Highlights
-
•
A unidimensional factor model best captured drug use and use disorder.
-
•
Prescription opioid and heroin use were neither separable nor distinctly associated.
-
•
Prescription opioid misuse was most strongly correlated with sedative misuse.
-
•
Heroin use was most strongly correlated with cocaine/crack use.
-
•
Nonopioid drug use should be included in prescription-to-heroin trajectory research.
Keywords: Prescription opioids, Heroin, Drug use, Substance use disorder, NESARC, Structural equation modeling
Abstract
Background
Prescription opioid misuse (POM) is often implicated in heroin initiation, despite evidence that POM does not predict heroin initiation any better than other drug use. Additionally, prescription misuse and illicit use behaviors tend to respectively “cluster” together. This study aimed to test a series of theory-driven factor models to explore how POM and heroin use are situated within the broader constellation of drug use that typically occurs alongside opioid (mis)use.
Methods
36,309 individuals from NESARC-III (56.31% female; mean age=45.63 [SD=17.53]) reported their lifetime (mis)use of prescription opioids, prescription stimulants, prescription sedatives, heroin, cannabis, cocaine/crack, illicit stimulants (e.g., methamphetamine), club drugs, hallucinogens, and inhalants, and were administered a DSM-5 substance use disorder (SUD) assessment. Bifactor, correlated factors, and one-factor confirmatory factor models were fit using all drug use/SUD variables and subsequently compared.
Results
POM was most strongly correlated with prescription sedative misuse; heroin use was most strongly correlated with cocaine/crack use. All factor models fit the data well. Highly correlated factors and patterns of factor loadings suggested that POM and heroin use were most parsimoniously captured within a general factor alongside all other forms of drug use. This was also the case for SUD. Additional analyses testing an alternate factor structure provided further support for unidimensionality.
Conclusions
POM and heroin use, as well as prescription- and heroin-based SUDs, were neither separable nor distinctly associated. Future research should account for other drug use more comprehensively rather than isolating POM as a primary risk factor in heroin use and use disorder.
1. Introduction
Opioid use, misuse, and overdose represent a significant health burden across the globe (Krausz et al., 2021). This is particularly pronounced in the United States (US), where the opioid crisis remains a major public health concern (Scholl et al., 2019; Wilson, 2020). Approximately 9.5 million people in the US aged 12 or older reported prescription opioid misuse (POM; i.e., use without a prescription, not as prescribed, or for a reason not medically indicated) and/or heroin use in the past year (SAMHSA, 2021). POM has been implicated in the initiation and perpetuation of the US opioid crisis, and the notion that POM leads to heroin use has become deeply engrained in the cultural perception of the opioid crisis (Volkow, 2014).
Missing in many examinations of the relationship between POM and heroin use is sufficient acknowledgement of the lack of specificity in the POM-heroin association. In almost no case is POM the only substance, or prescription drug, (mis)used prior to heroin initiation (Muhuri et al., 2013), yet POM is frequently, and often compellingly, implicated in narratives of heroin initiation (Compton et al., 2016; Mars et al., 2014; McCabe et al., 2021; Siegal et al., 2003). Though such findings tend to be conspicuously de-emphasized in studies aiming to explicate the POM-to-heroin trajectory, there is ample evidence that prior non-opioid drug use is also robustly associated with heroin use, including among individuals reporting POM (Thomas et al., 2022). For example, a prospective study of high school students found that the association between heroin initiation and prior POM was no stronger than the association between heroin initiation and prior use of other drugs (Kelley-Quon et al., 2019). Similarly, a prospective study of young adults reporting recent POM found that cocaine, LSD, sedative, MDMA/ecstasy, and stimulant use each predicted heroin initiation comparably to or more robustly than prescription-based opioid dependence. Further, POM to self-medicate a health condition was negatively related to heroin initiation (Carlson et al., 2016). Such findings suggest that POM may not add any predictive value above and beyond use of other drugs when predicting heroin use, and, as a result, that the nature of the relationship between POM and heroin use cannot be accurately captured without explicitly addressing other drug use behaviors.
In addition to the apparent lack of discriminant predictive value of POM in the context of heroin initiation, evidence suggests that POM tends to “cluster” more closely with other prescription misuse behaviors, while heroin use tends to “cluster” more closely with use of other illicit drugs. For example, rates of lifetime prescription sedative and tranquilizer misuse are significantly higher among those reporting POM without heroin use (43% and 45%) or both POM and heroin use (88% and 88%) as compared to those reporting heroin use without POM (16% and 14%) (Wu et al., 2011). Conversely, individuals reporting POM without heroin use report lower rates of lifetime cannabis (78%), cocaine (43%), inhalant (18%), and hallucinogen use (46%) than those reporting heroin use without POM (97%, 70%, 24%, and 68%, respectively) or both POM and heroin use (100%, 91%, 46%, and 97%, respectively) (Wu et al., 2011). Rates of past year cannabis and other non-heroin illicit drug use are also significantly lower among individuals reporting POM without heroin use (50% and 25%, respectively) than those reporting heroin use without POM (63% and 62%, respectively) (Rigg and Monnat, 2015). Findings from a latent class analysis of drug use mirror these patterns, identifying classes of 1) prescription misuse, characterized by POM, sedative misuse, and prescription stimulant misuse, with lower rates of other illicit drug use, and 2) illicit drug use, characterized by use of drugs such as illicit opioids (e.g., heroin), cocaine, hallucinogens, and inhalants, with lower rates of prescription misuse (Dash et al., 2021). These “clustering” patterns suggest that conceptualizing POM and heroin as elements subsumed by broader prescription misuse and illicit use factors, respectively, alongside other forms of drug use may be a valid approach to understanding the presentation of these behaviors (Dash et al., press; Kendler et al., 2007).
1.1. Present study
The findings described above underscore the importance of examining POM and heroin use within the greater context of other drug use that typically occurs among individuals engaged in opioid (mis)use (Pandika et al., 2022). Patterns observed in the extant literature suggest two possible ways that the association between POM and heroin use may be contextualized within the broader scope of drug use behaviors. First, studies showing a lack of differentiation in heroin use's association with POM versus use of other drugs potentially suggest a unidimensional structure of drug use behavior, such that a general liability undergirds the spectrum of drug use behavior across drug types. Second, studies showing a differentiable “clustering” of prescription misuse and illicit drug use potentially suggest a two-factor structure, such that prescription misuse and illicit drug use form distinct dimensions but remain meaningfully associated through some common mechanism. The present study aimed to critically evaluate the POM-heroin association by testing these dimensional conceptualizations of drug use via a series of confirmatory factor models. We anticipated that drug use would be best captured by a bifactor model reflecting 1) a prescription misuse factor influencing prescription opioid, stimulant, and sedative misuse, 2) an illicit drug use factor influencing heroin, cannabis, cocaine/crack, illicit stimulant, club drug, hallucinogen, and inhalant use, and 3) a general liability factor that accounts for the association between the prescription misuse and illicit use factors.
2. Material and methods
2.1. Participants and procedure
Data were drawn from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)-III. NESARC-III is a cross-sectional epidemiologic survey based on a representative sample of the civilian, non-institutionalized population age 18 and over. Between April 2012 and June 2013, 36,309 subjects participated in face-to-face interviews (56.31% female; 52.86% White, 21.39% Black, 1.41% American Indian/Alaska Native, 4.96% Asian, 19.38% Hispanic [any race]; mean age=45.63 [SD=17.53], range=18–90). NESARC-III procedures have been detailed extensively elsewhere (Grant et al., 2014).
2.2. Measures
Participants were interviewed using the Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5) (Grant et al., 2015). All participants were instructed to report lifetime drug use with the prompt: “Now I'd like to ask you about your experiences with medicines and other kinds of drugs that you may have used on your own- that is, without a doctor's prescription; in greater amounts, more often, or longer than prescribed, or for a reason other than a doctor said you should use them. People use these medicines and drugs on their own to feel more alert, to relax or quiet their nerves, to feel better, to enjoy themselves, to get high, or just to see how they work.” Substances queried included prescription opioids, prescription stimulants, prescription sedatives, heroin, cannabis, cocaine/crack, illicit stimulants, club drugs, hallucinogens, and inhalants. Examples of drug types (e.g., “painkillers, for example…methadone, codeine, Demerol, Vicodin, OxyContin, Percocet, Percodan, morphine”) and common slang terms (e.g., “heroin, for example…smack, black tar, poppy”) were included in the query for each drug. Participants were also administered a DSM-5 diagnostic assessment for lifetime use disorder of each drug that they endorsed using in their lifetime. Use disorders assessed included prescription opioid, prescription sedative, heroin, cannabis, cocaine/crack, any stimulant, club drug, hallucinogen, and inhalant. Participants who did not endorse use were coded as having no lifetime use disorder.
2.3. Analytic plan
2.3.1. Primary models
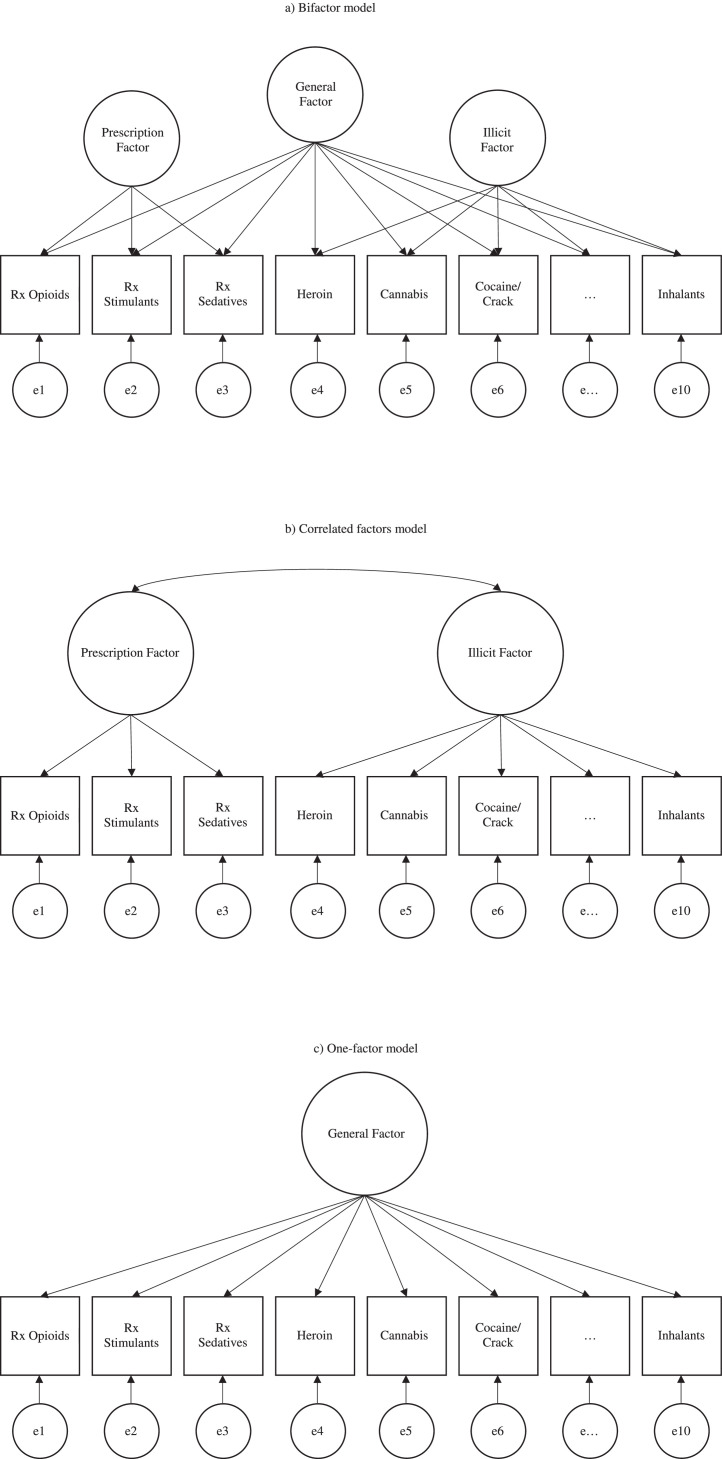
Analyses were conducted in Mplus version 8 (Muthén, 2017). Three confirmatory factor models were fit: 1) a bifactor model, 2) a correlated factors model, and 3) a one-factor model (see Fig. 1) (Caspi et al., 2014). Sex, age, race/ethnicity, and education level were included as covariates in all models; sampling weights, cluster, and stratification variables were included to account for the complex survey design of NESARC-III. The bifactor model tested the hypothesis that drug use behaviors reflect both general liability for drug use and more specific forms of drug use liability (i.e., prescription misuse and illicit use); that is, whether a common trait accounts for a substantive overlap in latent factors despite some degree of theoretically indicated orthogonal multidimensionality. In this model, general liability was represented by a factor that influenced all drug use variables and the specific factors reflected prescription misuse and illicit drug use. The prescription misuse factor included prescription opioid, stimulant, and sedative misuse; the illicit use factor included heroin, cannabis (recreational cannabis was illegal in nearly every state at the time of data collection), cocaine/crack, illicit stimulant, club drug, hallucinogen, and inhalant use. Each drug use variable loaded jointly onto the general liability factor and its associated specific factor. The correlated factors model was used to test the hypothesis that there are latent trait factors- in this case, a prescription misuse factor and an illicit use factor- each of which influences a subset of the drug use phenotypes and which may also be correlated. The one-factor model tested whether the specific factors are needed, or if drug use can be adequately represented as a unidimensional construct. This series of models was also fit to the drug use disorder variables.
Fig. 1.
Simplified depiction of three confirmatory factor models of drug (mis)use.
2.3.2. Alternate models
There is evidence that individuals may seek out particular types of drugs due to individual differences, such as personality, affect, and impulsivity (Dash et al., press; Mahu et al., 2019). Perhaps rather than conceptualizing drug (mis)use and use disorder as existing on prescription and illicit dimensions, these behaviors may be better modeled according to overlapping pharmacodynamic, physiologic, and subjective drug effects. To explore this alternate hypothesis, we ran an additional set of bifactor and correlated factors models on both drug use and use disorder phenotypes. These models tested three specific factors: “uppers” (stimulants, cocaine/crack), “downers” (prescription opioids, sedatives, heroin), and “all-arounders” (cannabis, club drugs, hallucinogens, inhalants) (Inaba and Cohen, 2014). This configuration permitted POM and heroin use to be modeled on the same specific factor, thereby providing an additional test of their relationship; that is, whether they capture a unique but overlapping liability that would indicate specificity in the POM-heroin association.
2.3.3. Supplemental models
In an effort to capture the full range of substances, we conducted a supplemental sequence of analyses that included alcohol and nicotine. Supplemental models mirrored the structure of the primary models (bifactor, correlated factors, and one-factor models for both substance use and substance use disorder), with alcohol and nicotine modeled on the “licit” factor. The purpose of this was to further test the viability of this dimensional model and to create more balanced factors as a means of mitigating overrepresentation of illicit drugs in the primary models.
2.3.4. Model evaluation
Determination of model fit was based on three fit indices (McDonald and Ho, 2002; Schreiber et al., 2006): the root mean-square error of approximation (RMSEA) (Steiger, 1990), the non-normed fit index (NNFI; i.e., Tucker-Lewis Index or TLI) (Tucker and Lewis, 1973) and the comparative fit index (CFI) (Bentler, 1990). Assessment of model fit was based on accepted cutoffs in the literature: RMSEA 〈0.05, TLI 〉 0.95, and CFI >0.95 (Chen et al., 2008; Hooper et al., 2008; Hu and Bentler, 1999; Yu, 2002). The chi square (χ2) statistic is reported per convention, but models were not rejected on the basis of a significant chi square due to its sensitivity to sample size. Chi-square difference tests for weighted least squares estimation were implemented via the difftest option in Mplus for the purpose of conducting formal model comparison, but were not relied upon to select the best-fitting model due to sensitivity to sample size, demonstrated bias toward bifactor models, and potential lack of power to detect model misspecification (Shi et al., 2018). In light of recommendations to avoid sole reliance on global fit indices to interpret bifactor models, we also evaluated patterns of factor loadings, wherein strong general and specific factor loadings in addition to good global fit would provide support for a bifactor structure (Bornovalova et al., 2020; Waldman et al., 2022).
3. Results
Prevalence rates of use and use disorder for each substance are presented in Table 1. The weighted estimate of any lifetime use drug was 36.57%. The weighted average number of drugs used was 0.85 (SE=0.02) in the full sample and 2.37 (SE=0.02) among respondents who endorsed any lifetime drug use. Weighted estimates for lifetime opioid (mis)use were 11.31% for POM and 1.61% for heroin use; weighted estimates for lifetime opioid use disorder (OUD) were 2.05% for prescription-based disorder and 0.48% for heroin-based disorder. Prevalence rates for number of drugs used in the lifetime and number of lifetime use disorders are available in Supplemental Table S1. Tetrachoric correlations between study variables are presented in Table 2. The correlation between POM and heroin use was robust (r = 0.70), but the magnitude of this correlation was similar to those of POM and heroin use with each of the other substance use variables. For POM, correlations with other substance use variables ranged from 0.59–0.85, with prescription sedative misuse being the strongest and cannabis use the weakest. For heroin use, correlations with other substance use variables ranged from 0.55–0.78, with cocaine/crack use being the strongest and illicit stimulant use the weakest. Similar results were obtained for the use disorder variables. The correlation between prescription- and heroin-based OUD was unsurprisingly robust (0.72), but this association was, again, not uniquely strong. For prescription-based OUD, correlations ranged from 0.53–0.81, with sedative use disorder being the strongest and cannabis use disorder the weakest. For heroin-based OUD, correlations ranged from 0.46–0.72, with prescription-based OUD being the strongest and cannabis use disorder the weakest.
Table 1.
Unweighted (weighted) percentage prevalence of substance use and substance use disorder in the NESARC-III sample.
| Total | Prevalence By Sex |
Prevalence By Race/Ethnicity |
||||||
|---|---|---|---|---|---|---|---|---|
| Drug Use Variable | Full Sample (N = 36,309) |
Men (n = 15,862) |
Women (n = 20,447) |
White (n = 19,194) |
Black (n = 7766) |
AI/IN (n = 511) |
Asian (n = 1801) |
Hispanic (n = 7037) |
| Lifetime (Mis)Use | ||||||||
| Prescription opioids | 11.28 (11.31) | 13.08 (12.98) | 9.89 (9.76) | 13.59 (12.80) | 9.50 (9.86) | 14.68 (14.07) | 5.29 (4.53) | 8.24 (8.10) |
| Prescription stimulants | 2.86 (3.22) | 3.71 (3.99) | 2.19 (2.51) | 4.31 (4.14) | 0.76 (0.91) | 3.35 (3.80) | 1.83 (1.68) | 1.42 (1.49) |
| Sedatives | 7.20 (7.49) | 8.20 (8.46) | 6.42 (6.59) | 9.62 (9.05) | 4.11 (4.34) | 9.80 (9.59) | 2.78 (2.64) | 4.94 (4.63) |
| Heroin | 1.65 (1.61) | 2.56 (2.42) | 0.93 (0.86) | 2.00 (1.90) | 1.50 (1.24) | 2.94 (2.18) | 0.56 (0.47) | 1.04 (0.99) |
| Cannabis | 31.10 (32.16) | 38.05 (38.07) | 25.72 (26.69) | 35.68 (35.71) | 30.12 (30.95) | 45.29 (45.12) | 14.48 (13.84) | 22.92 (22.92) |
| Cocaine/crack | 9.77 (9.96) | 13.15 (12.66) | 7.15 (7.46) | 11.86 (11.47) | 7.14 (6.98) | 15.13 (14.36) | 4.01 (3.70) | 8.07 (7.54) |
| Illicit stimulants | 3.63 (4.09) | 4.74 (5.11) | 2.76 (3.09) | 5.44 (5.24) | 0.76 (0.83) | 6.71 (6.96) | 2.11 (1.83) | 2.01 (1.93) |
| Club drugs | 4.39 (4.43) | 5.52 (5.20) | 3.51 (3.71) | 5.41 (5.02) | 2.60 (2.56) | 5.69 (4.95) | 3.28 (3.16) | 3.77 (3.68) |
| Hallucinogens | 8.49 (9.32) | 12.05 (12.60) | 5.73 (6.29) | 12.13 (11.59) | 2.91 (3.44) | 14.71 (14.80) | 3.67 (3.48) | 5.49 (5.55) |
| Inhalants | 2.82 (3.11) | 4.32 (4.53) | 1.66 (1.79) | 3.99 (3.85) | 0.88 (1.12) | 4.51 (4.98) | 1.61 (1.55) | 1.98 (1.79) |
| Lifetime Use Disorder | ||||||||
| Prescription opioids | 1.89 (2.05) | 2.08 (2.19) | 1.75 (1.93) | 2.50 (2.42) | 1.31 (1.55) | 3.52 (3.67) | 0.44 (0.42) | 1.14 (1.26) |
| Sedatives | 0.99 (1.06) | 1.04 (1.07) | 0.95 (1.06) | 1.42 (1.36) | 0.52 (0.44) | 1.17 (1.00) | 0.22 (0.24) | 0.54 (0.55) |
| Heroin | 0.44 (0.48) | 0.64 (0.71) | 0.27 (0.26) | 0.52 (0.57) | 0.33 (0.23) | 1.17 (0.95) | 0.17 (0.21) | 0.33 (0.31) |
| Cannabis | 6.17 (6.27) | 8.60 (8.37) | 4.29 (4.32) | 6.75 (6.65) | 6.81 (7.24) | 11.15 (11.53) | 3.22 (3.10) | 4.31 (4.45) |
| Cocaine/crack | 2.40 (2.40) | 3.15 (2.99) | 1.82 (1.85) | 2.74 (2.72) | 2.46 (2.27) | 4.11 (3.77) | 0.72 (0.61) | 1.71 (1.57) |
| Stimulants | 1.56 (1.71) | 1.76 (1.87) | 1.41 (1.57) | 2.33 (2.19) | 0.22 (0.30) | 4.31 (4.26) | 0.78 (0.76) | 0.95 (0.81) |
| Club drugs | 0.49 (0.48) | 0.66 (0.62) | 0.36 (0.36) | 0.59 (0.53) | 0.32 (0.38) | 0.98 (0.84) | 0.44 (0.48) | 0.37 (0.32) |
| Hallucinogens | 0.52 (0.60) | 0.78 (0.83) | 0.31 (0.38) | 0.77 (0.77) | 0.10 (0.14) | 1.76 (1.82) | 0.17 (0.13) | 0.28 (0.23) |
| Inhalants | 0.13 (0.16) | 0.21 (0.23) | 0.08 (0.10) | 0.18 (0.17) | 0.03 (0.03) | 0.20 (0.43) | 0.11 (0.15) | 0.14 (0.20) |
Note. AI/IN=American Indian/Alaska Native.
Table 2.
Tetrachoric correlations (standard errors) between study variables.
| Drug Use Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Prescription opioids | – | * | .81 (0.02) | .72 (0.03) | .53 (0.02) | .61 (0.02) | * | .65 (0.02) | .65 (0.03) | .69 (0.03) | .69 (0.05) |
| 2. Prescription stimulantsa | .64 (0.02) | – | * | * | * | * | * | * | * | * | * |
| 3. Sedatives | .85 (0.01) | .68 (0.01) | – | .67 (0.04) | .55 (0.02) | .64 (0.03) | * | .68 (0.02) | .71 (0.03) | .72 (0.03) | .69 (0.05) |
| 4. Heroin | .70 (0.02) | .56 (0.03) | .71 (0.02) | – | .46 (0.04) | .62 (0.04) | * | .57 (0.04) | .59 (0.06) | .57 (0.05) | .61 (0.07) |
| 5. Cannabis | .59 (0.01) | .68 (0.02) | .63 (0.01) | .67 (0.02) | – | .60 (0.02) | * | .57 (0.02) | .71 (0.03) | .74 (0.03) | .68 (0.04) |
| 6. Cocaine/crack | .66 (0.01) | .66 (0.01) | .71 (0.01) | .78 (0.02) | .84 (0.01) | – | * | .70 (0.02) | .70 (0.03) | .77 (0.03) | .67 (0.05) |
| 7. Illicit stimulantsa | .61 (0.02) | .81 (0.01) | .66 (0.01) | .55 (0.02) | .74 (0.02) | .73 (0.01) | – | * | * | * | * |
| 8. Stimulantsb | * | * | * | * | * | * | * | – | .69 (0.03) | .78 (0.03) | .71 (0.05) |
| 9. Club drugs | .65 (0.02) | .70 (0.02) | .66 (0.01) | .66 (0.02) | .76 (0.01) | .72 (0.01) | .58 (0.02) | * | – | .86 (0.02) | .73 (0.05) |
| 10. Hallucinogens | .67 (0.01) | .71 (0.02) | .71 (0.01) | .73 (0.02) | .87 (0.01) | .84 (0.01) | .75 (0.01) | * | .79 (0.01) | – | .83 (0.04) |
| 11. Inhalants | .62 (0.02) | .59 (0.02) | .65 (0.02) | .67 (0.02) | .73 (0.02) | .72 (0.01) | .63 (0.02) | * | .69 (0.02) | .79 (0.01) | – |
Note. (Mis)Use variables below the diagonal, use disorder variables above the diagonal.
measure only available for (mis)use.
measure only available for use disorder.
measure not available; all correlations significant, p<.001.
3.1. Prescription misuse and illicit use confirmatory factor models
Results of the three primary drug use models are presented in Table 3 (results for a bifactor model in which specific factors were permitted to correlate are presented in Supplemental Table S2). All models provided excellent fit to the data, though the bifactor model had the highest CFI and TLI and lowest RMSEA and chi square values. Difference testing also indicated superior fit of the bifactor model compared to the correlated factors (χ2(16)=420.28, p<.001) and one-factor model (χ2(24)=994.03, p<.001), as well as superior fit of the correlated factors model compared to the one-factor model (χ2(8)=500.80, p<.001). In the bifactor model, loadings on the general factor were high across all drug use variables (λ=0.699–0.919), with the highest loading for sedative misuse and the lowest loading for inhalant use. Loadings on the prescription factor were modest for both prescription opioid and sedative misuse (λ=0.280–0.380); unexpectedly, the loading for prescription stimulant misuse was negative (λ=−0.374). Loadings on the illicit use factor were moderate for cannabis, cocaine/crack use, club drug, hallucinogen, and inhalant use (λ=0.390–0.593), but less so for heroin (λ=0.284) and illicit stimulant use (λ=0.207). Thus, despite good global fit, several relatively weak loadings on the specific factors suggest that the bifactor model does not capture the data well.
Table 3.
Model fit statistics, standardized factor loadings, and factor correlations for models of drug use.
| Statistics, loadings, and correlations | Bifactor | Correlated Factors | One-Factor | |||
|---|---|---|---|---|---|---|
| Statistic | Model fit | Model fit | Model fit | |||
| Chi-square (WLSMV) | 755.933 | 1215.205 | 1691.237 | |||
| Degrees of freedom | 74 | 90 | 98 | |||
| Comparative fit index | 0.990 | 0.984 | 0.977 | |||
| Tucker-Lewis index | 0.985 | 0.979 | 0.973 | |||
| RMSEA (90% CI) | 0.016 (0.015–0.017) | 0.019 (0.018–0.019) | 0.021 (0.020–0.022) | |||
| Standardized factor loading (SE) | General | Prescription | Illicit | Prescription | Illicit | General |
| Prescription opioids | 0.860 (0.012) | 0.280 (0.033) | – | 0.878 (0.007) | – | 0.835 (0.008) |
| Prescription stimulants | 0.882 (0.013) | −0.374 (0.088) | – | 0.869 (0.012) | – | 0.813 (0.010) |
| Sedatives | 0.919 (0.010) | 0.380 (0.044) | – | 0.916 (0.006) | – | 0.859 (0.006) |
| Heroin | 0.767 (0.019) | – | 0.284 (0.030) | – | 0.835 (0.013) | 0.819 (0.013) |
| Cannabis | 0.702 (0.018) | – | 0.593 (0.021) | – | 0.906 (0.005) | 0.899 (0.005) |
| Cocaine/crack | 0.776 (0.015) | – | 0.454 (0.020) | – | 0.913 (0.004) | 0.906 (0.004) |
| Illicit stimulants | 0.805 (0.017) | – | 0.207 (0.031) | – | 0.842 (0.008) | 0.829 (0.008) |
| Club drugs | 0.767 (0.016) | – | 0.390 (0.021) | – | 0.875 (0.008) | 0.864 (0.008) |
| Hallucinogens | 0.782 (0.016) | – | 0.499 (0.019) | – | 0.940 (0.004) | 0.932 (0.004) |
| Inhalants | 0.699 (0.017) | – | 0.411 (0.025) | – | 0.820 (0.010) | 0.807 (0.010) |
| Factor correlation (95% CI) | – | – | – | .86 (0.84–0.87) | – | |
Note. Bold font indicates significant factor loading/correlation, p<.001.
In the correlated factors model, all items loaded strongly on their respective specific factors (λ=0.820–0.940). Sedative misuse showed the highest loading on the prescription factor (λ=0.916) and hallucinogen use showed the highest loading on the illicit factor (λ=0.940). Coupled with good global fit, this pattern suggests that the two correlated factors explain the structure of drug use well, though the correlation between the factors was quite high (r = 0.86, 95% CI [.84–0.87], p<.001), suggesting that they may not be truly separable.
The one-factor model showed uniformly high loadings across all drugs (λ=0.807–0.932), with the highest loading for hallucinogen use and the lowest loading for inhalant use. Taken together with the inconsistent factor loadings in the bifactor model, the high factor correlation in the correlated factors model, and the good global fit of the one-factor model, this pattern suggests that drug (mis)use may be most parsimoniously captured as a unidimensional construct.
3.2. Prescription misuse and illicit use disorder confirmatory factor models
It was not possible to precisely replicate the structure of the drug use models using the use disorder variables because stimulant use disorder was not disaggregated into prescription- and illicit-based disorders. Due to the negative loading of prescription stimulant misuse on the prescription misuse factor in the bifactor model of drug use, stimulant use disorder was placed on the illicit factor. With only two indicators remaining on the prescription factor, loadings were constrained to equality to achieve model identification.
Results of the three primary drug use disorder models are presented in Table 4 (results for a bifactor model in which specific factors were permitted to correlate are presented in Supplemental Table S3). All models provided good global fit to the data, though again difference testing indicated superior fit of the bifactor model compared to the correlated factors (χ2(15)=148.69, p<.001) and one-factor model (χ2(22)=292.79, p<.001), as well as superior fit of the correlated factors model compared to the one-factor model (χ2(7)=141.49, p<.001). In the bifactor model, loadings on the general factor were moderate to large across all drug use disorder variables (λ=0.596–0.863), with the highest loading for hallucinogen use disorder and the lowest loading for cannabis use disorder. Loadings on the prescription factor were modest but significant for both prescription-based OUD and sedative use disorder (λ=0.361–0.389). Loadings on the illicit factor were modest to moderate for cannabis (λ=0.625), hallucinogen (λ=0.375), and club drug use disorders (λ=0.369), but far less so for heroin (λ=−0.076, ns), stimulant (λ=0.142), cocaine/crack (λ=0.185), and inhalant use disorders (λ=0.266). This pattern suggests that the bifactor model does not explain the data well despite good global fit.
Table 4.
Model fit statistics, standardized factor loadings, and factor correlations for models of drug use disorder.
| Statistics, loadings, and correlations | Bifactor | Correlated Factors | One-Factor | |||
|---|---|---|---|---|---|---|
| Statistic | Model fit | Model fit | Model fit | |||
| Chi-square (WLSMV) | 151.024 | 264.586 | 381.109 | |||
| Degrees of freedom | 61 | 76 | 83 | |||
| Comparative fit index | 0.991 | 0.980 | 0.969 | |||
| Tucker-Lewis index | 0.985 | 0.975 | 0.963 | |||
| RMSEA (90% CI) | 0.006 (0.005–0.008) | 0.008 (0.007–0.009) | 0.010 (0.009–0.011) | |||
| Standardized factor loading (SE) | General | Prescription | Illicit | Prescription | Illicit | General |
| Prescription opioids | 0.804 (0.020) | 0.389 (0.032) | – | 0.901 (0.009) | – | 0.827 (0.012) |
| Sedatives | 0.834 (0.023) | 0.361 (0.033) | – | 0.901 (0.009) | – | 0.857 (0.015) |
| Heroin | 0.798 (0.031) | – | −0.076 (0.068) | – | 0.772 (0.029) | 0.760 (0.029) |
| Cannabis | 0.596 (0.026) | – | 0.625 (0.044) | – | 0.762 (0.015) | 0.751 (0.015) |
| Cocaine/crack | 0.793 (0.022) | – | 0.185 (0.066) | – | 0.820 (0.013) | 0.815 (0.013) |
| Stimulants | 0.813 (0.021) | – | 0.142 (0.062) | – | 0.832 (0.015) | 0.820 (0.015) |
| Club drugs | 0.831 (0.034) | – | 0.369 (0.074) | – | 0.919 (0.017) | 0.911 (0.018) |
| Hallucinogens | 0.863 (0.032) | – | 0.375 (0.073) | – | 0.951 (0.014) | 0.943 (0.014) |
| Inhalants | 0.825 (0.042) | – | 0.266 (0.090) | – | 0.882 (0.029) | 0.872 (0.029) |
| Factor correlation (95% CI) | – | .87 (0.84–0.89) | – | |||
Note. Loadings constrained to equality can vary slightly in standardized estimates; bold font indicates significant factor loading/correlation, p<.001; italic font indicates significant factor loading/correlation, p<.05.
In the correlated factors model, all items loaded strongly on their respective specific factors (λ=0.762–0.951). Prescription-based OUD and sedative use disorder both loaded strongly on the prescription factor (λ=0.901) and hallucinogen use disorder showed the highest loading on the illicit factor (λ=0.951). Coupled with good global fit, this pattern suggests that the two correlated factors explain the structure of drug use well. However, the prescription misuse and illicit use factors were, again, very highly correlated (r = 0.87, 95% CI [.84–0.89], p<.001), suggesting that they may not be truly separable.
The one-factor model again showed uniformly high loadings across all drugs (λ=0.751–0.943). As in the drug use models, a unidimensional solution appeared to most parsimoniously capture the drug use disorder construct.
3.3. Alternate confirmatory factor models
3.3.1. Uppers, downers, all-arounders: use factors
Results of the alternate drug use models are presented in Table 5 (results for a bifactor model in which specific factors were permitted to correlate are presented in Supplemental Table S4). Both models provided good global fit to the data. Again, difference testing indicated superior fit of the bifactor model compared to the correlated factors (χ2(13)=537.11, p<.001) and one-factor model (χ2(30)=1181.50, p<.001), as well as superior fit of the correlated factors model as compared to the one-factor model (χ2(17)=740.38, p<.001). In the bifactor model, loadings on the general factor were large across all drug use variables (λ=0.735–0.937), with the lowest loading for POM and the highest loading for cocaine/crack use. Loadings on the “uppers” factor were moderate for prescription stimulant misuse (λ=0.590) and illicit stimulant use (λ=0.328), but not for cocaine/crack use (λ=−0.078). Loadings on the “downers” factor were also modest to moderate (λ=0.174–0.623), with the highest loading for POM and the lowest loading for heroin use. Interestingly, loadings for POM and sedative misuse were 2.5–3.5 times the magnitude of the loading for heroin use, suggesting that POM may be “more similar” to sedative misuse than to heroin use. Loadings on the “all-arounders” factor were relatively modest (λ=0.176–0.443), with the highest loading for club drug use and the lowest loading for inhalant use.
Table 5.
Model fit statistics, standardized factor loadings, and factor correlations for alternate models of drug use.
| Statistics, loadings, and correlations | Bifactor | Correlated Factors | |||||
|---|---|---|---|---|---|---|---|
| Statistic | Model fit | Model fit | |||||
| Chi-square (WLSMV) | 540.909 | 959.605 | |||||
| Degrees of freedom | 68 | 81 | |||||
| Comparative fit index | 0.993 | 0.987 | |||||
| Tucker-Lewis index | 0.988 | 0.982 | |||||
| RMSEA (90% CI) | 0.014 (0.013–0.015) | 0.017 (0.016–0.018) | |||||
| Standardized factor loading (SE) | General | Uppers | Downers | All-Arounders | Uppers | Downers | All-Arounders |
| Prescription opioids | 0.735 (0.012) | – | 0.623 (0.028) | – | – | 0.882 (0.008) | – |
| Prescription stimulants | 0.749 (0.013) | 0.590 (0.038) | – | – | 0.834 (0.010) | – | – |
| Sedatives | 0.794 (0.009) | – | 0.448 (0.024) | – | – | 0.936 (0.006) | – |
| Heroin | 0.811 (0.014) | – | 0.174 (0.025) | – | – | 0.883 (0.014) | – |
| Cannabis | 0.842 (0.008) | – | – | 0.416 (0.012) | – | – | 0.913 (0.005) |
| Cocaine/crack | 0.937 (0.005) | −0.078 (0.020) | – | – | 0.936 (0.005) | – | – |
| Illicit stimulants | 0.799 (0.010) | 0.328 (0.029) | – | – | 0.848 (0.008) | – | – |
| Club drugs | 0.795 (0.011) | – | – | 0.443 (0.024) | – | – | 0.885 (0.008) |
| Hallucinogens | 0.906 (0.005) | – | – | 0.212 (0.015) | – | – | 0.948 (0.004) |
| Inhalants | 0.798 (0.012) | – | – | 0.176 (0.027) | – | – | 0.832 (0.010) |
| Factor correlation (95% CI) | |||||||
| Uppers | – | – | – | – | – | .83 (0.81–0.85) | .95 (0.94–0.97) |
| Downers | – | – | – | – | – | – | .81 (0.80–0.83) |
Note. Bold font indicates significant factor loading/correlation, p<.001.
In the correlated factors model, all items loaded strongly on their respective specific factors (λ=0.832–0.948), suggesting that these three factors also capture the data well. Cocaine/crack use showed the highest loading on the “uppers” factor (λ=0.937), sedative misuse showed the highest loading on the “downers” factor (λ=0.936), and hallucinogen use showed the highest loading on the “all-arounders” factor (λ=0.948). The three factors were very highly correlated (r = 0.81–0.95, ps<0.001).
While both of these alternative models appear statistically and theoretically sound, they do not appear to be appreciably superior to either the primary hypothesized models or the one-factor model. As such, the one-factor model once again presents the most parsimonious solution despite plausible validity of other multidimensional structures (see Table 3).
3.3.2. Uppers, downers, all-arounders: use disorder factors
Results of the alternate drug use disorder models are presented in Table 6 (results for a bifactor model in which specific factors were permitted to correlate are presented in Supplemental Table S5). Both models provided good global fit to the data. Again, difference testing also indicated superior fit of the bifactor model compared to the correlated factors (χ2(13)=41.81, p<.001) and one-factor model (χ2(29)=289.85, p<.001), as well as superior fit of the correlated factors model compared to the one-factor model (χ2(16)=261.32, p<.001). In the bifactor model, loadings on the general factor were large across all drug use variables (λ=0.666–0.920), with the lowest loading for cannabis use disorder and the highest loading for hallucinogen use disorder. Loadings on the “uppers” factor were small and nonsignificant (λ=0.097–0.100). Loadings on the “downers” factor were moderate (λ=0.379–0.562), with the highest loading for prescription-based OUD and the lowest loading for heroin-based OUD. Loadings on the “all-arounders” factor were all small and nonsignificant (λ=0.095–0.178), except for that of cannabis use disorder (λ=0.568). Overall, the pattern of loadings on the specific factors suggests that the bifactor configuration is not a sound solution.
Table 6.
Model fit statistics, standardized factor loadings, and factor correlations for alternate models of drug use disorder.
| Statistics, loadings, and correlations | Bifactor | Correlated Factors | |||||
|---|---|---|---|---|---|---|---|
| Statistic | Model fit | Model fit | |||||
| Chi-square (WLSMV) | 128.522 | 163.499 | |||||
| Degrees of freedom | 54 | 67 | |||||
| Comparative fit index | 0.992 | 0.990 | |||||
| Tucker-Lewis index | 0.986 | 0.985 | |||||
| RMSEA (90% CI) | 0.006 (0.005–0.008) | 0.006 (0.005–0.008) | |||||
| Standardized factor loading (SE) | General | Uppers | Downers | All-Arounders | Uppers | Downers | All-Arounders |
| Prescription opioids | 0.738 (0.024) | – | 0.562 (0.058) | – | – | 0.888 (0.011) | – |
| Sedatives | 0.784 (0.022) | – | 0.400 (0.044) | – | – | 0.917 (0.015) | – |
| Heroin | 0.672 (0.037) | – | 0.379 (0.057) | – | – | 0.796 (0.029) | – |
| Cannabis | 0.666 (0.021) | – | – | 0.568 (0.160) | – | – | 0.780 (0.015) |
| Cocaine/crack | 0.839 (0.021) | 0.100 (0.106) | – | – | 0.845 (0.012) | – | – |
| Stimulants | 0.850 (0.025) | 0.097 (0.102) | – | – | 0.845 (0.012) | – | – |
| Club drugs | 0.893 (0.029) | – | – | 0.166 (0.093) | – | – | 0.932 (0.016) |
| Hallucinogens | 0.920 (0.027) | – | – | 0.178 (0.093) | – | – | 0.961 (0.012) |
| Inhalants | 0.873 (0.040) | – | – | 0.095 (0.109) | – | – | 0.898 (0.028) |
| Factor correlation (95% CI) | |||||||
| Uppers | – | – | – | – | – | .85 (0.81–0.89) | .95 (0.92–0.98) |
| Downers | – | – | – | – | – | – | .81 (0.78–0.85) |
Note. Bold font indicates significant factor loading/correlation, p<.001.
In the correlated factors model, all items loaded strongly on their respective specific factors (λ=0.780–0.961). Cocaine/crack and stimulant use disorders loaded strongly on the “uppers” factor (λ=0.845), sedative use disorder showed the highest loading on the “downers” factor (λ=0.917), and hallucinogen use disorder loaded most strongly on the “all-arounders” factor (λ=0.961). The factors were, again, very highly correlated (r = 0.81–0.95). This pattern of model results provides further support for the unidimensional nature of drug use disorder (see Table 4).
3.4. Supplemental confirmatory factor models
3.4.1. Licit and illicit use factors
Fit indexes, standardized factor loadings, and factor correlations for the three supplemental substance use models are presented in Supplemental Table S6 (results for a bifactor model in which specific factors were permitted to correlate are presented in Supplemental Table S7). All models provided good fit to the data with acceptable global fit indexes, though the bifactor model again had the highest CFI and TLI and lowest RMSEA and chi square values. Difference testing also indicated superior fit of the bifactor model compared to the correlated factors (χ2(18)=1663.69, p<.001) and one-factor model (χ2(26)=2096.00, p<.001), as well as superior fit of the correlated factors model compared to the one-factor model (χ2(8)=584.68, p<.001). In the bifactor model, loadings on the general factor were quite large (λ=0.611–0.870), with lowest loadings for alcohol (λ=0.652) and nicotine use (λ=0.611). Loadings on the licit factor were quite small, with the exception of nicotine (λ=0.544), which seemed to essentially form its own factor; this may be because nicotine use was the only item for which a positive screen required repeated use (i.e., participants were screened positively for nicotine use if they endorsed 100 or more cigarette uses, 50 or more cigar uses, etc.), and thus reflected a higher level of use involvement than the other items. Interestingly, the loadings for the prescription misuse variables on the licit factor were all negative (λ=−0.386–0.122). Loadings on the illicit factor were more substantive (λ=0.356–0.505), with the exception of heroin (λ=0.184) and methamphetamine use (λ=0.098). This pattern of loadings is not reflective of an underlying bifactor structure despite good model fit. In the correlated factors model, items loaded highly on their respective factors (λ=0.816–0.937) with the exception of more moderate loadings for alcohol (λ=0.660) and nicotine use (λ=0.596) on the licit factor. The licit and illicit factors were very highly correlated (r = 0.90, 95% CI [.88–0.91], p<.001). The one-factor model showed uniformly high loadings across all drugs (λ=0.805–0.931), again with the exception of more moderate loadings for alcohol (λ=0.633) and nicotine use (λ=0.557). Taken together, this series of models may indicate that alcohol and nicotine use are be best modeled as their own factor, considering their relatively low loadings on common factors and seemingly opposing variance to prescription misuse behaviors.
3.4.2. Licit and illicit use disorder factors
Fit indexes, standardized factor loadings, and factor correlations for the three supplemental use disorder models are presented in Table S7 (results for a bifactor model in which specific factors were permitted to correlate are presented in Supplemental Table S8). The bifactor model appeared to provide best fit to the data; while the RMSEAs for the correlated factors and one-factor models indicated acceptable fit, the CFI and TLI values were below the established cutoff. Difference testing also indicated superior fit of the bifactor model compared to the correlated factors (χ2(17)=522.72, p<.001) and one-factor model (χ2(25)=569.80, p<.001), as well as superior fit of the correlated factors model compared to the one-factor model (χ2(8)=58.62, p<.001). In the bifactor model, loadings on the general factor were generally large (λ=0.721–0.887); alcohol and nicotine again displayed the lowest loadings (λ=0.721–0.731). Loadings on the licit factor were modest for all use disorders (λ=0.288–0.310) except alcohol (λ=−0.089). Loadings on the illicit factor were modest-to-moderate for all use disorders (λ=0.121–0.555) except heroin (λ=0.016, ns). Such a pattern again indicates the absence of an underlying bifactor structure despite superior model fit. In the correlated factors model, items loaded highly on their respective factors (λ=0.701–0.926). The licit and illicit factors were very highly correlated (r = 0.93, 95% CI [.90–0.95], p<.001). The one-factor model showed uniformly high loadings across drug use disorders (λ=0.764–0.922), but loadings for alcohol (λ=0.732) and nicotine (λ=0.686) use disorder were more modest. Overall, the pattern of findings was consistent with the substance use models.
4. Discussion
The present study aimed to provide insight into the relationship between POM and heroin use by testing a series of dimensional models of drug use and use disorder. Importantly, this study integrated a uniquely broad spectrum of drug use within which to contextualize POM and heroin use, both of which rarely occur in isolation from use of other non-opioid substances. Testing a series of novel, theory-driven configurations of plausible dimensional models of drug use while explicitly modeling non-opioid drug use- rather than treating it as a covariate or nuisance variable- we did not uncover compelling evidence for empirical differentiation of POM and heroin use as items subsumed by distinct but correlated prescription misuse and illicit use factors. Additionally, an alternate factor model informed by pharmacodynamic, physiologic, and subjective drug effects did not support the hypothesis that POM and heroin use form a shared factor distinct from most other forms of drug use. More specifically, our results did not suggest that POM operates as a unique risk factor in the context of heroin use, but rather that (mis)use of non-opioid drugs may be as valuable in predicting heroin use as is POM.
These findings are not unprecedented, though most studies investigating substance use in this type of latent variable framework have operationalized POM and heroin use as a single, aggregated opioid (mis)use variable and have included fewer forms of other drug use/use disorder. Consistent with the present results, this aggregate form of opioid (mis)use has been found to form a single substance use factor alongside other substance use in prior phenotypic (Pandika et al., 2022) and genomic studies (Hatoum et al., 2021). Twin studies have demonstrated similar findings, with aggregate opioid (mis)use loading on a single genetic factor shared with cannabis, cocaine, hallucinogen, sedative, and stimulant use, with no drug-specific genetic effects (Karkowski et al., 2000; Kendler et al., 2003). In fact, twin studies suggest that there may be no drug-specific genetic influence on any drug use disorders, including OUD (Kendler et al., 2003). Such patterns may explain the lack of specificity in prediction of heroin use from POM versus other forms of drug use. Across both phenotypic and genetically-informed studies, findings indicate that individual-specific environmental experiences, particularly partner and peer substance use, contribute to the use of one substance versus another, while the majority of variance in drug use and use disorder is attributable to nonspecific liability for any drug use (Kendler et al., 2003; Pandika et al., 2022). As such, existing universal prevention/intervention approaches targeting refusal self-efficacy, refusal skills, and normative feedback on peer drug use may be a feasible and efficacious way to address opioid (mis)use.
It is also worthwhile to note that these findings reflect many of the concerns regarding the bifactor model that have been increasingly raised in the literature (Bonifay et al., 2017; Waldman et al., 2022). The bifactor model tends to fit most possible data, which often reflects an artifact of overfitting as opposed to provision of a superior explanatory model (Bonifay and Cai, 2017; Bonifay et al., 2017). This was evinced in our model comparisons, wherein the bifactor model provided superior fit in all cases (p<.001), even those in which the pattern of factor loadings clearly did not display a pattern consistent with an underlying bifactor structure. Consonant with recent simulation studies, the bifactor models presented here had higher standard errors for factor loading estimates and specific factor loadings that were weaker and less interpretable than those in the correlated factors and one-factor models (Waldman et al., 2022). This study adds to the growing body of literature that suggests critical evaluation of the bifactor model in studies of human behavior is warranted.
4.1. Limitations
Despite the strength of NESARC-III as a large, nationally-representative epidemiologic study, it is not without limitations. National surveys may not be ideally equipped to accurately capture rates of heroin use at the population level, resulting in underestimation (Reuter et al., 2021). Relevant to this are issues of data censoring. That is, estimates of use prevalence may be biased due to factors such as incarceration and premature mortality (i.e., systematic exclusion of a subset of people who initiated use but could not be included in data collection), and initiation of use post-data collection (i.e., temporal limitations of capturing a respondent's complete pattern of use over the lifetime).
Though the most incisive way to understand the relationship between POM and heroin use is to study use over time, this approach was not feasible in these cross-sectional data. Future studies may consider longitudinal applications, network modeling, and cross-sectional survival analysis, which could extend the findings presented here by integrating salient factors such as age of onset and initiation sequence. Additionally, separate measures of prescription and illicit stimulant use disorder were not available, limiting our ability to build fully comparable models across use and use disorder. The use disorder models should be interpreted in light of the fact that the inclusion of individuals who do not use may have resulted in these models effectively differentiating use versus non-use rather than providing unique information about use disorder. This could potentially explain the similarity in results across the use and use disorder models, though unfortunately the nature of the multivariate analyses precluded exclusion of participants based on use status of any one, or of all, drugs. Finally, the analyses presented here should be considered exploratory given that we did not replicate factor structures in an independent sample. Despite these limitations, the present study provides a novel approach to understanding the relationship between POM and heroin use, and how they are situated within broader patterns of drug use.
4.2. Conclusions
POM and heroin use are often conceptualized as two sides of the same “opioid use” coin, with POM implicated as a step on the path to heroin use. However, studies aiming to address this topic often do so while insufficiently addressing the broader drug use context in which most opioid (mis)use occurs. The present study identified evidence for unidimensionality in both drug (mis)use and drug use disorder, which aligns with often overlooked findings showing that non-opioid drug use predicts heroin use at least as robustly as does POM. Simple explanations of the POM-to-heroin pathway, while intuitive, have proven to have deleterious downstream effects, including undertreatment of patient pain and uncertainty about best practices for opioid-based pain management among medical providers (Ebbert et al., 2018; Rose, 2018). A more nuanced approach to understanding how and under what conditions POM can confer risk for heroin use is requisite to balancing the potential negative sequalae of opioid use while avoiding an “over-correction” that ultimately results in unintended harm.
Role of funding source
This work was supported by the National Institute on Drug Abuse F31DA054701 (PI: Dash). NIDA had no role in study design; collection, analysis, or interpretation of data; in writing of the report; or in the decision to submit this article for publication.
This paper was prepared using a limited access dataset obtained from the National Institute on Alcohol Abuse and Alcoholism. This paper has not been reviewed or endorsed by NIAAA and does not necessarily represent the opinions of NIAAA, who is not responsible for the contents.
Contributors
GFD conceptualized the study, conducted statistical analyses, and wrote the first draft of the paper. IRG and WSS contributed to data acquisition, writing and editing of the manuscript, and jointly supervised the project in its entirety.
Declaration of Competing Interest
No conflict declared.
Footnotes
Author Note
The authors declare no conflict of interest.
This work was supported by the National Institute on Drug Abuse F31DA054701 (PI: Dash).
This paper was prepared using a limited access dataset obtained from the National Institute on Alcohol Abuse and Alcoholism. This paper has not been reviewed or endorsed by NIAAA and does not necessarily represent the opinions of NIAAA, who is not responsible for the contents.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dadr.2022.100123.
Appendix. Supplementary materials
References
- Bentler P.M. Comparative fit indexes in structural models. Psychol. Bull. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bonifay W., Cai L. On the complexity of item response theory models. Multivar. Behav. Res. 2017;52(4):465–484. doi: 10.1080/00273171.2017.1309262. [DOI] [PubMed] [Google Scholar]
- Bonifay W., Lane S.P., Reise S.P. Three concerns with applying a bifactor model as a structure of psychopathology. Clin. Psycholog. Sci. 2017;5(1):184–186. [Google Scholar]
- Bornovalova M.A., Choate A.M., Fatimah H., Petersen K.J., Wiernik B.M. Appropriate use of bifactor analysis in psychopathology research: appreciating benefits and limitations. Biol. Psychiatry. 2020;88(1):18–27. doi: 10.1016/j.biopsych.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R.G., Nahhas R.W., Martins S.S., Daniulaityte R. Predictors of transition to heroin use among initially non-opioid dependent illicit pharmaceutical opioid users: a natural history study. Drug Alcohol Depend. 2016;160:127–134. doi: 10.1016/j.drugalcdep.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Houts R.M., Belsky D.W., Goldman-Mellor S.J., Harrington H., Israel S., Meier M.H., Ramrakha S., Shalev I., Poulton R. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin. Psycholog. Sci. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Curran P.J., Bollen K.A., Kirby J., Paxton P. An empirical evaluation of the use of fixed cutoff points in RMSEA test statistic in structural equation models. Soc. Method. Res. 2008;36(4):462–494. doi: 10.1177/0049124108314720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton W.M., Jones C.M., Baldwin G.T. Relationship between nonmedical prescription-opioid use and heroin use. New Engl. J. Med. 2016;374(2):154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash G.F., Martin N.G., Agrawal A., Lynskey M.T., Slutske W.S. Typologies of illicit drug use in mid-adulthood: a quasi-longitudinal latent class analysis in a community-based sample of twins. Addiction. 2021;116(5):1101–1112. doi: 10.1111/add.15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash, G.F., Martin, N.G., Agrawal, A., Lynskey, M.T., & Slutske, W.S. (in press). Are prescription misuse and illicit drug up etiologically distinct? A genetically-informed analysis of opioids and stimulants. Psychol. Med.. doi: 10.1017/S0033291720005267. [DOI] [PMC free article] [PubMed]
- Dash, G.F., Martin, N.G., & Slutske, W.S. (in press). Big Five personality traits and illicit drug use: specificity in trait–drug associations. Psychol. Addict. Behav.. doi: 10.1037/adb0000793. [DOI] [PMC free article] [PubMed]
- Ebbert J.O., Philpot L.M., Clements C.M., Lovely J.K., Nicholson W.T., Jenkins S.M., Lamer T.J., Gazelka H.M. Attitudes, beliefs, practices, and concerns among clinicians prescribing opioids in a large academic institution. Pain Med. 2018;19(9):1790–1798. doi: 10.1093/pm/pnx140. [DOI] [PubMed] [Google Scholar]
- Hatoum A.S., Johnson E.C., Polimanti R., Zhou H., Walters R., Gelernter J., Edenberg H.J., Bogdan R., Agrawal A. The addiction genetic factor: a unitary genetic vulnerability characterizes substance use disorders and their associations with common correlates. Neuropsychopharmacology. 2021 doi: 10.1038/s41386-021-01209-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D., Coughlan J., Mullen M. 7th European Conference on research methodology for business and management studies. 2008. Evaluating model fit: a synthesis of the structural equation modelling literature. [Google Scholar]
- Hu L.t., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Eq. Model.: A Multidiscipl. J. 1999;6(1):1–55. [Google Scholar]
- Inaba D., Cohen W. 8th ed. CNS Publications; Medford, OR: 2014. Uppers, downers, All Arounders. [Google Scholar]
- Karkowski L.M., Prescott C.A., Kendler K.S. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. Am. J. Med. Genet. 2000;96(5):665–670. [PubMed] [Google Scholar]
- Kelley-Quon L.I., Cho J., Strong D.R., Miech R.A., Barrington-Trimis J.L., Kechter A., Leventhal A.M. Association of nonmedical prescription opioid use with subsequent heroin use initiation in adolescents. JAMA Pediatr. 2019;173(9) doi: 10.1001/jamapediatrics.2019.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Jacobson K.C., Prescott C.A., Neale M.C. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am. J. Psychiatry. 2003;160(4):687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Myers J., Prescott C.A. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch. Gen. Psychiatry. 2007;64(11):1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Krausz R.M., Westenberg J.N., Ziafat K. The opioid overdose crisis as a global health challenge. Curr. Opin. Psychiatry. 2021;34(4):405–412. doi: 10.1097/YCO.0000000000000712. [DOI] [PubMed] [Google Scholar]
- Mahu I., Conrod P., Barrett S., Sako A., Swansburg J., Lawrence M., Laroque F., Morin J., Chinneck A., Nogueira-Arjona R. Specificity of personality relationships to particular forms of concurrent substance use among methadone maintenance therapy clients. Addict. Behav. 2019;98 doi: 10.1016/j.addbeh.2019.106056. [DOI] [PubMed] [Google Scholar]
- Mars S.G., Bourgois P., Karandinos G., Montero F., Ciccarone D. Every ‘never'I ever said came true”: transitions from opioid pills to heroin injecting. Int. J. Drug Policy. 2014;25(2):257–266. doi: 10.1016/j.drugpo.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe S.E., Boyd C.J., Evans-Polce R.J., McCabe V.V., Schulenberg J.E., Veliz P.T. Pills to powder: a 17-year transition from prescription opioids to heroin among US adolescents followed into adulthood. J. Addict. Med. 2021;15(3):241–244. doi: 10.1097/ADM.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R.P., Ho M.-H.R. Principles and practice in reporting structural equation analyses. Psychol. Method. 2002;7(1):64–82. doi: 10.1037/1082-989x.7.1.64. [DOI] [PubMed] [Google Scholar]
- Muhuri P., Gfroerer J., Davies C. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Review. Subst. Abuse Ment. Health Serv. Administ. 2013;1:17. [Google Scholar]
- Muthén L. Los Angeles; CA, USA: 2017. Mplus Users Guide (Version 8) Muthén & Muthén. [Google Scholar]
- Pandika D., Bailey J.A., Oesterle S., Kuklinski M.R. Young adult opioid misuse indicates a general tendency toward substance use and is strongly predicted by general substance use risk. Drug Alcohol Depend. 2022;235 doi: 10.1016/j.drugalcdep.2022.109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter P., Caulkins J.P., Midgette G. Heroin use cannot be measured adequately with a general population survey. Addiction. 2021;116(10):2600–2609. doi: 10.1111/add.15458. [DOI] [PubMed] [Google Scholar]
- Rigg K.K., Monnat S.M. Comparing characteristics of prescription painkiller misusers and heroin users in the United States. Addict. Behav. 2015;51:106–112. doi: 10.1016/j.addbeh.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M.E. Are prescription opioids driving the opioid crisis? Assumptions vs facts. Pain Med. 2018;19(4):793–807. doi: 10.1093/pm/pnx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. (2021). Key substance use and mental health indicators in the United States: results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). https://www.samhsa.gov/data/
- Scholl L., Seth P., Kariisa M., Wilson N., Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. Morbid. Mortal. Wkly. Rep. 2019;67(5152):1419. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J.B., Nora A., Stage F.K., Barlow E.A., King J. Reporting structural equation modeling and confirmatory factor analysis results: a review. J. Educ. Res. 2006;99(6):323–338. [Google Scholar]
- Shi D., DiStefano C., McDaniel H.L., Jiang Z. Examining chi-square test statistics under conditions of large model size and ordinal data. Struct. Eq. Model.: A Multidiscipl. J. 2018;25(6):924–945. [Google Scholar]
- Siegal H.A., Carlson R.G., Kenne D.R., Swora M.G. Probable relationship between opioid abuse and heroin use. Am. Fam. Physician. 2003;67(5):942–945. [PubMed] [Google Scholar]
- Steiger J.H. Structural model evaluation and modification: an interval estimation approach. Multivar. Behav. Res. 1990;25(2):173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Thomas A.T., Fields K.G., Kaye A.D., Urman R.D. Factors associated with heroin use among those reporting prescription opioid misuse: results from a nationally representative sample. J. Opioid. Manag. 2022;18(3):243–255. doi: 10.5055/jom.2022.0716. [DOI] [PubMed] [Google Scholar]
- Tucker L.R., Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38(1):1–10. [Google Scholar]
- Volkow N.D. America's addiction to opioids: heroin and prescription drug abuse. Senate Caucus on Int. Narcot. Control. 2014;14:1–16. [Google Scholar]
- Waldman, I., King, C., Poore, H., Luningham, J., Zinbarg, R., Krueger, R., … Zald, D. (2022, October 20). Recommendations for Adjudicating Among Alternative Structural Models of Psychopathology. doi: 10.31234/osf.io/bksm7. [DOI]
- Wilson N. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 2020:69. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.-.T., Woody G.E., Yang C., Blazer D.G. How do prescription opioid users differ from users of heroin or other drugs in psychopathology: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Addict. Med. 2011;5(1):28–35. doi: 10.1097/ADM.0b013e3181e0364e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.-.Y. University of California; Los Angeles: 2002. Evaluating Cutoff Criteria of Model Fit Indices For Latent Variable Models With Binary and Continuous Outcomes. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.