Abstract
Sepsis predisposes the host to a number of infectious sequelae, particularly the development of nosocomial pneumonia. Mechanisms by which sepsis results in impairment of lung antibacterial host defense have not been well defined. Alveolar macrophages (AM) represent important immune effector cells of the lung airspace. In this study, we examined the effects of cecal ligation and puncture (CLP) on murine AM function ex vivo, including the expression of proinflammatory cytokines and AM phagocytic activity. AM were harvested from mice subjected to a sham operation and CLP 24 h after laparotomy, adherence purified, and challenged with lipopolysaccharide (LPS) or left unstimulated. Both unstimulated and LPS-stimulated AM from mice subjected to CLP (CLP mice) produced significantly smaller amounts of proinflammatory cytokines tumor necrosis factor alpha and interleukin (IL-12) and C-X-C chemokines KC and macrophage inflammatory protein 2 than similarly treated AM from animals subjected to a sham operation. Furthermore, AM isolated from CLP mice displayed a marked impairment in phagocytic activity, as determined by flow cytometry, with this defect persisting to 48 h post-CLP. Induction of peritoneal sepsis syndrome resulted in a time-dependent increase in IL-10 in plasma and peritoneal fluid. Interestingly, the impairment in AM proinflammatory-cytokine production and phagocytic activity observed in AM from CLP mice was partially reversed by the in vivo neutralization of IL-10 prior to AM harvest. These observations suggest that abdominal sepsis syndrome results in significant impairment in AM effector cell function, which is mediated, in part, by sepsis-induced expression of IL-10.
Sepsis is a complex systemic illness which is characterized by various degrees of hypotension, coagulopathy, and multiorgan dysfunction (4). Despite advances in supportive therapy, the mortality rate in patients from severe sepsis continues to be as high as 30 to 40%. The sepsis syndrome is associated with the unabated release of inflammatory mediators, including cytokines and chemokines, which often results in detrimental effects to the host (4, 15). The release of inflammatory molecules is regulated and counterbalanced by the coordinated expression of anti-inflammatory cytokines such as interleukin 10 (IL-10) (34).
IL-10 is an important anti-inflammatory cytokine and is one of the most potent produced. This cytokine is a 35-kDa protein, produced by the activated Th2 subset of CD4+ T cells, B cells, monocytes, keratinocytes, and bronchial epithelial cells. IL-10 plays an important role in down-regulating the expression of monocyte-derived tumor necrosis factor alpha (TNF-α), IL-1, and members of both the C-X-C and C-C chemokine families (5, 8, 23). Specifically, IL-10 has been shown to down-regulate lipopolysaccharide (LPS)-inducible mRNA expression of proinflammatory cytokines from monocytes/macrophages, including TNF-α, IL-1, and IL-12 and chemokines KC, macrophage inflammatory protein 1α (MIP-1α), and MIP-2 (6, 9, 13, 20). In addition, IL-10 inhibits the surface expression of major histocompatibility complex class II molecules, nitric oxide synthesis, and NF-κB nuclear translocation after LPS stimulation and causes the down-regulation of TNF-α receptors (16, 19, 35). Studies have identified IL-10 to be an important regulator of inflammation in a variety of inflammatory disease states, including sepsis (28). For example, increased blood IL-10 levels are found in septic patients, as well as healthy subjects challenged with endotoxin intravenously (21, 31). Importantly, the expression of IL-10 in sepsis is prolonged relative to the more rapid and transient expression of proinflammatory cytokines.
Sepsis patients have been shown to be highly susceptible to the development of nosocomial infection, particularly bacterial infection of the lung (7). The exact mechanism(s) for this phenomena remains unclear. However, dysregulation of blood monocyte function is believed to play an important role in this sepsis-mediated immunosuppression. Indeed, monocytes recovered from septic patients display a number of defects in regulatory cytokine production, antigen processing, and antigen presentation (10, 11, 24, 25, 33).
Few studies have examined the functional effects of systemic inflammation on tissue macrophages, and specifically the alveolar macrophages (AM). This is of considerable importance given that the AM represents the predominant resident immune effector cell within the alveolus. The AM is the initial phagocytic cell that comes in contact with inhaled pathogens. In addition, the AM can amplify the pulmonary inflammatory response through the production of various leukocyte chemotactic and activating cytokines. Previous work has provided evidence of abnormal function of AM isolated from animals during the postseptic period (26, 29).
The purpose of this study was to further define how sepsis alters AM function. A murine model of sepsis and cecal ligation and puncture (CLP) was employed to determine the effect of abdominal sepsis on AM effector cell function, including the ability to express proinflammatory cytokine mRNA and proteins, AM phagocytic activity, and AM apoptosis. Additional studies were performed to identify the contribution of endogenous IL-10 to sepsis-induced impairment in AM function.
MATERIALS AND METHODS
Reagents.
Polyclonal antimurine TNF-α, IL-10, IL-12, KC, and MIP-2 antibodies used in enzyme-linked immunosorbent assays (ELISAs) were produced by immunization of rabbits with murine recombinant cytokines in multiple intradermal sites with complete Freund's adjuvant. Carrier-free murine recombinant TNF-α, IL-10, IL-12, KC, and MIP-2 were purchased from R&D Systems, Minneapolis, Minn. In IL-10 neutralization experiments, 0.5 ml of control rabbit serum or anti-murine IL-10 serum was administered intraperitoneally (i.p.) 24 h after CLP. This antiserum contained an anti-IL-10 antibody titer of 106 and has been shown to be neutralizing both in vitro and in vivo (18, 34). Purified antibodies for ELISA were obtained by purification over an endotoxin-free protein A column. Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide-phosphatidylethanolamine for flow cytometry were purchased from Pharmingen (San Diego, Calif.).
Animals.
Specific-pathogen-free CD-1 mice (6- to 12-week-old females; Charles River Breeding Labs) were used in all experiments. CD-1 mice were chosen because the CLP model has been well characterized in this outbred strain and CLP has been shown to result in a dramatic increase in susceptibility to respiratory pathogens in CD-1 mice (29, 34). All mice were housed in specific-pathogen-free conditions within the animal care facility at the University of Michigan until the day of sacrifice.
Animal model.
The cecal ligation and 25-gauge puncture model was used as a model of systemic sepsis syndrome as previously described (34). In distinct contrast to CLP models using larger-gauge cecal punctures (19 gauge and larger), in which most animals rapidly develop bacteremia due to enteric organisms and in which death occurs as a result of polymicrobial sepsis (3), CLP using a 25-gauge needle results in the development of bacteremia in only 10 to 15% of animals (data not shown). However, this insult induces a marked septic response, with death occurring in approximately 20 to 30% of animals. To perform this procedure, pathogen-free female CD-1 mice were anesthetized with pentobarbital (Butler Company, Columbus, Ohio) at 50 mg/kg of body weight i.p. followed by inhaled methoxyflurane (Metafane; Pitman-Moore Inc, Mundelein, Ill.) as needed. In these mice (CLP mice), a 1- to 2-cm longitudinal incision to the lower-right quadrant of the abdomen was performed and the cecum was exposed. The distal one-third was ligated with 3-0 silk suture and punctured through and through with a 25-gauge needle. A small amount of the bowel contents was then extruded through the puncture site. The cecum was then replaced into the peritoneal cavity, and the incision was closed with surgical staples. In control animals, the cecum was exposed but not ligated or punctured and then returned to the abdominal cavity (sham operation). All mice were administered 1 ml of sterile saline subcutaneously for fluid resuscitation during the postoperative period.
BAL.
Bronchoalveolar lavage (BAL) was performed to obtain AM in pure culture for ex vivo studies. First, mice were euthanized by asphyxia in a high-CO2 environment. The trachea was then exposed and intubated using a 1.7-mm-outside-diameter polyethylene catheter. BAL was performed by instilling Dulbecco's phosphate-buffered saline (PBS) (Life Technologies, Grand Island, N.Y.) containing 5 mM EDTA in 1-ml aliquots. Fifteen milliliters of PBS was instilled per mouse, with approximately 10 ml of lavage fluid retrieved. Lavaged cells from each group of animals were pooled and counted after hypotonic lysis, and cytospins for determination of BAL differentials were prepared. Lavaged cells consisted of greater than 95% AM for each of the groups examined (data not shown).
AM culture conditions.
AM obtained from BAL were washed and resuspended in RPMI-Dulbecco modified Eagle medium without serum and with or without antibiotics (Life Technologies, Bethesda, Md.). For culture supernatants and isolation of RNA, cells were seeded at concentrations of 5 × 105 and 1 × 106 cells/ml, respectively, into six-well tissue culture plates (Costar, Cambridge, Mass.). The cells were then incubated with Escherichia coli 055:B5 LPS (Sigma Chemical Co., St. Louis, Mo.) for 16 h for ELISA and 2 h for mRNA analysis at 37°C under an atmosphere of 5% CO2. These time points were chosen because they represent the maximal accumulations of cytokine protein and mRNA, respectively. AM culture supernatants were harvested at specified time points and stored at −70°C until analyzed by ELISA. In separate experiments, adherent AM were washed and lysed for RNA isolation.
Murine cytokine ELISAs.
Murine TNF-α, IL-10, IL-12, KC, and MIP-2 were quantitated using a modification of a double-ligand method as previously described (34). Briefly, flat-bottom 96-well microtiter plates (Immuno-Plate I 96-F; Nunc, Roskilde, Denmark) were coated with 50 μl of rabbit antibody against the various cytokines (1 μg/ml in 0.6 M NaCl–0.26 M H3BO4–0.08 M NaOH, pH 9.6)/well for 16 h at 4°C and then washed with PBS (pH 7.5)–0.05% Tween 20 (wash buffer). Microtiter plate nonspecific binding sites were blocked with 2% bovine serum albumin in PBS and incubated for 90 min at 37°C. Plates were rinsed four times with wash buffer, and diluted (neat and 1:10) cell-free supernatants (50 μl) in duplicate were added, followed by incubation for 1 h at 37°C. Plates were washed four times, followed by the addition of 50 μl of biotinylated rabbit antibodies against the specific cytokines (3.5 μg/ml in PBS [pH 7.5]–0.05% Tween 20–2% fetal calf serum)/well, and plates were incubated for 30 min at 37°C. Plates were washed four times, streptavidin-peroxidase conjugate (Bio-Rad Laboratories, Richmond, Calif.) was added, and the plates were incubated for 30 min at 37°C. Plates were washed again four times, and chromogen substrate (Bio-Rad Laboratories) was added. The plates were incubated at room temperature to the desired extinction, and the reaction was terminated with 50 μl of 3 M H2SO4 solution/well. Plates were read at 490 nm in an ELISA reader. Standards were 1/2 log dilutions of recombinant murine cytokines from 1 pg/ml to 100 ng/ml. This ELISA method consistently detected murine cytokine concentrations above 25 pg/ml. In accordance with the standard protocol, the concentrations of cytokines in the AM-conditioned media, plasma, and peritoneal lavage fluid were derived from the linear portion of the ELISA curve for each respective cytokine. In some instances, serial dilutions were performed in order for values to fall on the linear portion of each standard curve. The ELISAs did not show a cross-reaction with IL-1, IL-2, IL-4, or IL-6. In addition, the ELISAs did not show a cross-reaction with other members of the murine chemokine family, including murine JE/monocyte chemoattractant protein 1, RANTES, growth-related gene α, and epithelial cell-derived neutrophil-activating protein 78.
Isolation and reverse transcriptase PCR amplification of AM total mRNA.
Total cellular RNA from AM was isolated as previously described (16). Briefly, total cellular RNA from AM was isolated, reversed transcribed into cDNA, and then amplified as previously described, using specific primers for TNF-α, MIP-2, KC, IL-10, and IL-12 p35 and p40 subunits, with β-actin primers serving as a control. The primers used had the sequences 5′-CCT-GTA-GCC-CAC-GTC-GTA-GC-3′ and 5′-TTG-ACC-TCA-GCG-CTG-AGT-TG-3′ for TNF-α, 5′-TGA-GCT-GCG-CTG-TCA-GTG-CCT-3′ and 5′-AGA-AGC-CAG-CGT-TCA-CCA-GGA-3′ for KC, 5′-TGC-CTG-AAG-ACC-CTG-CCA-AGG-3′ and 5′-GGT-AGC-CTT-GCC-TTT-GTT-CAG-3′ for MIP-2, 5′-CTA-TGC-TGC-CTG-CTC-TTA-3′ and 5′-ATG-GCC-TTG-TAG-ACA-CCT-3′ for IL-10, 5′-ACC-TGC-TGA-AGA-CCA-CAG-AT-3′ and 5′-GAT-TCT-GAA-GTG-CTG-CGT-TG-3′ for IL-12 p35, 5′-ATG-TTG-TAG-AGG-TGG-ACT-3′ and 5′-GGA-CTG-CTA-CTG-CTC-TTG-AT-3′ for IL-12 p40, and 5′-ATG-GAT-GAC-GAT-ATC-GCT-C-3′ and 5′-GAT-TCC-ATA-CCC-AGG-AAG-G-3′ for β-actin. These gave amplified products of approximately 380 bp for TNF-α, 256 bp for KC, 355 bp for MIP-2, 455 for IL-10, 314 for IL-12 p35, 384 bp for IL-12 p40, and 812 bp for β-actin. The amplification buffer contained 50 mM KCl, 10 mM Tris-HCl (pH 8.3), and 2.5 mM MgCl. A specific oligonucleotide primer was added to the buffer, along with 5 μl of the reverse-transcribed cDNA samples. The cDNA was amplified after determining the optimal number of cycles. After amplification, the sample was separated on a 2% agarose gel containing 0.3 mg (0.003%) of ethidium bromide/ml and bands were visualized and photographed using UV transillumination.
Plasma and peritoneal fluid IL-10 analysis.
At designated time points, the mice were euthanized by asphyxia in a high-CO2 environment. The peritoneal cavity was lavaged with 1 ml of sterile saline, and this peritoneal lavage fluid was collected. Blood was collected in heparinized syringes by cardiac puncture via the right ventricle and centrifuged, and the plasma fraction was then collected. Peritoneal lavage and plasma were then stored at −20°C for assessment of cytokine levels.
Phagocytic assay.
BAL fluid was centrifuged at 551 × g for 10 min at 4°C. The pellet was resuspended in RPMI-Dulbecco modified Eagle medium without serum or antibiotics. Cells were counted in a hemocytometer using trypan blue exclusion as an index of viability. Test tubes (Falcon; Becton Dickinson, Paramus, N.J.) were seeded with 2 × 105 cells/200 μl for each condition. Phagocytosis was performed using the Phagotest kit (Orpegen, Heidelberg, Germany). Ten microliters of mixed precooled FITC-labeled opsonized bacteria (E. coli-FITC) (109/ml; 0°C) was added to the test tubes, and the test tubes were vortexed. Tubes were incubated in the dark for 2 h at 37°C under an atmosphere of 5% CO2, with a control sample (without E. coli-FITC) remaining on ice. At the end of the incubation time, all samples were placed in ice water and 2 μl of an ice-cold quenching solution (Orpegen) was added to each sample. Then 200 μl of washing solution (Orpegen) was added to each sample. Samples were centrifuged at 551 × g for 10 min at 4°C. The supernatant was discarded. All samples were washed again. Phagocytosis was then immediately assessed by flow cytometry (EPICS Profile II; Coulter Electronics Inc., Miami, Fla.). The proportion of phagocytizing cells was assessed.
Apoptosis assay.
AM apoptosis was determined by flow-cytometric analysis of surface expression of phosphatidylserine. AM were obtained from BAL 24 h post-CLP or sham surgery and then stained with annexin V-FITC and propidium iodide-phosphatidylethanolamine (Pharmingen) according to the manufacturer's protocol. Cells that stain for annexin V only are undergoing early apoptosis, whereas cells that stain for both annexin V and propidium iodide are undergoing late apoptosis. Cells were analyzed without fixation by flow cytometry within 1 h of staining.
Statistical analysis.
Data were expressed as means ± standard errors of the means (SEM). Statistical significance was determined using the unpaired Student t test. All calculations were performed on the Prism, version 3.0, statistical program (Graphpad Software, Inc., San Diego, Calif.). Values of P that were <0.05 were considered significant.
RESULTS
Effect of sepsis on AM cytokine production.
To establish the effect of sepsis on AM cytokine production, AM were lavaged from CD-1 mice 24 h after mice under went CLP or sham surgery and then were incubated for 18 h in the presence or absence of LPS at 1 μg/ml. Dose-response curves were performed to determine the optimal concentration of LPS to be used in these experiments. The 24-h time point for AM harvest was chosen because maximal immunosuppression occurs at 24 h post-CLP (34). Supernatants were then assessed for the presence of TNF-α, IL-10, IL-12, KC, and MIP-2 by ELISA. These cytokines were chosen for study because each of these cytokines has been shown to regulate protective innate responses in murine bacterial pneumonia models. As shown in Fig. 1, both resting and LPS-stimulated AM from septic mice produced smaller amounts of TNF-α, IL-12, KC, and MIP-2 than did AM isolated from control mice. When challenged with LPS, AM isolated from septic mice produced 30.1, 53, 53.5, and 52.8% smaller amounts of TNF-α, IL-12, KC, and MIP-2, respectively, than did LPS-stimulated AM from mice subjected to a sham operation (sham operation mice) (P < 0.05 for all cytokines). Interestingly, no IL-10 was detected from AM recovered from either sham operation or CLP mice cultured with or without LPS (data not shown).
FIG. 1.
Production of TNF-α, IL-12, KC, and MIP-2 by AM from septic (CLP) and control (sham) mice left unstimulated and stimulated in vitro with LPS after 18 h of culture. Data are expressed as means ± SEM obtained from three separate experiments, each performed with a pool of cells collected from 15 to 20 mice. ∗, P < 0.05 compared to sham operation animals.
Cytokine mRNA levels from unstimulated and LPS-stimulated AM were determined by reverse transcriptase-polymerase chain reaction. In this study, AM were lavaged at 24 h following CLP and then cultured in the presence or absence of LPS (1 μg/ml) for 2 h and cytokine mRNA levels were determined. Resting and LPS-stimulated AM obtained from septic mice expressed substantially decreased amounts of TNF-α, IL-12 p40, KC, and MIP-2 mRNA, compared to similarly treated AM obtained from sham operation animals (Fig. 2). No IL-10 mRNA was detected from resting or LPS-challenged AM recovered from either sham operation or CLP animals (data not shown).
FIG. 2.
Effect of sepsis on TNF-α, IL-12 p40, KC, and MIP-2 mRNA expression from unstimulated and LPS-stimulated murine AM after 2 h in culture. Each lane represents 106 AM. All cDNAs were amplified by 35 cycles of PCR for unstimulated AM and 25, 30, 25, 20, and 25 cycles of PCR for MIP-2, KC, IL-12 p40, TNF-α, and β-actin (both unstimulated and stimulated AM), respectively, for LPS-stimulated AM.
Effect of sepsis on AM phagocytic function ex vivo.
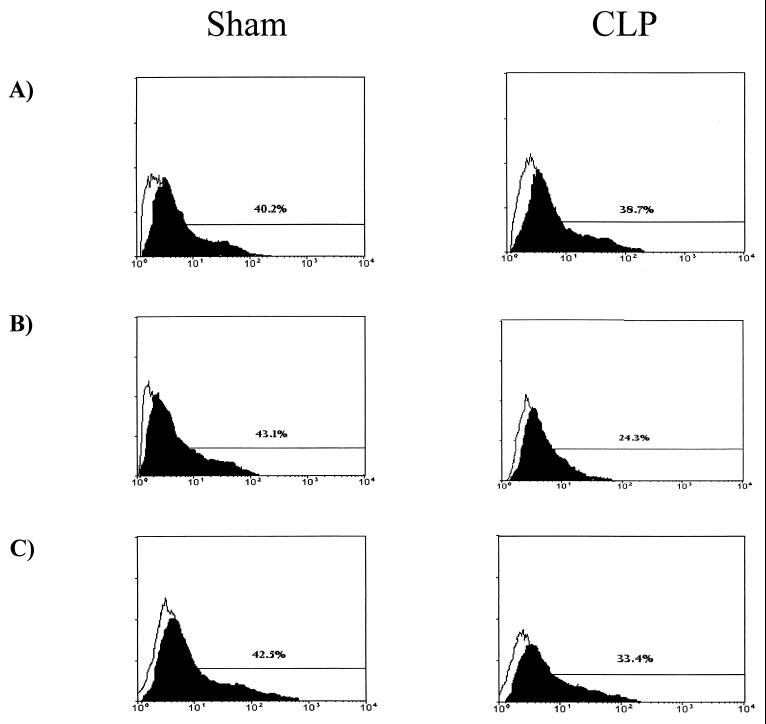
To assess the effect of abdominal sepsis on AM antimicrobial activity, AM were isolated from CLP and sham operation mice and phagocytic activity was determined. In these experiments, CD-1 mice underwent sham surgery or CLP and then BAL was performed at various time points postsurgery. AM were incubated with FITC-labeled E. coli, and then flow cytometry was performed to assess qualitative differences in ingestion of bacteria between AM from septic and control mice. As shown in Fig. 3, no statistically significant differences in AM phagocytic activity was detected in cells obtained from CLP or sham operation animals at 6 h postsurgery (37.4% ± 5.3% phagocytic activity for AM from CLP mice versus 42.6% ± 4.4% for AM from control mice; P = 0.22). However, by 24 h, a marked impairment in the ability to ingest FITC-labeled E. coli was noted in AM recovered from CLP mice 24 h post-CLP, compared to that for AM recovered from sham operation mice at 24 h (22.6% ± 4.9% phagocytic activity for AM from CLP mice versus 40.4% ± 5.3% for AM from control mice; P < 0.05). This defect in phagocytic activity persisted to 48 h post-CLP (data not shown; P < 0.05), with nearly complete recovery of phagocytic function by 72 h post-CLP (33.2% ± 3.3% phagocytic activity for AM from CLP mice versus 39.8% ± 4.3% for AM from control mice; P = 0.20).
FIG. 3.
Effect of sepsis on AM phagocytic function at 6 (A), 24 (B), and 72 (C) post-CLP. AM from control (sham) and septic (CLP) CD-1 mice were incubated with FITC-labeled E. coli (solid profile) and compared with AM without incubation with FITC-labeled E. coli (open profile). Histograms are representative of five independent flow-cytometric analyses.
Induction of IL-10 in plasma and peritoneal fluid following CLP.
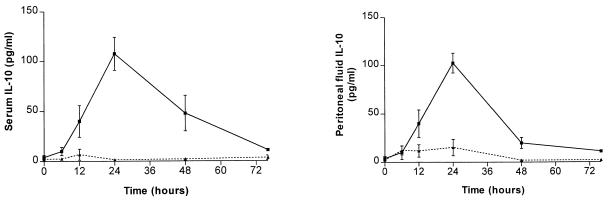
Given that IL-10 is a potent anti-inflammatory cytokine which has been shown to be elevated in patients with sepsis, immunoreactive IL-10 was measured in plasma and peritoneal lavage at baseline (pre-CLP) and at 6, 12, 24, 48, and 76 h post-CLP. An IL-10 concentration over baseline was induced in the plasma as early as 12 h, peaking at 24 h, with a gradual decline at 48 h and return to baseline by 76 h (Fig. 4). Likewise, measurement of IL-10 from peritoneal lavage fluid demonstrated peak levels by 24 h, with a return to baseline by 76 h. Interestingly, the temporal expression of IL-10 closely correlated with the time course of impaired AM effector cell activity.
FIG. 4.
Plasma and peritoneal lavage fluid IL-10 concentrations, from CLP mice (solid line) and sham operation mice (dashed line), expressed as means ± SEM. Experimental n = 4 to 6 per time point.
Effect of IL-10 neutralization in vivo on AM cytokine production ex vivo.
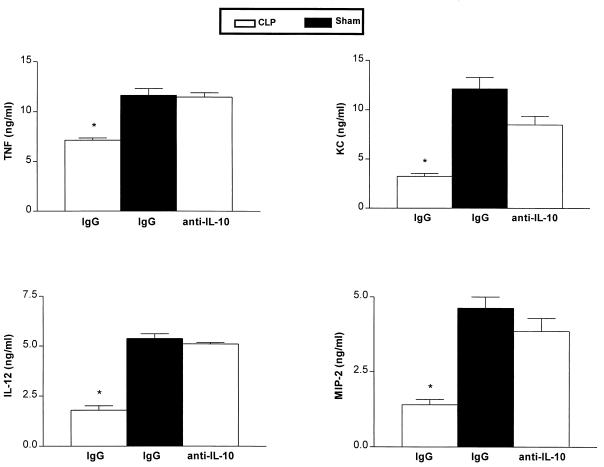
After demonstrating impairments in AM proinflammatory cytokine production and phagocytosis, which occurred in association with the enhanced and persistent elevation of IL-10 in CLP mice, we next examined the effects of in vivo IL-10 neutralization on AM function ex vivo. In these studies, mice were treated with rabbit anti-IL-10 antisera or control preimmune serum i.p. 24 h following CLP and then AM were harvested by BAL 8 h later. Treatment with anti-IL-10 was delayed, because we have previously shown that neutralization of IL-10 at the time of CLP results in an increase in sepsis-induced mortality (29). AM were adherence purified and then cultured in the presence or absence of LPS for 18 h. As shown in Fig. 5, AM obtained from mice passively immunized with anti-IL-10 antisera showed a significant increase in the ability to produce TNF-α and IL-12, and to a lesser extent KC and MIP-2, compared to AM obtained from CLP mice treated with preimmune serum in vivo (P < 0.05 for all cytokines).
FIG. 5.
Effect of in vivo IL-10 neutralization on the production of TNF-α, IL-12, KC, and MIP-2 from AM cultured ex vivo. ∗, P < 0.05 compared with AM from CLP animals receiving control serum. Experimental n = 15 to 20 per group. Results were combined from two separate experiments. IgG, immunoglobulin G.
Effect of IL-10 neutralization in vivo on AM phagocytic activity ex vivo.
To assess the role of IL-10 in down-regulating AM antimicrobial activity during abdominal sepsis syndrome, neutralization of IL-10 in vivo was performed, followed by isolation of AM from CLP and sham operation mice for assessment of phagocytic activity ex vivo. In these studies, CD-1 mice underwent sham surgery or CLP and then 24 h later were administered either rabbit anti-IL-10 serum or control serum i.p. Eight hours following antibody administration, AM were recovered and then immediately cocultured with FITC-labeled E. coli and flow-cytometric analysis was performed. Compared to what was found for AM recovered from CLP mice pretreated with control serum, treatment of CLP mice with anti-IL-10 serum resulted in a significant improvement of AM phagocytic activity (20.8% ± 2.7% phagocytic activity for AM from CLP mice given control serum versus 32.8% ± 3.5% for AM from CLP mice given anti-IL-10 serum; P < 0.05) (Fig. 6).
FIG. 6.
Effect of IL-10 neutralization on phagocytic activity of AM obtained from CLP mice administered control serum (A) or anti-IL-10 serum (B) i.p. 24 h after the septic event. AM from septic (CLP) mice were incubated both with (solid profile) and without (open profile) FITC-labeled E. coli. Histograms are representative of five independent flow-cytometric analyses.
Effect of CLP on AM apoptosis.
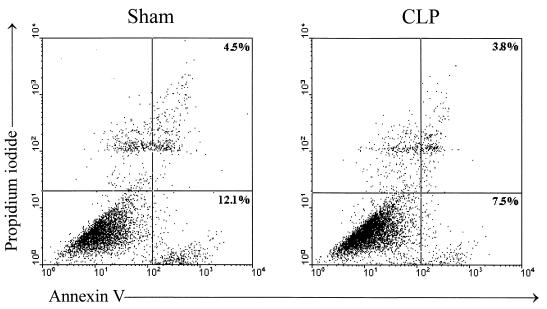
Previous studies have shown enhanced apoptosis of immune effector cells during systemic sepsis syndrome (1, 2, 14, 17, 36). Therefore, we next determined whether enhanced apoptosis would partially explain the impaired AM effector cell function observed in sepsis. CD-1 mice underwent sham surgery or CLP, and then 24 h later BAL was performed and cells were immediately stained with annexin V and propidium iodide as indicators of cellular apoptosis and death, respectively. Flow-cytometric analysis was performed on cells within 1 h of their being stained, and the percentages of apoptotic cells were determined. There was no statistically significant difference between the number of apoptotic AM recovered from CLP mice and the number recovered from animals 24 h after undergoing sham surgery (Fig. 7). In fact, there was a trend toward a lower percentage of early apoptotic cells isolated from the CLP mice than from controls (8.3% ± 5.4% versus 13.1% ± 6.1%, respectively; P = 0.08). These results were confirmed by electron microscopy, as the majority of freshly isolated AM from control and septic mice failed to demonstrate morphologic evidence of either apoptosis or cellular necrosis, and no differences between the two groups was noted (data not shown).
FIG. 7.
Effect of CLP on AM apoptosis and cell death. Shown is a flow-cytometric analysis of AM at 24 h post-CLP that were stained with annexin V-FITC (as an indicator of apoptosis) and propidium iodide (as an indicator of cell death). One representative experiment from four independent flow-cytometric analyses is depicted, showing 12% early-apoptotic cells (lower right) and 4.5% late-apoptotic cells (upper right), in AM obtained from sham operation animals, whereas 7.5% early-apoptotic and 3.8% late-apoptotic cells were noted in AM from CLP mice.
DISCUSSION
A number of functional defects in leukocytes isolated from sepsis patients have been characterized, particularly in blood monocytes. These defects include alterations in antigen-presenting ability, diminished HLA-DR expression, and dysregulated cytokine production (11, 24, 25, 33). Interestingly, impaired monocyte function has important clinical ramifications, as high mortality rates have been observed in patients displaying evidence of sepsis-induced monocyte deactivation (10). In the present study, we observed that the effects of sepsis are not limited to the circulating pool of blood monocytes but are also observed in more-differentiated tissue macrophages. Specifically, we detected quantitative defects in the ability of AM from septic mice to produce important proinflammatory cytokines, as well as defects in AM phagocytic activity. The suppression of AM effector cell activity was transient and reversible, with nearly complete restoration of phagocytic function by 72 h after the onset of abdominal sepsis.
The mechanism of sepsis-induced AM deactivation has not been completely elucidated. However, IL-10 appears to be a major endogenous mediator of these effects. First, the up-regulation of IL-10 in response to CLP temporally correlated with the duration of AM suppression. In addition, we found that the delayed administration of the IL-10 antibody in vivo resulted in partial restoration of AM effector cell function. Our results are consistent with previous studies which demonstrated significant improvement in clearance of intrapulmonary Pseudomonas aeruginosa in septic mice treated with anti-IL-10 antiserum in vivo (29). The suppression of proinflammatory cytokine production occurred in concert with decreased cytokine mRNA levels. The global dsyregulation of proinflammatory cytokine mRNA synthesis is consistent with interference of NF-κB-dependent signal transduction, which has shown to be required for the gene expression of these cytokines and which is inhibited by IL-10 (35).
We employed a novel technique of quantitating ingestion of FITC-labeled opsonized bacteria by flow cytometry to demonstrate impaired phagocytosis of E. coli by AM recovered from septic mice. Our results are consistent with previously published observations in a rat model of sepsis (26). We have extended these observations to demonstrate a temporal window of phagocytic responses and to demonstrate the importance of IL-10 as a mediator of this response. While it is known that IL-10 can inhibit the phagocytic responses of other leukocyte populations, the mechanism by which IL-10 inhibits AM phagocytosis remains unclear (18). We have examined the expression of several important cell surface molecules involved in the phagocytic response, including CD54, CD11b, CD11c, and CD16/32 but were unable to detect differences in expression between AM from the septic and control groups (data not shown). The effect of sepsis on the cell surface expression of other candidate molecules is the focus of ongoing studies.
We observed time-dependent expression of IL-10 in response to CLP. A potential source of IL-10 was felt to be the AM themselves. However, we were unable to detect expression of IL-10 mRNA or protein from either resting or LPS-stimulated AM in culture. Our results are similar to those of Salez and associates, who found no synthesis of IL-10 by AM at baseline or in response to LPS at both the protein and mRNA level (27). Even though murine AM do not appear to produce IL-10, it is known that murine macrophages can bind and express cell surface IL-10, and expression of surface IL-10 has been associated with decreased macrophage bactericidal activity (12). Therefore it is likely that AM, while not a source of IL-10, can bind and are influenced by this cytokine. IL-10 most likely reaches the alveolus via the systemic circulation from cellular sources outside the lung. However, potential sources of IL-10 within the lung include T and B cells, NK cells, and lung epithelial cells (5).
Prostaglandin E2 (PGE2) is a known inhibitor of proinflammatory cytokine expression from monocytes, as well as an inducer of IL-10 production (30, 32). Furthermore, indomethacin has been shown to partially reverse monocyte deactivation induced by endotoxin. Therefore, an elevation of this eicosanoid could account for many of the observed changes in cytokine expression. While we have detected significant increases in PGE2 in both lung and blood in mice after i.p. administration of endotoxin, we did not observe any increase in PGE2 levels in either plasma or lung homogenates at any time after 25-gauge CLP. Moreover, we observed a striking reduction in PGE2 production from LPS-stimulated AM from CLP mice, compared to similarly treated AM obtained from sham operation animals (unpublished data). Therefore, it is unlikely that PGE2 plays a significant role in mediating the observed changes.
Studies were performed to determine whether sepsis led to an acceleration of AM apoptosis or cell death, thereby contributing to several of the defects observed. Of note, prior studies have shown that the sepsis syndrome can induce apoptosis of T cells, neutrophils, and lung alveolar epithelial cells (1, 2, 14, 17, 22, 36). Indeed, the apoptosis of selected T-cell populations is believed to contribute to the T2-phenotype responses that predominate in the postseptic period. However, we did not find accelerated apoptosis or cell death of AM during the postseptic period. In fact, a trend toward protection from apoptosis was noted.
In summary, our results indicate that systemic sepsis syndrome results in impairment of AM effector cell function that is partially mediated by the enhanced expression of IL-10. Neutralization of IL-10 leads to restoration of AM function and therefore may serve as an important adjunct in overcoming sepsis-induced immunosuppresion, promoting more-appropriate host innate responses to respiratory pathogens.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL58200, HL57243, and P50HL60289.
REFERENCES
- 1.Ayala A, Chung C S, Xu Y X, Evans T A, Redmond K M, Chaudry I H. Increased inducible apoptosis in CD4+ T lymphocytes during polymicrobial sepsis is mediated by Fas ligand and not endotoxin. Immunology. 1999;97:45–55. doi: 10.1046/j.1365-2567.1999.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayala A, Xu Y X, Ayala C A, Sonefeld D E, Karr S M, Evans T A, Chaudry I H. Increased mucosal B-lymphocyte apoptosis during polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated process. Blood. 1998;91:1362–1372. [PubMed] [Google Scholar]
- 3.Baker C C, Chaudry I H, Gaines H O, Baue A E. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 4.Bone R C. Sepsis and its complications: the clinical problem. Crit Care Med. 1994;22:S8–S11. [PubMed] [Google Scholar]
- 5.Bonfield T L, Konstan M W, Burfeind P, Panuska J R, Hilliard J B, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–261. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg P, Osnes L, Ovstebo R, Joo G B, Westvik A B, Kierulf P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med. 1996;184:51–60. doi: 10.1084/jem.184.1.51. . (Erratum, 184:2075.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier J C, Offenstadt G, Regnier B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–974. [PubMed] [Google Scholar]
- 8.Cassatella M A, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke C J, Hales A, Hunt A, Foxwell B M. IL-10-mediated suppression of TNF-alpha production is independent of its ability to inhibit NF kappa B activity. Eur J Immunol. 1998;28:1719–1726. doi: 10.1002/(SICI)1521-4141(199805)28:05<1719::AID-IMMU1719>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Docke W D, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk H D, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 11.Faist E, Markewitz A, Fuchs D, Lang S, Zarius S, Schildberg F W, Wachter H, Reichart B. Immunomodulatory therapy with thymopentin and indomethacin. Successful restoration of interleukin-2 synthesis in patients undergoing major surgery. Ann Surg. 1991;214:264–275. doi: 10.1097/00000658-199109000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming S D, Campbell P A. Macrophages have cell surface IL-10 that regulates macrophage bactericidal activity. J Immunol. 1996;156:1143–1150. [PubMed] [Google Scholar]
- 13.Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiramatsu M, Hotchkiss R S, Karl I E, Buchman T G. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Karzai W, Reinhart K. Sepsis: definitions and diagnosis. Int J Clin Pract Suppl. 1998;95:44–48. [PubMed] [Google Scholar]
- 16.Kasama T, Strieter R M, Lukacs N W, Burdick M D, Kunkel S L. Regulation of neutrophil-derived chemokine expression by IL-10. J Immunol. 1994;152:3559–3569. [PubMed] [Google Scholar]
- 17.Keel M, Ungethum U, Steckholzer U, Niederer E, Hartung T, Trentz O, Ertel W. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356–3363. [PubMed] [Google Scholar]
- 18.Laichalk L L, Danforth J M, Standiford T J. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol Med Microbiol. 1996;15:181–187. doi: 10.1111/j.1574-695X.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 19.Leeuwenberg J F, Jeunhomme T M, Buurman W A. Slow release of soluble TNF receptors by monocytes in vitro. J Immunol. 1994;152:4036–4043. [PubMed] [Google Scholar]
- 20.Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gerard C, Delvaux A, De Groote D, Abramowicz D, Velu T, Goldman M. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994;24:1167–1171. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 21.Marchant A, Deviere J, Byl B, De Groote D, Vincent J L, Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994;343:707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- 22.Matute-Bello G, Liles W C, Steinberg K P, Kiener P A, Mongovin S, Chi E Y, Jonas M, Martin T R. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 23.Moore K W, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 24.Munoz C, Carlet J, Fitting C, Misset B, Bleriot J P, Cavaillon J M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Investing. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk H D. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson J D, Fry D E, van Arsdall L, Flint L M., Jr Delayed pulmonary clearance of gram-negative bacteria: the role of intraperitoneal sepsis. J Surg Res. 1979;26:499–503. doi: 10.1016/0022-4804(79)90040-4. [DOI] [PubMed] [Google Scholar]
- 27.Salez L, Singer M, Balloy V, Creminon C, Chignard M. Lack of IL-10 synthesis by murine alveolar macrophages upon lipopolysaccharide exposure. Comparison with peritoneal macrophages. J Leukoc Biol. 2000;67:545–552. doi: 10.1002/jlb.67.4.545. [DOI] [PubMed] [Google Scholar]
- 28.Standiford T J. Anti-inflammatory cytokines and cytokine antagonists. Curr Pharm Des. 2000;6:633–649. doi: 10.2174/1381612003400533. [DOI] [PubMed] [Google Scholar]
- 29.Steinhauser M L, Hogaboam C M, Kunkel S L, Lukacs N W, Strieter R M, Standiford T J. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162:392–399. [PubMed] [Google Scholar]
- 30.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Poll T, de Waal Malefyt R, Coyle S M, Lowry S F. Antiinflammatory cytokine responses during clinical sepsis and experimental endotoxemia: sequential measurements of plasma soluble interleukin (IL)-1 receptor type II, IL-10, and IL-13. J Infect Dis. 1997;175:118–122. doi: 10.1093/infdis/175.1.118. [DOI] [PubMed] [Google Scholar]
- 32.van der Pouw Kraan T C, Boeije L C, Smeenk R J, Wijdenes J, Aarden L A. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volk H D, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller J M, Docke W D, Kox W J. Monocyte deactivation—rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22(Suppl. 4):S474–S481. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 34.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel S L. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang P, Wu P, Siegel M I, Egan R W, Billah M M. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 36.Wang S D, Huang K J, Lin Y S, Lei H Y. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]