Abstract
Background:
Laparoscopic cholecystectomy (LC) has become the procedure of choice for the management of symptomatic gallstone disease. In LC, the surgeons encountered difficulties with acutely inflamed or gangrenous gallbladder (GB), dense adhesions at Calot’s triangle, fibrotic and contracted GB, and cholecystoenteric fistula. Depending on the difficulty faced during the surgery, the outcome of LC may vary from abandoning the procedure or partial cholecystectomy to conversion into open cholecystectomy. Complications related to biliary tract or adjoining structures or vessels may also occur. Our aim was to assess the different preoperative factors in patients of cholelithiasis and ascertain the validity of the scoring system devised by Randhawa and Pujahari in preoperatively predicting the difficult LC in our hospital scenario.
Materials and Methods:
This hospital-based observational study was conducted in the Department of General Surgery for a period of 2 years. All diagnosed cases of cholelithiasis admitted for elective LC during the study period in our hospital were included in the study.
Results:
In total, 154 patients, aged≥50 years, history of hospitalization for acute cholecystitis (AC), body mass index of 25 kg/m2 and more, abdominal scar, palpable GB, GB wall thickness ≥4 mm, pericholecystic collection, impacted stone found to be significant factors to predict difficult LC preoperatively. Endoscopic retrograde cholangiopancreatography and pancreatitis were found as independent risk factor for difficult LC.
Conclusion:
We recommend that the scoring system should be regularly used as a protocol for predicting difficulty levels preoperatively in LC. It can help to decide the surgical approach, counsel the patients, and reduce the complication rate, rate of conversion, and overall medical cost. The scoring system proposed by Randhawa and Pujahari is effective but has some lacunae.
Keywords: Cholelithiasis, difficult laparoscopic cholecystectomy, easy laparoscopic cholecystectomy, scoring system
Introduction
Cholelithiasis was first described in 1420 by a Florentine pathologist, Antonio Benivenius.[1] Further, Jean-Louis Petit, the founder of gallbladder (GB) surgery in 1733, had suggested the removal of gall stones and drainage of GB for gallstone disease.[2]
The first open cholecystectomy was performed on July 15, 1882, by a German surgeon Carl Johann August Langenbuch at the Lazarus Krankenhaus. Phillip Mouret performed the first laparoscopic cholecystectomy (LC) in 1987.[3] In India, Professor Tehempton E Udwadia from Mumbai performed the first LC in 1990 and presented the paper at the 10th world congress of digestive surgery in New Delhi.[4]
LC has become the procedure of choice for the management of symptomatic gallstone disease.[5] In LC, the surgeons encountered difficulties with acutely inflamed or gangrenous GB, dense adhesions at Calot’s triangle, fibrotic and contracted GB, and cholecystoenteric fistula.[6] There are many risk factors identified that make laparoscopic surgery difficult such as male sex, old age, obesity, attacks of acute cholecystitis (AC), previous abdominal surgery, and certain ultrasonographic findings, that is, thickened GB wall, distended GB, pericholecystic fluid collection, and impacted stone at GB neck.[7] According to a similar study by Lee et al.,[8] the risk factors for conversion included age >65 years, male sex, patients with previous upper abdominal surgery, and a documented history of AC.
Depending on the difficulty faced during the surgery, the outcome of LC may vary from abandoning the procedure or partial cholecystectomy to conversion into open cholecystectomy. Complications related to the biliary tract or adjoining structures or vessels may also occur.
If surgeons get an indication preoperatively then they may schedule the time and team for the operation appropriately. Patients predicted to have a high risk should be scheduled for longer hospitalization and more intensive postoperative care. This may also help the hospital administration to plan and predict admissions and bed vacancies more efficiently. Different methods have been suggested from time to time using different criteria, further adding to the controversy.
Our aim was to assess the different preoperative factors in patients with cholelithiasis and ascertain the validity of the scoring system devised by Randhawa and Pujahari[9] in preoperatively predicting the difficult LC in our hospital scenario.
Materials and Methods
Study setting
This hospital-based observational study was conducted in the Department of General Surgery after due permission from the Institutional Ethical Committee for a period of 2 years. All diagnosed cases of cholelithiasis admitted for elective LC during the study period in our hospital were included in the study and were operated by an experienced laparoscopic surgeon (operated LC for at least 5 years) in a single unit.
Inclusion criteria
All patients undergoing elective LC
Exclusion criteria
Patients having AC or suspected/proven malignancy and conversion due to technical problems.
Sample size
The sample size was calculated using, Med-Calc9.0.1 software for the area under the receiver-operating characteristic (ROC) curve for our study to compare the effective and non-effective scoring.
For alpha error or level of significance of 0.05 and for the power of study of 80% (or beta error of 20%) with ROC as 0.82.
The value of null hypothesis as 0.9 (if we assume our H = 0 the scoring system cannot predict the difficulty preoperatively, and we want to reject it completely so that we can prove that the proposed score can actually predict the difficulty the value of null hypothesis should be 0.9).
Then our sample size was 152.
But the total number of patients that followed us till the completion of the study was 154. Therefore, the final study sample size was 154.
Method for data collection
Patients were evaluated by detailed history and clinical examination. The diagnosis of cholelithiasis was established on ultrasonography and they underwent preoperative workup. The preoperative scoring based on the scoring system given by Randhawa and Pujahari[9] were calculated [Table 1].
Table 1.
Preoperative scoring system to preoperatively predict difficulty in laparoscopic cholecystectomy[9]
| Parameters | Score | Maximum score | ||
|---|---|---|---|---|
| History | Age | < 50 years | 0 | 1 |
| ≥50 years | 1 | |||
| Sex | Male | 1 | 1 | |
| Female | 0 | |||
| Hospitalization history for acute cholecystitis | Yes | 4 | 4 | |
| No | 0 | |||
| Clinical | BMI Weight/Height KG/Metre2 | <25 | 0 | 2 |
| 25–27.5 | 1 | |||
| >27.5 | 2 | |||
| Abdominal Scar | No | 0 | 2 | |
| Infraumbilical | 1 | |||
| Supraumbilical | 2 | |||
| Palpable gall bladder | Yes | 1 | 1 | |
| No | 0 | |||
| Sonography | Wall thickness | Thin (<4 mm) | 0 | 2 |
| Thick (≥4 mm) | 2 | |||
| Pericholecystic collection | Yes | 1 | 1 | |
| No | 0 | |||
| Impacted stone | Yes | 1 | 1 | |
| No | 0 |
Preoperative score up to 5 was defined as easy, 6–10 as difficult, and 11–15 as very difficult. By this, we preoperatively defined the difficulty level.
The timing was noted from the first port site incision till the closure of the last ports. All the intraoperative events were recorded. All cases received standard postoperative care and follow-up.
Intraoperative grading done on basis of difficulty levels into easy, difficult, and very difficult LC [Table 2].
Table 2.
Intraoperative grading of difficulty of LC[9]
| Intraoperative parameters | Grading |
|---|---|
| Time taken <60 min; no bile spillage; no injury to duct or artery | Easy |
| Time taken 60–120 min and/or bile or stone spillage and/or injury to duct | Difficult |
| Time taken >120 min or conversion | Very difficult |
Comparison was done between preoperative prediction and intra-operative finding (grading).
Statistical analysis
The presentation of the categorical variables was done in the form of numbers and percentages (%). On the contrary, the quantitative data were presented as the mean ± standard deviation (SD) and median with 25th and 75th percentiles (interquartile range). The data normality was checked by using the Kolmogorov–Smirnov test. For cases in which the data were not normal, we used nonparametric tests.
For quantitative variables, Mann–Whitney test (for two groups) and the Independent t test were used. For qualitative variables, chi-square test or Fisher’s exact test was used. Inter-rater kappa agreement was used to find out the strength of agreement between preoperative prediction and intraoperative grading. Sensitivity, specificity, PPV (positive predictive value), and NPV (negative predictive value) of preoperative score findings were calculated.
Multivariate logistic regression was used to find out independent risk factors. The data entry was done in the Microsoft EXCEL spreadsheet, and the final analysis was done with the use of Statistical Package for Social Sciences (SPSS) software, IBM manufacturer, Chicago, USA, version 21.0. For statistical significance, a value of P < 0.05 was considered statistically significant.
Results and Analysis
A total of 154 patients were included in the study as per inclusion and exclusion criteria.
The patient’s age in our study was from 20 to 75 years with a mean age of 42.16 years. The maximum number of patients were of the age group 31–40 years 54 (31.08%) [Table 3].
Table 3.
Distribution of participants on basis of gender and age
| Age (years) | Female (n = 74) | Male (n = 80) | Total |
|---|---|---|---|
| 20–30 | 12 (16.22%) | 13 (16.25%) | 25 (16.23%) |
| 31–40 | 23 (31.08%) | 31 (38.75%) | 54 (35.06%) |
| 41–50 | 15 (20.27%) | 17 (21.25%) | 32 (20.78%) |
| 51–60 | 21 (28.38%) | 17 (21.25%) | 38 (24.68%) |
| >60 | 3 (4.05%) | 2 (2.50%) | 5 (3.25%) |
| Total | 74 (100%) | 80 (100%) | 154 (100%) |
Approximately 70.78% of patients were below 50 years of age (n = 109). Approximately 45 patients were of 50 years or above comprising 29.22%.
The number of female patients was 74 (48.05%) and male patients was 80 (51.95%). The number of males was more than females with a ratio of 1.08:1 [Table 3].
In total, 95 (61.69%) patients had no history of prior hospitalisation for AC, whereas 59 (38.31%) patients required hospitalisation for AC. None of them were operated in early phase of AC.
The number of patients with body mass index (BMI) below 25 was 93 (60.39%), whereas patients with BMI between 25 and 27.5 were 32 (20.78%) and with BMI above 27.5 was 29 (18.83%). The majority of the patients were with BMI under 25 kg/m². The mean BMI of our study group was 24.3 kg/m2.
Out of 154 patients, only 39 (25.33%) patients had a history of previous abdominal surgery. Of them, 37 (24.03%) had infraumbilical abdominal scar and 2 (1.30%) had the supraumbilical abdominal scar.
In 154 patients, 33 (21.43%) had palpable GB.
On ultrasonography, 98 (63.64%) patients had a thickness of GB wall less than 4 mm, whereas 56 (36.36%) patients had a 4 mm or more thickness of GB wall. A total of 140 (90.91%) patients had no pericholecystic collection but 14 (9.09%) patients had pericholecystic collection. In total, 131 (85.06%) patients did not show any impacted stone, whereas 23 (14.94%) patients showed impacted stone on ultrasonography.
Out of 154 patients, 92 (59.74%) patients had a preoperative score of 0 to 5 (easy LC), 41 (26.62%) patients had a preoperative score between 6 and 10 (difficult LC) and 21 (13.64%) patients had preoperative scores 11 to 15 (very difficult LC) [Table 4].
Table 4.
Distribution on the basis of preoperative score for laparoscopic cholecystectomy
| Preoperative score | Preoperative prediction of laparoscopic cholecystectomy | Frequency | % |
|---|---|---|---|
| Up to 5 | Easy | 92 | 59.74% |
| 6–10 | Difficult | 41 | 26.62% |
| 11–15 | Very difficult | 21 | 13.64% |
| Total | 154 | 100% |
On basis of intraoperative parameters, 80 (51.95%) patients had easy LC, 46 (29.87%) patients had difficult LC, and 28 (18.18%) patients had very difficult LC [Graph 1].
Graph 1.
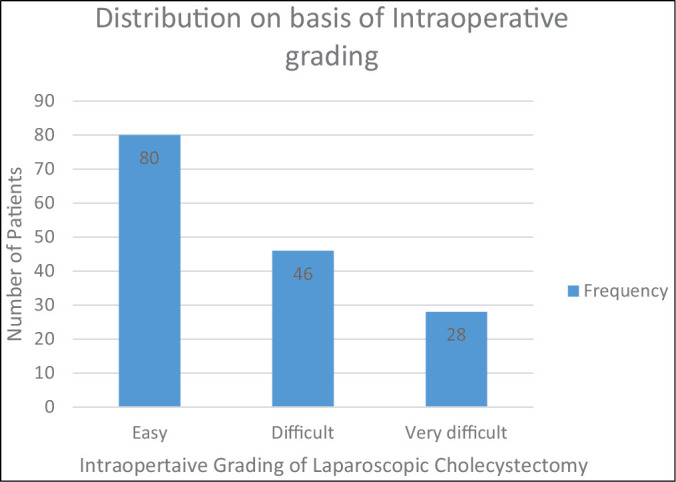
Distribution on basis of intraoperative grading
Intraoperatively 7 (4.55%) patients had bile or stone spillage, whereas 1 (0.65%) patient had bile duct injury. In 24 (15.58%) patients, LC was converted to open cholecystectomy [Table 5].
Table 5.
Distribution on the basis of preoperative score and intraoperative outcome of laparoscopic cholecystectomy (LC)
| S. no. | Preoperative score | Intraoperative grading of LC | Time taken intraoperatively (min) | Conversion to open | Bile/stone spillage | Bile duct/Artery injury | Reasons for mismatch preop score and intraoperative grading | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| <60 | 60 to 120 | >120 | ERCP (E) | Pancreatitis (P) | E + P | Dense adhesions | ||||||||
| 1 | 0–5 (easy) | 92 | Easy | 80 | 80 | – | – | – | – | – | 0 | 0 | 0 | 0 |
| Difficult | 8 | 0 | 8 | 0 | - | 1 | 0 | 3 | 2 | 2 | 1 | |||
| Very difficult | 4 | 0 | 3 | 1 | 4 | 0 | 0 | 1 | 0 | 2 | 1 | |||
| Total | 92 | |||||||||||||
| 2 | 6–10 | 41 | Easy | 0 | – | – | – | – | – | – | – | – | – | – |
| (difficult) | Difficult | 38 | 0 | 38 | 0 | - | 1 | 1 | 0 | 0 | – | 0 | ||
| Very difficult | 3 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 2 | – | 1 | |||
| Total | 41 | |||||||||||||
| 3 | 11–15 | 21 | Easy | 0 | – | – | – | – | – | – | – | – | – | – |
| (very | Difficult | 0 | – | – | – | – | – | – | – | – | – | – | ||
| difficult) | Very difficult | 21 | 0 | 16 | 5 | 18 | 5 | 0 | 0 | 0 | – | 0 | ||
| Total | 41 | |||||||||||||
| Total | 154 | 154 | 24 | 7 | 1 | 4 | 4 | 4 | 3 | |||||
The mean operative for cholecystectomy was 73.56 min with a range of 40–140 min. 80 (51.95%) patients LC was finished in less than 60 min, 67 (43.51%) patients’ LC was finished in 60–120 min, and 7 (4.54%) patients cholecystectomy surgery was finished in more than 120 min.
Preoperative score for predicting difficult LC has the following features: 83.78% sensitivity with a 95% confidence interval of 73.39% to 91.33%; 100% specificity with a 95% confidence interval of 95.49% to 100.00%; and 100% PPV with a 95% confidence interval of 94.22% to 100.00%. It has an 86.96% NPV with a 95% confidence interval of 78.32% to 93.07%. Preoperative score for predicting difficult LC has 92.21% diagnostic accuracy [Table 6].
Table 6.
Sensitivity, specificity, PPV, and NPV of preoperative score for predicting difficult laparoscopic cholecystectomy
| Preoperative score diagnostic accuracy | Values |
|---|---|
| Sensitivity (95% CI) | 83.78% (73.39% to 91.33%) |
| Specificity (95% CI) | 100% (95.49% to 100.00%) |
| Area under curve (AUC) (95% CI) | 0.92 (0.86 to 0.96) |
| Positive predictive value (95% CI) | 100% (94.22% to 100.00%) |
| Negative predictive value (95% CI) | 86.96% (78.32% to 93.07%) |
| Diagnostic accuracy | 92.21% |
Fisher’s exact test and Mann–Whitney test were used for preoperative score and it was found there is a significant association between preoperative score and difficult LC. It means our chosen preoperative score significantly predicts difficult LC [Table 7].
Table 7.
Association of preoperative score with difficult laparoscopic cholecystectomy
| Preoperative score | Easy (n = 80) | Difficult (n = 74) | Total | P Value | Significant/not significant |
|---|---|---|---|---|---|
| Preoperative prediction | |||||
| Easy (0–5) | 80 (86.96%) | 12 (13.04%) | 92 (100%) | <.0001‡ | Significant |
| Difficult (6–15) | 0 (0%) | 62 (100%) | 62 (100%) | ||
| Preoperative score | |||||
| Mean ± SD | 0.88 ± 0.7 | 8.16 ± 3.28 | 4.38 ± 4.33 | <.0001† | Significant |
| Median (25th–75th percentile) | 1(0–1) | 8(7–11) | 2(1–8) | ||
| Range | 0–3 | 0–13 | 0–13 |
‡ Fisher’s exact test
† Mann–Whitney test
*For statistical purpose difficult and very difficult cases are counted together
In 15 cases, the preoperative score and intraoperative grading did not match. Out of them, four patients had a history of endoscopic retrograde cholangiopancreatography (ERCP), in which there was difficult and very difficult LC. Four patients with a history of pancreatitis, and LC was also difficult to very difficult in contrast to preoperative score. Four patients had a history of ERCP as well as pancreatitis and LC was difficult or very difficult in contrast to preoperative score. In three cases preoperative score predicted easy LC, but we found difficult to very difficult LC because of more time taken or conversion to open cholecystectomy intraoperatively due to dense adhesions at Calot’s triangle and between GB and surrounding structures with no factor predicting difficulty in LC preoperatively [Tables 5 and 8].
Table 8.
Distribution on the basis of P value of other variables
| Other variables | Easy (n = 80) | Difficult (n = 74) | Total | P Value | Significant/nNot Significant |
|---|---|---|---|---|---|
| ERCP | |||||
| No | 80 (54.79%) | 66 (45.21%) | 146 (100%) | 0.002‡ | Significant |
| Yes | 0 (0%) | 8 (100%) | 8 (100%) | ||
| Pancreatitis | |||||
| No | 80 (54.79%) | 66 (45.21%) | 146 (100%) | 0.002‡ | Significant |
| Yes | 0 (0%) | 8 (100%) | 8 (100%) | ||
| Time more taken due to dense adhesions | |||||
| No | 80 (52.98%) | 71 (47.02%) | 151 (100%) | 0.109‡ | Not significant |
| Yes | 0 (0%) | 3 (100%) | 3(100%) |
‡ Fisher’s exact test
Multivariate logistic regression was used to find out the independent risk factors of the above variables for difficult LC and it was found that a history of ERCP and pancreatitis are independent risk factors for difficult LC [Table 9].
Table 9.
Multivariate logistic regression to find out independent risk factors of difficult laparoscopic cholecystectomy
| Variable | Beta coefficient | Standard error | P Value | Odds ratio | Odds ratio lower bound (95%) | Odds ratio upper bound (95%) |
|---|---|---|---|---|---|---|
| Difficult | 2.955 | 2.630 | 0.261 | 19.201 | 0.111 | 3326.205 |
| ERCP | 3.853 | 1.836 | 0.036 | 47.132 | 1.289 | 1723.463 |
| Pancreatitis | 5.541 | 2.000 | 0.006 | 254.844 | 5.053 | 12853.871 |
Discussion
Cholecystectomy is the most commonly performed surgery and after its introduction in 1985, LC has been termed as gold standard management for the disease of gallstone. Through the years LC has become a relatively safe procedure though occasionally it can be difficult due to certain reasons. Due to various difficulties faced while performing the LC, approximately 3%–35% of attempted LC have to be converted to the open procedure.[10,11] Preoperative assessment using clinical and radiological tools to predict the possibility of difficulty in carrying out LC can help in counseling patients.
In our study, age ≥50 years was found to be a significant factor that results in difficult LC. It correlates with different studies available in the literature.[8,12,13,14,15,16]
The possible reason for difficult cholecystectomy among patients with age ≥50 years could be that with age there is increased possibility of multiple attacks of AC and also increased frequency of abdominal surgeries. Therefore, there is an increased probability of fibrosis and adhesions in the hepatic hilum.[16] Similarly, studies in the western world in the past have implicated ages more than 65 years with difficulty in dissection of Calot’s triangle and adhesiolysis.
Generally, cholelithiasis is three times more common in females than men.[17] But in our study, the male:female ratio was 1.08:1 which may be due to the relatively small sample size limited to single-centre catering to labourer population.
Many studies have found the male gender as a significant factor that results in difficult LC.[8,13,14,15,16,18] In male patients, there is more intense inflammation and fibrosis that lead to more dense adhesions and further making dissection more difficult in LC.[16] There is a more frequent association with a severe form of the disease, that is, both acute and chronic cholecystitis and because of a higher percentage of intra-abdominal and visceral adipose tissue in men than women. Men are also less likely to seek medical attention than women.[13] In our study, there were difficult LC cases in male gender but statistically that was not significant. A small sample size could be the reason for this variation from the literature.
In our study, we found the history of hospitalisation for AC is a significant factor for predicting pre-operatively difficult LC. This may be due to difficult anatomy due to repeated cholecystitis causing adhesions of GB with adjacent organs.[19] Each attack of cholecystitis increases the GB wall thickness and the GB becomes scarred and fibrosed. It also increases the adhesions at Calot’s triangle and between GB and fossa.[10] Dense adhesions cause difficult handling of GB and difficulty in dissection at Calot’s triangle making LC difficult. Many studies have shown that a history of hospitalisation for AC is a significant factor for difficult LC.[5,9,10,12,16,19,20,21,22,23]
In our study, we found BMI 25 or more as a significant factor for predicting pre-operatively difficult LC by chi-square test (P < 0.0001) and independent t-test (P < 0.0001). Port placement in an obese patient takes a longer time due to thick abdominal wall. Dissection at the Calot’s triangle is also technically difficult due to the obscure anatomy because of larger intraperitoneal fat and difficulty in manipulating the instruments through an highly thick abdominal wall.[10] Similar results were seen in different studies in the literature.[9,12,13,16,19]
In our study, we found previously abdominal scar is a significant factor for predicting preoperatively difficult LC. Supra-umbilical surgical scars led to the difficult creation of pneumoperitoneum and difficulty in accessing of the peritoneal cavity.[24] Previous upper abdominal surgery scar is associated with the higher rate of adhesions, an increased risk of operative complications, a greater conversion rate, a prolonged operating time and longer stay.[6] Abdominal scars (signifying previous abdominal surgeries) may cause the intraperitoneal adhesions formation that may cause an increased possibility of injury and bleeding during the placement of umbilical port.[6] Authors in different studies also found that previously abdominal surgery scar to be associated with difficult LC.[8,10,16,18]
Palpable GB was a significant factor for predicting preoperatively difficult LC. Palpable GB could be due to a distended GB, mucocele GB, thick-walled GB, inflammation of GB (AC) or due to adhesions between the GB and the omentum.[10] Distended GB without inflammation may even have difficulty in holding GB intraoperatively and may need time to aspirate before removing from the port.[19] Similar results were seen in different studies.[5,9,10,12,20,21]
In our study, we found that GB wall thickness 4 mm or more on ultrasonography is a significant factor for predicting pre-operatively difficult LC. GB wall thickness is related to the inflammation or fibrosis that follows previous attacks of AC and thus may reflect the difficulty in the delineation of the anatomy during surgery.[19] The presence of a thick GB wall may cause grasping and manipulation of GB difficult. This makes dissection at Calot’s triangle and the GB bed to be the difficult and limits the extent of anatomical definition. Singh and Ohri[6] in their study also found that there is a statistically significant association of difficulty in GB grasping in pericholecystic inflammation and in distended GB. Similar results were found in different studies.[5,8,9,12,14,18,19,20,21,22,23,25]
In our study, we found the pericholecystic collection of GB on ultrasonography as a significant factor for predicting laparoscopic difficult cholecystectomy pre-operatively. In cases of pericholecystic fluid presence, there is an inflamed field with adhesions. The achievement of the critical view of safety (CVS) requires complete dissection of the fat and fibrous tissue in the Calot’s triangle which cannot be performed easily in an inflamed field.[24] Similar results were found in different studies.[5,8,10,20,22]
In our study, we found impacted stone on ultrasonography as a significant factor for predicting preoperatively difficult LC. Impacted stone makes it difficult holding of the GB.[19] While performing LC, stone impacted at neck of GB poses few technical problems due to distension of the GB as it is with thick-walled GB. It is difficult to grasp the GB neck and we did not get adequate retraction for performing dissection at the Calot’s triangle.[6] Similar results were found in different studies.[5,10,12,20,21]
In our study, we found that Randhawa and Pujahari’s[9] scoring system significantly predicts pre-operatively difficult LC by Fisher’s exact test (P < 0.0001) and Mann Whitney test (P < 0.0001).
In their studies, Randhawa and Pujahari,[9] Agarwal et al.,[10] Khetan and Yeola,[23] and Kumar and Baderiya[12] used Randhawa and Pujahari scoring system (the scoring system that we used) and it was able to predict preoperatively difficult LC significantly. It can be used effectively for preoperative prediction of difficult LC and further planning of surgery and post-operative care.
In our study, we found the past history of ERCP is a significant factor for predicting preoperatively difficult LC by fisher’s exact (P < 0.002). Multivariate logistic regression was done and it was concluded that past history of ERCP is an independent risk factor to predict difficult LC pre-operatively with P = 0.036. We found dense adhesions at Calot’s triangle and thickened GB in patients who had a past history of ERCP.
Raza and Venkata[20] found difficult LC in post-ERCP patients and advised modified Randhawa and Pujahari scoring system including post-ERCP as one additional parameter in the scoring system. Vivek et al.[16] and Nassar et al.[14] also found difficult LC in post-ERCP patients. These all studies had advised for scoring of 2 for past history of ERCP and 0 score for no ERCP history.
In our study, we concluded that the past history of pancreatitis is a significant factor for predicting pre-operatively difficult LC by fisher’s exact (P < 0.002). Multivariate logistic regression was done and it was concluded that the past history of pancreatitis is the independent risk factor to predict difficult LC pre-operatively with P = 0.006. We found dense adhesions at Calot’s triangle, between GB and surrounding structures and thickened GB in patients who had past history of pancreatitis. Vivek et al.[16] also found difficult LC in patients with peri-pancreatic fluid (suggesting pancreatitis) and given 1 score for pancreatitis and 0 for no history of pancreatitis.
Conclusion
We recommend that scoring system should be regularly used as a protocol for predicting difficulty level preoperatively in LC. It can help to decide the surgical approach, counsel the patients, reduce the complication rate, rate of conversion, and overall medical cost. Scoring system proposed by Randhawa and Pujahari is effective but has some lacunae. Keeping other factors (like pancreatitis and ERCP) in mind and incorporating them along with this scoring system can help the surgeon to plan the surgery with better vision of challenges that can come intraoperatively. However large sample size studies should be done to further evaluate these scoring systems.
Financial support and sponsorship
Not applicable.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–9. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 2.Beal JM. Historical perspective of gallstone disease. Surg Gynecol Obstet. 1984;158:181–9. [PubMed] [Google Scholar]
- 3.Reynolds W., Jr The first laparoscopic cholecystectomy. Jsls. 2001;5:89–94. [PMC free article] [PubMed] [Google Scholar]
- 4.Soper NJ, Stockmann PT, Dunnegan DL, Ashley SW. Laparoscopic cholecystectomy. The new ‘gold standard’? Arch Surg. 1992;127:917–21. doi: 10.1001/archsurg.1992.01420080051008. discussion 921–3. [DOI] [PubMed] [Google Scholar]
- 5.Bhondave S, Dash N, Thipse MV, Gadekar J. Proposed diagnostic scoring system to predict difficult laparoscopic cholecystectomy. J Med Sci Clin Res. 2017;50:31683–8. [Google Scholar]
- 6.Singh K, Ohri A. Difficult laparoscopic cholecystectomy: A large series from north India. Indian J Surg. 2006;68:205–08. [Google Scholar]
- 7.Abdel Baki NA, Motawei MA, Soliman KE, Farouk AM. Pre-operative prediction of difficult laparoscopic cholecystectomy using clinical and ultrasonographic parameters. JMRI. 2006;27:102–7. [Google Scholar]
- 8.Lee NW, Collins J, Britt R, Britt LD. Evaluation of preoperative risk factors for converting laparoscopic to open cholecystectomy. Am Surg. 2012;78:831–3. [PubMed] [Google Scholar]
- 9.Randhawa JS, Pujahari AK. Preoperative prediction of difficult lap chole: A scoring method. Indian J Surg. 2009;71:198–201. doi: 10.1007/s12262-009-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal N, Singh S, Khichy S. Preoperative prediction of difficult laparoscopic cholecystectomy: A scoring method. Niger J Surg. 2015;21:130–3. doi: 10.4103/1117-6806.162567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaton KW, Braddon FE, Mountford RA, Hughes AO, Emmett PM. Symptomatic and silent gall stones in the community. Gut. 1991;32:316–20. doi: 10.1136/gut.32.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar V, Baderiya VK. Assessment of preoperative patient factors in early prediction of difficult laparoscopic cholecystectomy: A single institution study. Int J Surg Sci. 2019;3:223–5. [Google Scholar]
- 13.Kumar A, Chabra A, Bhushan B. Evaluation of various pre-operative parameters for prediction of difficult laparoscopic cholecystectomy. J Adv Med Dent Scie Res. 2017;5:91–5. [Google Scholar]
- 14.Nassar AHM, Hodson J, Ng HJ, Vohra RS, Katbeh T, Zino S, et al. CholeS Study Group, West Midlands Research Collaborative. Predicting the difficult laparoscopic cholecystectomy: development and validation of a pre-operative risk score using an objective operative difficulty grading system. Surg Endosc. 2020;34:4549–61. doi: 10.1007/s00464-019-07244-5. [DOI] [PubMed] [Google Scholar]
- 15.Kanaan SA, Murayama KM, Merriam LT, Dawes LG, Prystowsky JB, Rege RV, et al. Risk factors for conversion of laparoscopic to open cholecystectomy. J Surg Res. 2002;106:20–4. doi: 10.1006/jsre.2002.6393. [DOI] [PubMed] [Google Scholar]
- 16.Vivek MA, Augustine AJ, Rao R. A comprehensive predictive scoring method for difficult laparoscopic cholecystectomy. J Minim Access Surg. 2014;10:62–7. doi: 10.4103/0972-9941.129947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rattner DW, Ferguson C, Warshaw AL. Factors associated with successful laparoscopic cholecystectomy for acute cholecystitis. Ann Surg. 1993;217:233–6. doi: 10.1097/00000658-199303000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kama NA, Kologlu M, Doganay M, Reis E, Atli M, Dolapci M. A risk score for conversion from laparoscopic to open cholecystectomy. Am J Surg. 2001;181:520–5. doi: 10.1016/s0002-9610(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 19.Acharya A, Adhikari SK. Preoperative scoring system to predict difficulty laparoscopic cholecystectomy. Postgraduate J NAMS. 2012;12:45–50. [Google Scholar]
- 20.Raza M, Venkata RM. Predicting difficulty in laparoscopic cholecystectomy preoperatively using a scoring system. Int Surg J. 2019;6:957–62. [Google Scholar]
- 21.Gupta N, Ranjan G, Arora MP, Goswami B, Chaudhary P, Kapur A, et al. Validation of a scoring system to predict difficult laparoscopic cholecystectomy. Int J Surg. 2013;11:1002–6. doi: 10.1016/j.ijsu.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Nidoni R, Udachan TV, Sasnur P, Baloorkar R, Sindgikar V, Narasangi B. Predicting difficult laparoscopic cholecystectomy based on clinicoradiological assessment. J Clin Diagn Res. 2015;9:PC09–12. doi: 10.7860/JCDR/2015/15593.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khetan AK, Yeola M. Preoperative prediction of difficult laparoscopic cholecystectomy using a scoring system. Int Surg J. 2017;4:3388–91. [Google Scholar]
- 24.Le VH, Smith DE, Johnson BL. Conversion of laparoscopic to open cholecystectomy in the current era of laparoscopic surgery. Am Surg. 2012;78:1392–5. [PubMed] [Google Scholar]
- 25.Khan IA, El-Tinay OE. Laparoscopic cholecystectomy for acute cholecystitis. Can preoperative factors predict conversion? Saudi Med J. 2004;25:299–302. [PubMed] [Google Scholar]


