Abstract
Background
Women with HIV (WWH) face heightened risks of heart failure; however, insights on immune/inflammatory pathways potentially contributing to left ventricular (LV) systolic dysfunction among WWH remain limited.
Setting
Massachusetts General Hospital, Boston, Massachusetts.
Methods
Global longitudinal strain (GLS) is a sensitive measure of LV systolic function, with lower cardiac strain predicting incident heart failure and adverse heart failure outcomes. We analyzed relationships between GLS (cardiovascular magnetic resonance imaging) and monocyte activation (flow cytometry) among 20 WWH and 14 women without HIV.
Results
WWH had lower GLS compared to women without HIV (WWH vs. women without HIV: 19.4±3.0 vs. 23.1±1.9%, P<0.0001). Among the whole group, HIV status was an independent predictor of lower GLS. Among WWH (but not among women without HIV), lower GLS related to a higher density of expression of HLA-DR on the surface of CD14+CD16+ monocytes (ρ = -0.45, P = 0.0475). Further, among WWH, inflammatory monocyte activation predicted lower GLS, even after controlling for CD4+ T-cell count and HIV viral load.
Conclusions
Additional studies among WWH are needed to examine the role of inflammatory monocyte activation in the pathogenesis of lower GLS and to determine whether targeting this immune pathway may mitigate risks of heart failure and/or adverse heart failure outcomes.
Trial registration
Clinical trials.gov registration: NCT02874703.
Introduction
People with HIV (PWH) on antiretroviral therapy (ART) face a two-fold increased risk of heart failure as compared to people without HIV [1–5]. PWH also have worse heart failure outcomes, including higher rates of heart failure hospitalization and cardiovascular mortality [6, 7]. Persistent monocyte activation is thought to contribute to heightened heart failure risk and adverse heart failure outcomes among PWH [5]. In vitro and animal studies have implicated monocytes and macrophages in pathologic cardiac remodeling through pro-inflammatory and pro-fibrotic phenotypes which promote myocardial fibrosis. Myocardial fibrosis, in turn, can have downstream effects on cardiac function and ultimately can progress to heart failure [8]. Notably, a recent meta-analysis suggests that risks of HIV-associated heart failure are higher in women vs. men [1]. Among PWH, sex-specific differences in heart failure risk may relate to sex-differences in systemic immune activation/inflammation [9]. Few studies, however, have focused on elucidating relevant immune pathways contributing to heart failure risk specifically among women with HIV (WWH).
Previous studies employing cardiovascular imaging techniques to interrogate heart failure risks among predominantly male cohorts of people with vs. without HIV suggest that PWH have lower global longitudinal strain (GLS), or cardiac strain [10–12]. GLS is a more sensitive measure of cardiac systolic function than ejection fraction and a lower GLS has been shown to be a robust predictor of adverse cardiac outcomes in different populations [13–15]. The value of GLS is a simple parameter that expresses longitudinal shortening of the cardiac muscle in systole as a percentage of the baseline length in diastole [16]. GLS provides meaningful information on heart failure risk, with general-population studies demonstrating that lower GLS predicts both incident heart failure and adverse heart failure outcomes [15, 17–19]. No prior studies have investigated GLS or immune correlates specifically among women with vs. without HIV, as we set out to do here. We leveraged data from a translational physiology study which included detailed immune phenotyping and cardiovascular magnetic resonance imaging (MRI) among matched cohorts of women with and without HIV with no known cardiovascular disease (CVD) [20].
Materials and methods
Study design and participants
Women with vs. without HIV, age 45 to 70 years, without a history CVD or diabetes were recruited from the Greater Boston Area, as previously described [20]. WWH were group-matched based on age and body mass index (BMI) to women without HIV. Twenty WWH and 14 women without HIV underwent immune and cardiovascular phenotyping, including cardiovascular MRI imaging. Data on GLS in this cohort have not previously been published. All participants provided written informed consent. This study was approved by the Massachusetts General Brigham Institutional Review Board and is registered on clinicaltrials.gov (NCT02874703).
Cardiovascular MRI
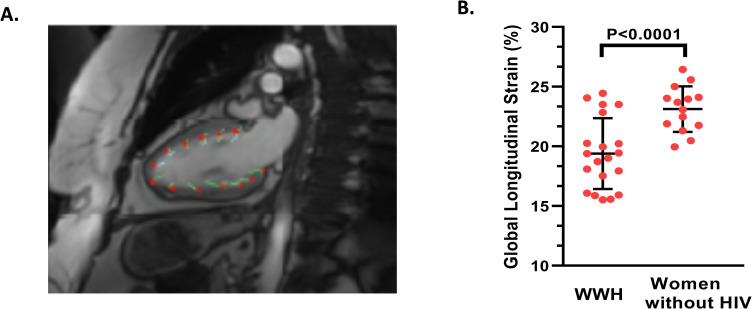
The cardiovascular magnetic resonance imaging protocol was performed on a Siemens Skyra 3 Tesla MR scanner (Erlangen, Germany), as previously described [20]. Electrocardiogram (ECG)-gated b-SSFP cine sequences were acquired for long-axis 2-, 3- and 4- chamber views as well as a short axis stack. Imaging specifications included: 40 frames/cardiac cycle, pixel spacing 0.8mm x 0.8mm, 8mm slice thickness, as well as inter-slice gap, (time to echo) TE 1.5ms, (time to repetition) TR 3ms. Images were analyzed using Medis Q-strain software (Medis Medical Imaging Systems, Leiden, the Netherlands) by two readers blinded to HIV status (Fig 1A). GLS was characterized as follows: Feature tracking was performed in the end-diastole, and in the end-systole using Medis. The left ventricle was tracked at the endo- and epicardial borders in 2- and 4- chamber long axis views, with subsequent averaging of peak strain values to derive GLS. Tracking accuracy was visually reviewed and, if needed, corrections were made to the initial contours only. This procedure was repeated three times to determine an average GLS for each study participant. While GLS is a negative percentage, as it is calculated based on the longitudinal shortening of the myocardium during contraction, absolute percentage values are shown here to simplify data presentation. Please see S1 File for additional details.
Fig 1. Global longitudinal strain on cardiovascular MRI among women with versus without HIV.
A. Representative cardiovascular MRI image from a study participant illustrating longitudinal feature tracking of a 2- chamber cine image and analyzed using QStrain, the Medis strain software. B. Women with HIV had lower GLS compared to women without HIV. The mean and standard deviation are shown for each respective group. GLS is expressed as an absolute percentage value. Abbreviations: HIV, human immunodeficiency virus; MRI, magnetic resonance imaging; WWH, women with HIV.
Laboratory assessments and immune parameters
Flow cytometric assessments of monocyte subpopulations were performed from cryopreserved peripheral blood mononuclear cells (PBMCs) using a BD FACsAria and analyzed using FlowJo software 8.7.1 (Treestar, Ashland, OR) [20]. The absolute number and relative percentages of monocyte subpopulations (classical, inflammatory/ intermediate and non-classical/patrolling/homing) were determined. The mean fluorescence intensity (MFI) of cell-surface receptors on various monocyte subpopulations was also determined [20]. Among WWH, CD4+ T cell counts were collected prospectively as part of their screen visit, while HIV viral load assessments were collected on the day of their imaging procedures.
Statistical analysis
Our primary endpoint was the between-group difference in cardiac strain, quantified by the MRI-derived measure of GLS. Parameters associated with global longitudinal strain were assessed among the whole group and among sub-groups of women with vs. without HIV. Bivariate analyses were performed using either a Pearson’s or Spearman’s correlation coefficient, as appropriate. Multivariable regression modeling among the whole group was performed using global longitudinal strain as the dependent variable and atherosclerotic cardiovascular disease (ASCVD) risk score and HIV status as independent variables. Among the WWH, multivariable regression modeling was performed using GLS as the dependent variable and CD4+ T-cell count, HIV viral load, and immune parameters identified through correlational analysis as independent variables. Statistical analyses were performed using JMP Pro software (versions 16.0; SAS Institute).
Results
Demographic, cardiovascular, and immune parameters among women with vs. without HIV
Key demographic, cardiovascular, and immune parameters among women with vs. without HIV in this cohort have been previously published [20] and are presented in S1 Table for reference. Briefly, women with vs. without HIV were similar in age and BMI. WWH had evidence of diastolic dysfunction and higher left ventricular mass index, as well as elevated levels of select immune/inflammatory indices. The expression of the major histocompatibility complex (MHC) class II cell surface receptor, human-leukocyte-associated antigen-D Related (HLA-DR), on the surface of CD14+CD16+ (inflammatory) monocytes was higher among WWH compared to women without HIV [24600.5 (16933.0, 28725.8) vs. 14025.0 (11489.5, 20020.0), P = 0.001].
Relationships between GLS and other measures of cardiac structure and function
WWH exhibited lower GLS as compared to women without HIV (GLS: 19.4±3.0 vs 23.1±1.9%, respectively, P<0.0001; Fig 1B). Among the whole group, after controlling for 10-year ASCVD risk score, HIV status independently predicted lower GLS (Overall Model: R2 = 0.38, P = 0.0008; HIV status: β-estimate = -1.98, P = 0.0002; Table 1A). Among the whole group, lower GLS related to lower left ventricular ejection fraction (LVEF) (r = 0.40, P = 0.02), as expected given that GLS is a measure of systolic function. Among the whole group, lower GLS was also related to lower left atrial passive EF (r = 0.38, P = 0.03) and higher left ventricular mass (ρ = -0.36, P = 0.03). Among the whole group, GLS, however, did not relate to myocardial fibrosis (r = 0.22, P = 0.23).
Table 1. Multivariable regression modeling for global longitudinal strain among (a) whole group and (b) women with HIV.
| a. Multivariable regression modeling for GLS among whole group | |||
| Whole Model R2 = 0.38, P = 0.0008 | |||
| Covariate | β-estimate | β-SE | P-value |
| HIV status (positive) | -1.98 | 0.46 | 0.0002 |
| ASCVD Risk Score (%) | 0.02 | 0.13 | 0.87 |
| b. Multivariable regression modeling for GLS among women with HIV | |||
| Whole Model R2 = 0.40, P = 0.0499 | |||
| Covariate | β-estimate | β-SE | P-value |
| CD4+ T-cell count (cells/mm3) | 0.0009 | 0.002 | 0.60 |
| HIV viral load (copies/mL) | -0.02 | 0.01 | 0.12 |
| Expression of HLA-DR on CD14+CD16+ monocytes (MFI) | -0.0002 | 0.00007 | 0.02 |
In multivariable modeling among the whole group, HIV status remained an independent predictor of lower GLS even after controlling for ASCVD Risk Score. In multivariable modeling among women with HIV, the expression of HLA-DR on CD14+CD16+ (inflammatory) monocytes remained an independent predictor of lower GLS even after controlling for the HIV-specific parameters of CD4+ T-cell count and HIV viral load.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; β-estimate, beta-estimate; β-SE, beta-standard error; CD4, cluster of differentiation 4; CD14, cluster of differentiation 14; CD16, cluster of differentiation 16; GLS, global longitudinal strain; HIV, Human Immunodeficiency Virus; HLA-DR, human-leukocyte-associated antigen-D Related; MFI, mean fluorescence index
Relationship between GLS and immune parameters
Among the whole group and among WWH (but not among women without HIV), lower GLS related to a higher density of expression of HLA-DR on the surface of CD14+CD16+ (inflammatory) monocytes, reflective of monocyte activation (Whole Group: ρ = -0.53, P = 0.001; WWH: ρ = -0.45, P = 0.0475). Further, among WWH, the density of expression of HLA-DR on the surface of CD14+CD16+ monocytes remained an independent predictor of lower GLS even after controlling for CD4+ T-cell count and HIV viral load (Whole Model: R2 = 0.40, P = 0.0499; CD14+CD16+ MFI of HLA-DR: β-estimate = -0.0002, P = 0.02; Table 1B). Among the whole group, lower GLS related to higher levels of soluble CD14 (ρ = -0.46, P = 0.006), but not to levels of soluble CD163 (r = 0.26, P = 0.14) and MCP-1/CCL2 (r = 0.15, P = 0.41). Among the whole group and among sub-groups, GLS did not relate to the absolute and percentage of CD14+CD16- (classical) monocytes (Absolute CD14+CD16- monocytes: Whole Group: ρ = 0.08, P = 0.68, WWH: ρ = -0.09, P = 0.70; women without HIV: ρ = 0.47, P = 0.10 and Percentage of CD14+CD16- monocytes: Whole Group: ρ = -0.24, P = 0.18, WWH: ρ = 0.02, P = 0.93; women without HIV: ρ = -0.12, P = 0.69), CD14+CD16+ (inflammatory/intermediate) monocytes (Absolute CD14+CD16+ monocytes: Whole Group: ρ = 0.11, P = 0.53, WWH: ρ = -0.06, P = 0.80; women without HIV: ρ = 0.26, P = 0.39 and Percentage of CD14+CD16+ monocytes: Whole Group: ρ = 0.17, P = 0.34, WWH: ρ = 0.08, P = 0.75; women without HIV: ρ = -0.13, P = 0.67), and CD14-CD16+ (non-classical/patrolling/homing) monocytes (Absolute CD14-CD16+ monocytes: Whole Group: ρ = 0.23, P = 0.20, WWH: ρ = -0.15, P = 0.52; women without HIV: ρ = 0.48, P = 0.10 and Percentage of CD14-CD16+ monocytes: Whole Group: ρ = 0.28, P = 0.12, WWH: ρ = 0.06, P = 0.79; women without HIV: ρ = 0.27, P = 0.36).
Relationship between GLS and ART use among WWH
Among our cohort of WWH on ART, GLS did not relate to the duration of ART use (ρ = -0.01, P = 97).
Discussion
In our study, WWH without known CVD had lower GLS, even after controlling for traditional CVD risk factors. Among the whole group and WWH (but not among women without HIV), the density of expression of HLA-DR on the surface of CD14+CD16+ (inflammatory) monocytes, reflective of inflammatory monocyte activation, related inversely to GLS and remained a predictor of lower GLS among WWH, even after controlling for HIV-specific factors. Together, these data suggest that among WWH, inflammatory monocyte activation – through effects on cardiac muscle function – may play a role in heightened heart failure risk.
In our study, women with (vs. without) HIV had lower GLS, which presages and predicts heart failure and adverse heart failure outcomes [18, 19]. These findings are directionally consistent with findings from a prior study among a predominantly-male cohort employing cardiovascular MRI [12]. Of note, a study of individuals with vs. without HIV in Uganda employing speckle-tracking echocardiography also revealed lower GLS among PWH, 60% of whom were women [21]. In our cohort of United States (U.S.) women, HIV infection predicted lower GLS even after controlling for ASCVD risk score, which encompasses age, sex, race, blood pressure, lipid levels, smoking status, history of diabetes, and current anti-hypertensive treatment. This finding suggests a potential pathway – beyond traditional CVD risk factors – through which HIV may augment risks of heart failure and adverse heart failure outcomes in this vulnerable population.
Higher density of expression of HLA-DR on the surface of CD14+CD16+ (inflammatory) monocytes remained a strong predictor of lower GLS among WWH in our study cohort, even after controlling for HIV-specific parameters. Inflammatory monocytes have a pro-inflammatory cytokine signature [22] and highly express the MHC class II receptor HLA-DR on their surface [23]. A higher density of expression of HLA-DR on the surface of inflammatory monocytes reflects a greater degree of monocyte activation. A prior U.S. study employing cardiovascular MRI to interrogate heart failure risk among a predominately male cohort of individuals with vs. without HIV revealed that lower radial strain (another cardiovascular MRI-derived measure of cardiac systolic function) was associated with higher systemic levels of the marker of monocyte activation, MCP-1, specifically among the sub-group with HIV [11]. While this study supports our own findings, it also differs in several respects including the measure of cardiac strain used (radial strain vs. longitudinal strain) and the study population (predominately male cohort of PWH vs. WWH). Future studies on sex-specific relationships between immune activation and lower GLS among PWH across regions are needed.
Evaluating sex-specific immune mechanisms of CVD risk among WWH is crucial given sex-differences in immune responses to HIV infection and sex-differences in relationships between immune indices and CVD risk surrogates among PWH. In response to HIV infection, women demonstrate higher levels of toll-like receptor signaling [9] and T-cell activation [24]. Sex-differences in the immune response to HIV infection may contribute to heightened levels of immune/inflammatory markers among WWH. In a recent study of ART-treated PWH in the U.S., WWH demonstrated higher levels of systemic markers of immune activation compared to men with HIV, even after controlling for ASCVD risk [25]. Further, select immune parameters associated significantly with CVD risk surrogates among WWH but not among men with HIV [25]. Conversely, in our current study, we did not find higher levels of inflammatory monocytes among WWH on ART compared to women without HIV, while among predominately male cohort of PWH on ART in separate study recruited from the same geographic region and similar entry criteria, we did find higher levels of inflammatory monocytes among PWH on ART [26]. To elucidate immune mechanisms contributing to heightened CVD risk specifically among WWH, we need more studies which either focus exclusively on WWH or which robustly enroll WWH, thereby enabling the performance of appropriately powered sex-stratified analyses.
Our study was limited by the cross-sectional design, which precludes inferences on causality. The participants in our study were recruited from a U.S. metropolitan area, which may limit the generalizability of our results to other regions. Our study was also limited by its relatively small sample size and the potential influence of unmeasured confounders on the between-group differences on GLS. A key strength of our study was the focus on WWH, a population underrepresented in CVD risk research. Further, our data were prospectively collected using cardiovascular MRI and detailed metabolic/immune phenotyping procedures. Lastly, our participants reflected the racial/ethnic diversity of the demographic of WWH in the Greater Boston area.
Conclusion
In our study, we applied cardiovascular MRI to quantify GLS in matched cohorts of US women with vs. without HIV. WWH (compared to age- and BMI- group matched women without HIV) had lower GLS; and HIV status was an independent predictor of lower GLS, controlling for traditional CVD risk. Inflammatory monocyte activation, in turn, related to lower GLS among WWH but not among women without HIV. Future studies are needed to determine if inflammatory monocyte activation (independent of CD4+T-cell count and HIV viral load) may contribute etiologically to lower cardiac strain among WWH on ART. Further, while in our study duration of ART use did not relate to lower GLS, future studies exploring the potential role of ART on inflammation and downstream adverse cardiac modeling among WWH are needed. Additional studies are also needed to investigate whether targeting inflammatory monocyte activation among WWH may prevent progression of subclinical LV systolic dysfunction to heart failure and adverse heart failure outcomes in this at-risk population.
Supporting information
Normally distributed variables are presented as mean ± standard deviation (SD); non-normally distributed data are presented as median (interquartile range; IQR). P-values were determined by student’s two-tailed t-test, Wilcoxon rank-sum test, and chi-square test for normally distributed, non-normally distributed, and categorical variables, respectively. There were no significant differences in baseline characteristics among women with versus without HIV. Select systemic markers of monocyte activation (MCP-1, sCD14, and sCD163) were higher among women with HIV versus without HIV. The expression of HLA-DR on the surface of CD14+CD16+ (inflammatory) monocytes, reflective of inflammatory monocyte activation, was higher among women with versus without HIV. *Lower limit of detection for the HIV viral load assay employed was 20 copies/mL. Values of 19 copies/mL were imputed when viral load was undetectable. **Expression of HLA-DR on monocyte subpopulations could not be obtained for one woman without HIV. Abbreviations: ART, anti-retroviral therapy; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CCR2, C-C chemokine receptor type 2; CCR5, C-C chemokine receptor type 5; CD4, cluster of differentiation 4; CD14, cluster of differentiation 14; CD16, cluster of differentiation 16; CXCL10, C-X-C motif chemokine 10; HDL-C, high-density lipoprotein cholesterol; HCV, hepatitis C virus; HIV, Human Immunodeficiency Virus; HLA-DR, human-leukocyte-associated antigen-D Related; INSTI, integrase inhibitor; LDL-C, low-density lipoprotein cholesterol; LMP, last menstrual period; MCP-1, monocyte chemoattractant protein 1; MFI, mean fluorescence intensity; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PBMCs, peripheral blood mononuclear cells; PIs, protease inhibitors; sCD14, soluble CD14; sCD163, soluble CD163; WHIV, women with HIV; WHR, waist to hip ratio.
(DOCX)
Additional details on the assessment of global longitudinal strain using cardiovascular MRI can be found in our Supplemental Methods.
(DOCX)
Acknowledgments
We thank the participants in this study and the Nursing Staff of the Massachusetts General Hospital (MGH) Translation and Clinical Research Center. We also thank Jacob Calkins, Mary O’Hara, and Larry White from the MGH Martinos Center for Biomedical Imaging.
Meetings: Data on cardiac strain from this original investigation including a smaller cohort of participants were presented in the Conference on Retroviruses and Opportunistic Infections in Boston, Massachusetts, United States in March of 2018. Further, data from this original investigation were presented as an oral presentation during the International Workshop on Women and HIV in April of 2022 (virtual conference) in Utrecht, Netherlands.
Data Availability
Our study data set even if de-identified contains sensitive information regarding the HIV status of our participants. Given this sensitive information together with the relatively small sample size for this study (which recruited only from the Greater Boston Area), we have concerns about public sharing of our data set. Requests for de-identified data could be made by contacting our Massachusetts General Hospital Institutional Review Board at partnersirb@partners.org and referring to Protocol 2015P000200.
Funding Statement
This project was funded through a Collaborative Feasibility award from the NIH/Harvard Center for AIDS Research [P30AI060354] to MVZ and TGN. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. MT is supported by the NIH/ National Heart, Lung, and Blood Institute (NHLBI) grant [1K23HL147799-01] and the American Heart Association-Harold Amos Medical Research Faculty Development Program by the Robert Wood Johnson Foundation. ZDD is supported by the ÚNKP-22-4-II-SE new national excellence program of the ministry for innovation and technology from the source of the National Research, Development, and Innovation Fund. LTF is supported by the NIH/National Institute of Child Health and Human Development (NICHD) grant [5K23HD100266-03]. T.H.B. has received support from the NIH/NHLBI grant [R01HL141132]. TGN is supported by the Michael and Kathryn Park Chair in Cardiology, the Hassenfeld Scholar Award, a gift from Paul and Christina Kasolinas, and a gift from A. Curt Greer and Pamela Kohlberg, and grants from the NIH/NHLBI [R01HL137562, R01HL130539, and K24HL150238]. MVZ is supported by grants from the NIH/NHLBI [R01HL137562] and [R01HL146267] and from the NIH/NIAID [1K24AI157882]. This work was also supported by the Nutrition Obesity Research Center at Harvard [DK040561] and by grants to the Harvard Clinical and Translational Science Center from the National Center for Research Resources [8 UL 1TR000170 and 1 UL 1TR001102].
References
- 1.Chen Y, Gao Y, Zhou Y, Li X, Wang H, Polonsky TS, et al. Human Immunodeficiency Virus Infection and Incident Heart Failure: A Meta-Analysis of Prospective Studies. J Acquir Immune Defic Syndr. 2021;87(1):741–9. Epub 2021/01/26. doi: 10.1097/QAI.0000000000002629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinstein MJ, Steverson AB, Ning H, Pawlowski AE, Schneider D, Ahmad FS, et al. Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. J Am Heart Assoc. 2018;7(21):e009985. Epub 2018/12/21. doi: 10.1161/JAHA.118.009985 ; PubMed Central PMCID: PMC6404176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yen YF, Ko MC, Yen MY, Hu BS, Wang TH, Chuang PH, et al. Human Immunodeficiency Virus Increases the Risk of Incident Heart Failure. J Acquir Immune Defic Syndr. 2019;80(3):255–63. Epub 2018/12/12. doi: 10.1097/QAI.0000000000001917 . [DOI] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2(5):536–46. Epub 2017/04/07. doi: 10.1001/jamacardio.2017.0264 2615262 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toribio M, Neilan TG, Zanni MV. Heart Failure among People with HIV: Evolving Risks, Mechanisms, and Preventive Considerations. Curr HIV/AIDS Rep. 2019;16(5):371–80. Epub 2019/09/05. doi: 10.1007/s11904-019-00458-1 [pii]. ; PubMed Central PMCID: PMC6822682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvi RM, Afshar M, Neilan AM, Tariq N, Hassan M, Gerber J, et al. Heart failure and adverse heart failure outcomes among persons living with HIV in a US tertiary medical center. Am Heart J. 2019;210:39–48. Epub 2019/02/08. doi: 10.1016/j.ahj.2019.01.002 ; PubMed Central PMCID: PMC6441629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janjua SA, Triant VA, Addison D, Szilveszter B, Regan S, Staziaki PV, et al. HIV Infection and Heart Failure Outcomes in Women. J Am Coll Cardiol. 2017;69(1):107–8. Epub 2017/01/07. doi: S0735-1097(16)36914-5 [pii] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2016;93:149–55. Epub 2015/11/26. doi: 10.1016/j.yjmcc.2015.11.015 ; PubMed Central PMCID: PMC4846552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis. 2014;209 Suppl 3:S86–92. Epub 2014/06/27. doi: 10.1093/infdis/jiu175 [pii]. ; PubMed Central PMCID: PMC4157516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alenezi F, Bloomfield GS, Okeke NL, Velagapudi P, Abudaqa L, Ijioma N, et al. Global Longitudinal Strain and Immune Status in Patients Living With Human Immunodeficiency Virus. Am J Cardiol. 2019;124(6):966–71. Epub 2019/08/03. doi: 10.1016/j.amjcard.2019.06.013 ; PubMed Central PMCID: PMC6887515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, et al. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. J Infect Dis. 2015;212(10):1544–51. Epub 2015/05/13. doi: 10.1093/infdis/jiv274 [pii]. ; PubMed Central PMCID: PMC4621251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128(8):814–22. Epub 2013/07/03. doi: 10.1161/CIRCULATIONAHA.113.001719 [pii]. . [DOI] [PubMed] [Google Scholar]
- 13.Sengelov M, Jorgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, et al. Global Longitudinal Strain Is a Superior Predictor of All-Cause Mortality in Heart Failure With Reduced Ejection Fraction. JACC Cardiovasc Imaging. 2015;8(12):1351–9. Epub 2015/11/19. doi: 10.1016/j.jcmg.2015.07.013 . [DOI] [PubMed] [Google Scholar]
- 14.Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Burrage M, Leano R, et al. Left Ventricular Global Longitudinal Strain (GLS) Is a Superior Predictor of All-Cause and Cardiovascular Mortality When Compared to Ejection Fraction in Advanced Chronic Kidney Disease. PLoS One. 2015;10(5):e0127044. Epub 2015/05/16. doi: 10.1371/journal.pone.0127044 ; PubMed Central PMCID: PMC4433230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JJ, Park JB, Park JH, Cho GY. Global Longitudinal Strain to Predict Mortality in Patients With Acute Heart Failure. J Am Coll Cardiol. 2018;71(18):1947–57. Epub 2018/05/05. doi: 10.1016/j.jacc.2018.02.064 . [DOI] [PubMed] [Google Scholar]
- 16.Flachskampf FA, Blankstein R, Grayburn PA, Kramer CM, Kwong RYK, Marwick TH, et al. Global Longitudinal Shortening: A Positive Step Towards Reducing Confusion Surrounding Global Longitudinal Strain. JACC Cardiovasc Imaging. 2019;12(8 Pt 1):1566–7. Epub 2019/08/10. doi: 10.1016/j.jcmg.2019.03.032 ; PubMed Central PMCID: PMC7561032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adamo L, Perry A, Novak E, Makan M, Lindman BR, Mann DL. Abnormal Global Longitudinal Strain Predicts Future Deterioration of Left Ventricular Function in Heart Failure Patients With a Recovered Left Ventricular Ejection Fraction. Circ Heart Fail. 2017;10(6). Epub 2017/06/01. doi: 10.1161/CIRCHEARTFAILURE.116.003788 ; PubMed Central PMCID: PMC5505492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100(21):1673–80. Epub 2014/05/27. doi: 10.1136/heartjnl-2014-305538 . [DOI] [PubMed] [Google Scholar]
- 19.Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16(12):1301–9. Epub 2014/09/12. doi: 10.1002/ejhf.154 ; PubMed Central PMCID: PMC4672867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanni MV, Awadalla M, Toribio M, Robinson J, Stone LA, Cagliero D, et al. Immune Correlates of Diffuse Myocardial Fibrosis and Diastolic Dysfunction Among Aging Women With Human Immunodeficiency Virus. J Infect Dis. 2020;221(8):1315–20. Epub 2019/05/18. doi: 10.1093/infdis/jiz184 ; PubMed Central PMCID: PMC7325805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buggey J, Yun L, Hung CL, Kityo C, Mirembe G, Erem G, et al. HIV and pericardial fat are associated with abnormal cardiac structure and function among Ugandans. Heart. 2020;106(2):147–53. Epub 2019/09/21. doi: 10.1136/heartjnl-2019-315346 ; PubMed Central PMCID: PMC7927634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1. Epub 2014/01/09. doi: 10.1186/2050-7771-2-1 ; PubMed Central PMCID: PMC3892095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16–31. Epub 2011/06/10. doi: 10.1182/blood-2010-12-326355 . [DOI] [PubMed] [Google Scholar]
- 24.Scully EP, Gandhi M, Johnston R, Hoh R, Lockhart A, Dobrowolski C, et al. Sex-Based Differences in Human Immunodeficiency Virus Type 1 Reservoir Activity and Residual Immune Activation. J Infect Dis. 2019;219(7):1084–94. Epub 2018/10/30. doi: 10.1093/infdis/jiy617 ; PubMed Central PMCID: PMC6784502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanni MV, Foldyna B, Williams K, Burdo TH, McCallum S, Looby SE, et al., editors. Sublinical atherosclerosis and immune activation among US females versus males with HIV Conference on Retroviruses and Opportunistic Infections 2022; Denver, Colorado 2022. [Google Scholar]
- 26.Toribio M, Wilks MQ, Hedgire S, Lu MT, Cetlin M, Wang M, et al. Increased Macrophage-Specific Arterial Infiltration Relates to Non-calcified Plaque and Systemic Immune Activation in People with HIV. J Infect Dis. 2022. Epub 2022/07/21. doi: 10.1093/infdis/jiac301 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Normally distributed variables are presented as mean ± standard deviation (SD); non-normally distributed data are presented as median (interquartile range; IQR). P-values were determined by student’s two-tailed t-test, Wilcoxon rank-sum test, and chi-square test for normally distributed, non-normally distributed, and categorical variables, respectively. There were no significant differences in baseline characteristics among women with versus without HIV. Select systemic markers of monocyte activation (MCP-1, sCD14, and sCD163) were higher among women with HIV versus without HIV. The expression of HLA-DR on the surface of CD14+CD16+ (inflammatory) monocytes, reflective of inflammatory monocyte activation, was higher among women with versus without HIV. *Lower limit of detection for the HIV viral load assay employed was 20 copies/mL. Values of 19 copies/mL were imputed when viral load was undetectable. **Expression of HLA-DR on monocyte subpopulations could not be obtained for one woman without HIV. Abbreviations: ART, anti-retroviral therapy; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CCR2, C-C chemokine receptor type 2; CCR5, C-C chemokine receptor type 5; CD4, cluster of differentiation 4; CD14, cluster of differentiation 14; CD16, cluster of differentiation 16; CXCL10, C-X-C motif chemokine 10; HDL-C, high-density lipoprotein cholesterol; HCV, hepatitis C virus; HIV, Human Immunodeficiency Virus; HLA-DR, human-leukocyte-associated antigen-D Related; INSTI, integrase inhibitor; LDL-C, low-density lipoprotein cholesterol; LMP, last menstrual period; MCP-1, monocyte chemoattractant protein 1; MFI, mean fluorescence intensity; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PBMCs, peripheral blood mononuclear cells; PIs, protease inhibitors; sCD14, soluble CD14; sCD163, soluble CD163; WHIV, women with HIV; WHR, waist to hip ratio.
(DOCX)
Additional details on the assessment of global longitudinal strain using cardiovascular MRI can be found in our Supplemental Methods.
(DOCX)
Data Availability Statement
Our study data set even if de-identified contains sensitive information regarding the HIV status of our participants. Given this sensitive information together with the relatively small sample size for this study (which recruited only from the Greater Boston Area), we have concerns about public sharing of our data set. Requests for de-identified data could be made by contacting our Massachusetts General Hospital Institutional Review Board at partnersirb@partners.org and referring to Protocol 2015P000200.