Abstract
A monoclonal antibody (MAb), designated MAb 8E7 (immunoglobulin G3), specific for Moraxella catarrhalis lipooligosaccharide (LOS) was evaluated for its functional activity in vitro and in a mouse model of colonization. Enzyme-linked immunosorbent assay (ELISA) demonstrated that the MAb 8E7 could be prepared to a high titer against LOS of the homologous strain 035E, and that it had bactericidal activity. MAb 8E7 reacted with M. catarrhalis serotype A and C LOSs but not serotype B LOS, as measured by ELISA and Western blotting. On the basis of published structures of LOSs, this suggests that the epitope recognized by MAb 8E7 is directed to a common sequence of either α-GlcNAc-(1→2)-β-Glc-(1→ at the branch substituting position 4 of the trisubstituted Glc residue or a terminal tetrasaccharide α-Gal-(1→4)-β-Gal-(1→4)-α-Glc-(1→2)-β-Glc-(1→ at the branch substituting position 6 of the trisubstituted Glc residue. In a whole-cell ELISA, MAb 8E7 reacted with 70% of the 30 wild-type strains and clinical isolates tested. Immuno-electron microscopy demonstrated that MAb 8E7 reacted with a cell surface-exposed epitope of LOS on strain O35E. MAb 8E7 inhibited the adherence of strain O35E to Chang conjunctival epithelial cells by 90%. Passive immunization with MAb 8E7 could significantly enhance the clearance of strain O35E from mouse lungs in an aerosol challenge mouse model. This enhanced bacterial clearance was inhibited when MAb 8E7 was absorbed by M. catarrhalis serotype A LOS, indicating that the M. catarrhalis LOS-directed antibody may play a major role in the enhancement of M. catarrhalis clearance from lungs. These data suggest that MAb 8E7, which recognizes surface-exposed LOS of M. catarrhalis, is a protective antibody against M. catarrhalis.
Moraxella catarrhalis is an important and common cause of otitis media in children as well as bronchopulmonary infection in the elderly, especially those with underlying pulmonary disease or a compromised immune system (4, 5, 26). Sporadic cases of conjunctivitis, meningitis, endocarditis, ophthalmia neonatorum, keratitis, urethritis, peritonitis, and septicemia have also been reported to be due to M. catarrhalis (25). In addition, an extremely high carriage rate (56%) has been reported for healthy children (11, 12, 18). Although the pathogenesis of M. catarrhalis infection is not fully understood, it is generally agreed that it has several virulence factors involved in colonization and adherence that enable it to evade the normal host defense mechanisms and that allow it to cause disease.
The lipooligosaccharide (LOS) from M. catarrhalis is a major component of the outer membrane and is a potential virulence factor (7, 13). LOS consists of an oligosaccharide and lipid A and is similar to the lipopolysaccharide (LPS) of gram-negative enteric pathogens, but it lacks the O-antigenic side chain of repeating units characteristic of classical LPS. LOS has been demonstrated to be an important virulence factor for such gram-negative bacteria as Neisseria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae (6, 21, 30). It is thought to participate in pathogenesis through the destruction of the mucosal barrier, the initiation of a severe inflammatory response, the promotion of or resistance to the immune defense, and the damaging of tissues and organs. LOS has also been shown to be an important adherence factor for Haemophilus ducreyi and N. gonorrhoeae (2, 36), but little is known about the role of M. catarrhalis LOS in the adherence of epithelium or in pathogenesis.
At present, we only have limited knowledge about which antigens confer protective immunity against M. catarrhalis infection. Components on the surface of the bacterium, such as the outer membrane proteins, and LOS have the potential to interact directly with the host immune system and to be targets for these specific antibodies. However, the role of M. catarrhalis LOS as a target for these functional antibodies has not been evaluated. Serological studies have shown that M. catarrhalis LOS is conserved among clinical isolates, and only three major LOS serotypes (A, B, and C) have been identified using rabbit antisera (35). The chemical structures of the oligosaccharide portions of the three LOS serotypes have been determined by mass spectrometry and nuclear magnetic resonance spectroscopy (8–10, 23). Of the clinical isolates that have been typed, most belong to serotype A (61.3%). Of the remainder, 28.8% are serotype B and 5.3% are serotype C. In addition, serum antibodies to LOS appear in patients with M. catarrhalis infections (28), and the human convalescent sera have bactericidal activity against M. catarrhalis (31). Recently, we reported that serotype A LOS-based conjugate vaccines were safe and immunogenic in animals. Antibodies elicited by these vaccines were bactericidal and enhanced clearance of M. catarrhalis from the lungs of mice (14, 19). This suggests that LOS not only is a potential virulence factor but also may be a protective antigen.
Monoclonal antibodies (MAbs) are useful tools for studying bacterial virulence factors, identifying immunological epitopes, and assessing host immune responses. Two kinds of MAbs against M. catarrhalis LOS have been reported; however, the functions of these MAbs were unknown (27, 29). In this study, a new MAb, 8E7, was examined to determine whether the epitope it recognized on LOS was surface exposed. In addition, experiments were designed to evaluate some of its biological functions, including inhibition of bacterial adherence to epithelial cells, bactericidal activity, and enhancement of pulmonary clearance of bacteria in an aerosol mouse model.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Strains O35E and TTA24 were described previously (33). Strains 8176, 8193, 23246, 25238, 25239, 25240, 43167, 43618, 43627, 43628, and 49143 were purchased from the American Type Culture Collection (ATCC), Manassas, Va. Strains 3292, 26391, 16394, 26395, 26397, 26400, and 26404 were obtained from the Culture Collection of the University of Göteborg (CCUG), Department of Clinical Bacteriology, Göteborg, Sweden. Ten other clinical isolates (M1 to M10) were kindly provided by Goro Mogi at Oita Medical University (Oita, Japan). All strains were grown overnight on chocolate agar at 37°C with 5% CO2-enriched air. Several isolated colonies from each strain were transferred to new plates and incubated for 3.5 to 4 h (mid-logarithmic phase) for the studies that followed.
Purification of M. catarrhalis LOS.
LOSs from strains O35E and 25238 (serotype A) were purified from cell powder by phenol-water extraction and then column purification as described previously (14, 15). The protein or nucleic acid contents of the LOSs were less than 1%. LOSs from strain 26397 (serotype B) and 26404 (serotype C) were purified from wet cells by the phenol-water extraction method as described previously (16). The protein contents ranged from 1.4 to 2.3% and nucleic acid contents ranged from 0.9 to 1.1%.
Production of MAb 8E7.
MAb 8E7 (immunoglobulin G3 [IgG3]) against strain O35E LOS was generated using outer membrane vesicles of strain O35E, kindly provided by Eric Hansen (17). Ascites was produced by the intraperitoneal inoculation of BALB/c mice with cloned hybridoma cells. The ascites was used for the entire study, since MAb 8E7 purified by using affinity columns lost 80 to 90% of its original LOS-binding activity.
ELISA.
Titers against LOS with MAb 8E7 and with sera from mice passively immunized with MAb 8E7 were determined by enzyme-linked immunosorbent assay (ELISA) (3, 14) using 96-well plates (Immuno I) coated with LOSs of M. catarrhalis (10 μg/ml). A mouse antiserum against strain O35E was used as a positive control. An irrelevant mouse ascites generated from nontypeable H. influenzae strain 9274 served as a negative control and gave optical density readings of less than 0.1. For the whole-cell ELISA, bacteria were suspended in phosphate buffered-saline (PBS) to an optical density of 65% transmission at 540 nm. The wells of the plates were coated with 70 μl of the bacterial suspension and incubated at 37°C overnight to dry. Thereafter the procedures were the same as described for the LOS ELISA.
Western blot analysis.
Two-hundred nanograms of each LOS was subjected to discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a Mini Protean II gel system (Bio-Rad, Richmond, Calif.) using 1.5-mm spacers with 15% polyacrylamide in the separating gel. After electrophoresis at 100 V for 2 h, the LOSs were transferred onto a pure nitrocellulose membrane (Bio-Rad) using a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad) at 250 mA for 6 h. After being blocked with 3% bovine serum albumin in PBS for 1 h, the membrane was incubated with MAb 8E7 (1:1,000) for 3 h followed by incubation with goat anti-mouse IgG labeled with horseradish peroxidase (1:1,000) (Sigma Chemical Co., St. Louis, Mo.) for 2 h. The membrane was developed using 4-chloro-1-naphthol (Sigma). A duplicate gel was silver stained for LOS after SDS-PAGE (32).
Bactericidal assay.
MAb 8E7 and an irrelevant mouse ascites were inactivated at 56°C for 30 min and measured for bactericidal activity against strain O35E by a complement-mediated bactericidal assay described previously (14), except that a guinea pig serum (5 μl per well) was used as the source of complement (Calbiochem-Novabiochem Corp., San Diego, Calif.). The bactericidal titer was determined to be the last dilution of serum causing at least 50% killing.
Negative staining and immuno-electron microscopy of bacteria.
Strain O35E was suspended on a slide in a small amount of saline. Formvar-coated 200-mesh grids (Electron Microscopy Sciences, Fort Washington, Pa.) were floated on the bacterial suspensions for 1 min and blotted dry. The grids were sequentially incubated at room temperature with 0.5% bovine serum albumin in 0.01 M PBS for 30 min with a 1:100 dilution of MAb 8E7 or with the irrelevant mouse ascites for 15 min and then with 1:100 gold (5-nm diameter)-conjugated goat anti-mouse IgG (EY Laboratories Inc., San Mateo, Calif.) for 60 min. Between steps, the grids were blotted and washed three times with PBS. After the final washing, the bacteria were negatively stained with a mixture of equal volumes of 2% ammonium acetate (Mallinckrodt Chemical, Inc., Paris, Ky.) and 2% ammonium molybdate (Sigma). The grids were viewed with a JEOL-200SX transmission electron microscope (JEOL Ltd., Tokyo, Japan).
Bacterial adherence assay and inhibition with MAb 8E7.
The Chang conjunctival epithelial cell line (HCEC, CCL-20.2) was obtained from the ATCC. The cell line was cultured in Media 199 (Life Technologies Inc., Rockville, Md.) supplemented with 10% fetal bovine serum, 200 U of penicillin per ml, and 50 μg of streptomycin per ml, (Life Technologies Inc.). A total of 105 cells were seeded into each well of a six-well tissue culture plate (containing cover slips) and incubated for 24 h at 37°C with 5% CO2 to obtain a monolayer of cells on the slips. The slips were washed with warm PBS before performing the bacterial adherence assay. Strain O35E was preincubated with either a 1:100 dilution of MAb 8E7 or the irrelevant mouse ascites for 30 min at 37°C. After that, 6 × 107 CFU of bacteria were added to each well and incubated for 45 min at 37°C. After five washes with warm PBS to remove nonadherent bacteria, the slips were fixed with methanol for 10 min. After two additional washes with PBS at room temperature, the slips were stained with Giemsa (Sigma) for 20 min and mounted on microscope slides. The slides were viewed and photographed by using a light microscope equipped with a camera (Leica Microscopy and Scientific Instruments Group, Heerbrugy, Switzerland) at a magnification of ×400. The number of bacteria adherent to 100 cells was counted.
Animals and passive immunization.
Female BALB/c mice (10 weeks of age) were obtained from Taconic Farms Inc. (Germantown, N.Y.). The mice were housed in an animal facility according to the National Institutes of Health guidelines for animal studies (protocol no. 850-98). MAb 8E7 was absorbed with serotype A or B LOS by incubating the antibody with 200 μg of LOS per ml at 37°C for 1 h and then leaving it at 4°C overnight. The mixtures of LOS and MAb 8E7 were centrifuged at 13,793 × g for 10 min to remove the MAb-bound LOS. The supernatants were then passed through a filter (0.2-μm pore size) and tested for the remaining MAb activity by ELISA before administration. MAb 8E7 was diluted to 1, 5, or 25% with saline, whereas LOS-absorbed MAb 8E7 and the irrelevant mouse ascites were only diluted to 25%. One milliliter of the diluted antibodies was administered intraperitoneally to each mouse 17 h prior to the bacterial aerosol challenge.
Bacterial aerosol challenge and measurement of pulmonary clearance of M. catarrhalis.
The bacterial suspension was adjusted to 3 × 107 CFU/ml with PBS determined by optical density of 65% transmission at 540 nm and confirmed for accuracy by counting the CFU on the plates after overnight growth. The bacteria were stored on ice until use. Bacterial aerosol challenge was carried out with an inhalation exposure system (Glas-col, Terre Haute, Ind.) as described previously (20). Five mice from each challenge group were euthanatized with an overdose of Metophane (Mallinckrodt Veterinary Inc., Mundelein, Ill.), and the lungs were removed under sterile conditions 6-h postchallenge. Blood samples were collected for antibody quantification. Lungs were homogenized in 5 ml of PBS for 1 min using a tissue homogenizer (Stomacher lab system model 80; Seward, London, United Kingdom). Each homogenate was diluted serially in PBS and 50 μl of the homogenate, and the diluted samples were plated on chocolate agar plates. The plates were incubated at 37°C in 5% CO2 overnight, and the bacterial colonies were counted.
Statistical analysis.
The viable bacteria were expressed as the mean CFU of n independent observations ± the standard deviation. Geometric means of bactericidal titers and reciprocal antibody IgG titers were expressed. Significance was determined using Mann-Whitney nonparametric analysis.
RESULTS
Isotype and specificity of MAb 8E7.
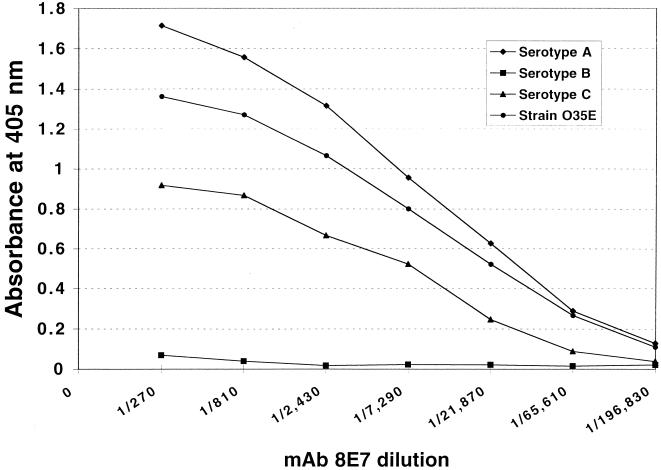
The isotype of MAb 8E7 was found to be an IgG3, and the ELISA titer for the ascites fluid was 1:196,830 against the strain O35E LOS. The specificity of the antibody against the LOSs was analyzed by ELISA and Western blotting. MAb 8E7 bound to the LOSs of strain O35E, ATCC 25238 (serotype A), and CCUG 26404 (serotype C) but not CCUG 26397 (serotype B) (Fig. 1 and 2). Binding activity of MAb 8E7 to the serotype A LOS was stronger than that of serotype C. MAb 8E7 was tested in the whole-cell ELISA for reactivity with 30 wild-type and clinical strains (Table 1). The results showed that it reacted with 21 of the 30 tested strains (70%). Of the 30 strains, only 8 were of known serotype, and of those, MAb 8E7 bound to serotypes A and C but not B. Furthermore, the titer of MAb 8E7 for serotype A was higher than that for C. When the bactericidal activity of MAb 8E7 against the homologous strain O35E was examined, the bactericidal titer of MAb 8E7 was 1:16, while the control titer was less than 1:2.
FIG. 1.
ELISA binding reactivities of MAb 8E7 with LOSs from three serotype strains and a homologous strain of M. catarrhalis. Each point represents the average optical density from duplicate wells recorded at 405 nm.
FIG. 2.
Silver-stained SDS-PAGE patterns (A) and Western blots (B) of LOSs with MAb 8E7. Lanes 1 and 2 contain 0.2 μg of each LPS from Salmonella minnesota, with Ra and Rc mutants as markers. Lanes 3 through 6 contain 0.2 μg of each LOS from strains 25238 (serotype A), 26397 (serotype B), 26404 (serotype C), and 035E.
TABLE 1.
Binding reactivities of MAb 8E7 to M. catarrhalis strains determined by whole-cell ELISA
| Straina | Serotype | Titerb |
|---|---|---|
| 25238 | A | 2,430 |
| 26394 | A | 2,430 |
| 26395 | A | 2,430 |
| 3292 | B | 30 |
| 26397 | B | 10 |
| 26400 | B | 30 |
| 26391 | C | 810 |
| 26404 | C | 810 |
| O35E | —c | 810 |
| TTA24 | — | 810 |
| 8176 | — | 2,430 |
| 8193 | — | 810 |
| 23246 | — | 10 |
| 25239 | — | 10 |
| 25240 | — | 2,430 |
| 43167 | — | 810 |
| 43618 | — | 810 |
| 43627 | — | 30 |
| 43628 | — | 10 |
| 49143 | — | 2,430 |
| M1 | — | 810 |
| M2 | — | 810 |
| M3 | — | 2,430 |
| M4 | — | 810 |
| M5 | — | 810 |
| M6 | — | 270 |
| M7 | — | 10 |
| M8 | — | 810 |
| M9 | — | 810 |
| M10 | — | 30 |
Whole cells of 30 M. catarrhalis strains (about 108 to 109) were used as coating antigens.
Titers of MAb 8E7 against M. catarrhalis LOS were expressed as the reciprocals of the dilutions.
—, unknown.
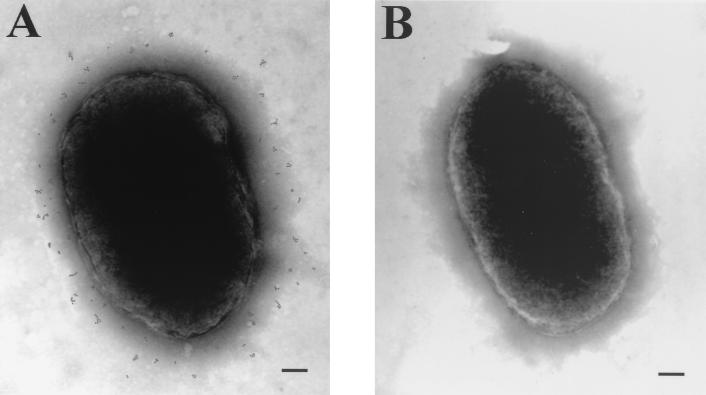
Immuno-electron microscopy of bacteria with MAb 8E7.
Immuno-electron microscopy was done on fixed whole cells to determine if MAb 8E7 recognized a surface-exposed epitope on the intact bacterium. Figure 3 shows cells of strain O35E incubated with MAb 8E7 or the irrelevant mouse ascites followed by exposure to protein A colloidal gold. MAb 8E7 bound to the surface of the bacterium, as evidenced by the electron-dense gold particles scattered across the surface of the cell (Fig. 3A). The irrelevant ascites did not bind to the surface of this organism (Fig. 3B). This finding confirmed the surface localization of the LOS epitope recognized by MAb 8E7.
FIG. 3.
Binding of 8E7 to the surface of strain O35E as visualized by immuno-electron microscopy. Cells of this strain were incubated with 8E7 (A) or the irrelevant ascites (B) at a 1:100 dilution. Gold particles are 5 nm in diameter.
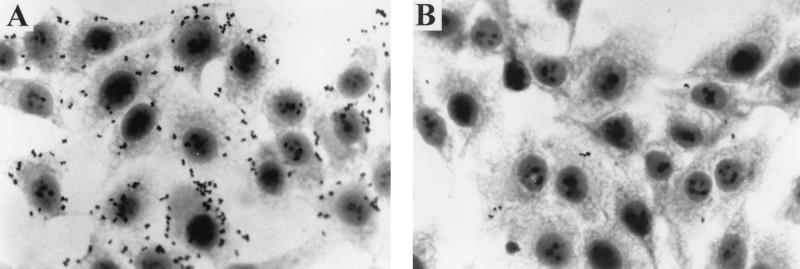
Effect of MAb 8E7 on adherence to epithelial cells.
To investigate the role of LOS in the adherence of M. catarrhalis to epithelial cells, MAb 8E7 was examined for its effect on the adherence of strain O35E to the Chang conjunctival epithelial cells (Fig. 4). Bacteria were incubated with epithelial cells in the presence of MAb 8E7 or the irrelevant mouse ascites. In the presence of the irrelevant mouse ascites, the bacteria were observed to attach to the surface of the Chang conjunctival epithelial cells and to the interstitial spaces between the cells (Fig. 4A). In contrast, preincubation of bacteria with MAb 8E7 inhibited bacterial attachment to the epithelial cells by 90%, from 10 bacteria to 1 bacterium/cell (Fig. 4B).
FIG. 4.
Inhibition of strain O35E adherence to Chang conjunctival epithelial cells by MAb 8E7. The bacteria were incubated with the irrelevant mouse ascites (A) or with MAb 8E7 (B) at a 1:100 dilution. Magnification, ×100.
Effect of MAb 8E7 on bacterial clearance from mouse lungs.
The above results indicated that the epitope on the outer surface of strain O35E that was recognized by MAb 8E7 is exposed and accessible. Furthermore, they indicated that LOS was involved in the bacterial attachment to the epithelium. This prompted us to evaluate MAb 8E7 in a mouse aerosol challenge model and to determine whether this antibody could enhance clearance of M. catarrhalis in vivo. Mice were immunized intraperitoneally with 1 ml of 1, 5, or 25% MAb 8E7 or of 25% irrelevant mouse ascites and were challenged with 3 × 107 CFU of strain O35E per ml. As shown in Table 2, the number of bacteria recovered at 6 h postchallenge was significantly reduced for the groups treated with 25 and 5% MAb 8E7 compared to the control group (P < 0.01). No difference was seen between the 1% MAb 8E7-treated group and the control group. The ELISA results confirmed that the MAb 8E7-treated groups had higher titers against LOS than the control group. Meanwhile, to further investigate whether MAb 8E7 was responsible for the enhancement of bacterial clearance from the lungs, we used serotype A and B LOSs to absorb MAb 8E7. The serotype A LOS was used because it has the strongest reactivity with MAb 8E7 (Fig. 1). After absorption, the LOS ELISA titer dropped from 1:196,830 to 1:270 for MAb absorbed by serotype A LOS. There was less of a reduction for MAb absorbed by serotype B LOS, from 1:196,830 to 1:98,415. The mice that received an intraperitoneal injection of 1 ml of 25% serotype A LOS-absorbed MAb 8E7 failed to enhance the pulmonary clearance of the bacteria compared with the control group (Table 2). However, the mice that received serotype B LOS-absorbed MAb 8E7 showed a similar clearance rate (65%) compared with the mice that received 25 or 5% MAb 8E7.
TABLE 2.
Effect of passive immunization with different doses of MAb 8E7 on bacterial recovery of strain O35E in mouse lungsa
| Dose (1 ml/mouse) | Anti-LOS IgG titer (range)b | Bacterial recovery (CFU/lung)c | Bacterial reduction (%)d |
|---|---|---|---|
| 25% MAb 8E7 | 17,556 (7,290–21,870) | 6.6 × 102 ± 0.3 × 102e | 65 |
| 5% MAb 8E7 | 1,009 (810–2,430) | 6.4 × 102 ± 0.4 × 102e | 66 |
| 1% MAb 8E7 | 174 (90–270) | 1.3 × 103 ± 0.2 × 103 | 31 |
| 25% LOSf-absorbed MAb 8E7 | 19 (10–30) | 1.5 × 103 ± 0.3 × 103 | 21 |
| 25% Irrelevant mouse ascites | 10 | 1.9 × 103 ± 0.5 × 103 | 0 |
Mice were challenged with 3 × 107 CFU of strain O35E per ml in the nebulizer, and their lungs were collected at 6 h postchallenge.
Levels of serum antibody against M. catarrhalis LOS were detected by ELISA and expressed as the reciprocal of the geometric mean for five mice per group.
Means ± standard deviations for five mice.
Compared to 25% irrelevant mouse ascites.
P < 0.01 compared to 25% irrelevant mouse ascites.
Serotype A LOS.
DISCUSSION
Recently, several MAbs against LOSs from M. catarrhalis were developed by two research groups. Oishi et al. described a MAb reactive with a common epitope of M. catarrhalis LOS (27). This MAb could bind to live bacterial cells and culture supernatants from a total of 34 strains of M. catarrhalis, including 12 strains of different LOS serotypes. Rahman et al. described six MAbs raised against the three major serotypes of M. catarrhalis LOSs (29). MAbs selected from serotype A and C LOSs reacted with all three LOS serotypes, but the MAb prepared against serotype B LOS was serotype B specific. The common structure among the three serotypes is a terminal tetrasaccharide α-Gal-(1→4)-β-Gal-(1→4)-α-Glc-(1→2)-β-Glc-(1→ at the branch substituting position 6 of the trisubstituted Glc residue. The terminal trisaccharide also occurs in the parts of glycolipids on the surface of human epithelial cells of mucous membranes (22). The above non-type-specific MAbs were shown to bind to the terminal disaccharide, α-Gal-(1→4)-β-Gal, based on their reactivity with a panel of synthetic glycoproteins and glycolipids.
We have examined MAb 8E7, which reacted with the serotype A and C LOSs but not with serotype B LOS. A comparison of these LOS structures has allowed us to locate a MAb 8E7-specific epitope. Besides the terminal tetrasaccharide described above, another common structure of serotypes A and C is α-GlcNAc-(1→2)-β-Glc-(1→ at the branch substituting position 4 of the trisubstituted Glc residue (8, 9). This epitope is located in the terminal of serotype A LOS but is an internal part of the serotype C LOS. This structure is not found in human tissues. Further study is warranted to identify the protective epitope of MAb 8E7 by structural analysis and saccharide inhibition studies.
Bacterial adherence to the surface of epithelial cells plays an important role in colonization and is generally believed to be the first step in the pathogenesis of both systemic and mucosal infections. Our results showed that the binding of MAb 8E7 significantly abrogated the adherence of strain 035E to Chang conjunctival epithelial cells. This suggests that LOS is an adhesin. However, another possibility also exists. The epitope recognized by MAb 8E7 may not be directly involved in adherence; instead, the MAb's effect on bacterial attachment may be due to steric hindrance (34) or charge shift (1). Further study, possibly with a deletion of LOS from the outer membrane of M. catarrhalis by gene mutation, will be needed to evaluate if LOS itself is an adhesin and to determine what role it plays in bacterial adherence.
One of our primary interests is the immune response to LOS. In particular, we are interested in the potential of LOS as a vaccine antigen. The results of passive immunization with MAb 8E7 indicated that the number of bacteria recovered from the lungs of the immunized mice is significantly less than that seen in the control mice. The enhancement of bacterial clearance following passive immunization occurred in a dose-dependent manner and was abrogated by absorption of the MAb 8E7 with serotype A LOS. These results indicate that M. catarrhalis LOS-directed antibodies played a major role in the enhancement of clearance of a homologous strain from mouse lungs. It has been reported that serum IgG can enter the alveolar spaces even under normal conditions (24). This suggests that MAb 8E7 entered the alveolar spaces and exerted its protective effect by blocking bacterial adherence of the epithelium.
In summary, a bactericidal MAb 8E7 recognizes surface-exposed LOS of M. catarrhalis, inhibits adherence of the bacterium to epithelial cells, and enhances bacterial clearance from the lungs in an aerosol challenge mouse model. Therefore, M. catarrhalis LOS is a promising vaccine candidate and deserves further study.
ACKNOWLEDGMENTS
We are grateful to Eric Hansen for providing MAb 8E7 and M. catarrhalis strains, Goro Mogi for providing M. catarrhalis clinical isolates, and Takashi Hirano for performing the Western blotting.
REFERENCES
- 1.Ahmed K, Nakagawa T, Nakano Y, Martinez G, Ichinose A, Zheng C H, Akaki M, Aikawa M, Nagatake T. Attachment of Moraxella catarrhalis occurs to the positively charged domains of pharyngeal epithelial cells. Microb Pathog. 2000;28:203–209. doi: 10.1006/mpat.1999.0342. [DOI] [PubMed] [Google Scholar]
- 2.Alfa M J, DeGagne P. Attachment of Haemophilus ducreyi to human foreskin fibroblasts involves LOS and fibronectin. Microb Pathog. 1997;22:39–46. doi: 10.1006/mpat.1996.0089. [DOI] [PubMed] [Google Scholar]
- 3.Barenkamp S J, Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992;60:1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluestone C D. Otitis media and sinusitis in children. Role of Branhamella catarrhalis. Drugs. 1986;31(Suppl. 3):132–141. doi: 10.2165/00003495-198600313-00029. [DOI] [PubMed] [Google Scholar]
- 5.Boyle F M, Georghiou P R, Tilse M H, McCormack J G. Branhamella (Moraxella) catarrhalis: pathogenic significance in respiratory infections. Med J Aust. 1991;154:592–596. doi: 10.5694/j.1326-5377.1991.tb121219.x. [DOI] [PubMed] [Google Scholar]
- 6.DeMaria T F, Apicella M A, Nichols W A, Leake E R. Evaluation of the virulence of nontypeable Haemophilus influenzae lipooligosaccharide htrB and rfaD mutants in the chinchilla model of otitis media. Infect Immun. 1997;65:4431–4435. doi: 10.1128/iai.65.11.4431-4435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doern W J. Animal models of otitis media: other pathogens. Pediatr Infect Dis J. 1989;81(Suppl.):S45–S47. [PubMed] [Google Scholar]
- 8.Edebrink P, Jansson P E, Rahman M M, Widmalm G, Holme T, Rahman M. Structural studies of the O-antigen oligosaccharides from two strains of Moraxella catarrhalis serotype C. Carbohydr Res. 1995;266:237–261. doi: 10.1016/0008-6215(94)00276-l. [DOI] [PubMed] [Google Scholar]
- 9.Edebrink P, Jansson P E, Rahman M M, Widmalm G, Holme T, Rahman M, Weintraub A. Structural studies of the O-polysaccharide from the lipopolysaccharide of Moraxella (Branhamella) catarrhalis serotype A (strain ATCC 25238) Carbohydr Res. 1994;257:269–284. doi: 10.1016/0008-6215(94)80040-5. [DOI] [PubMed] [Google Scholar]
- 10.Edebrink P, Jansson P E, Widmalm G, Holme T, Rahman M. The structures of oligosaccharides isolated from the lipopolysaccharide of Moraxella catarrhalis serotype B, Strain CCUG 3292. Carbohydr Res. 1996;295:127–146. doi: 10.1016/s0008-6215(96)90132-9. [DOI] [PubMed] [Google Scholar]
- 11.Ejlertsen T, Thisted E, Ebbesen F, Olesen B, Renneberg J. Branhamella catarrhalis in children and adults. A study of prevalence, time of colonisation, and association with upper and lower respiratory tract infections. J Infect. 1994;29:23–31. doi: 10.1016/s0163-4453(94)94979-4. [DOI] [PubMed] [Google Scholar]
- 12.Faden H, Waz M J, Bernstein J M, Brodsky L, Stanievich J, Ogra P L. Nasopharyngeal flora in the first three years of life in normal and otitis-prone children. Ann Otol Rhinol Laryngol. 1991;100:612–615. doi: 10.1177/000348949110000802. [DOI] [PubMed] [Google Scholar]
- 13.Fomsgaard J S, Fomsgaard A, Hoiby N, Bruun B, Galanos C. Comparative immunochemistry of lipopolysaccharides from Branhamella catarrhalis strains. Infect Immun. 1991;59:3346–3349. doi: 10.1128/iai.59.9.3346-3349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu X X, Chen J, Barenkamp S J, Robbins J B, Tsai C M, Lim D J, Battey J. Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect Immun. 1998;66:1891–1897. doi: 10.1128/iai.66.5.1891-1897.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu X X, Tsai C M. Purification of rough-type lipopolysaccharides of Neisseria meningitidis from cells and outer membrane vesicles in spent media. Anal Biochem. 1991;196:311–318. doi: 10.1016/0003-2697(91)90472-6. [DOI] [PubMed] [Google Scholar]
- 16.Gu X X, Tsai C M, Apicella M A, Lim D J. Quantitation and biological properties of released and cell-bound lipooligosaccharides from nontypeable Haemophilus influenzae. Infect Immun. 1995;63:4115–4120. doi: 10.1128/iai.63.10.4115-4120.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helminen M E, Maciver I, Latimer J L, Cope L D, McCracken G H, Hansen E J. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–2010. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homoe P, Prag J, Olsen C B, Farholt S. Nasopharyngeal bacteria found on blood agar plates from healthy children in Greenland. Int J Circumpolar Health. 1998;57:32–39. [PubMed] [Google Scholar]
- 19.Hu W G, Chen J, Battey J F, Gu X X. Enhancement of clearance of bacteria from murine lungs by immunization with detoxified lipooligosaccharide from Moraxella catarrhalis conjugated to proteins. Infect Immun. 2000;68:4980–4985. doi: 10.1128/iai.68.9.4980-4985.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W G, Chen J, Collins F M, Gu X X. An aerosol challenge mouse model for Moraxella catarrhalis. Vaccine. 1999;18:799–804. doi: 10.1016/s0264-410x(99)00335-7. [DOI] [PubMed] [Google Scholar]
- 21.Kahler C M, Stephens D S. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin) Crit Rev Microbiol. 1998;24:281–334. doi: 10.1080/10408419891294216. [DOI] [PubMed] [Google Scholar]
- 22.Mandrell R E, Griffiss J M, Macher B A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988;168:107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masoud H, Perry M B, Brisson J R, Uhrin D, Richards J C. Structural elucidation of the backbone oligosaccharide from the lipopolysaccharide of Moraxella catarrhalis serotype A. Can J Chem. 1994;72:1466–1477. [Google Scholar]
- 24.McGehee J L, Radolf J D, Toews G B, Hansen E J. Effect of primary immunization on pulmonary clearance of nontypable Haemophilus influenzae. Am J Respir Cell Mol Biol. 1989;1:201–210. doi: 10.1165/ajrcmb/1.3.201. [DOI] [PubMed] [Google Scholar]
- 25.Murphy T F. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267–279. doi: 10.1128/mr.60.2.267-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicotra B, Rivera M, Luman J I, Wallace R J., Jr Branhamella catarrhalis as a lower respiratory tract pathogen in patients with chronic lung disease. Arch Intern Med. 1986;146:890–893. [PubMed] [Google Scholar]
- 27.Oishi K, Tanaka H, Sonoda F, Borann S, Ahmed K, Utsunomiya Y, Watanabe K, Nagatake T, Vaneechoutte M, Verschraegen G, Matsumoto K. A monoclonal antibody reactive with a common epitope of Moraxella (Branhamella) catarrhalis lipopolysaccharides. Clin Diagn Lab Immunol. 1996;3:351–354. doi: 10.1128/cdli.3.3.351-354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman M, Holme T, Jonsson I, Krook A. Lack of serotype-specific antibody response to lipopolysaccharide antigens of Moraxella catarrhalis during lower respiratory tract infection. Eur J Clin Microbiol Infect Dis. 1995;14:297–304. doi: 10.1007/BF02116522. [DOI] [PubMed] [Google Scholar]
- 29.Rahman M, Jonsson A B, Holme T. Monoclonal antibodies to the epitope alpha-Gal-(1-4)-beta-Gal-(1- of Moraxella catarrhalis LPS react with a similar epitope in type IV pili of Neisseria meningitidis. Microb Pathog. 1998;24:299–308. doi: 10.1006/mpat.1997.0191. [DOI] [PubMed] [Google Scholar]
- 30.Song W, Ma L, Chen R, Stein D C. Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J Exp Med. 2000;191:949–960. doi: 10.1084/jem.191.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka H, Oishi K, Sonoda F, Iwagaki A, Nagatake T, Matsumoto K. Biochemical analysis of lipopolysaccharides from respiratory pathogenic Branhamella catarrhalis strains and the role of anti-LPS antibodies in Branhamella respiratory infections. J Jpn Assoc Infect Dis. 1992;66:709–715. doi: 10.11150/kansenshogakuzasshi1970.66.709. [DOI] [PubMed] [Google Scholar]
- 32.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 33.Unhanand M, Maciver I, Ramilo O, Arencibia-Mireles O, Argyle J C, McCracken G H, Hansen E J. Pulmonary clearance of Moraxella catarrhalis in an animal model. J Infect Dis. 1992;165:644–650. doi: 10.1093/infdis/165.4.644. [DOI] [PubMed] [Google Scholar]
- 34.van den Berg B M, Beekhuizen H, Mooi F R, van Furth R. Role of antibodies against Bordetella pertussis virulence factors in adherence of Bordetella pertussis and Bordetella parapertussis to human bronchial epithelial cells. Infect Immun. 1999;67:1050–1055. doi: 10.1128/iai.67.3.1050-1055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaneechoutte M, Verschraegen G, Claeys G, van den Abeele A M. Serological typing of Branhamella catarrhalis strains on the basis of lipopolysaccharide antigens. J Clin Microbiol. 1990;28:182–187. doi: 10.1128/jcm.28.2.182-187.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weel J F, Hopman C T, van Putten J P. Stable expression of lipooligosaccharide antigens during attachment, internalization, and intracellular processing of Neisseria gonorrhoeae in infected epithelial cells. Infect Immun. 1989;57:3395–3402. doi: 10.1128/iai.57.11.3395-3402.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]