Abstract
Streptococcal toxic shock syndrome (STSS) is a highly lethal, acute-onset illness that is a subset of invasive streptococcal disease. The majority of clinical STSS cases have been associated with the pyrogenic toxin superantigens (PTSAgs) streptococcal pyrogenic exotoxin A or C (SPE A or C), although cases have been reported that are not associated with either of these exotoxins. Recent genome sequencing projects have revealed a number of open reading frames that potentially encode proteins with similarity to SPEs A and C and to other PTSAgs. Here, we describe the cloning, expression, purification, and functional characterization of a novel exotoxin termed streptococcal pyrogenic exotoxin J (SPE J). Purified recombinant SPE J (rSPE J) expressed from Escherichia coli stimulated the expansion of both rabbit splenocytes and human peripheral blood lymphocytes, preferentially expanded human T cells displaying Vβ2, -3, -12, -14, and -17 on their T-cell receptors, and was active at concentrations as low as 5 × 10−6 μg/ml. Furthermore, rSPE J induced fevers in rabbits and was lethal in two models of STSS. Biochemically, SPE J had a predicted molecular weight of 24,444 and an isoelectric point of 7.7 and lacked the ability to form the cystine loop structure characteristic of many PTSAgs. SPE J shared 19.6, 47.1, 38.8, 18.1, 19.6, and 24.4% identity with SPEs A, C, G, and H, streptococcal superantigen, and streptococcal mitogenic exotoxin Z-2, respectively, and was immunologically cross-reactive with SPE C. The characterization of a seventh functional streptococcal PTSAg raises important questions relating to the evolution of the streptococcal superantigens.
Streptococcus pyogenes (group A streptococcus) produces a variety of exotoxins, including the streptococcal pyrogenic exotoxins (SPEs; scarlet fever toxins) that have been implicated in severe invasive streptococcal diseases such as streptococcal toxic shock syndrome (STSS) and scarlet fever (reviewed in references 29, 37, 51, and 54). The SPEs belong to a larger group of pyrogenic toxin superantigens (PTSAgs) (5) that stimulate T cells by binding both invariant regions on major histocompatibility complex (MHC) class II molecules and specific Vβ chains of the T-cell receptor; this activity has been termed superantigenicity (36). The ability of PTSAgs to bind to the T-cell receptor (TCR) is independent of the peptide contained in the groove of MHC class II molecules, and since there are relatively few TCR Vβ regions, the frequency of T cells responding to PTSAg exposure exceeds that of conventional peptide antigens by several orders of magnitude (reviewed in reference 35). Extensive T-cell proliferation results in massive cytokine release, which is believed to contribute to the most severe effects of STSS.
Structural and immunological characterization of the SPEs have revealed that they are low-molecular-weight proteins (24,000 to 28,000) that are relatively heat and protease resistant. Although only SPEs A and C are generally considered to be associated with STSS (7–9, 16, 17, 41, 45, 55, 58), distinct SPE serotypes are now known to include serotypes A (23, 63), B (14), C (12), F (46, 64), G (48), and H (48), as well as the streptococcal superantigen (SSA) (40) and multiple streptococcal mitogenic exotoxin Z (SMEZ) serotypes (25, 48, 49). However, unlike the other SPE serotypes, SPEs B and F have enzymatic activity (protease and DNase, respectively) (13, 18, 27), neither has sequence homology to the other SPEs, and SPE B is known to not share structural similarity with the other SPE serotypes (24).
It has been shown that several clinical strains of S. pyogenes from patients with STSS produce neither SPE A nor SPE C (6, 10, 25, 42, 44, 62). This observation raises the possibility that uncharacterized PTSAgs, related to SPE A or SPE C, are capable of causing STSS. Consistent with this hypothesis, it has been demonstrated that novel streptococcal superantigens are in fact made by virulent streptococci (1, 40). Furthermore, selective depletion of certain Vβ T-cell subsets from patients with STSS that did not correlate with Vβ patterns from known superantigens has been demonstrated (62). Importantly, this study also determined that many of the strains did not possess the genes for SPE A, SPE C, or SSA.
Two novel streptococcal superantigens (SPE G and SPE H) were recently identified and characterized by Proft et al. (48), aided by the S. pyogenes genomic database at the University of Oklahoma. These authors also identified SMEZ-2, a novel superantigen very similar to the previously described SMEZ (25). The crystal structures of SPE H and SMEZ-2 were also recently reported, and both conformed to the generic bacterial superantigen folding pattern (1). This group demonstrated that SMEZ, unlike other superantigens (at least to our current knowledge), is highly polymorphic in that 22 different smez alleles were identified. This research also revealed that although the polymorphisms in the SMEZ serotypes (now serotypes 1 through 22) maintained their Vβ T-cell subset stimulatory profiles (Vβ4 and Vβ8), they resulted in antigenic variants (48).
During the characterization of SPEs G, H, and SMEZ-2, a partial gene sequence of a putative PTSAg was also identified. This putative gene was named speJ, although it was not characterized further (48). The present study describes the cloning, expression, and immunological characterization of this novel PTSAg, termed SPE J, and we show that this toxin has properties similar to those of already-characterized PTSAgs, including mitogenic activity for rabbit and human T cells, Vβ-specific superantigenic activity, and lethal activity in two models of STSS. SPE J represents a seventh streptococcal superantigen, most similar to SPE C, and raises interesting questions about the evolution of the streptococcal superantigens.
MATERIALS AND METHODS
Cloning of speJ.
During the characterization of SPEs G and H, only a partial region of speJ was identified (48). Since this time, the complete sequence of the S. pyogenes SF370 genome, including the remainder of speJ, has been released from the Streptococcal Genome Sequencing Project at the University of Oklahoma. From this sequence, we predicted the full-length translation product of speJ, which showed significant homology to other known SPEs. The protein sequences of SPEs A, C, G, H, and J were aligned to provide a basis to predict the signal peptide cleavage site of SPE J (Fig. 1). From this prediction, DNA primers (5′-GCGCCCCATGGATAGTGAAAATATTAAAGAC-3′ and 5′-GCGCGGATCCTTATTTAGTCCAAAGGTAAATATC-3′; Sigma-Genosys, The Woodlands, Tex.) were designed containing the restriction enzyme recognition sequences for NcoI and BamHI (underlined in the primer sequences) to amplify speJ lacking coding sequence for the putative signal peptide. Taq DNA polymerase (Qiagen, Inc., Valencia, Calif.) was used for PCR amplification from DNA extracted from S. pyogenes strains SF370 and HMC-2. Amplified speJ was digested with the restriction enzymes NcoI and BamHI (Roche Molecular Biochemicals, Indianapolis, Ind.) and cloned into the pET-28 (Novagen, Inc., Madison, Wis.) expression vector using Escherichia coli XL1-Blue (Stratagene, La Jolla, Calif.) as the host. The DNA sequence of the cloned gene was determined by the University of Minnesota Advanced Genetic Analysis Center, and both PCR products (from strains SF 370 and HMC 2) were identical.
FIG. 1.
Amino acid alignment of all known streptococcal superantigens. Identical residues conserved over all seven superantigens are shown in black, and highly conserved residues are shaded in gray.
Expression of recombinant SPE J.
All reagents and glassware used for toxin purification and biological assays were maintained pyrogen free. Recombinant SPE J (rSPE J) was produced in E. coli BL21(DE3) (Novagen) containing the recombinant plasmid pET-28::speJ. Cells were grown in dialyzable beef heart medium (52) at 37°C containing 50 μg of kanamycin (Sigma Chemical Co., St. Louis, Mo.) per ml for plasmid maintenance and induced with 0.2 mM isopropyl-β-d-galactopyranoside (IPTG) (Sigma) when the absorbance (600 nm) was approximately 0.5. After 3 to 4 h, rSPE J was both released from the E. coli cells and precipitated with 4 volumes of ethanol. Concentrated crude protein containing rSPEJ was resolubilized in water, and two consecutive separations via flatbed isoelectric focussing (pH 3-10 ampholytes and then pH 6-8 ampholytes, Amersham Pharmacia Biotech AB, Uppsala, Sweden) were done. Isoelectric focusing revealed an rSPE J protein with an isoelectric point of approximately 7.7. Dialysis against distilled water to remove ampholytes completed the purification of rSPE J. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 12% gels (31) and Western immunoblotting (4) were used to estimate the molecular weight, the homogeneity of purified toxin, and the serologic cross-reactivity with SPEs A and C.
Antibody production.
Antibody was obtained by immunizing an American Dutch Belted rabbit (Birchwood Farms, Red Wing, Minn.) with 25 μg of rSPE J in phosphate-buffered saline (PBS; 0.005 M NaPO4, 0.15 M NaCl; pH 7.2) and Freund incomplete adjuvant (final volume of 1 ml) (Difco Laboratories, Detroit, Mich.) on days 0, 14, and 28. The rabbit was bled on days 21 and 35, and the resultant anti-SPE J serum was used for Western blot analysis.
Mitogenic activity.
The mitogenicity of rSPE J was determined via incorporation of [3H]thymidine into rabbit splenocytes and human peripheral blood mononuclear cells (PBMCs), as described previously (3, 38). RPMI 1640 medium (Gibco-BRL, Grand Island, N.Y.) supplemented with 2% fetal calf serum (FCS; Gibco), 100 U of penicillin (Mediatech Cellgro) per ml, 100 μg of streptomycin (Mediatech Cellgro) per ml, and 2 mM l-glutamine (Mediatech Cellgro) was used to culture American Dutch Belted rabbit splenocytes and human PBMCs; lymphocytes were used at a concentration of 2 × 105 cells per well in 200-μl volumes. Serial 10-fold dilutions of rSPE J (beginning at 10 μg/well) were added to the wells, and each dilution was assayed in quadruplicate; control wells received lymphocytes only. Equivalent concentrations of purified SPE A or SPE C prepared as previously described (38, 50) were added to the wells for comparison. Plates were incubated at 37°C for 72 h in 7% CO2, and then 1 μCi of [3H]thymidine (Amersham Corp., Arlington Heights, Ill.) was added to each well. After another 18 h, cells were harvested onto fiberglass filters (Whatman, Maidstone, England), and incorporation of the [3H]thymidine was determined using a scintillation counter.
Vβ profile determination by fluorescence-activated cell sorter (FACS) analysis.
Separate preparations of PBMCs obtained from three normal human donors were isolated from heparinized venous blood by density gradient sedimentation over Ficoll-Hypaque (Histopaque; Sigma). In each case, cells were washed three times in Hanks balanced salt solution (HBSS; Mediatech Cellgro, Herndon, Va.) and resuspended in medium for cell culture. PBMCs (106 cells/ml) were cultured in RPMI 1640 (Mediatech Cellgro) supplemented with 10% heat-inactivated FCS (Gemini Bioproducts, Woodland, Calif.), 20 mM HEPES buffer (Mediatech Cellgro), 100 U of penicillin (Mediatech Cellgro) per ml, 100 μg of streptomycin (Mediatech Cellgro) per ml, and 2 mM l-glutamine (Mediatech Cellgro). Cells were cultured in the presence of either anti-CD3 (20 ng/ml) or rSPE J (100 ng/ml) for 3 days, washed, and allowed to grow for an additional day in the presence of interleukin-2 (50 U/ml) before washing and staining them for immunofluoresence analysis of the Vβ T-cell repertoire as previous described (33, 34, 56).
For flow cytometry studies, PBMCs were washed in HBSS and resuspended at 10 × 106 cells/ml in a staining solution (PBS with 5% FCS [Gemini Bioproducts], 1% immunoglobulin [Alpha Therapeutic Corp., Los Angeles, Calif.], 0.02% sodium azide [Sigma]). Cells were stained in 96-well, round-bottomed plates with a panel of biotinylated monoclonal antibodies against human Vβ2, -3, -5.1, -5.2, -7, -8, -11, -12, -13.1, -13.2, -14, -16, -17, -20, -21.3, and -22 (Immunotech, Westbrook, Maine), Vβ9 and -23 (Pharmingen, San Diego, Calif.), and Vβ6.7 fluorescein isothiocyanate (FITC; Endogen, Woburn, Mass.); the cells were then incubated for 30 min at 37°C in the dark. After the incubation period, the cells were washed twice with washing buffer (PBS, 2% FCS [Gemini Bioproducts], 0.02% sodium azide [Sigma]) by centrifugation at 300 × g for 5 min at 4°C. The cell pellets were resuspended in staining solution and incubated with anti-CD3 allophycocyanin (APC), anti-CD4 phycoerythrin (Becton Dickinson, San Jose, Calif.), anti-CD8 (FITC) (Becton Dickinson), and a streptavidin peridinin chlorophyl protein (PerCP) conjugate (Becton Dickinson) for 30 min at 4°C. Stained cells were again washed twice in washing buffer and once in 0.02% sodium azide (Sigma) in PBS by centrifugation at 300 × g for 5 min at 4°C. Finally, cells were fixed in 300 μl of 1% (vol/vol) formaldehyde (Polysciences, Warrington, Pa.) in 1× PBS. Analysis was performed using four-color flow cytometry (FACSCalibur; Becton Dickinson) as described elsewhere (56). List mode multiparameter data files (each file with forward scatter, side scatter, and four fluorescent parameters) were analyzed using CellQuest software (Becton Dickinson). Analysis of activated populations was performed with the light scatter gate set on the T-cell blast population. Negative control reagents were used to verify the staining specificity of experimental antibodies.
Pyrogenicity and lethal models of toxic shock syndrome.
American Dutch Belted rabbits were used to establish the pyrogenicity and capacity for enhancement of susceptibility to the lethal effects of endotoxin of rSPE J. Rabbits were injected with either rSPE J or toxic shock syndrome toxin 1 (TSST-1) as a positive control in the marginal ear veins (5 μg/kg), and temperatures were recorded at both 2 and 4 h in order to assay the pyrogenicity.
The ability of rSPE J to enhance host susceptibility to lethal endotoxin shock (endotoxin enhancement) was assayed via injection of rSPE J as described for the pyrogenicity model, followed by intravenous injection of endotoxin from Salmonella enterica serovar Typhimurium (10 μg/kg, 1/50 lethal dose, 50% endpoint) at the 4-h time point. Animals were monitored for symptoms of STSS, and mortality was recorded over a 2-day period.
The miniosmotic pump model of STSS (32, 47) was used to assess the lethality of the rSPE J in a situation that is presumed to resemble infection with exotoxin-producing S. pyogenes. The miniosmotic pumps are designed to release a constant amount of exotoxin into the subcutaneous tissue for 7 days. Three American Dutch Belted rabbits were anesthetized with ketamine and xylazine (Phoenix Pharmaceuticals, Inc., St. Joseph, Mo.), and miniosmotic pumps (Alza Pharmaceuticals, Palo Alto, Calif.) prefilled with 200 or 500 μg of rSPE J or 200 μg of TSST-1 in 200 μl of PBS were implanted subcutaneously in the left flank. Rabbits were monitored for signs of STSS, and mortality was recorded over a 15-day period.
Sequence accession number.
The nucleotide and protein sequence corresponding to the SPE J protein was deposited with GenBank under accession no. AF321000.
RESULTS
Cloning and purification of rSPE J.
The complete speJ gene was identified from the S. pyogenes SF370 genomic sequence, based on previous work (48) and homology to known PTSAgs. The predicted translation product of speJ was a polypeptide of 232 amino acids with a molecular weight of 27,171 and contained only one cysteine residue. Because PTSAgs are secreted toxins that contain signal peptides, we examined this protein for this feature. Typical signal peptides have three domains that are structurally conserved based on hydrophobicity, charge or size of the amino acids (61). The amino terminus is short and positively charged, followed by a central and longer, hydrophobic domain, and a carboxy domain, normally characterized by small side chain amino acids at the −3 and −1 positions relative to the cleavage site. It was apparent that SPE J contained the first two domains, although a cleavage site could not immediately be predicted for this protein. Therefore, we aligned SPE J with known streptococcal PTSAgs and predicted a possible cleavage site based on homology to known signal peptides (Fig. 1).
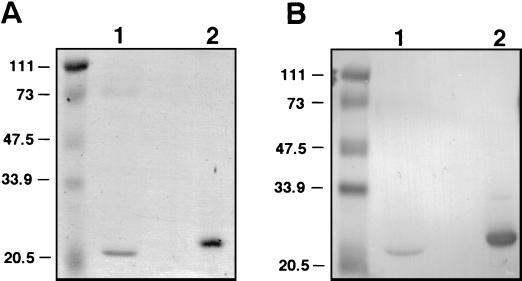
The region of speJ predicted to encode mature SPE J was amplified by PCR and cloned into the pET-28 E. coli expression vector. The sequences of speJ from strains SF370 and HMC-2 were identical. rSPE J was expressed and purified, and the functional activities were determined. SDS-PAGE revealed an apparently pure preparation of rSPE J (Fig. 2A). Western blot analysis with polyclonal antibodies raised against rSPE J revealed cross-reactivity with SPE C (Fig. 2B) but not SPE A (not shown).
FIG. 2.
SDS-PAGE (A) and Western blot analysis (B) of rSPE J and SPE C with antisera raised against rSPE J. Lanes 1 and 2 contain approximately 5 μg of purified SPE C and rSPE J, respectively. Molecular mass markers are shown in kilodaltons.
Superantigenicity.
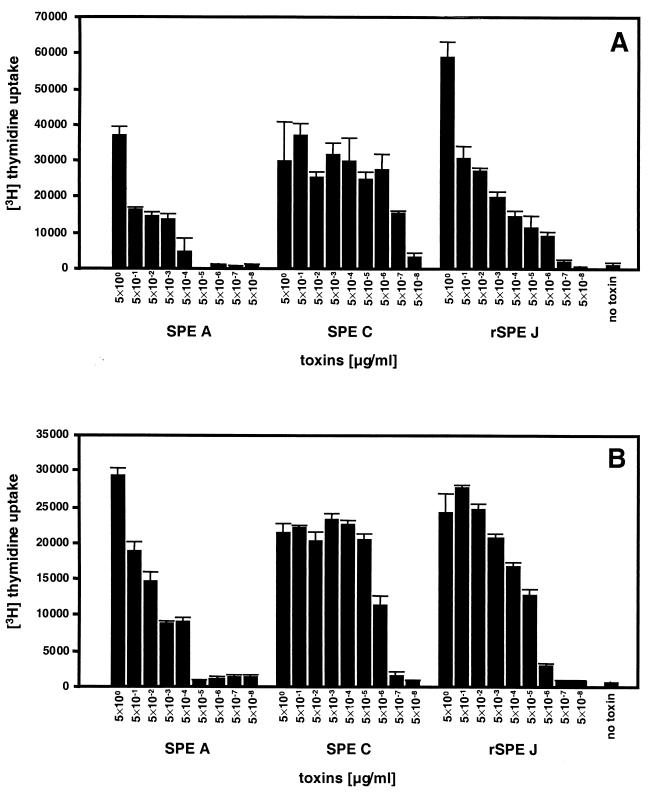
One of the characteristics of SPEs is the ability to induce marked T-cell proliferation as superantigens. rSPE J was a potent lymphocyte mitogen, as measured by the incorporation of [3H]thymidine into both rabbit splenocytes (Fig. 3A) and human PBMCs (Fig. 3B). rSPE J caused proliferation of rabbit splenocytes and human PBMCs at concentrations as low as 5 × 10−6 μg/ml. The proliferation observed for rSPE J was comparable to the proliferation caused by identical concentrations of SPE C, which showed significant activity at 5 × 10−7 μg/ml for rabbit splenocytes and 5 × 10−6 μg/ml for human PBMCs, where as SPE A was active at 5 × 10−4 μg/ml for both of these cell types.
FIG. 3.
Mitogenic activity of recombinant SPE J for rabbit splenocytes (A) and human PBMCs (B). Cells (2 × 105/well) were incubated with toxins at 37°C in 7% CO2 for 3 days. Cells were then labeled with 1 μCi of [3H]thymidine, and DNA was harvested after another 24 h of incubation. The counts per minute were determined by scintillation counting and plotted at each concentration of protein. The bars represent the mean ± the standard error for experiments done in quadruplicate. Negative controls represent cells incubated without toxin.
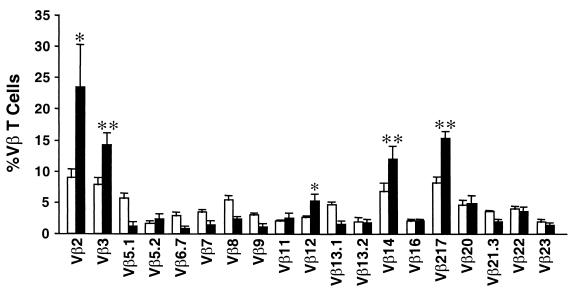
Although nonspecific mitogens are capable of inducing lymphocyte proliferation in culture, superantigens preferentially stimulate T cells with specific Vβ regions on their TCRs. In order to verify superantigenicity, the TCR Vβ stimulation profile of rSPE J-treated human lymphocytes was determined. T cells bearing Vβ2, -3, -12, -14, or -17 were preferentially stimulated by rSPE J (Fig. 4). Consistent with superantigen activation, both CD4+ and CD8+ T cells were stimulated, although CD4+ cells represented the majority of stimulated cells (73.9%).
FIG. 4.
TCR Vβ profiles of T cells stimulated with rSPE J. The bars represent the percentage of total T cells stimulated with rSPE J (solid bars) compared to stimulation with anti-CD3 (open bars) expressed as the means ± the standard error obtained for three individuals; each individual was analyzed separately. ∗, P < 0.05; ∗∗, P < 0.01 (when stimulation by rSPE J was compared to stimulation by anti-CD3 antibodies) as determined by the paired Student's t test.
Fever and lethality activity.
Another characteristic of the rSPEs is their ability to cause fever and enhance the susceptibility of rabbits to intravenously administered endotoxin. Three rabbits were given a sublethal intravenous bolus of rSPE J or TSST-1, and their temperatures were recorded at both 2 and 4 h (Table 1). At 4 h, temperatures had risen 0.9°C, indicating that rSPE J had pyrogenic activity; in this model, fevers are considered significant if the average was greater than 0.5°C (53). Challenge with a sublethal intravenous bolus of endotoxin at 4 h after injection of rSPE J resulted in lethality in three of five rabbits (Table 1), indicating that rSPE J is capable of enhancing susceptibility to endotoxin-induced shock. Comparable results were obtained for rabbits challenged with TSST-1 and then endotoxin.
TABLE 1.
Pyrogenic and lethal activities of rSPE J
| Toxin | Pyrogenic activitya
|
Lethal models (no. of animals dead/total no.)
|
|||
|---|---|---|---|---|---|
| Temp increase (2 h) (°C) | Temp increase (4 h) (°C) | Endotoxin enhancementb | Miniosmotic pumpc with:
|
||
| 200 μg | 500 μg | ||||
| TSST-1 | 1.3 | 1.7 | 3/5 | 3/3 | NDd |
| SPE J | 0.2 | 0.9 | 3/5 | 0/3 | 2/3 |
Three rabbits were used for each pyrogenicity assay. An amount (5 μg/kg) was injected intravenously at 0 h. Temperature changes of 0.5°C or greater are considered significant in this model (53).
Mortality was recorded for a 48-h period following the injection of endotoxin.
Mortality was recorded for 10 days following subcutaneous implantation of a miniosmotic pump.
ND, not determined.
Slow subcutaneous release of PTSAg (as provided by a miniosmotic pump) is presumed to mimic toxin release by an exotoxin-producing strain of S. pyogenes. The miniosmotic pump model of STSS (32, 47) results in lethal activity without the administration of exogenous endotoxin. The three rabbits implanted with 200 μg of rSPE J in miniosmotic pumps did not succumb to the effects of rSPE J after 15 days; however, a dose of 500 μg was lethal in two of three rabbits in this model. Rabbits that received 200 μg of TSST-1 as a control also succumbed.
Phylogenetic analysis of all known streptococcal and staphylococcal PTSAgs.
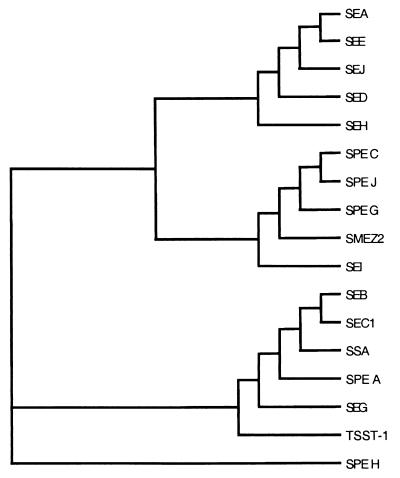
Using CLUSTAL W (59) we aligned the protein sequences of all known PTSAgs including SPE serotypes A, C, G, H, and J, SSA and SMEZ-2, and staphylococcal enterotoxin (SE) serotypes A, B, C1, D, E, G, H, I, and J, as well as TSST-1, to create a phylogenetic tree of this large family of toxins (Fig. 5). This analysis showed three main branches, except for SPE H which is less related to the other PTSAgs. SPE J was most similar to SPEs C and G and to SMEZ-2. Interestingly, two of the branches contained both streptococcal and staphylococcal PTSAgs.
FIG. 5.
Phylogenetic tree of all known streptococcal and staphylococcal pyrogenic toxin superantigens. The tree was created using the CLUSTAL W program (59) for PTSAg alignment. Three main branches are shown with SPE H representing an individual branch.
DISCUSSION
In this study, rSPE J was cloned from two clinical strains of S. pyogenes isolated from patients with STSS. These strains are known to contain the genes for SPEs A and B and SPEs B and C, respectively, and are now known to also contain the gene for SPE J. The speJ gene was identical in both strains, and the mature protein had a predicted molecular weight of 24,444 and an isoelectric point of 7.6. These values are consistent with those obtained by experimental measurement. Although related to all PTSAgs in the primary sequence, SPE J was most similar to SPE C, with 47.1% identity. Because SPE J contains only one cysteine residue, this toxin does not contain a cystine loop structure that is seen in many PTSAgs, such as SPE A and the SEs A, B, C, D, and E. Finally, epitopes on SPE J cross-reacted with epitopes on SPE C as determined by Western blotting. It is possible that previous studies which determined SPE C production by antibody could also have been detecting SPE J, although we currently do not know whether SPE J is produced by clinical strains of group A streptococci. These data also suggest that persons with immunity to SPE C may also be immune to SPE J.
Biologically, PTSAgs share several activities, including pyrogenicity, superantigenicity, the ability to enhance the susceptibility of the host to the lethal effects of endotoxin, and the ability to induce lethality of rabbits when administered via subcutaneously implanted miniosmotic pumps. rSPE J shares these activities with other PTSAgs. The toxin was pyrogenic and lethal in rabbits (although not as toxic as TSST-1 when administered via miniosmotic pumps) and stimulated proliferation of both rabbit and human lymphocytes. It has not been determined if SPE G and H, SSA, or SMEZ serotypes have pyrogenic or lethal activity. However, because all known structures conform to the typical superantigen fold (1, 57) and act as superantigens (25, 40, 48, 49), it is likely they also share the lethal phenotypes. Interestingly, rSPE J stimulated a unique pattern of human T cells bearing the TCR subsets Vβ2, -3, -12, -14, and -17. Despite the highest homology with SPE C, the Vβ pattern stimulated by rSPE J was most similar to that of SPE A, which stimulates Vβ2, -12, -14, and -15, whereas SPE C stimulates Vβ1, -2, -5.1, and -10 (60). It would be of interest to determine which residues in SPE C and SPE J are responsible for the different Vβ specificities. Both SPE C and SPE J were extremely potent in stimulating rabbit splenocytes and human PBMCs compared to SPE A, which was active above ca. 2 higher orders of magnitude in concentration. The basis for this difference is currently unknown, although Proft et al. (48) have reported activity at similar concentrations with SMEZ. These three toxins (SPE C, SPE J, and SMEZ) are also phylogenetically similar (Fig. 5), indicating that some structural feature of this group of toxins may account for their potency.
The characterization of a seventh, distinct streptococcal PTSAg raises some interesting questions regarding the evolution of the potent toxins. It is currently unclear as to why there are so many functional PTSAgs present in both group A streptococci and S. aureus. The extensive homology among streptococcal and staphylococcal superantigens suggests that they share a common ancestor, either before the evolutionary divergence of the two organisms or as a result of horizontal gene transfer. The two prototypic streptococcal superantigens, SPE A and SPE C, are both encoded on functional phage (11, 20–23, 26, 39, 41), and lateral movement by converting bacteriophage has been demonstrated for both speA (19, 63, 65) and speC (11). Comparison of the phylogenetic tree constructed from all known streptococcal and staphylococcal bacterial superantigens reveals three main evolutionary branches, not including SPE H (Fig. 5). Interestingly, two of these main branches contain both streptococcal and staphylococcal superantigens. Based on this analysis, these toxins may have at one time resided in the same host or were freely exchanged between hosts. It is possible that the PTSAg genes undergo rearrangement during phage transfer into a new host, giving rise to the distinct serotypes. The speA and speC genes are variable characteristics in different S. pyogenes strains, which is consistent with them being encoded on phage. Some of the other streptococcal superantigen genes are probably variable traits (speH, ssa, and speJ), while some are apparently not (speG and smeZ). In a recent study of 103 isolates from New Zealand, only 24% possessed the speH gene, while all possessed some form of smeZ (49). Consistent with this observation, speG and smeZ do not appear to be genetically linked to phage in the S. pyogenes SF370 genome.
From an evolutionary understanding, it is unlikely that these toxins have evolved to induce life-threatening illness in humans. We believe that these potent toxins must provide their bacterial host with a distinct, yet largely unrecognized evolutionary advantage during infection. This reasoning may help to explain the multiple serotypes of the streptococcal superantigens and why some of the toxins, such as SMEZ (49), undergo apparent antigenic variation. Although SPE A and C have historically been associated with invasive streptococcal disease, we believe that the presence of a specific PTSAg gene is not the most appropriate association to examine. Instead, the expression level of any one of these potent toxins may determine their ability to cause severe streptococcal disease such as STSS and scarlet fever. Due to the presence of at least seven PTSAgs in group A streptococci, it is possible that other toxins within this group will be discovered. Evidence also suggests that PTSAgs may be present in other species. Compounds from group G streptococci have been partially characterized that are mitogenic and can produce fevers and induce lethal activity in the endotoxin hypersensitivity model of toxic shock syndrome (2). Since numerous cases of STSS caused by group C (15, 28, 43) and group G (15, 30) streptococci have been reported, it is possible that other novel superantigens are involved. Further study of this important family of toxins will improve our understanding of superantigen biology and evolution and may ultimately lead to improved treatments for severe streptococcal disease.
ACKNOWLEDGMENTS
This investigation was supported by U.S. Public Health Service research grants HL36611, AR41256, and HL37260 from the National Institutes of Health.
We thank Heather Donahue for technical assistance, Jeremy Yarwood for critical reading of the manuscript, and Timothy Leonard for assistance with photography. We also acknowledge the Streptococcal Genome Sequencing Project, University of Oklahoma (funded by USPHS/NIH grant AI38406) and B. A. Roe, S. P. Linn, L. Song, X. Yuan, S. Clifton, R. E. McLaughlin, M. McShan, and J. Ferretti.
REFERENCES
- 1.Arcus V L, Proft T, Sigrell J A, Baker H M, Fraser J D, Baker E N. Conservation and variation in superantigen structure and activity highlighted by the three-dimensional structures of two new superantigens from Streptococcus pyogenes. J Mol Biol. 2000;299:157–168. doi: 10.1006/jmbi.2000.3725. [DOI] [PubMed] [Google Scholar]
- 2.Assimacopoulos A P, Stoehr J A, Schlievert P M. Mitogenic factors from group G streptococci associated with scarlet fever and streptococcal toxic shock syndrome. Adv Exp Med Biol. 1997;418:109–114. doi: 10.1007/978-1-4899-1825-3_27. [DOI] [PubMed] [Google Scholar]
- 3.Barsumian E L, Schlievert P M, Watson D W. Nonspecific and specific immunological mitogenicity by group A streptococcal pyrogenic exotoxins. Infect Immun. 1978;22:681–688. doi: 10.1128/iai.22.3.681-688.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake M S, Johnston K H, Russell-Jones G J, Gotschlich E C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 5.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 6.Chatellier S, Ihendyane N, Kansal R G, Khambaty F, Basma H, Norrby-Teglund A, Low D E, McGeer A, Kotb M. Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect Immun. 2000;68:3523–3534. doi: 10.1128/iai.68.6.3523-3534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleary P P, Kaplan E L, Handley J P, Wlazlo A, Kim M H, Hauser A R, Schlievert P M. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet. 1992;339:518–521. doi: 10.1016/0140-6736(92)90339-5. [DOI] [PubMed] [Google Scholar]
- 8.Cockerill F R, III, MacDonald K L, Thompson R L, Roberson F, Kohner P C, Besser-Wiek J, Manahan J M, Musser J M, Schlievert P M, Talbot J, Frankfort B, Steckelberg J M, Wilson W R, Osterholm M T. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA. 1997;277:38–43. [PubMed] [Google Scholar]
- 9.Demers B, Simor A E, Vellend H, Schlievert P M, Byrne S, Jamieson F, Walmsley S, Low D E. Severe invasive group A streptococcal infections in Ontario, Canada: 1987–1991. Clin Infect Dis. 1993;16:792–800. doi: 10.1093/clind/16.6.792. [DOI] [PubMed] [Google Scholar]
- 10.Descheemaeker P, Van Loock F, Hauchecorne M, Vandamme P, Goossens H. Molecular characterisation of group A streptococci from invasive and non-invasive disease episodes in Belgium during 1993–1994. J Med Microbiol. 2000;49:467–471. doi: 10.1099/0022-1317-49-5-467. [DOI] [PubMed] [Google Scholar]
- 11.Goshorn S C, Schlievert P M. Bacteriophage association of streptococcal pyrogenic exotoxin type C. J Bacteriol. 1989;171:3068–3073. doi: 10.1128/jb.171.6.3068-3073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goshorn S C, Schlievert P M. Nucleotide sequence of streptococcal pyrogenic exotoxin type C. Infect Immun. 1988;56:2518–2520. doi: 10.1128/iai.56.9.2518-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauser A R, Schlievert P M. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J Bacteriol. 1990;172:4536–4542. doi: 10.1128/jb.172.8.4536-4542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauser A R, Stevens D L, Kaplan E L, Schlievert P M. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J Clin Microbiol. 1991;29:1562–1567. doi: 10.1128/jcm.29.8.1562-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirose Y, Yagi K, Honda H, Shibuya H, Okazaki E. Toxic shock-like syndrome caused by non-group A beta-hemolytic streptococci. Arch Intern Med. 1997;157:1891–1894. [PubMed] [Google Scholar]
- 16.Hoge C W, Schwartz B, Talkington D F, Breiman R F, MacNeill E M, Englender S J. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. A retrospective population-based study. JAMA. 1993;269:384–389. [PubMed] [Google Scholar]
- 17.Holm S E, Norrby A, Bergholm A M, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988–1989. J Infect Dis. 1992;166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki M, Igarashi H, Yutsudo T. Mitogenic factor secreted by Streptococcus pyogenes is a heat-stable nuclease requiring His122 for activity. Microbiology. 1997;143:2449–2455. doi: 10.1099/00221287-143-7-2449. [DOI] [PubMed] [Google Scholar]
- 19.Johnson L P, L'Italien J J, Schlievert P M. Streptococcal pyrogenic exotoxin type A (scarlet fever toxin) is related to Staphylococcus aureus enterotoxin B. Mol Gen Genet. 1986;203:354–356. doi: 10.1007/BF00333979. [DOI] [PubMed] [Google Scholar]
- 20.Johnson L P, Schlievert P M. Group A streptococcal phage T12 carries the structural gene for pyrogenic exotoxin type A. Mol Gen Genet. 1984;194:52–56. doi: 10.1007/BF00383496. [DOI] [PubMed] [Google Scholar]
- 21.Johnson L P, Schlievert P M. A physical map of the group A streptococcal pyrogenic exotoxin bacteriophage T12 genome. Mol Gen Genet. 1983;189:251–255. doi: 10.1007/BF00337813. [DOI] [PubMed] [Google Scholar]
- 22.Jonhson L P, Schlievert P M, Watson D W. Transfer of group A streptococcal pyrogenic exotoxin production to nontoxigenic strains of lysogenic conversion. Infect Immun. 1980;28:254–257. doi: 10.1128/iai.28.1.254-257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson L P, Tomai M A, Schlievert P M. Bacteriophage involvement in group A streptococcal pyrogenic exotoxin A production. J Bacteriol. 1986;166:623–627. doi: 10.1128/jb.166.2.623-627.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagawa T F, Cooney J C, Baker H M, McSweeney S, Liu M, Gubba S, Musser J M, Baker E N. Crystal structure of the zymogen form of the group A Streptococcus virulence factor SpeB: an integrin-binding cysteine protease. Proc Natl Acad Sci USA. 2000;97:2235–2240. doi: 10.1073/pnas.040549997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamezawa Y, Nakahara T, Nakano S, Abe Y, Nozaki-Renard J, Isono T. Streptococcal mitogenic exotoxin Z, a novel acidic superantigenic toxin produced by a T1 strain of Streptococcus pyogenes. Infect Immun. 1997;65:3828–3833. doi: 10.1128/iai.65.9.3828-3833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapur V, Nelson K, Schlievert P M, Selander R K, Musser J M. Molecular population genetic evidence of horizontal spread of two alleles of the pyrogenic exotoxin C gene (speC) among pathogenic clones of Streptococcus pyogenes. Infect Immun. 1992;60:3513–3517. doi: 10.1128/iai.60.9.3513-3517.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapur V, Topouzis S, Majesky M W, Li L L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 28.Keiser P, Campbell W. ‘Toxic strep syndrome’ associated with group C Streptococcus. Arch Intern Med. 1992;152:882–884. doi: 10.1001/archinte.152.4.882a. [DOI] [PubMed] [Google Scholar]
- 29.Kotb M. Bacterial pyrogenic exotoxins as superantigens. Clin Microbiol Rev. 1995;8:411–426. doi: 10.1128/cmr.8.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kugi M, Tojo H, Haraga I, Takata T, Handa K, Tanaka K. Toxic shock-like syndrome caused by group G Streptococcus. J Infect. 1998;37:308–309. doi: 10.1016/s0163-4453(98)92510-5. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Lee P K, Deringer J R, Kreiswirth B N, Novick R P, Schlievert P M. Fluid replacement protection of rabbits challenged subcutaneous with toxic shock syndrome toxins. Infect Immun. 1991;59:879–884. doi: 10.1128/iai.59.3.879-884.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung D Y, Gately M, Trumble A, Ferguson-Darnell B, Schlievert P M, Picker L J. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–753. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung D Y, Travers J B, Giorno R, Norris D A, Skinner R, Aelion J, Kazemi L V, Kim M H, Trumble A E, Kotb M, et al. Evidence for a streptococcal superantigen-driven process in acute guttate psoriasis. J Clin Investig. 1995;96:2106–2112. doi: 10.1172/JCI118263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Llera A, Malchiodi E L, Mariuzza R A. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–466. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 36.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 37.McCormick J K, Schlievert P M. Toxins and superantigens of group A streptococci. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: American Society for Microbiology; 2000. pp. 43–52. [Google Scholar]
- 38.McCormick J K, Tripp T J, Olmsted S B, Matsuka Y V, Gahr P J, Ohlendorf D H, Schlievert P M. Development of streptococcal pyrogenic exotoxin C vaccine toxoids that are protective in the rabbit model of toxic shock syndrome. J Immunol. 2000;165:2306–2312. doi: 10.4049/jimmunol.165.4.2306. [DOI] [PubMed] [Google Scholar]
- 39.McShan W M, Tang Y F, Ferretti J J. Bacteriophage T12 of Streptococcus pyogenes integrates into the gene encoding a serine tRNA. Mol Microbiol. 1997;23:719–728. doi: 10.1046/j.1365-2958.1997.2591616.x. [DOI] [PubMed] [Google Scholar]
- 40.Mollick J A, Miller G G, Musser J M, Cook R G, Grossman D, Rich R R. A novel superantigen isolated from pathogenic strains of Streptococcus pyogenes with amino-terminal homology to staphylococcal enterotoxins B and C. J Clin Investig. 1993;92:710–719. doi: 10.1172/JCI116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musser J M, Hauser A R, Kim M H, Schlievert P M, Nelson K, Selander R K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci USA. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakashima K, Ichiyama S, Iinuma Y, Hasegawa Y, Ohta M, Ooe K, Shimizu Y, Igarashi H, Murai T, Shimokata K. A clinical and bacteriologic investigation of invasive streptococcal infections in Japan on the basis of serotypes, toxin production, and genomic DNA fingerprints. Clin Infect Dis. 1997;25:260–266. doi: 10.1086/514543. [DOI] [PubMed] [Google Scholar]
- 43.Natoli S, Fimiani C, Faglieri N, Laurenzi L, Calamaro A, Frasca A M, Arcuri E. Toxic shock syndrome due to group C streptococci. A case report. Intensive Care Med. 1996;22:985–989. doi: 10.1007/BF02044129. [DOI] [PubMed] [Google Scholar]
- 44.Newton D, Norrby-Teglund A, McGeer A, Low D E, Schlievert P M, Kotb M. Novel superantigens from streptococcal toxic shock syndrome Streptococcus pyogenes isolates. Adv Exp Med Biol. 1997;418:525–529. doi: 10.1007/978-1-4899-1825-3_123. [DOI] [PubMed] [Google Scholar]
- 45.Norgren M, Norrby A, Holm S E. Genetic diversity in T1M1 group A streptococci in relation to clinical outcome of infection. J Infect Dis. 1992;166:1014–1020. doi: 10.1093/infdis/166.5.1014. [DOI] [PubMed] [Google Scholar]
- 46.Norrby-Teglund A, Newton D, Kotb M, Holm S E, Norgren M. Superantigenic properties of the group A streptococcal exotoxin SpeF (MF) Infect Immun. 1994;62:5227–5233. doi: 10.1128/iai.62.12.5227-5233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parsonnet J, Gillis Z A, Richter A G, Pier G B. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect Immun. 1987;55:1070–1076. doi: 10.1128/iai.55.5.1070-1076.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proft T, Moffatt S L, Berkahn C J, Fraser J D. Identification and characterization of novel superantigens from Streptococcus pyogenes. J Exp Med. 1999;189:89–102. doi: 10.1084/jem.189.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proft T, Moffatt S L, Weller K D, Paterson A, Martin D, Fraser J D. The streptococcal superantigen SMEZ exhibits wide allelic variation, mosaic structure, and significant antigenic variation. J Exp Med. 2000;191:1765–1776. doi: 10.1084/jem.191.10.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roggiani M, Stoehr J A, Olmsted S B, Matsuka Y V, Pillai S, Ohlendorf D H, Schlievert P M. Toxoids of streptococcal pyrogenic exotoxin A are protective in rabbit models of streptococcal toxic shock syndrome. Infect Immun. 2000;68:5011–5017. doi: 10.1128/iai.68.9.5011-5017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlievert P M, Kotb M Y, Stevens D L. Streptococcal superantigens: streptococcal toxic shock syndrome. In: Cunningham M W, Fujinami R S, editors. Effects of microbes on the immune system. Philadelphia, Pa: The Williams & Wilkins Co.; 2000. pp. 25–39. [Google Scholar]
- 52.Schlievert P M, Shands K N, Dan B B, Schmid G P, Nishimura R D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 53.Schlievert P M, Watson D W. Group A streptococcal pyrogenic exotoxin: pyrogenicity, alteration of blood-brain barrier, and separation of sites for pyrogenicity and enhancement of lethal endotoxin shock. Infect Immun. 1978;21:753–763. doi: 10.1128/iai.21.3.753-763.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens D L. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu Rev Med. 2000;51:271–288. doi: 10.1146/annurev.med.51.1.271. [DOI] [PubMed] [Google Scholar]
- 55.Stevens D L, Tanner M H, Winship J, Swarts R, Ries K M, Schlievert P M, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 56.Strickland I, Hauk P J, Trumble A E, Picker L J, Leung D Y. Evidence for superantigen involvement in skin homing of T cells in atopic dermatitis. J Investig Dermatol. 1999;112:249–253. doi: 10.1046/j.1523-1747.1999.00502.x. [DOI] [PubMed] [Google Scholar]
- 57.Sundberg E, Jardetzky T S. Structural basis for HLA-DQ binding by the streptococcal superantigen SSA. Nat Struct Biol. 1999;6:123–129. doi: 10.1038/5809. [DOI] [PubMed] [Google Scholar]
- 58.Talkington D F, Schwartz B, Black C M, Todd J K, Elliott J, Breiman R F, Facklam R R. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect Immun. 1993;61:3369–3374. doi: 10.1128/iai.61.8.3369-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomai M A, Schlievert P M, Kotb M. Distinct T-cell receptor V beta gene usage by human T lymphocytes stimulated with the streptococcal pyrogenic exotoxins and pep M5 protein. Infect Immun. 1992;60:701–705. doi: 10.1128/iai.60.2.701-705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe-Ohnishi R, Low D E, McGeer A, Stevens D L, Schlievert P M, Newton D, Schwartz B, Kreiswirth B, Kotb M. Selective depletion of V beta-bearing T cells in patients with severe invasive group A streptococcal infections and streptococcal toxic shock syndrome. Ontario Streptococcal Study Project. J Infect Dis. 1995;171:74–84. doi: 10.1093/infdis/171.1.74. [DOI] [PubMed] [Google Scholar]
- 63.Weeks C R, Ferretti J J. Nucleotide sequence of the type A streptococcal exotoxin (erythrogenic toxin) gene from Streptococcus pyogenes bacteriophage T12. Infect Immun. 1986;52:144–150. doi: 10.1128/iai.52.1.144-150.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yutsudo T, Murai H, Gonzalez J, Takao T, Shimonishi Y, Takeda Y, Igarashi H, Hinuma Y. A new type of mitogenic factor produced by Streptococcus pyogenes. FEBS Lett. 1992;308:30–34. doi: 10.1016/0014-5793(92)81043-l. [DOI] [PubMed] [Google Scholar]
- 65.Zabriskie J. The role of temperate bacteriophage in the production of erythrogenic toxin by group A streptococci. J Exp Med. 1964;119:761–780. doi: 10.1084/jem.119.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]