Abstract
Background
Hypercoagulability is a common complication seen in COVID-19 infection. However, arterial thrombosis such as acute limb ischemia (ALI) is far less common. Data on the incidence and nature of arterial thromboembolic complications in patients with COVID-19 is limited, originating from a few case reports and case series. Data in the African continent are very scarce.
Method
This is a case series of 10 patients with COVID-19 who developed ALI while on treatment at Eka Kotebe General Hospital, Addis Ababa, Ethiopia. All patients with ALI and COVID-19 admitted between February 1, 2021, and December 31, 2021, were retrospectively identified and reviewed. COVID-19 was confirmed by RT-PCR and ALI was confirmed by Doppler ultrasound and/or computed tomography angiography in the presence of clinical suspicion.
Results
A total of 3098 patients were hospitalized with confirmed COVID-19 during the study period. In a series of 10 patients, 8 (80%) males with a median age of 53.5 years were included. All except one (10%) had one or more risk factors for ALI and one had a ‘possible’ case of vaccine-induced thrombotic thrombocytopenia (VITT) associated with ALI. All were admitted with severe COVID-19 and most (80%) developed ALI during hospitalization (median of seven days from admission). The median duration between COVID-19 and ALI symptom onset was 14.5 days (IQR, 11–15). The majority (60%) were taking therapeutic anticoagulation at the time of ALI onset which is the standard of care for patients with severe disease. Five (50%) were successfully revascularized (median time of 3.5 days) and the rest underwent amputation. All survived and were discharged improved.
Conclusion
ALI can occur in the context of COVID-19 even while a patient is on therapeutic dose anticoagulation and in the absence of traditional risk factors. It is wise to be vigilant of this complication for timely intervention and better treatment outcomes.
Keywords: Acute limb ischemia, COVID-19, Case series, Anticoagulation, VITT
Abbreviations: VITT, vaccine-induced thrombotic thrombocytopenia
1. Background
Hypercoagulability is one of the complications seen in the Coronavirus 2019 disease (COVID-19) that is associated with significant morbidity and mortality. COVID-19 predisposes to all three components of Virchow's triad. i.e., endothelial dysfunction via direct viral infection through angiotensin-converting enzyme 2 (ACE-2) receptor, patients are usually hospitalized and immobilized for a prolonged duration, cytokine release associated hypercoagulability and hypoxia. In addition, as with other critically ill patients, patients with COVID-19 may be treated with vasopressor agents that can lead to small vessel thrombosis, acral ischemia, and digital gangrene [1].
Thrombotic complications in patients with COVID-19 present in different ways, most commonly with venous thromboembolism during inpatient as well as outpatient periods. Although far less common, arterial events are related to thrombosis of the extremity, cerebral, coronary, and visceral arteries [1].
Acute limb ischemia (ALI), which is a medical emergency, is a sudden severe decrease in the perfusion to an extremity, usually <2 weeks in duration. Thus, early recognition of ALI and timely intervention can help reduce morbidity and mortality in patients with COVID-19. The typical clinical features of acute limb ischemia include the 6 Ps (pain, pallor, poikilothermia, pulselessness, paresthesia, and paralysis).
According to the Society for Vascular Surgery/International Society for Clinical Vascular Surgery (SVS/ISVS), the severity of ALI is staged by the presence and degree of sensory and motor function loss as well as doppler findings. This classification (i.e., Rutherford staging) determines the urgency and type of diagnostic evaluation and intervention (Table 1 ) [9,10].
Table 1.
Rutherford classification of ALI (SVS/SICVS) [10].
| Category | Description/prognosis | Findings |
Doppler signals |
||
|---|---|---|---|---|---|
| Sensory loss | Muscle weakness | Arterial | Venous | ||
| I. Viable | Not immediately threatened | None | None | Audible | Audible |
| II. Threatened | |||||
| a. Marginally | Salvageable if promptly treated | Minimal (toes) or none | None | Inaudible | Audible |
| b. Immediately | Salvageable with immediate revascularization | More than toes, associated with rest pain | Mild, moderate | Inaudible | Audible |
| III. Irreversible | Major tissue loss or permanent nerve damage is inevitable | Profound, anesthetic | Profound, paralysis (rigor) | Inaudible | Inaudible |
The diagnosis of ALI is mainly clinically based on physical examination results, including measurement of extremity pressures (e.g., ankle-brachial index, wrist brachial index, if distal Doppler signals are present), in combination with the patient's history (symptom duration, pain severity, and sensory-motor loss).
We describe here a case series of ten patients with COVID-19-associated acute limb ischemia, all treated at Eka Kotebe General Hospital, the premiere COVID-19 treatment center in Addis Ababa, Ethiopia. We aimed to report our institutional experience, i.e., the incidence, characteristics, and clinical outcome of COVID-19 patients who developed ALI during hospitalization, and compare it to findings from resource-rich countries.
2. Methods
We presented a single-center, retrospective chart review of patients who were admitted to the hospital with COVID-19 and had ALI between February 1, 2021, and December 31, 2021. Data about patient demographics, co-morbidities, and outcomes were collected. COVID-19 was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) in all and for a patient diagnosed to have ALI based on clinical presentation, Doppler ultrasound, and CT Angiography are used to identify the site of occlusion. Continuous variables are presented as median (interquartile range [IQR]), using Excel 2021. Categorical variables are presented as absolute numbers (n) and proportions (%).
3. Results
A total of 3098 patients, with a median age of 50 years (IQR, 33–65), were hospitalized with confirmed COVID-19 during the study period. Of those, 1393 (45%) were females and 797 (25.7%) required ICU admission. ICU mortality rate was 58% using complete case analysis during the study period. Ten patients (0.32% incidence) with a median age of 53.5 years (IQR, 53–63) developed ALI during the study period (Table 3 in supplementary data). Two of the 10 patients (20%) were females. Two were overweight and two were obese. Type 2 diabetes mellitus (T2DM) was the most common comorbidity (70%) followed by dyslipidemia and hypertension, each present in four patients (40%), and two were ex-smokers (Tables 3 and 4 in supplementary data). Only one was vaccinated for COVID-19 (two doses of AstraZeneca).
All presented with COVID-19 symptoms before the ALI. All were admitted with severe COVID-19 and six (60%) had possible superimposed bacterial pneumonia supported by imaging studies (CXR and/or CT) in addition to compatible clinical features. The relative frequency of COVID-19 symptoms was cough (100%), shortness of breath (60%), fever and easy fatiguability (50%), anorexia (30%), and loss of smell and taste sensation (20%). The median duration between COVID-19 and ALI symptom onset, and between admission and ALI was 14.5 days (IQR, 11–15) and 7 days (IQR, 3.25–8), respectively.
The site of occlusion was infra-popliteal (50%) in five cases (bilateral dorsalis pedis, anterior and posterior tibialis in three of them), popliteal (50%) followed by superficial femoral artery (30%), and one case (10%) of deep femoral artery, common iliac artery, external iliac artery, brachial artery and coronary artery each. Most (60%) were already on a therapeutic dose of anticoagulation (35,000 IU UFH/24 h in two divided doses SC) for severe COVID-19 and 20% were on prophylactic anticoagulation at the time of the event. None had any form of bleeding or over-anticoagulation.
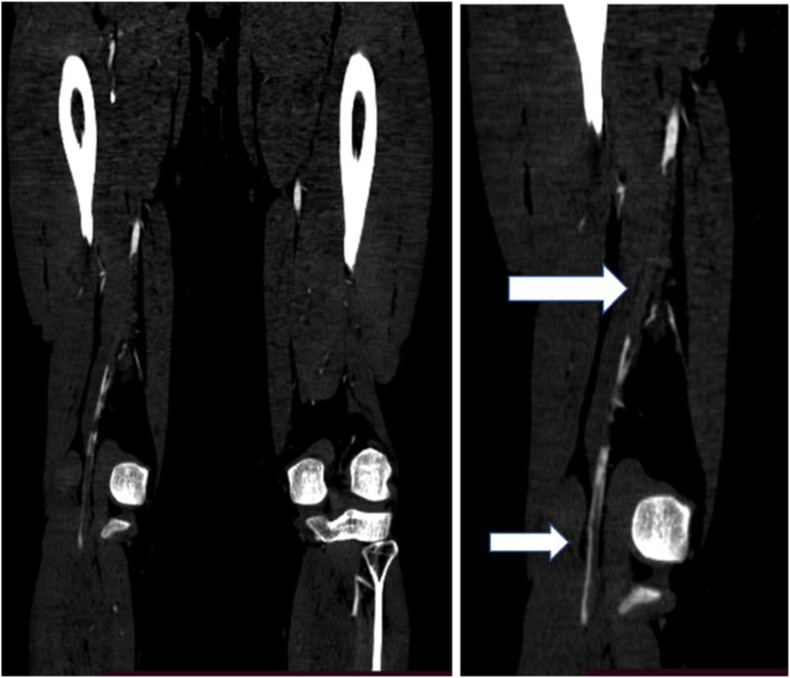
The severity of the ALI was staged IIa in three patients, IIb in four patients, and III in four patients, according to Rutherford classification. Five (50%) were successfully revascularized by open thrombectomy while the rest underwent major amputation during hospitalization. One patient underwent amputation within one month of hospital discharge. Recurrent thrombosis occurred in two patients after thrombectomy, 3 days later (same site) in one patient and 3 weeks later (at a different site) in the other patient. All survived hospital discharge (Table 2 ). Filling defects on lower extremity CT angiogram of a patient (patient 2) is shown below (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5 ).
Table 2.
Summary of characteristics of patients with acute limb ischemia.
| Variable | Value |
|---|---|
| Demographics | |
| Female sex, n (%) | 2 (20%) |
| Age (years), median (IQR) | 53.5 (53–63) |
| Risk factors, n (%) | |
| Diabetes Mellitus (type 2) | 7 (70%) |
| Hypertension | 4 (40%) |
| Smoking | 2 (20%) |
| Obesity | 2 (20%) |
| Dyslipidemia | 4 (40%) |
| Clinical severity of COVID-19 | |
| Asymptomatic | 0 (0%) |
| Mild | 0 (0%) |
| Moderate | 0 (0%) |
| Severe | 10 (100%) |
| Critical | 0 (0%) |
| Time from COVID-19 symptom onset to ALI symptom onset (days), median (IQR) | 14.5 days (11–15) |
| Grade of ALI (Rutherford Classification), n (%) | |
| I | 0 (0%) |
| IIa | 3 (30%) |
| IIb | 4 (40%) |
| III | 4 (40%) |
| Management of ALI, n (%) | |
| Systemic anticoagulation with UFH alone | 0 (0%) |
| Thrombectomy (open) and systemic anticoagulation with UFH (therapeutic dose) | 5 (50%) |
| Amputation (disarticulation) | 5 (50%) |
| Outcome, n (%) | |
| Revascularization | 5 (50%) |
| Amputation | 5 (50%) |
| In-hospital mortality | 0 (0%) |
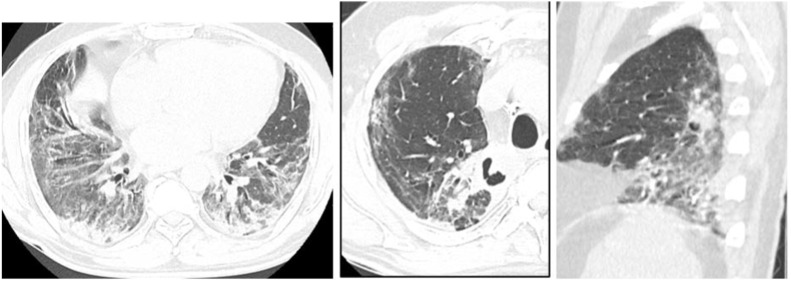
Fig. 1.
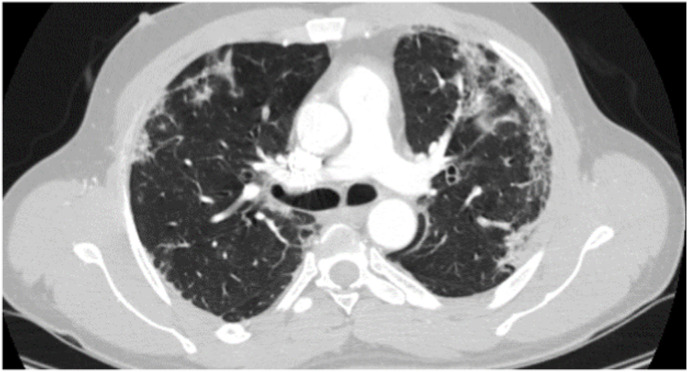
Contrast chest CT showing bilateral lungs subpleural and peri-bronchovascular patchy consolidation, ground-glass opacity, and crazy paving pattern with subpleural fibrosis consistent with severe COVID-19 pneumonia (patient 2).
Fig. 2.
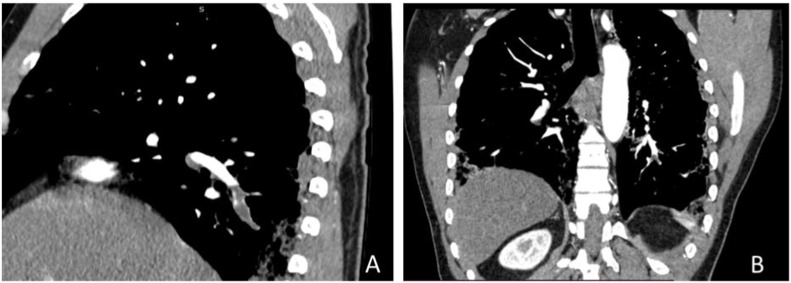
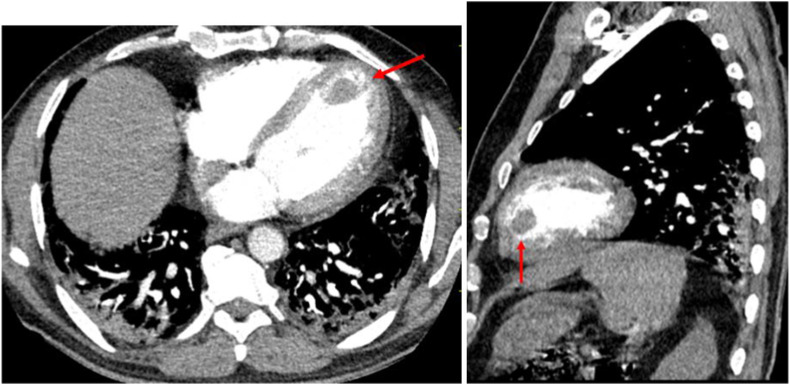
Contrast CT showing Right (A) and left (B) basal segmental pulmonary artery central filling defect (acute pulmonary embolism) of the same patient.
Fig. 3.
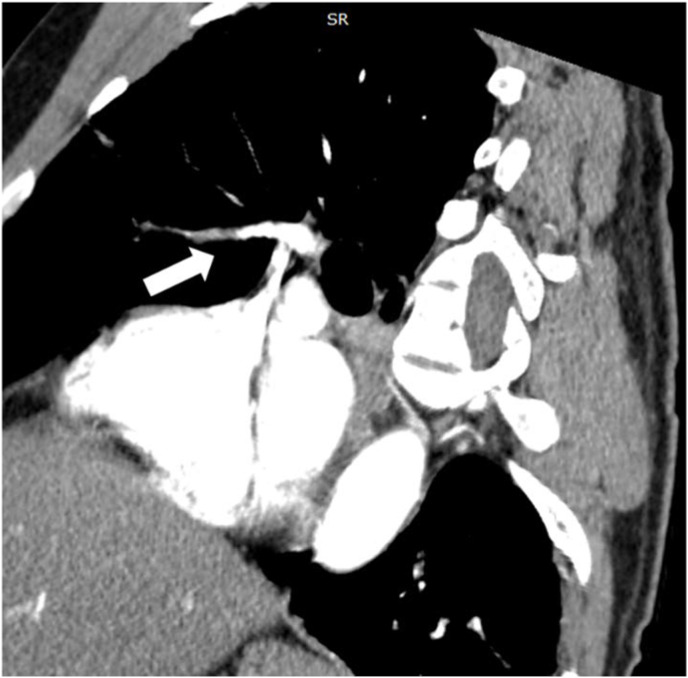
Contrast chest CT showing the anterior filling defect (arrow) of the same patient.
Fig. 4.
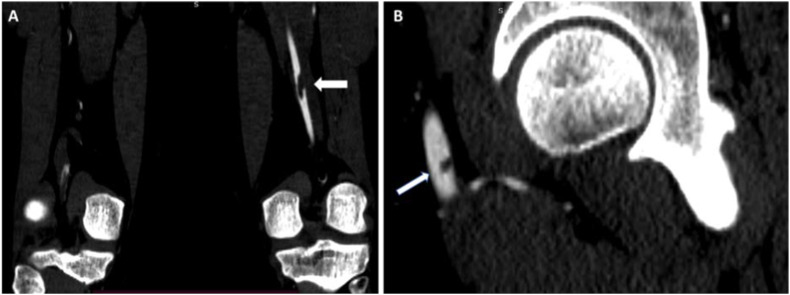
A lower extremity CT angiogram showing a filling defect in the left proximal popliteal artery (A) and a small filling defect in the left CFA (B) of the same patient.
Fig. 5.
CT angiogram showing long segment filling defect in the right popliteal artery with a thin rim of contrast (arrows) of the same patient.
4. Discussion
Among COVID-19 patients requiring admission, data shows ALI occurred in 3 to 15% of such patients [2,3]. By comparison, the rate of ALI in the general population is approximately 10–15 per 100,000 per year and includes embolic, thrombotic, and traumatic etiologies [1]. There is no data on the prevalence of ALI in Ethiopia but data from the Global Burden of Diseases (GBD) study and a systematic review of prevalence surveys showed that the rate of increase of ALI prevalence between 1990 and 2010 was faster in low- and middle-income countries than in developed nations [13]. Out of 3098 hospitalized patients in Eka Kotebe General hospital (EKGH), the largest COVID-19 treatment center in the country, there were ten cases of ALI (i.e., an incidence of around 0∙32%). However, this is lower than other countries’ studies [[2], [3], [4]].
As with ALI in the general population, the lower extremity is affected more commonly than the upper extremity in patients with COVID-19. In our study, 90% had ALI in the lower extremity and 10% in the upper extremity with concomitant coronary thrombosis and DVT seen in 10% each. This is similar to one of the largest case series in New York [2,3].
The most common risk factors were DM found in 70%, followed by HTN and dyslipidemia each found in 40% of cases and 20% were obese. This is also similar to several case series in the US and Italy [2,3,5]. However, in our study, on average, patients were less than 60 years of age (mean 56.6 years) with BMI <25 and none had CKD (Table 2).
Surprisingly, COVID-19-associated ALI is also seen in young, healthy patients with no identified risk factor [1]. This was also evident in case three of our study where the patient lacked any of the traditional risk factors except for his age (60 years old). Here, it is good to note that evaluation for cardiovascular (embolic) causes is useful when risk factors are not present. Acute thrombosis can also be a presenting feature for patients with COVID-19 before the onset of other symptoms. For example, in a study by Etkin et al. [2] in the New York area, about twenty-two (45%) patients presented with signs of acute arterial ischemia and were subsequently diagnosed with COVID-19 and about 27% of patients had no prior medical history.
COVID-19-associated ALI has also occurred in patients receiving thromboprophylaxis. Six out of ten (60%) were receiving therapeutic dose anticoagulation at the time of ALI onset (Table 3). Here, it is also good to note among patients who developed ALI whilst on a therapeutic dose of UFH, all but one had a suboptimal level of anticoagulation (i.e., below the target level of aPTT) at the time of ALI. A similar finding was seen in one study in Turkey [[11], [14]]. This requires reexamining the efficacy of administering subcutaneous unfractionated heparin, especially in COVID-19 patients as well as considering rare conditions such as heparin resistance. However, this is significantly lower in other studies [[5], [6], [7]]. In one Cochrane meta-analysis, the authors concluded that there is no evidence of a difference between subcutaneous versus intravenous UFH for preventing VTE recurrence, VTE-related or all-cause mortality, and major bleeding. But the quality of the evidence was low and arterial thrombosis was not studied [17]. Others believe aPTT value may not reliably reflect the true anti-Xa properties of UFH in such inflammatory condition of COVID-19 [18].
Even though the majority of patients (55%) develop ischemia during hospitalization, it can occur in patients with mild symptoms of COVID-19, concurrently or even following recovery [8]. As shown in Table 3, in our study, we found out that having Rutherford grade IIb and beyond is usually but not necessarily associated with an increased risk of amputation while having grade IIa and below is strongly linked with an increased chance of successful revascularization (Table 3).
The median duration between COVID-19 diagnosis and ALI symptoms was 14.5 days (IQR, 11–15) and this is similar to a study from Turkey [11]. In addition, except for two cases, all other patients developed ALI after admission to a COVID-19 treatment center, with a median duration of seven days after admission (Table 2).
Based on the classification used by Pavord et al. in our study, one out of ten patients (10%) developed a ‘possible’ case of VITT-associated ALI and this is similar to the study published on NEJM [12].
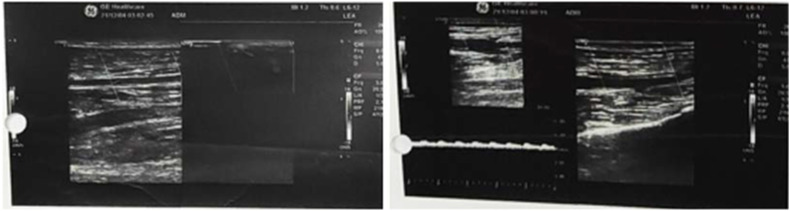
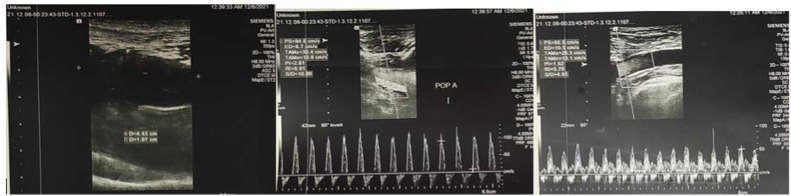
Duplex ultrasound and CT angiography are the most common techniques used in clinical practice to help localize the site and extent of occlusion. In resource-limited set-ups such as Ethiopia, however, such imaging modalities may not be readily accessible and may require advanced patient transfer off-site which might not be feasible due to the patient's condition. Similarly, in a review of a small cohort of 16 patients with acute limb ischemia, only 8 patients underwent confirmatory imaging studies [5]. Overall, patients with viable or marginally threatened limbs (stage I and IIa) might have adequate time for vascular imaging before the intervention, however, patients with an immediately threatened limb (stage IIb and above) require more urgent evaluation, typically in the operating room. Doppler ultrasound of a patient (patient 6) before and after thrombectomy is shown below (Fig. 6, Fig. 7, Fig. 8, Fig. 9 ).
Fig. 6.
Patient six Doppler U/S showing partial arterial intraluminal acute thrombosis with the hemodynamic effect of dampened arterial flow velocity from the proximal right SFA up to peroneal and AT artery (before thrombectomy).
Fig. 7.
Follow-up doppler U/S showing intramuscular cuff hematoma of the above patient three days after thrombectomy.
Fig. 8.
Contrast chest CT showing bilateral lower lobes subpleural and peri-bronchovascular GGO and subpleural fibrosis and bilateral upper lobes cavitating consolidation suggestive of superimposed bacterial pneumonia (patient six).
Fig. 9.
Left ventricular apical area small thrombus (red arrow) of patient six. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Adequate anticoagulation and timely vascular surgery consultation are crucial to the successful treatment of ALI. For patients with ALI, it is better to initiate therapeutic anticoagulation with intravenous unfractionated heparin (UFH) (bolus followed by continuous infusion), unless there are significant contraindications such as active or major bleeding in the prior 24–48 h, recent surgery, or contraindications to the use of heparin (e.g., suspected heparin-induced thrombocytopenia [HIT]) [3].
Based on the patient's overall stability and limb viability, a decision needs to be made on whether intervention is proper. Because of the severe respiratory problems with COVID-19, critically ill patients may not even be candidates for revascularization [1,2,9]. Options for revascularization include the following: open thrombectomy; endovascular revascularization may include catheter-directed thrombolysis or percutaneous mechanical thrombectomy. The procedure that results in the quickest restoration of blood flow with the least risk should be chosen.
Following intervention for ALI, based on general treatment outcomes in patients with ALI, patients with COVID-19 should be maintained on therapeutic anticoagulation and transitioned to oral anticoagulation with or without an antiplatelet agent. Oral anticoagulation is required to reduce recurrent ischemic limb events and amputation in the future since some patients with COVID-19-related ALI develop recurrent thrombosis after invasive interventions (e.g., thrombectomy, thrombolysis) that were initially technically successful [15]. This is seen in patient four of our case study who developed thrombosis twice after the first successful thrombectomy, each after 72hrs of the last thrombectomy. Even though it was possible to salvage the limb, the functionality of the limb was significantly compromised on post-discharge follow-up (Table 2). Patient seven had also recurrent thrombosis at a different site and eventually underwent amputation (Table 3) .
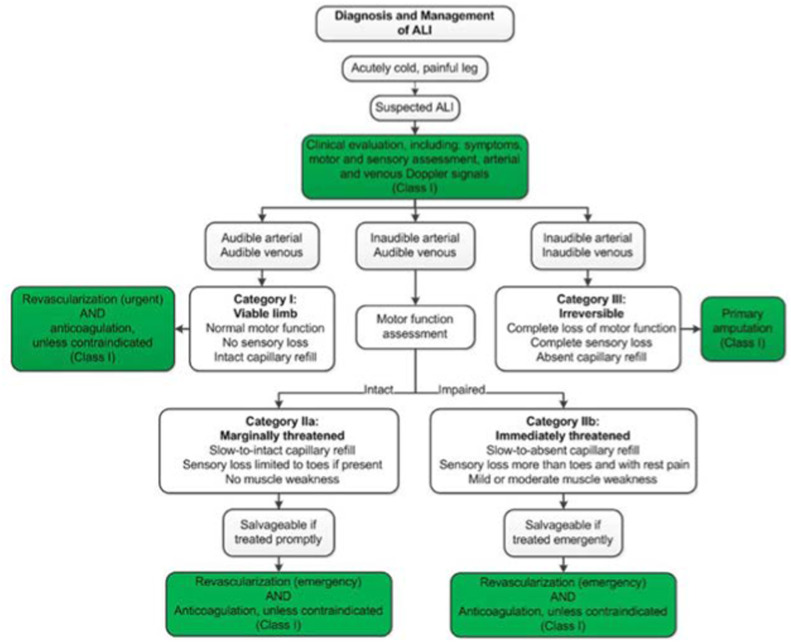
Even though data specific to postprocedural anticoagulation is sparse, in one Italian review, 17 of 20 patients with ALI underwent revascularization, which was successful in 12 patients. The use of continuous postoperative heparin was associated with increased survival. Even though more data on anticoagulation for COVID-19-associated ALI are forthcoming, the authors concluded that continuous intravenous heparin might improve surgical treatment efficacy, limb salvage, and overall survival [3]. As mentioned above, this is especially important in our study where adequate anticoagulation was not achieved in the majority of the cases. Fig. 10 below summarizes the diagnosis and management of ALI (taken from the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity PAD).
Fig. 10.
Below summarizes the diagnosis and management of ALI (taken from the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity PAD).
Overall, the mortality rate of COVID-19 for those who require hospitalization is over 20% [16]. However, among patients who develop ALI, mortality rates are as high as 50% [3,5]. In a review of 571 COVID-19 patients, the risk of death was nearly three-fold higher in patients who had arterial thrombotic events (hazard ratio 2∙96, 95% CI 1∙4-4∙7) [1]. Fortunately, all patients in our study survived despite all having severe COVID-19 infections.
The rate of major amputation (e.g., below-knee amputation, above-knee amputation) is higher in patients with COVID-19-associated ALI (7–35%) than ALI in non-COVID-19 patients (6 and 23%) [2,3,5]. This is especially high in our case study with half (50%) ending up in amputation.
We acknowledge the following limitations of the study: the limited number of patients and the observational, descriptive nature of the study renders the establishment of causality impossible. Asymptomatic ALI cases may have been missed since a radiological investigation was only performed in the presence of clinical suspicion. These limitations are also seen in similar published studies [11,18].
5. Conclusion
There was a delay in revascularization therapy (median of 3.5 days) resulting in poor treatment outcomes which were mainly due to imaging, laboratory, equipment (e.g., Fogarty catheter), and vascular specialist shortages.
Even young and otherwise healthy patients with COVID-19 may develop ALI despite the use of prophylactic or therapeutic anticoagulation. It is also prudent to not only have a low threshold for initiating therapeutic anticoagulation, especially in severe COVID-19 but also regularly follow coagulation profiles and check the adequacy of anticoagulation.
Authors’ contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Funding
There was no specific funding for this project.
Conflict of interest
All authors declare no conflict of interest.
Ethics approval
Ethical approval was obtained for the publication of this case series from the Research Ethical Review Committee at Eka Kotebe General Hospital, Addis Ababa (IRB reference number Eka/150/5/53 on Dec 21, 2021).
Consent for publication
Informed written consent was obtained from the patients and their family members for the publication of this case series and accompanying images.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Tariku Assefa Soboka (MD, Intensivist, ICU head at EKGH) and the rest of the ICU team as well as the Medical Record department at EKGH. The authors also thank the patients and their families for consenting to the publication of the article.
Handling Editor: Dr P Emmanouil
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tru.2022.100128.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Detail of ALI patients’ characteristics and outcome
Detail of ALI patients’ characteristics and outcome (Continued)
References
- 1.Adam Cuker, MS Flora Peyvandi, Lawrence LK Leung, Jennifer S Tirnauer et al. COVID-19: Hypercoagulability. https://www.uptodate.com/. Accessed on December 9, 2021.
- 2.Etkin Y., Conway A.M., Silpe J., et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City area. Ann. Vasc. Surg. 2021;70:290. doi: 10.1016/j.avsg.2020.08.085. Epub 2020 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellosta R., Luzzani L., Natalini G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J. Vasc. Surg. 2020;72(6):1864. doi: 10.1016/j.jvs.2020.04.483. Epub 2020 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nabil A., Nawaf S., Hamza J., et al. Acute Lower Limb Ischemia in Patients Infected with COVID-19. Dove press. 2021;vol. 14:833–839. doi: 10.2147/IJGM.S301462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llonzo N., Rao A., Safir S., Vouyouka A., Phair J., Baldwin M., et al. Acute thrombotic manifestation of COVID-19 infection: experience at a Large New York City Health system. J. Vasc. Surg. 2020 doi: 10.1016/j.jvs.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Roquetaillade C., Chousterman B.G., Tomasoni D., et al. Unusual arterial thrombotic events in Covid-19 patients. Int. J. Cardiol. 2021;323:281. doi: 10.1016/j.ijcard.2020.08.103. Epub 2020 Sep. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin D.O., Jensen A., Khan M., et al. Arterial thromboembolic complications in COVID-19 in low-risk patients despite prophylaxis. Br. J. Haematol. 2020;190(1):e11. doi: 10.1111/bjh.16792. Epub 2020 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horani G., Kaur K., Patel P., et al. Acute limb ischemia as the initial severe presentation in COVID-19. Cureus. 2021;13(3) doi: 10.7759/cureus.14226. Epub 2021 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhard-Herman, Gornik H.L., Barrett C., et al. AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2016;69(11):e71. doi: 10.1161/cir.0000000000000470. 2017. [DOI] [PubMed] [Google Scholar]
- 10.Rutherford R.B., Baker J.D., Ernst C., et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J. Vasc. Surg. 1997;26(3):517. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 11.Topcu A.C., Ozturk-Altunyurt G., Akman D., et al. Acute limb ischemia in hospitalized COVID-19 patients. Ann. Vasc. Surg. 2021;74:88. doi: 10.1016/j.avsg.2021.03.003. Epub 2021 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavord S., Scully M., Hunt B.J., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N. Engl. J. Med. 2021;385(18):1680. doi: 10.1056/NEJMoa2109908. Epub 2021 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton Richard. GBD 2010: understanding disease, injury, and risk. Lancet. December 15, 2012;380 doi: 10.1016/S0140-6736(12)62133-3. issue 9859. [DOI] [PubMed] [Google Scholar]
- 14.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9. doi: 10.1016/j.thromres.2020.04.024. Epub 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell W.B., Ridler B.M., Szymanska T.H. Two-year follow-up after acute thromboembolic limb ischemia: the importance of anticoagulation. Eur. J. Vasc. Endovasc. Surg. 2000;19(2):169. doi: 10.1053/ejvs.1999.0999. [DOI] [PubMed] [Google Scholar]
- 16.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, Comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson L., Strachan J. Subcutaneous unfractionated heparin for the initial treatment of venous thromboembolism. Cochrane Database Syst. Rev. 2017;Issue 2 doi: 10.1002/14651858.CD006771.pub3. CD006771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Touzani Soumaya, Zahra Haddari Fatima, El Bouazzaoui Abderrahim, et al. Acute limb ischemia in critically ill COVID-19 patients: a case series and literature review. JMSR. 2021;VII(3):917–922. doi: 10.46327/msrjg. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detail of ALI patients’ characteristics and outcome
Detail of ALI patients’ characteristics and outcome (Continued)
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.