Abstract
Gastrointestinal disease caused by Shiga toxin-producing Escherichia coli (STEC) is frequently complicated by life-threatening toxin-induced systemic sequelae, including the hemolytic uremic syndrome. We previously constructed a recombinant bacterium displaying a Shiga toxin receptor mimic on its surface which neutralized Shiga toxins with very high efficiency. Moreover, oral administration of the live bacterium completely protected mice from challenge with virulent STEC. In this study, we investigated the protective capacity of formaldehyde-killed receptor mimic bacteria, as these are likely to be safer for administration to humans. The killed bacteria completely protected STEC-challenged mice when administered three times daily; incomplete protection was achieved using two doses per day. Commencement of therapy could be delayed for up to 48 h after challenge without diminishing protection, depending on the virulence of the challenge strain. Thus, administration of this agent early in the course of human STEC disease may prevent progression to life-threatening complications.
Shiga toxin (Stx)-producing strains of Escherichia coli (STEC) cause diarrhea and hemorrhagic colitis in humans, which can be complicated by systemic sequelae such as the hemolytic uremic syndrome (HUS). HUS is a life-threatening condition characterized by a triad of microangiopathic hemolytic anemia, thrombocytopenia, and renal failure, and it is a leading cause of acute renal failure in children (5, 8, 9, 14). STEC infection can also result in a variant form of HUS, sometimes referred to as thrombotic thrombocytopenic purpura. This diarrhea-associated disease is more common in adults than in children. Pathological features are essentially the same, but it differs from the typical form of HUS in that patients are more often febrile and have marked neurological involvement (5). The severe gastrointestinal symptoms as well as the systemic complications associated with STEC infections are principally caused by Stx, which is a sine qua non of virulence. During infections, STEC bacteria colonize the gut and release Stx into the gut lumen; the bacteria do not invade the gut mucosa, but toxin is absorbed into the circulation and targets tissues displaying the appropriate glycolipid receptor (particularly the microvasculature of the gut, kidneys, and brain) (6).
Development of rapid and sensitive methods for early diagnosis of STEC infection has created a window of opportunity for therapeutic intervention. Indeed, STEC infection may be detected almost a week before symptoms of HUS become apparent (12–14). Furthermore, increased awareness during major outbreaks will result in more patients presenting during the prodromal stage. Contacts of persons with proven or suspected STEC infection could also be treated. Unfortunately, antibiotic therapy is contraindicated for STEC infection, because it increases free Stx in the gut lumen by releasing cell-associated toxin and inducing toxin gene expression (14, 17). Thus, adsorption or neutralization of Stx in the gut is a potentially important alternative therapeutic strategy. STEC strains associated with human disease produce one or more of the recognized types of Stx (designated Stx1, Stx2, Stx2c, and Stx2d). Although they differ in amino acid sequence and in some biological properties, all of these Stx types recognize the same glycolipid receptor, globotriaosyl ceramide (Gb3), which has the structure Galα[1→4]Galβ[1→4]Glc-ceramide (6). In a recent study we exploited this specificity to develop a recombinant bacterium expressing a mimic of the Gb3 oligosaccharide on its surface (11). This involved insertion of a plasmid (pJCP-Gb3) carrying two Neisseria galactosyltransferase genes, lgtC and lgtE (3), in a derivative of E. coli R1 (CWG308) which has a waaO mutation in the outer core lipopolysaccaride (LPS) biosynthesis locus such that a truncated LPS core terminating in glucose is produced (4). Expression of lgtC and lgtE resulted in the linkage of Galα[1→4]Galβ[1→4] onto the terminal glucose. This bacterium adsorbed and neutralized Stx1, Stx2, Stx2c, and Stx2d with very high efficiency in vitro. Moreover, oral administration of the live recombinant bacterium was 100% protective in a streptomycin-treated mouse model of STEC-induced renal damage (11). Oral administration of this novel agent to individuals diagnosed with, or at risk of, STEC infection has the potential to adsorb and neutralize free Stx in the gut lumen, thereby preventing absorption of toxin into the bloodstream and the concomitant life-threatening systemic sequelae associated with STEC disease in humans. However, oral administration of live genetically manipulated organisms to humans is potentially controversial and is likely to be subjected to rigorous scrutiny by regulatory authorities. In our previous study we demonstrated that formaldehyde-killed E. coli CWG308:pJCP-Gb3 was also capable of binding and neutralizing Stx in vitro (11). Here we examine the capacity of oral administration of killed recombinant cells to protect mice from otherwise fatal challenge with a highly virulent STEC strain. We have also examined the effect of delaying commencement of therapy on protective efficacy.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli CWG308 (4), provided by Chris Whitfield, and construction of plasmid pJCP-Gb3 (11) have been described previously. The Stx2-producing O113:H21 STEC strains 97MW1 and 98NK2 (13) are both clinical isolates from the Women's and Children's Hospital, North Adelaide, South Australia, Australia. Spontaneous streptomycin-resistant derivatives of these strains used in challenge experiments were isolated by in vitro exposure to the drug. All E. coli strains were routinely grown in Luria-Bertani (LB) medium (7) with or without 1.5% Bacto Agar. Where appropriate, streptomycin or kanamycin was added to the growth medium at a concentration of 50 μg/ml.
Formaldehyde treatment of E. coli.
E. coli CWG308 or E. coli CWG308:pJCP-Gb3 cells were grown overnight in LB broth supplemented with isopropyl-β-d-thiogalactopyranoside (20 μg/ml) and, for CWG308:pJCP-Gb3, kanamycin (50 μg/ml). Cells were harvested by centrifugation, washed, and resuspended in phosphate-buffered saline (PBS) at a density of 1010 CFU/ml (equivalent to 20 mg [dry weight] of cells per ml). Formaldehyde was added to a final concentration of 1% (vol/vol), and the suspension was held at 4°C for 16 h. Cells were then washed twice with PBS to remove the formaldehyde and resuspended at the same density in sterile PBS. Complete killing of the E. coli suspensions was confirmed by culture. Suspensions were stored at 4°C for up to 2 weeks before use.
In vivo protection studies.
The streptomycin-treated mouse model of STEC-induced renal injury has been described previously (11, 16). Male 5 to 6-week-old BALB/c mice were given oral streptomycin (5 mg/ml in drinking water) for 24 h before oral challenge with 108 CFU of the streptomycin-resistant STEC, suspended in 50 μl of 20% sucrose. Successful colonization of each mouse, and maintenance at a level of at least 109 CFU/g, was confirmed by quantitative culture of feces on MacConkey agar supplemented with streptomycin. Mice were then given oral doses of approximately 8 mg (dry weight) of either CWG308 or CWG308:pJCP-Gb3 (formaldehyde killed) freshly resuspended in 60 μl of 20% sucrose–10% NaHCO3, twice or three times daily for up to 12 days. Oral streptomycin was continued throughout the experiment. The survival times of mice in each of the groups were recorded. The differences in survival rate between STEC-challenged mice treated with killed CWG308 or CWG308:pJCP-Gb3 were analyzed using the Fisher exact test. Kidneys were also removed from selected mice and fixed in formalin, and hematoxylin-and-eosin-stained sections were examined for histological evidence of renal injury.
RESULTS
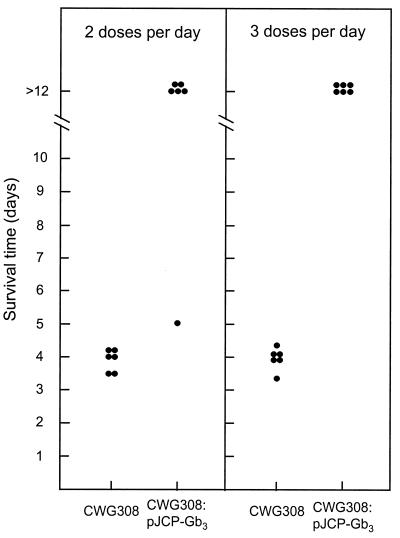
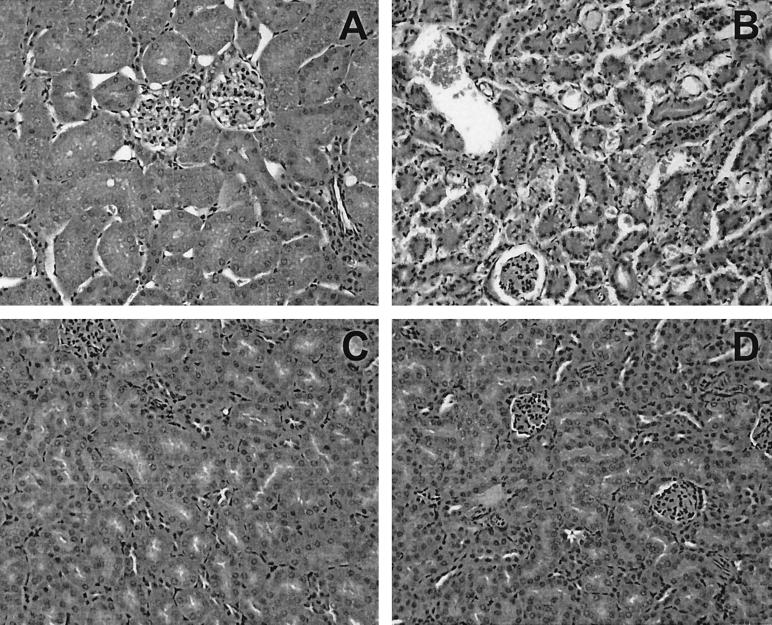
In an initial experiment, we examined the degree of protection against the highly virulent STEC strain 97MW1 afforded by oral administration of formaldehyde-killed CWG308:pJCP-Gb3. Four groups of six mice were challenged with 97MW1 and then treated with either killed CWG308 or CWG308:pJCP-Gb3. The dose administered (approximately 8 mg [dry weight]) was the same as that used in our previous study for live bacteria, and this was given either twice daily (i.e., every 12 h, as in our previous study [11]) or three times daily (every 8 h), commencing immediately after challenge. Figure 1 shows that all STEC-challenged mice treated with CWG308 died, with a median survival time of approximately 4 days. Five of the six mice which were treated with CWG308:pJCP-Gb3 twice daily survived; all six mice which received three doses per day were alive and well at the termination of the experiment. For both of these groups, the survival rate was significantly better than that of the corresponding control group treated with CWG308 (P < 0.005). Histological examination of the renal cortex of kidneys removed from CWG308-treated mice revealed extensive Stx-mediated tubular necrosis consistent with that seen in previous studies (10, 16). In contrast, the renal cortex of kidneys of unchallenged healthy mice or those removed at the end of the experiment from STEC-challenged mice treated with CWG308:pJCP-Gb3 showed no signs of tubular necrosis (Fig. 2). The lack of obvious renal damage in the CWG308:pJCP-Gb3-treated mice is remarkable, given that high levels of STEC (109 to 1010 CFU per g) had been maintained in the gut throughout the 12 days of the experiment.
FIG. 1.
Protective efficacy of formaldehyde-killed receptor mimic bacteria. Groups of six streptomycin-treated mice were challenged with 97MW1 and then treated orally twice or three times daily with E. coli CWG308 or CWG308:pJCP-Gb3 (see Materials and Methods). The survival time of each mouse is shown.
FIG. 2.
Histological confirmation of STEC-induced renal injury. Kidneys were removed from an uninfected control mouse (A), a CWG308-treated mouse which died 4 days after challenge with 97MW1 (B), and two different CWG308:pJCP-Gb3-treated mice which were alive and well 12 days after challenge with 97MW1 (C and D). Kidneys were fixed, sectioned, and stained with hematoxylin and eosin as described in Materials and Methods and examined by light microscopy at a magnification of ×400.
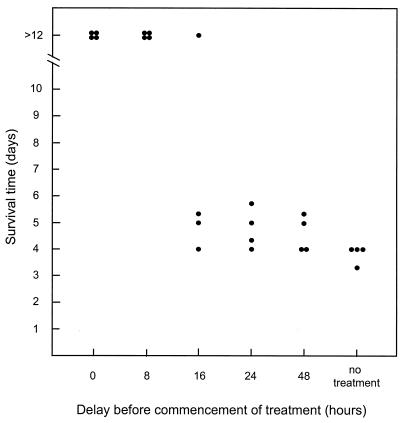
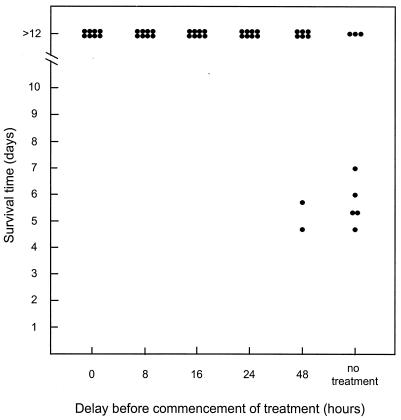
We then investigated the impact of delaying commencement of therapy on survival of STEC-challenged mice. Six groups of four mice were challenged with 97MW1. For five of the groups, therapy with formaldehyde-killed CWG308:pJCP-Gb3 (8 mg [dry weight] administered every 8 h) was commenced immediately or after a delay of 8, 16, 24, or 48 h; the sixth group did not receive treatment. As shown in Fig. 3, all mice in the untreated group died within 4 days. All of the mice also died when treatment with CWG308:pJCP-Gb3 was commenced 24 or 48 h after challenge, although the median survival time was slightly longer. Only one mouse survived when therapy was commenced after 16 h, but all mice survived when treatment commenced either immediately or 8 h after challenge with 97MW1 (P < 0.025, compared with the untreated group). 97MW1 is a highly virulent O113:H21 STEC strain which grows rapidly in the mouse gut and carries three stx2-related genes (13). While levels of expression in vivo were not determined, it is presumed to be capable of releasing large amounts of Stx2 into the gut lumen within hours of infection, and this may contribute to the rapidly fulminant course of disease. In humans, the lag between acquisition of STEC infection and onset of HUS may be as much as 2 weeks, and so the above model may underestimate the extent to which commencement of treatment can be delayed. Accordingly, we conducted an experiment similar to that described above, using a somewhat less virulent STEC challenge strain, 98NK2. This strain, also O113:H21, is closely related to 97MW1, on the basis of pulsed-field gel electrophoretic analysis of genomic DNA, but it carries only one stx2 gene (13). Nevertheless, the two strains produce similar levels of Stx in vitro; the toxin titer in culture lysates (determined by Vero cell cytotoxicity assay) was 8.2 × 106 tissue culture cytotoxic doses per ml. Using 98NK2 as the challenge strain, five of eight untreated mice died, with a median survival time of 6 days (Fig. 4). In contrast, all eight mice survived in the groups in which treatment with CWG308:pJCP-Gb3 commenced either 0, 8, 16, or 24 h after challenge (P < 0.025). Six of the eight mice also survived when treatment commenced 48 h after challenge.
FIG. 3.
Effect of delayed therapy with CWG308:pJCP-Gb3 on survival of mice challenged with 97MW1. Groups of four mice were challenged with 97MW1, and treatment with CWG308:pJCP-Gb3 was commenced either immediately, after a delay of 8, 16, 24, or 48 h, or not at all. Survival time of each mouse is indicated.
FIG. 4.
Effect of delayed therapy with CWG308:pJCP-Gb3 on survival of mice challenged with 98NK2. Groups of eight mice were challenged with 98NK2, and treatment with CWG308:pJCP-Gb3 was commenced either immediately, after a delay of 8, 16, 24, or 48 h, or not at all. Survival time of each mouse is indicated.
DISCUSSION
The results of this study unequivocally demonstrate that oral administration of formaldehyde-killed recombinant bacteria expressing a mimic of the Stx receptor protects mice from otherwise fatal challenge with a highly virulent STEC strain. The dose of bacteria used was similar to that employed in our previous study involving live recombinant cells (11). Thus, the capacity to survive in the gut is not an essential feature of this novel therapeutic agent. However, in order to maintain 100% protection, it was necessary to administer formaldehyde-killed cells three times rather than twice daily. The slight reduction in protective efficacy observed with twice-daily administration is probably a consequence of clearance of the toxin-binding agent between doses. Estimates of the gut transit time for mice are of the order of 8 h, and so at lower treatment frequencies, mice may be unprotected for the latter portion of each treatment period. The protective efficacy of the killed recombinant cells is encouraging, because such a product is likely to have a more expeditious regulatory passage to human trials compared with live genetically manipulated bacteria, which currently are somewhat controversial.
Commencement of therapy immediately after challenge was 100% protective, but in the human setting such early intervention will be possible only for contacts of patients with confirmed cases, who have not yet, or have only just, become infected with STEC. When the highly virulent challenge strain 97MW1 was used in our mouse model, delaying commencement of therapy with formaldehyde-killed CWG308:pJCP-Gb3 by 16 or more h resulted in loss of protection. However, the window of opportunity for treatment was extended to 24 to 48 h when a less virulent challenge strain (98NK2) was used. 98NK2 is closely related to 97MW1 and has a similar gut colonization capacity in the mouse model, as judged by quantitative culture of feces (result not presented). The principal difference between the two strains is that 97MW1 has three stx2 genes whereas 98NK2 has only one. Nevertheless 98NK2 has high human virulence and was the first locus for a enterocyte effacement (LEE)-negative STEC strain to be associated with an outbreak of HUS (13). Moreover, 98NK2 is more virulent in the mouse model than most O157:H7 STEC strains.
Although the median survival time of unprotected mice challenged with 98NK2 was 6 days, compared with only 4 days for those challenged with 97MW1, this still represents a significant time compression relative to the kinetics of human disease. In the mouse model, streptomycin treatment eliminates endogenous gut flora prior to challenge, and the STEC strains do not have to compete with other organisms. Under these circumstances, the numbers of STEC in the gut increase very rapidly to 109 to 1010 CFU per g of feces. Thus, the host could potentially be exposed to very high levels of Stx in the lumen almost from the outset, such that an ultimately lethal dose is absorbed into the circulation relatively early in the course of infection. In human disease, ingested doses of STEC are usually very low, and the pathogen must establish colonization in competition with endogenous flora. Thus, the time lag between actual infection and onset of systemic complications such as HUS is probably up to 2 weeks. In view of these considerations, it seems probable that a significantly broader window exists for treatment of human infections. This is supported by the preliminary findings of a phase II clinical trial of a synthetic Stx-binding agent Synsorb-Pk for the prevention of progression of STEC disease in children from diarrhea to HUS. Treatment was associated with a 40% reduction in progression if commenced within 3 days of onset of gastrointestinal symptoms (2). However, the number of patients was low, and a statistically significant difference between treatment and placebo groups was not demonstrable. The practical difficulties of conducting such efficacy trials are considerable, particularly given the low incidence of sporadic STEC cases and the unpredictability of outbreaks. The need to target patients in the early stage of illness is also complicated by the inevitable delays associated with laboratory confirmation of STEC infection; retrospective exclusion of patients whose stool samples ultimately prove to be negative for STEC can upset randomization.
In our previous study (11) we demonstrated that the in vitro Stx-binding capacity of CWG308:pJCP-Gb3 was 10,000 times better than that reported by others for Synsorb-Pk (1, 15). For this reason, we would anticipate improved in vivo performance in humans relative to Synsorb-Pk, although this can be determined only in a large-scale clinical trial. Another important consideration is that the Stx-binding bacterium is likely to be extremely cheap to produce on a large scale, and the formaldehyde treatment should preserve it such that it has a long shelf life, particularly in dried form. Low cost and long shelf life will permit presumptive treatment of persons with suspected STEC disease, pending the results of laboratory analysis of fecal samples. This is an important consideration, since the findings of this study indicate that early commencement of therapy will be essential to prevent progression of disease to life-threatening systemic complications.
ACKNOWLEDGMENTS
This work was supported by grants from the National Health and Medical Research Council of Australia and the Women's and Children's Hospital Research Foundation.
REFERENCES
- 1.Armstrong G D, Fodor E, Vanmaele R. Investigation of Shiga-like toxin binding to chemically synthesized oligosaccharide sequences. J Infect Dis. 1991;164:1160–1167. doi: 10.1093/infdis/164.6.1160. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong G D, McLaine P N, Rowe P C. Clinical trials of Synsorb-Pk in preventing hemolytic-uremic syndrome. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: American Society for Microbiology; 1998. pp. 374–384. [Google Scholar]
- 3.Gotschlich E C. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipopolysaccharide. J Exp Med. 1994;180:2181–2190. doi: 10.1084/jem.180.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrichs D E, Yethon J A, Amor P A, Whitfield C. The assembly system for the outer core portion of R1- and R4-type lipopolysaccharides of Escherichia coli. J Biol Chem. 1998;273:29497–29505. doi: 10.1074/jbc.273.45.29497. [DOI] [PubMed] [Google Scholar]
- 5.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingwood C A. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 1996;4:147–153. doi: 10.1016/0966-842x(96)10017-2. [DOI] [PubMed] [Google Scholar]
- 7.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 8.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien A D, Holmes R K. Shiga and Shiga-like toxins. Microbiol Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paton A W, Bourne A J, Manning P A, Paton J C. Comparative toxicity and virulence of Escherichia coli clones expressing variant and chimeric Shiga-like toxin type II operons. Infect Immun. 1995;63:2450–2458. doi: 10.1128/iai.63.7.2450-2458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paton A W, Morona R, Paton J C. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat Med. 2000;6:265–270. doi: 10.1038/73111. [DOI] [PubMed] [Google Scholar]
- 12.Paton A W, Ratcliff R, Doyle R M, Seymour-Murray J, Davos D, Lanser J A, Paton J C. Molecular microbiological investigation of an outbreak of hemolytic uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J Clin Microbiol. 1996;34:1622–1627. doi: 10.1128/jcm.34.7.1622-1627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paton A W, Woodrow M C, Doyle R M, Lanser J A, Paton J C. Molecular characterization of a Shiga-toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3357–3361. doi: 10.1128/jcm.37.10.3357-3361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeda T, Yoshino K, Adachi E, Sato Y, Yamagata K. In vitro assessment of a chemically synthesized Shiga toxin receptor analog attached to chromosorb P (Synsorb Pk) as a specific absorbing agent of Shiga toxin 1 and 2. Microbiol Immunol. 1999;43:331–337. doi: 10.1111/j.1348-0421.1999.tb02413.x. [DOI] [PubMed] [Google Scholar]
- 16.Wadolkowski E A, Burris J A, O'Brien A D. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1990;58:2438–2445. doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, McDaniel A D, Wolf L E, Keusch G T, Waldor M K, Acheson D W. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis. 2000;181:664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]