Abstract
Listeria monocytogenes-infected phagocytes are present in the bloodstream of experimentally infected mice, but whether they play a role in central nervous system (CNS) invasion is unclear. To test whether bacteria within infected leukocytes could establish CNS infection, experimentally infected mice were treated with gentamicin delivered by surgically implanted osmotic pumps. Bacterial inhibitory titers in serum and plasma ranged from 1:16 to 1:256, and essentially all viable bacteria in the bloodstream of treated mice were leukocyte associated. Nevertheless, CNS infection developed in gentamicin-treated animals infected intraperitoneally or by gastric lavage, suggesting that intracellular bacteria could be responsible for neuroinvasion. This was supported by data showing that 43.5% of bacteria found with blood leukocytes were intracellular and some colocalized with F-actin, indicating productive intracellular parasitism. Experiments using an L. monocytogenes strain containing a chromosomal actA-gfpuv-plcB transcriptional fusion showed that blood leukocytes were associated with intracellular and extracellularly bound green fluorescent protein-expressing (GFP+) bacteria. Treatment with gentamicin decreased the numbers of extracellularly bound GFP+ bacteria significantly but did not affect the numbers of intracellular GFP+ bacteria, suggesting that the latter were the result of intercellular spread of GFP+ bacteria to leukocytes. These data demonstrate that infected leukocytes and the intracellular L. monocytogenes harbored within them play key roles in neuroinvasion. Moreover, they suggest that phagocytes recruited to infected organs such as the liver or spleen are themselves parasitized by intercellular spread of L. monocytogenes and then reenter the bloodstream and contribute to the systemic dissemination of bacteria.
Listeria monocytogenes is a facultative intracellular bacterium that infects the central nervous system (CNS) of humans and domesticated animals (21, 22). Most human infections result from ingestion of contaminated food and typically manifest as febrile gastroenteritis (25). Immunosuppressed hosts, however, are much more likely to develop invasive listeriosis marked by bacteremia, CNS infection, and death (24, 27). Although the precise mechanism(s) used by L. monocytogenes for entering the CNS are not clear, current theory indicates that neuroinvasive bacteria in general can enter the CNS by several different routes (37). These include invasion of microvascular endothelial cells, invasion of epithelial cells of the choroid plexus, and passage of bacteria through intercellular junctions. In addition, bacteria that are capable of intracellular survival can enter the CNS via phagocyte-facilitated infection, the major steps of which are adhesion of infected phagocytes to endothelium followed by cell-to cell spread of bacteria to endothelial cells and/or migration of infected phagocytes into the CNS (10).
Experimental L. monocytogenes infection of mice shows that bacteria enter the CNS following prolonged bacteremia (2). Because L. monocytogenes bacteremia is composed of cell-free bacteria as well as infected phagocytes, more than one neuroinvasive mechanism may be used (8). L. monocytogenes has a well-described ability to invade endothelial cells, including brain microvascular endothelial cells, suggesting that this is one possible means of entering the CNS (12, 17, 29, 36). A role for phagocyte-facilitated invasion of the CNS by L. monocytogenes is also plausible. This is suggested by data showing that cell-associated bacteria are virulent, as demonstrated by their intercellular spread to endothelial cells in vitro and their ability to cause systemic disease when transferred to other mice (8). In addition, neurons are more easily infected by cell-to-cell spread of L. monocytogenes from macrophages than by direct invasion by cell-free bacteria (7). L. monocytogenes-infected monocytes also have been indentified in the CNS of infected mice, but it is unclear whether these cells were infected in the periphery and then had migrated into the CNS or whether they had migrated into the CNS and then had phagocytosed bacteria (33).
The experiments presented here tested whether L. monocytogenes-infected phagocytes could establish CNS infection in mice. For this, extracellular bacteria were killed during experimental infection of mice with gentamicin that was delivered by surgically implanted osmotic pumps. The results show that leukocytes containing intracellular bacteria were present in the bloodstream and that CNS infection developed despite bactericidal levels of gentamicin. Intracellular parasitism of circulating phagocytes was documented at the single-cell level using an L. monocytogenes strain containing a chromosomal actA-gfpuv-plcB transcriptional fusion. Data obtained using this bacterial strain suggest that leukocytes are parasitized by cell-to-cell spread of bacteria rather than by phagocytosis of bacteria from the extracellular milieu. This suggests that inflammatory phagocytes are parasitized after they have been recruited into infected parenchymal tissues. Once infected, the phagocytes reenter the bloodstream and play crucial roles in systemic dissemination and neuroinvasion.
MATERIALS AND METHODS
Bacteria.
L. monocytogenes strains EGD and 10403s were stored in brain heart infusion broth (Difco, Detroit, Mich.) at 109 CFU/ml at −70°C. For experiments, 10 μl of stock culture was inoculated into 4 ml of broth and cultured overnight at 37°C with shaking.
Construction of an actA-gfpuv-plcB transcriptional gene fusion mutant in L. monocytogenes 10403S.
Plasmid pNF333 contains a transcriptional fusion of gfp to the actA gene of L. monocytogenes (23), as well as flanking L. monocytogenes chromosomal regions, for introduction of the actA-gfp-plcB fusion into the L. monocytogenes chromosome via homologous recombination (13). Primers GFP-1 and GFP-2A (13) were used to amplify gfpuv coding sequences from plasmid pGFPuv (Clontech Laboratories Inc., Palo Alto, Calif.) by PCR and to introduce a gram-positive ribosome binding site derived from SD1 of ermC upstream of gfpuv (5). The PCR-amplified product was digested with XbaI and PstI and subcloned into pNF333 in place of the original gfp allele to yield plasmid pNF579. pNF579 was introduced into L. monocytogenes 10403S by electroporation, and transformants were isolated by growth at 30°C on brain heart infusion agar containing 10 μg of chloramphenicol per ml (30). L. monocytogenes NF-L512, containing the actA-gfpuv-plcB transcriptional fusion in single copy on the bacterial chromosome, was isolated from the pNF579 transformants as previously described (4, 14).
Mice.
Female (C57BL/6 × DBA/2)F1 mice were purchased from Jackson Laboratory (Bar Harbor, Maine). The animals were housed in microisolator cages and given food and water ad libitum. They were 10 to 16 weeks of age and weighed 20 to 25 g when used in the experiments.
Implantation of osmotic pumps.
Alzet osmotic pumps model 1007D (Alza Corp. Newark, Del.) were filled with gentamicin sulfate at 80 mg/ml (Sigma Chemical Co., St. Louis, Mo.) in phosphate-buffered saline (PBS), and pump operation was initiated prior to implantation. Mice were anesthetized by sequential injections of 0.1 mg of xylazine and 2.0 to 2.5 mg of ketamine (both from Vedco, Inc., St. Joseph, Mo.). The fur was cleansed with 70% ethanol, the lower back was shaved with a razor, and a horizontal incision was made. A sterile osmotic pump was inserted into a subcutaneous pouch, and the incision was closed with sterile skin clips. Activated pumps were in the animal 90 to 120 min prior to infection.
Mouse infection.
Infected resident peritoneal cells were harvested by peritoneal lavage 60 min after intraperitoneal (i.p.) injection of 2 × 107 CFU of L. monocytogenes as previously described (8). Unbound bacteria were removed by washing the peritoneal cells twice followed by centrifugation through a layer of 30% sucrose (11). The cells were suspended at 2.0 × 106 to 2.5 × 106/ml in PBS, and then 0.2-ml volumes were injected i.p. into recipient animals. Bacterial CFU associated with the peritoneal cells were quantified by serial dilution in distilled water and plating on agar.
At the indicated times, infected mice were euthanized and then exsanguinated by cardiac puncture. The liver, spleen, and brain were aseptically removed and homogenized in 2 ml of sterile PBS. Bacterial CFU were quantified by serial 10-fold dilutions in sterile distilled water and plating on agar. To dilute gentamicin, blood was diluted 1:100; this was followed by serial 10-fold dilutions and plating on agar. To quantify bacterial CFU associated with peripheral blood leukocytes (PBL), mice were anticoagulated by i.p. injection of 25 U of heparin (Sigma) 10 min prior to exsanguination. An aliquot of whole blood was cultured to quantify total bacteria, the remainder was diluted into 10 ml of Hanks balanced salt solution without Mg2+ or Ca2+, and the cells were washed twice. The erythrocytes were lysed, and the leukocytes were centrifuged through 30% sucrose to remove unbound bacteria. Isolated PBL were suspended in sterile distilled water to the original volume of whole blood, and bacterial CFU were quantified as before. The percentage of bacterial CFU in whole blood that was collected in the PBL fraction was calculated as 100 × (CFU of bacteria per milliliter associated with leukocytes)/(CFU of bacteria per milliliter in whole blood).
Immunosuppression was accomplished by daily i.p. injections of 2 mg of hydrocortisone sodium succinate (Upjohn Co., Kalamazoo, Mich.) and 2 mg of cyclosporin A (Sandoz Pharmaceutical Corp., East Hanover, N.J.) beginning the day prior to infection (28, 35). In these animals, the osmotic pumps were loaded with 40 mg of gentamicin per ml due to the potential for severe renal toxicity and the decreased clearance of gentamicin caused by two nephrotoxic drugs (gentamicin and cyclosporin A). Immunosuppressed mice were infected per os by gastric lavage with 0.1 ml of PBS containing 109 bacteria delivered through a 24-gauge stainless steel gavage needle 2 to 4 h after pump placement.
Determination of inhibitory and bactericidal titers.
Serum and plasma were collected from blood by centrifugation for 20 min at 2,000 × g at 4°C and then were stored at −20°C until inhibitory and bactericidal titers were determined. For this, samples of serum or plasma were serially diluted 1:1 into 100 μl of BHI broth in 96-well plates. For a control, a known amount of gentamicin was added to normal mouse serum or to fetal calf serum (FCS) to a final concentration of 2 μg/ml and then the serum was diluted as described above. Each well was inoculated with 10 μl of a log-phase culture which contained approximately 103 CFU of L. monocytogenes. The plate was incubated overnight at 37°C, and bacterial growth in wells was determined by the presence of turbidity. The presence or absence of viable bacteria in nonturbid wells was determined by overnight culture of 10-μl samples on blood agar. The serum/plasma inhibitory titer was defined as the highest dilution in which no bacterial growth was detected by turbidity after overnight incubation. The serum/plasma bactericidal titer was defined as the highest titer that showed no bacterial growth on agar. The gentamicin MIC for the L. monocytogenes strain used in these experiments was 0.063 to 0.125 μg of gentamicin/ml, similar to the value reported by Blanot et al. for this strain (3).
Immunofluorescence microscopy of leukocyte-associated bacteria.
PBL were cytocentrifuged onto glass coverslips and then fixed with 2% paraformaldehyde. Discrimination between extracellular and intracellular bacteria was performed as previously described (12). Fixed cells were incubated with rabbit anti-L. monocytogenes antiserum (Difco) followed by Texas Red-conjugated goat anti-rabbit immunoglobulin G secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.). Next the cells were permeabilized with 0.2% Triton X-100 (Sigma) and then incubated with anti-L. monocytogenes antiserum followed by fluorescein isothiocyanate conjugated secondary antibody. As a result, extracellular bacteria fluoresce red and green whereas intracellular bacteria fluoresce green only. The coverslips were mounted on glass slides, and the numbers of red and green bacteria associated with 50 to 150 infected cells per coverslip were counted by fluorescence microscopy under oil immersion (magnification, ×1,000) with an Olympus BX-40 epifluorescence microscope. The numbers of intracellular bacteria per cell were calculated as total bacteria (green) per cell minus extracellular bacteria (red) per cell. In experiments in which the fluorescence of green fluorescent protein (GFP) was used as a marker, fixed cells were incubated only with anti-L. monocytogenes antiserum followed by Texas Red-conjugated secondary antibody. Thus, extracellular GFP− bacteria fluoresced red, extracellular GFP+ bacteria appeared yellow (red plus green), and intracellular GFP+ bacteria fluoresced green. Staining for F-actin-coated bacteria was performed using Bodipy 581/591 phalloidin or Alexa 568 phalloidin (Molecular Probes, Eugene, Oreg.) as previously described (12). Confocal images were collected on a Leica TCS NT confocal microscope using argon (488 nm) and krypton (568 nm) laser stimulation at the Flow Cytometry and Confocal Microscopy Laboratory, Warren Medical Research Institute, University of Oklahoma Health Sciences Center.
RESULTS
Inhibitory titers of gentamicin in vivo.
Preliminary experiments showed that serum inhibitory titers of 1:8 to 1:16, and bactericidal titers of 1:4 to 1:8 were achieved 24 h after implantation of osmotic pumps containing gentamicin. Interestingly, concentrations of gentamicin of <0.063 to 0.125 μg/ml, which did not inhibit bacterial growth in broth medium, did inhibit growth when ≥25% (by volume) normal mouse serum or fetal calf serum was added to the broth. Whether this was due to interactions between gentamicin and serum proteins such as lysozyme or β-lysin is not known (1). However, it does suggest that the inhibitory activity of gentamicin may be greater in vivo than it is in vitro. The inhibitory titers from infected mice ranged from 1:16 to 1:256. Higher titers may have resulted from decreased renal clearance of gentamicin due to dehydration and/or drug-induced nephrotoxicity.
Gentamicin slows experimental infection but does not prevent brain invasion.
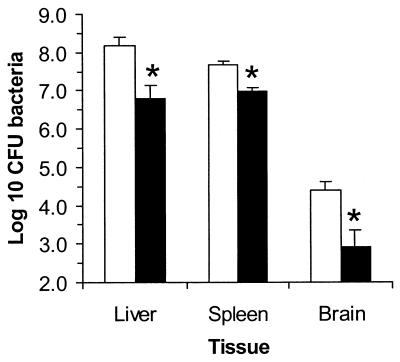
Gentamicin-treated mice had significantly lower bacterial loads in the liver, spleen, and brain than did control (untreated) mice 72 h after infection with 3 to 5 50% lethal doses of L. monocytogenes (Fig. 1). Because brain infection is a relatively late event during L. monocytogenes infection of mice (2), organs were not harvested from subsequent groups of animals until they showed signs of advanced illness. Using this approach, control mice were euthanized 3 to 4 days postinfection whereas gentamicin-treated mice were euthanized 4 to 6 days postinfection. The bacterial load in the bloodstream remained significantly lower in the antibiotic-treated group, 4.96 ± 0.32 log10 CFU/ml (mean ± standard error of the mean [SEM]), than in untreated animals, 6.21 ± 0.13 log10 CFU/ml (P < 0.01). In contrast, the bacterial load in the brains of treated mice was similar to that in the brains of untreated mice, 5.47 ± 0.65 and 5.18 ± 0.68 log10 CFU/brain, respectively (n = 6). These data show that continuous infusion of gentamicin produced levels in the blood that were sufficient to kill extracellular bacteria and slowed the progression of the infection in vivo but that bacteria still infected the brain.
FIG. 1.
Gentamicin slows the progression of experimental L. monocytogenes infection. Mice were (solid bars) or were not (open bars) treated with gentamicin, and then were infected with 3 to 5 50% lethal doses of L. monocytogenes. The animals were euthanized 3 days after infection, and the bacteria were quantified by serial dilution and plating. Results shown are the mean log10 CFU of bacteria per organ ± SEM from nine mice in each group. Statistically significant differences by Student's t test (P < 0.01) between control and gentamicin-treated groups are indicated by asterisks.
Next we tested whether CNS infection also followed oral infection. For this, mice were immunosuppressed by daily injections of 2 mg each of hydrocortisone plus cyclosporin A, beginning 18 h prior to gastric lavage with 109 CFU of L. monocytogenes. Similar to control animals, gentamicin-treated animals developed fatal illness with large numbers of bacteria in their livers and spleens (data not shown), as well as bacteremia (5.38 ± 0.26 log10 CFU of bacteria/ml of whole blood) and brain infection (5.49 ± 0.46 log10 CFU of bacteria/brain) (n = 4).
Killing of cell-free bacteria and presence of intracellular bacteria in the bloodstream of gentamicin-treated mice.
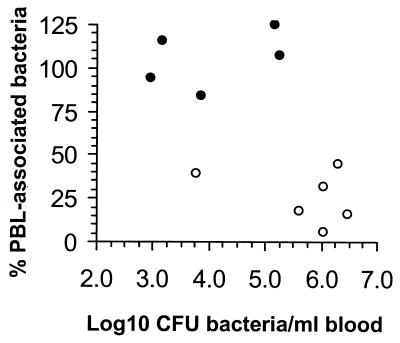
To confirm that gentamicin eliminated viable cell-free bacteria from the bloodstream, the percentage of bacteria in whole blood that were cell associated was determined in treated and untreated mice. Gentamicin-treated mice had lower total bacterial counts in whole blood than did control mice (Fig. 2). In control mice, 25.8% ± 6.1% (mean ± SEM) of the bacteria in whole blood were recovered in association with PBL. By comparison, 105% ± 7.4% of the bacteria recovered from gentamicin-treated mice were in the PBL fraction (P < 0.001). Residual gentamicin in the blood could have contributed to the apparent isolation of more CFU bacteria in the cell fraction than in whole blood by inhibiting bacterial growth on the agar plates despite an initial 100-fold dilution prior to plating. Other experiments showed that there was no change in the MIC of gentamicin for brain isolates from nine antibiotic-treated animals compared with the value for the same number of control animals, indicating that CNS infection in treated animals was not due to the emergence of gentamicin-resistant bacteria (data not shown).
FIG. 2.
Gentamicin eliminates cell-free L. monocytogenes from the bloodstream. Gentamicin-treated (●) and untreated (○) mice were infected by i.p. injection of L. monocytogenes-infected peritoneal cells. The CFU of bacteria in whole blood and in isolated blood leukocytes adjusted to the original volume of whole blood were quantified. The percentage of bacteria from whole blood that were associated with the leukocyte fraction are shown as a function of the CFU of bacteria per milliliter of whole blood from individual animals.
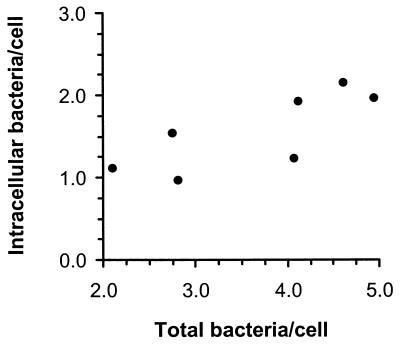
To confirm the presence of intracellular bacteria at the single-cell level, the intracellular and extracellular bacteria associated with PBL were quantified by fluorescence microscopy. Infected cells had 3.6 ± 0.4 bacteria/cell (mean ± SEM, n = 7), 43.5% ± 3.6% of which were intracellular, and most cells had a combination of intra- and extracellularly bound bacteria. The number of intracellular bacteria per infected cell increased significantly as the total number of cell-associated bacteria increased (P < 0.05 by Spearman nonparametric correlation) (Fig. 3). In addition, immunofluorescence with a fluorochrome-labeled phalloidin showed that bacteria were associated with F-actin comet tails in a small number of cells, indicating active intracellular parasitism. Taken together, these data suggest that CNS infection in treated animals was established by viable bacteria harbored within infected leukocytes.
FIG. 3.
Infected blood leukocytes are associated with intracellular and extracellular bacteria. Blood leukocytes were isolated from L. monocytogenes-infected mice and cytocentrifuged onto coverslips. Intracellular and extracellular bacteria were differentially stained and numbers of each were determined by fluorescence microscopy. Results shown are the mean number of intracellular bacteria per cell and total bacteria per cell from seven mice.
Use of a L. monocytogenes strain containing an actA-gfp-plcB fusion to study intracellular parasitism of circulating phagocytes in vivo.
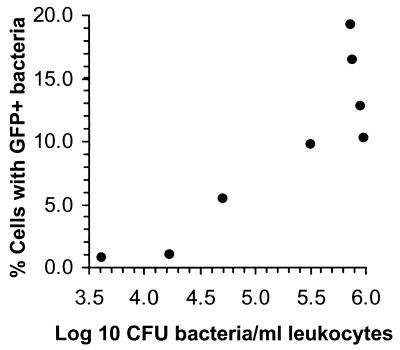
To study the role of intracellular parasitism of blood leukocytes further, we used an L. monocytogenes strain, NF-L512, that expresses gfpuv under the control of the actA promoter so that fluorescence is detected after bacteria have escaped from phagosomes (13). Preliminary studies showed that this strain was comparably virulent to the parent strain, 10403s (data not shown). GFP+ bacteria associated with PBL were easily identified by fluorescence microscopy, and the percentage of PBL harboring GFP+ bacteria increased with increasing numbers of bacteria in the leukocyte fraction (P < 0.05 by Spearman nonparametric correlation) (Fig. 4). GFP+ bacteria were also found associated with F-actin in some cells, demonstrating active intracellular parasitism (Fig. 5). Discrimination between intracellular and extracellular cell-associated bacteria irrespective of GFP fluorescence showed that 38.1% ± 4.5% of total bacteria (GFP+ and GFP−) were intracellular. By comparison, 59.6% ± 4.7% of GFP+ bacteria were intracellular, whereas GFP+ bacteria comprised only 18.3% ± 4.1% of total bacteria that were bound extracellularly to the leukocytes (Table 1). Thus, the intracellular environment was enriched 1.6-fold for GFP expression whereas the extracellular environment was relatively depleted of them.
FIG. 4.
Association of GFP+ L. monocytogenes with circulating leukocytes. Blood leukocytes were isolated from gentamicin-treated mice infected with NF-L512, and the CFU of bacteria associated with them was determined. Leukocytes were cytocentrifuged onto coverslips, the number of leukocytes that were and were not associated with GFP+ bacteria was determined by fluorescence microscopy, and the percentage of leukocytes associated with GFP+ bacteria was calculated. Each point represents a single animal.
FIG. 5.
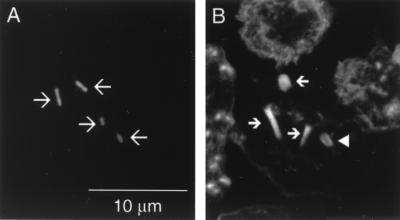
Intracellular parasitism of blood leukocytes. Leukocytes from NF-L512-infected mice were cytocentrifuged onto coverslips and stained with Alexa 568 phalloidin to reveal F-actin. Data were collected on a Leica TCS NT confocal microscope and are shown as gray-scale representations of GFP (A) and F-actin (B) of the same image. GFP+ bacteria (large arrows) are clearly associated with polarized F-actin tails (small arrows) of various lengths, or a surrounding F-actin cloud (arrowhead).
TABLE 1.
Gentamicin does not inhibit intracellular parasitism of circulating phagocytesa
| Treatment | No. of bacteria/cellb
|
% of extracellular GFP+ bacteriabc | % of GFP+ bacteria that are intracellularbd | ||
|---|---|---|---|---|---|
| Extracellular GFP− | Extracellular GFP+ | Intracellular GFP+ | |||
| None (control) | 3.33 ± 0.41 | 0.87 ± 0.27 | 1.08 ± 0.23 | 18.3 ± 4.1 | 59.6 ± 4.5 |
| Gentamicin | 2.88 ± 0.37 | 0.18 ± 0.05 | 1.20 ± 0.35 | 6.7 ± 1.0 | 85.8 ± 2.3 |
| P | 0.82 | 0.005 | 0.95 | 0.001 | <0.001 |
Mice without (control) and with gentamicin-containing osmotic pumps were infected with L. monocytogenes NF-L512. Leukocytes were harvested 3 to 5 days postinfection, and the intracellular and extracellular bacteria were quantified by fluorescence microscopy.
Results presented are the mean ± SEM for five or six animals in each group. P values are for control versus gentamicin-treated groups (Student's two-tailed t-test).
Calculated as (extracellular GFP+ bacteria/total extracellular bacteria) × 100.
Calculated as (intracellular GFP+ bacteria/total extracellular bacteria) × 100.
When NF-L512-infected mice were also treated with gentamicin, there was no significant change in the percentage of total cell-associated bacteria that were intracellular (data not shown), nor was the distribution of GFP+ bacteria among infected cells altered (Fig. 6). Importantly, gentamicin did not decrease the mean number of intracellular GFP+ bacteria per cell, suggesting that intracellular parasitism was not affected by antibiotic treatment (Table 1). In untreated mice the numbers of extracellular and intracellular GFP+ bacteria per cell increased in parallel with a heavier bacterial load. By comparison, extracellular GFP+ bacteria remained at a low constant level in gentamicin-treated mice despite the presence of increasing numbers of intracellular GFP+ bacteria (Fig. 7). This resulted in a fivefold reduction in mean extracellularly bound GFP+ bacteria compared with controls, and as a consequence there was significant increase in the percentage of intracellular GFP+ bacteria (Table 1). However, fluorescence microscopy could not distinguish intracellular GFP+ bacteria that were cytosolic (parasitic) from phagocytosed GFP+ bacteria that were phagosomal and being killed. Thus, it is possible that the numbers of cytosolic bacteria were increased in the presence of gentamicin compared with its absence, due to a relative shift in the compartments in which intracellular bacteria actually reside. Nevertheless, these data confirm productive intracellular parasitism of circulating phagocytes and suggest that they were infected by intercellular spread of bacteria, a process not affected by gentamicin.
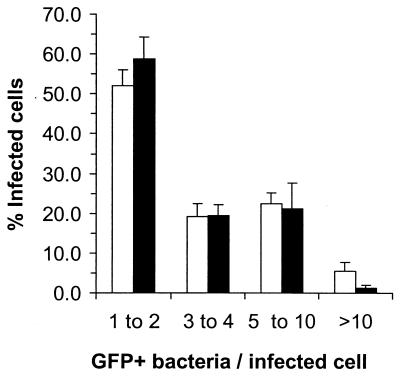
FIG. 6.
The distribution of GFP+ bacteria among infected leukocytes is not altered by treatment with gentamicin. Gentamicin-treated (solid bars) and untreated (open bars) mice were infected with NF-L512. Leukocytes were isolated, and the numbers of GFP+ bacteria associated with them were determined by fluorescence microscopy. Data shown are the mean percentages of cells associated with the indicated numbers of bacteria from six (gentamicin-treated) or seven (control) animals. Error bars indicate SEM.
FIG. 7.
The association of blood leukocytes with extracellular GFP+ bacteria, but not intracellular GFP+ bacteria, is inhibited by treatment with gentamicin. Leukocytes were isolated from gentamicin-treated (●) and control (○) mice infected with L. monocytogenes strain NF-L512. Extracellular bacteria were labeled with anti-Listeria antiserum, and the numbers of intracellular and extracellular GFP+ bacteria associated with leukocytes were determined by fluorescence microscopy. Data shown are the mean number extracellular GFP+ bacteria per cell and the mean intracellular GFP+ bacteria per cell. Each point represents a single animal.
A final set of experiments used an L. monocytogenes strain with gfpuv on an isopropyl-β-d-thiogalactopyranos (IPTG)-inducible plasmid to test the stability of GFP fluorescence when GFP+ bacteria were killed by gentamicin and when fluorescent bacteria were grown in medium in the absence of an inducing signal. These showed that fluorescence was detectable up to 4 h after exposure to 50 μg of gentamicin per ml and after removal of IPTG (data not shown). This result suggests that GFP+ bacteria which are released into the extracellular milieu can replicate and produce fluorescent daughter cells for at least two generations. By comparison, newly released GFP+ bacteria are killed in gentamicin-treated animals. They remain fluorescent but do not increase numerically. The different outcomes for bacteria released into the extracellular compartment are most probably responsible for the smaller numbers of extracellularly bound GFP+ bacteria in gentamicin-treated mice than in untreated mice.
DISCUSSION
The ability of extracellular bacteria to invade the CNS from the bloodstream has been made clear by in vitro and in vivo models of infection (37). However, the role of intracellular bacteria in neuroinvasion has not yet been established. The goal of the experiments presented here was to test whether L. monocytogenes-infected phagocytes could be primarily responsible for infecting the CNS. To do this, extracellular bacteria were killed by a continuous infusion of gentamicin during experimental infection of mice. Gentamicin is rapidly bactericidal against extracellular L. monocytogenes, but it crosses cell membranes relatively poorly and does not achieve a bactericidal concentration in the cytoplasm, where L. monocytogenes is located during the majority of its intracellular life cycle (19, 32). Thus gentamicin can be used to kill extracellular L. monocytogenes while allowing intracellular replication and cell-to-cell spread to continue unabated (see, for example, references 12, 15, 18, and 31).
Continuous infusion of gentamicin produced concentrations in serum at least 10-fold greater than the MIC for the strain of L. monocytogenes used for infection and effectively killed extracellular bacteria in the bloodstream. This was documented by finding that essentially all viable bacteria recovered from the blood of antibiotic-treated mice were leukocyte associated compared with approximately 25% in untreated animals. Fluorescence microscopy showed that 38 to 44% of cell-associated bacteria were actually intracellular and thus protected from gentamicin. The relatively high percentage of extracellularly bound bacteria was somewhat surprising, particularly given that the circulating phagocytes are probably activated by the inflammatory milieu. In the experiments reported here, it is most likely that extracellular bacteria are recently bound by the phagocyte but not yet internalized. Nevertheless, because bacteria bound extracellularly were also exposed to bactericidal concentrations of gentamicin, it is likely that most, if not all, of the viable leukocyte-associated bacteria from treated mice were intracellular.
In vivo studies using intermittent administration (every 12 h) of gentamicin in L. monocytogenes-infected rodents showed that this antibiotic did not significantly decrease the bacterial load in the spleen (18), the bloodstream (20), or the brain (3). By comparison, we found that a continuous infusion of gentamicin did significantly decrease bacterial loads in the liver, brain, and spleen compared with those in untreated mice killed at the same time. Nevertheless, treated mice did die of infection, but 1 to 3 days later than control mice did, by which time the bacterial loads in the liver and brain had increased and were similar to those of moribund, untreated mice euthanized earlier. These data suggest that extracellular L. monocytogenes accelerates the infective process over that of intracellular bacteria alone, through invasion of other cells locally and/or by dissemination to distant sites including the CNS. In addition to killing extracellular bacteria, it is possible that gentamicin increased the killing of phagocytosed bacteria by inflammatory leukocytes, as previously reported in an in vitro study (9). This, too, could contribute to the delayed kinetics of bacterial infection in treated animals.
The development of CNS infection in gentamicin-treated animals strongly suggests that it was initiated by intracellular bacteria harbored within circulating phagocytes. In vitro studies demonstrate two different mechanisms by which this could occur. The first is through adhesion of infected mononuclear phagocytes to brain endothelial cells, followed by spread of intracellular bacteria from phagocytes to the endothelium (12, 16). These bacteria presumably go on to invade deeper structures through continued cell-to-cell spread. The second mechanism involves the carriage of intracellular bacteria across the blood-brain or blood-cerebrospinal fluid barriers by transmigrating phagocytes. The results reported here are compatible with either mechanism. In addition, it is possible that bacteria bound extracellularly to leukocytes could be transported to the CNS and perhaps across the blood-brain or blood-cerebrospinal fluid barrier during leukocyte migration.
To test whether bacteria within PBL were engaged in a parasitic relationship with the cell, we used genetically engineered L. monocytogenes strains that contain the gfpuv gene controlled by the actA promoter. The actA gene is essential for virulence and is required for the F-actin-based motility of intracytoplasmic L. monocytogenes (6). Data obtained from experiments with cultured macrophages show that actA expression is upregulated approximately 200 to 500-fold during intracellular growth compared with extracellular growth in broth medium and that actA is not expressed when bacteria are contained in phagosomes (13, 26). Interestingly, we found that cell-associated GFP+ bacteria were positioned extracellularly as well as intracellularly. GFP expressing extracellular bacteria could represent an uncoupling of actA expression from the intracellular signals that trigger it. However, the finding that only 18.3% of all extracellular bacteria were GFP+ suggests that indiscriminate extracellular actA expression did not occur. Similarly, data showing that the intracellular environment was enriched for GFP+ bacteria are consistent with preferential intracellular expression. It is most likely that extracellular GFP+ bacteria had been released from the cytoplasm of parasitized cells into the extracellular milieu and then were bound by phagocytes.
More important is the question of how circulating phagocytes become associated with intracellular GFP+ bacteria, and two general pathways are likely. One is phagocytosis of extracellular bacteria. This includes GFP+ bacteria that continue to fluoresce after internalization, as well as GFP-negative bacteria that eventually may escape from phagosomes and then express GFP intracellularly. However, the finding that gentamicin killed most or all extracellular bacteria but did not decrease the numbers of intracellular GFP+ bacteria per cell suggests that phagocytosis of extracellular bacteria contributed little, if at all, to intracellular parasitism. This interpretation is reasonable, given that phagocytes from infected mice are activated and can kill phagocytosed L. monocytogenes, particularly when opsonized with complement, as would be the case for cell-free bacteria (11). However because of technical limitations in determining the precise intracellular compartment of GFP+ bacteria, it is possible that phagocytosis does lead to intracellular parasitism in untreated animals. Nevertheless, experiments with gentamicin-treated mice showed that was not the only pathway which could give rise to parasitized phagocytes. The other means by which phagocytes become associated with intracellular GFP+ bacteria involves intercellular spread of GFP+ bacteria that then continue in a parasitic life cycle. This pathway is gentamicin insensitive and, given the essential role of F-actin based motility in L. monocytogenes pathogenesis in vivo, most probably operates in the absence of gentamicin as well (6, 32). Because intercellular spread of bacteria is unlikely to have happened in the circulation, it is most likely that the phagocytes became infected in the parenchyma of organs such as the liver and spleen and perhaps also the bone marrow.
These data suggest a model of trafficking of bacteria within phagocytes that begins with recruitment of inflammatory cells to foci of infection (10). There, they phagocytose and kill extracellular bacteria but are themselves infected by cell-to-cell spread of bacteria from parenchymal cells. Infected phagocytes reenter the bloodstream, perhaps through the mechanism of reverse migration (34), and then transport intracellular bacteria to the CNS. Central infection can be initiated by cell-to-cell spread of bacteria from infected leukocytes to the endothelium or by migration of infected leukocytes to the CNS.
ACKNOWLEDGMENTS
We are grateful to C. Gentry for helpful discussions about pharmacokinetics, to R. Greenfield for careful review of the manuscript, and to J. Henthorn of the Warren Medical Research Institute for assistance with confocal microscopy.
This work was supported in part by NIH grants AI46651 to D.A.D. and AI41816 to N.E.F.
REFERENCES
- 1.Asensi V, Fierer J. Synergistic effect of human lysozyme plus ampicillin or beta-lysin on the killing of Listeria monocytogenes. J Infect Dis. 1991;163:574–578. doi: 10.1093/infdis/163.3.574. [DOI] [PubMed] [Google Scholar]
- 2.Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–336. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 3.Blanot S, Boumaila C, Berche P. Intracerebral activity of antibiotics against Listeria monocytogenes during experimental rhombencephalitis. J Antimicrob, Chemother. 1999;44:565–568. doi: 10.1093/jac/44.4.565. [DOI] [PubMed] [Google Scholar]
- 4.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denoya C D, Bechhofer D H, Dubnau D. Translational autoregulation of ermC 23S rRNA methyltransferase expression in Bacillus subtilis. J Bacteriol. 1986;168:1133–1141. doi: 10.1128/jb.168.3.1133-1141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wachter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dramsi S, Levi S, Triller A, Cossart P. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infect Immun. 1998;66:4461–4468. doi: 10.1128/iai.66.9.4461-4468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drevets D A. Dissemination of Listeria monocytogenes by infected phagocytes. Infect Immun. 1999;67:3512–3517. doi: 10.1128/iai.67.7.3512-3517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drevets D A, Canono B P, Leenen P J, Campbell P A. Gentamicin kills intracellular Listeria monocytogenes. Infect Immun. 1994;62:2222–2228. doi: 10.1128/iai.62.6.2222-2228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drevets D A, Leenen P J. Leukocyte-facilitated entry of intracellular pathogens into the central nervous system. Microbes Infect. 2000;2:1609–1618. doi: 10.1016/s1286-4579(00)01317-4. [DOI] [PubMed] [Google Scholar]
- 11.Drevets D A, Leenen P J, Campbell P A. Complement receptor type 3 (CD11b/CD18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J Immunol. 1993;151:5431–5439. [PubMed] [Google Scholar]
- 12.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freitag N E, Jacobs K E. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aequorea victoria. Infect Immun. 1999;67:1844–1852. doi: 10.1128/iai.67.4.1844-1852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freitag N E, Portnoy D A. Dual promoters of the Listeria monocytogenes prfA transcriptional activator appear essential in vitro but are redundant in vivo. Mol Microbiol. 1994;12:845–853. doi: 10.1111/j.1365-2958.1994.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 15.Gregory S H, Sagnimeni A J, Wing E J. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J Immunol. 1996;157:2514–2520. [PubMed] [Google Scholar]
- 16.Greiffenberg L, Goebel W, Kim K S, Weiglein I, Bubert A, Engelbrecht F, Stins M, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greiffenberg L, Sokolovic Z, Schnittler H J, Spory A, Bockmann R, Goebel W, Kuhn M. Listeria monocytogenes-infected human umbilical vein endothelial cells: internalin-independent invasion, intracellular growth, movement, and host cell responses. FEMS Microbiol Lett. 1997;157:163–170. doi: 10.1111/j.1574-6968.1997.tb12768.x. [DOI] [PubMed] [Google Scholar]
- 18.Hof H, Guckel H. Lack of synergism of ampicillin and gentamicin in experimental listeriosis. Infection. 1987;15:40–41. doi: 10.1007/BF01646117. [DOI] [PubMed] [Google Scholar]
- 19.Hof H, Nichterlein T, Kretschmar M. Management of listeriosis. Clin Microbiol Rev. 1997;10:345–357. doi: 10.1128/cmr.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K S. In vitro and in vivo studies of imipenem-cilastatin alone and in combination with gentamicin against Listeria monocytogenes. Antimicrob Agents Chemother. 1986;29:289–293. doi: 10.1128/aac.29.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorber B. Listeriosis. Clin Infect Dis. 1997;24:1–9. doi: 10.1093/clinids/24.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Low J C, Donachie W. A review of Listeria monocytogenes and listeriosis. Vet J. 1997;153:9–29. doi: 10.1016/s1090-0233(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 23.Marshall J, Molloy R, Moss G W, Howe J R, Hughes T E. The jellyfish green fluorescent protein: a new tool for studying ion channel expression and function. Neuron. 1995;14:211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 24.McLauchlin J. Human listeriosis in Britain, 1967–85, a summary of 722 cases. 2. Listeriosis in non-pregnant individuals, a changing pattern of infection and seasonal incidence. Epidemiol Infect. 1990;104:191–201. doi: 10.1017/s0950268800059355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mead P S, Slutsker L, Dietz V, McCaig L F, Bresee J S, Shapiro C, Griffin P M, Tauxe R V. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moors M A, Levitt B, Youngman P, Portnoy D A. Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect Immun. 1999;67:131–139. doi: 10.1128/iai.67.1.131-139.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mylonakis E, Hohmann E L, Calderwood S B. Central nervous system infection with Listeria monocytogenes. 33 years' experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 1998;77:313–336. doi: 10.1097/00005792-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 28.North R J. The action of cortisone acetate on cell-mediated immunity to infection. Suppression of host cell proliferation and alteration of cellular composition of infective foci. J Exp Med. 1971;134:1485–1500. doi: 10.1084/jem.134.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parida S K, Domann E, Rohde M, Muller S, Darji A, Hain T, Wehland J, Chakraborty T. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol Microbiol. 1998;28:81–93. doi: 10.1046/j.1365-2958.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 30.Park S F, Stewart G S. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene. 1990;94:129–132. doi: 10.1016/0378-1119(90)90479-b. [DOI] [PubMed] [Google Scholar]
- 31.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Portnoy D A, Jones S. The cell biology of Listeria monocytogenes infection (escape from a vacuole) Ann NY Acad Sci. 1994;730:15–25. doi: 10.1111/j.1749-6632.1994.tb44235.x. [DOI] [PubMed] [Google Scholar]
- 33.Prats N, Briones V, Blanco M M, Altimira J, Ramos J A, Dominguez L, Marco A. Choroiditis and meningitis in experimental murine infection with Listeria monocytogenes. Eur J Clin Microbiol Infect Dis. 1992;11:744–747. doi: 10.1007/BF01989983. [DOI] [PubMed] [Google Scholar]
- 34.Randolph G J, Beaulieu S, Lebecque S, Steinman R M, Muller W A. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 35.Strauss R, Heymer B, Hof H. Effects of cyclosporin A on experimental infection with Listeria monocytogenes. Clin Exp Immunol. 1985;62:491–498. [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson S L, Drevets D A. Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. J Infect Dis. 1998;178:1658–1666. doi: 10.1086/314490. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J R, Tuomanen E. Molecular and cellular mechanisms for microbial entry into the CNS. J Neurovirol. 1999;5:591–603. doi: 10.3109/13550289909021288. [DOI] [PubMed] [Google Scholar]