi M, Fujiwara S, Kohno N: White blood cell count, especially neutrophil count, as a predictor of hypertension in a Japanese population. Hypertens Res 31: 1391–1397, 2008. 10.1291/hypres.31.1391 [DOI] [PubMed] [Google Scholar]
- 96.Liu X, Zhang Q, Wu H, Du H, Liu L, Shi H, Wang C, Xia Y, Guo X, Li C, Bao X, Su Q, Sun S, Wang X, Zhou M, Jia Q, Zhao H, Song K, Niu K: Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am J Hypertens 28: 1339–1346, 2015. 10.1093/ajh/hpv034 [DOI] [PubMed] [Google Scholar]
- 97.Araos P, Figueroa S, Amador CA: The role of neutrophils in hypertension. Int J Mol Sci 21: 8536, 2020. 10.3390/ijms21228536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Veglia F, Sanseviero E, Gabrilovich DI: Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol 21: 485–498, 2021. 10.1038/s41577-020-00490-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V: Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 22: 238–244, 2010. 10.1016/j.coi.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 100.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V: Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 116: 2777–2790, 2006. 10.1172/JCI28828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kondělková K, Vokurková D, Krejsek J, Borská L, Fiala Z, Ctirad A: Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove) 53: 73–77, 2010. 10.14712/18059694.2016.63 [DOI] [PubMed] [Google Scholar]
- 102.Kirabo A, Fontana V, de Faria APC, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, Harrison DG: DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656, 2014. 10.1172/JCI74084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A: Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep 21: 1009–1020, 2017. 10.1016/j.celrep.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu X, Rudemiller NP, Privratsky JR, Ren J, Wen Y, Griffiths R, Crowley SD: Classical dendritic cells mediate hypertension by promoting renal oxidative stress and fluid retention. Hypertension 75: 131–138, 2020. 10.1161/HYPERTENSIONAHA.119.13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Beusecum JP, Barbaro NR, McDowell Z, Aden LA, Xiao L, Pandey AK, Itani HA, Himmel LE, Harrison DG, Kirabo A: High salt activates CD11c + antigen-presenting cells via SGK (serum glucocorticoid kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension 74: 555–563, 2019. 10.1161/HYPERTENSIONAHA.119.12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu X, Zhang J, Wen Y, Ren J, Griffiths R, Rudemiller NP, Ide S, Souma T, Crowley SD: Type 1 angiotensin receptors on CD11c-expressing cells protect against hypertension by regulating dendritic cell-mediated T cell activation. Hypertension 79: 1227–1236, 2022. 10.1161/HYPERTENSIONAHA.121.18734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han Y-L, Li Y-L, Jia L-X, Cheng J-Z, Qi Y-F, Zhang H-J, Du J: Reciprocal interaction between macrophages and T cells stimulates IFN-γ and MCP-1 production in Ang II-induced cardiac inflammation and fibrosis. PLoS One 7: e35506, 2012. 10.1371/journal.pone.0035506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Markó L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Müller DN: Interferon-γ signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension 60: 1430–1436, 2012. 10.1161/HYPERTENSIONAHA.112.199265 [DOI] [PubMed] [Google Scholar]
- 109.Garcia AG, Wilson RM, Heo J, Murthy NR, Baid S, Ouchi N, Sam F: Interferon-γ ablation exacerbates myocardial hypertrophy in diastolic heart failure. Am J Physiol Heart Circ Physiol 303: H587–H596, 2012. 10.1152/ajpheart.00298.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kimura A, Ishida Y, Furuta M, Nosaka M, Kuninaka Y, Taruya A, Mukaida N, Kondo T: Protective roles of interferon-γ in cardiac hypertrophy induced by sustained pressure overload. J Am Heart Assoc 7: e008145, 2018. 10.1161/JAHA.117.008145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR: Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498, 2009. 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rosshart SP, Herz J, Vassallo BG, Hunter A, Wall MK, Badger JH, McCulloch JA, Anastasakis DG, Sarshad AA, Leonardi I, Collins N, Blatter JA, Han SJ, Tamoutounour S, Potapova S, Foster St Claire MB, Yuan W, Sen SK, Dreier MS, Hild B, Hafner M, Wang D, Iliev ID, Belkaid Y, Trinchieri G, Rehermann B: Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365: eaaw4361, 2019. 10.1126/science.aaw4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jama HA, Kaye DM, Marques FZ: The gut microbiota and blood pressure in experimental models. Curr Opin Nephrol Hypertens 28: 97–104, 2019. 10.1097/MNH.0000000000000476 [DOI] [PubMed] [Google Scholar]
- 114.de la Visitación N, Robles-Vera I, Toral M, Gómez-Guzmán M, Sánchez M, Moleón J, González-Correa C, Martín-Morales N, O’Valle F, Jiménez R, Romero M, Duarte J: Gut microbiota contributes to the development of hypertension in a genetic mouse model of systemic lupus erythematosus. Br J Pharmacol 178: 3708–3729, 2021. 10.1111/bph.15512 [DOI] [PubMed] [Google Scholar]
- 115.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J: Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5: 14, 2017. 10.1186/s40168-016-0222-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Avery EG, Bartolomaeus H, Maifeld A, Marko L, Wiig H, Wilck N, Rosshart SP, Forslund SK, Müller DN: The gut microbiome in hypertension: Recent advances and future perspectives. Circ Res 128: 934–950, 2021. 10.1161/CIRCRESAHA.121.318065 [DOI] [PubMed] [Google Scholar]
- 117.Divac N, Naumović R, Stojanović R, Prostran M: The role of immunosuppressive medications in the pathogenesis of hypertension and efficacy and safety of antihypertensive agents in kidney transplant recipients. Curr Med Chem 23: 1941–1952, 2016. 10.2174/0929867323666151221150052 [DOI] [PubMed] [Google Scholar]
- 118.Morales JM: Influence of the new immunosuppressive combinations on arterial hypertension after renal transplantation. Kidney Int Suppl 62: S81–S87, 2002. 10.1046/j.1523-1755.62.s82.16.x [DOI] [PubMed] [Google Scholar]
- 119.Ferro CJ, Edwards NC, Hutchison C, Cockwell P, Steeds RP, Savage CO, Townend JN, Harper L: Does immunosuppressant medication lower blood pressure and arterial stiffness in patients with chronic kidney disease? An observational study. Hypertens Res 34: 113–119, 2010. 10.1038/hr.2010.193 [DOI] [PubMed] [Google Scholar]
- 120.Chen J, Bundy JD, Hamm LL, Hsu CY, Lash J, Miller ER, Thomas G, Cohen DL, Weir MR, Raj DS, Chen HY, Xie D, Rao P, Wright JT, Rahman M, He J. Inflammation and apparent treatment-resistant hypertension in patients with chronic kidney disease. Hypertension 73: 785–793, 2019. 10.1161/HYPERTENSIONAHA.118.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bomfim GF, Cau SBA, Bruno AS, Fedoce AG, Carneiro FS: Hypertension: A new treatment for an old disease? Targeting the immune system. Br J Pharmacol 176: 2028–2048, 2019. 10.1111/bph.14436 [DOI] [PMC free article] [PubMed] [Google Scholar]