Background:
Electromagnetic navigational bronchoscopy (ENB) has been shown to have variable diagnostic accuracy for the assessment of peripheral pulmonary nodules. This may be because of discrepancies between the preplanned computed tomography of chest target lesion location versus actual target location (computed tomography-to-body divergence), and the lack of a continuous navigational image. The ILLUMISITE (Medtronic, Minneapolis, MN) is a newly developed ENB platform that utilizes tomosynthesis, an imaging technology that can visualize the target location using fluoroscopy (F-ENB). This new system also allows for intraprocedural positional correction and continuous navigation guidance during sampling to overcome these limitations and improve diagnostic yield. We report our first experience in a single center, single proceduralist using this new technology.
Methods:
We conducted a retrospective, single center, single operator study reviewing 72 consecutive patients (78 nodules) over a 3-month period. We investigated the overall diagnostic yield and diagnostic yield by nodule location, size, and sedation type using this new F-ENB system.
Results:
The overall diagnostic yield was 87% and pnemothoraces occurred in 2/78 procedures. We did not find any statistically significant difference when comparing pulmonary nodule location, size or sedation method utilized (P=0.231, 0.338, and 0.112, respectively). Sixty-nine percent of the pulmonary nodules biopsied were 2 to 3 cm in size. The average distance corrected after tomosynthesis visualization was 15.4 mm (0.4 to 29.8 mm).
Conclusion:
We report our initial experience with the ILLUMISITE system using fluoroscopic tomosynthesis-assisted visualization with continuous navigational guidance at our institution. This new technology allows the operator to correct for better target lesion alignment and real time positional correction and may improve diagnostic yields with minimal complications for evaluation of peripheral pulmonary nodules.
Key Words: bronchoscopy, navigational bronchoscopy, lung nodule, lung cancer, fluoroscopy, S-ENB and F-ENB
BACKGROUND
Electromagnetic navigational bronchoscopy (ENB) has been utilized for the evaluation of peripheral pulmonary nodules (PPN) for several years.1 Reported diagnostic yields have varied from 38% to 94%.2–4 Two significant limitations that may affect the diagnostic yield with ENB guided biopsy are the occurrence of computed tomography (CT) scan to body divergence (CTBD) and the lack of continuous image guidance tissue sampling during the procedure. The CTBD refers to the difference that occurs between the location of the target lesion on the preprocedural CT scan of the chest and the location of the target lesion during the actual procedure (Fig. 1) and has been reported to take place in up to 32% of ENB cases.5,6 Aboudara et al reported a 25% increase (54% to 79%) in diagnostic yield after correction of the CTBD with a newer unique feature of the SuperDimension V 7.2 (Medtronic, Minneapolis, MN) fluoroscopy ENB (F-ENB) system that utilizes a fluoroscopic digital tomosynthesis image compared with the prior standard-ENB (S-ENB) SuperDimension V 7.1 (Medtronic) system in the evaluation of PPN.7 A recent study by Katsis et al,8 using F-ENB system, showed an overall diagnostic yield of 77.4%.
FIGURE 1.
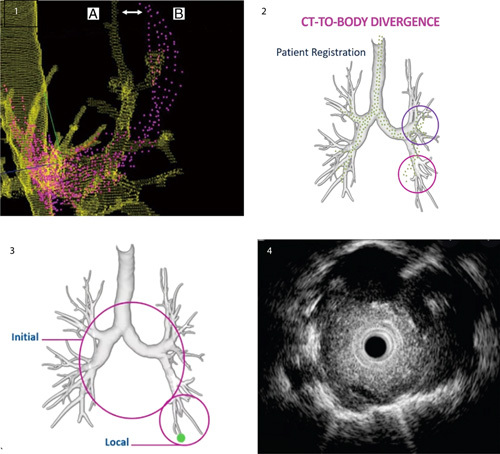
Image 1: computed tomography (CT)-to-body divergence (CTBD) (white arrow) is defined as the difference from the lung roadmap. Image 2: that is done before the procedure (A, green) and the real time patient anatomy (B, purple) is CT-to-body divergence.
The development of the new ILLUMISITE platform system may increase diagnostic yield by integrating a (F-ENB) fluoroscopic navigational tomosynthesis technology, which can correct for CTBD and uses continuous navigational guidance that results in a continuous ENB image during bronchoscopy sampling (Fig. 2). This may result in less sampling error and higher diagnostic yield. We report our first experience in terms of diagnostic yield and safety with this new system in the evaluation of PPN.
FIGURE 2.
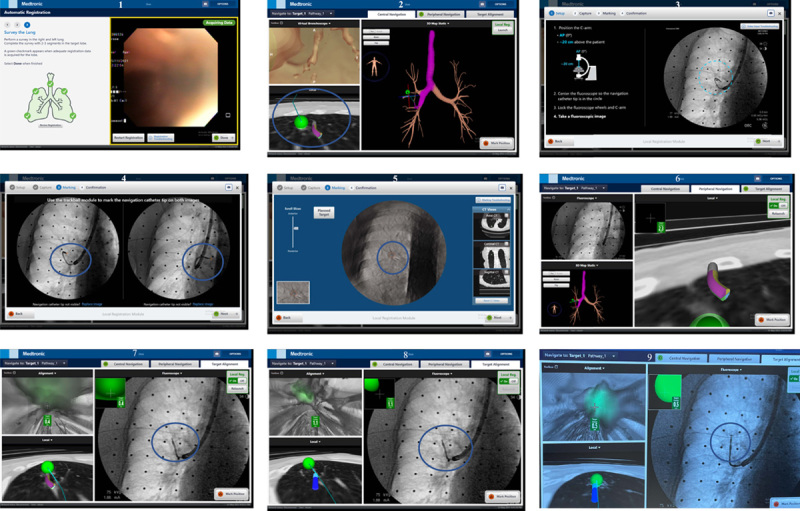
Overview and steps for using new electromagnetic navigational bronchoscopy system with fluoroscopy tomosynthesis-assisted visualization (local registration), intraprocedural positional correction and continuous navigation guidance for a right upper lobe 1.1 cm peripheral pulmonary nodule.
METHODS
The protocol was approved by the Institutional Review Board of our institution (UMCIRB 15-002257). We conducted a retrospective, single academic center, single operator study investigating the safety and diagnostic yield of the new F-ENB system for the assessment of PPN.
We collected data on 72 consecutive patients that required ENB for the evaluation of 78 PPN over a 3-month time (December 2019 to February 2020). The indication for sampling of the PPN in our study was predetermined from past medical history, physical examination, signs and symptoms, pulmonary nodule size, presence, or absence of mediastinal and/or hilar adenopathy (using 2017 Fleischner criteria guidelines or ACR Lung Rads criteria). Clinical stage I-IV was determined based of CT scan imaging, using the 8th edition of primary tumor, regional lymph nodes, distant metastasis staging of lung cancer, as noted in Table 1. Patients with suspected clinical stage I to IV were all sampled because these lesions were accessible by bronchoscopy. After the ENB procedure, all patients had a mediastinal and hilar lymph node interrogation through endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) (BF-UC160F-OL8; Olympus, Tokyo, Japan), if the lymph nodes were >5 mm as measured on ultrasound imaging.9 Herth et al published a study in CHEST 2008 which showed that EBUS-TBNA can be utilized to for clinical stage I lung cancers and found that malignancy was present in 9% of their patients with a prior normal mediastinum on CT scan or PET scans and upstaged these patients from Stage I to Stage II or III, thus affecting prognosis and treatment strategies.10,11 At our institution we have found similar results with our patient population, so a staging EBUS-TBNA is always performed after a diagnosis of lung cancer is determined by rapid on-site cytologic evaluation (rapid onset evaluation) from a PPN.12 We have adopted the philosophy of sampling the primary tumor as well as the mediastinum since ∼5% of our samples have demonstrated more than two cell types between the lymph nodes and primary lesions.
TABLE 1.
Demographics, Location and Size of Nodule, Pathologic Diagnosis, Distance From Pleura, Target Correction, Sedation Types and Complications
| Demographics | N (%) |
|---|---|
| Total patients enrolled | 72 (—) |
| Total pulmonary nodules | 78 (—) |
| Sex | |
| Male | 48/72 (66.7) |
| Female | 24/72 (33.3) |
| Age | |
| Range | 37-96 |
| Median | 69 |
| Location of nodule | |
| Right upper lobe | 32/78 (40.1) |
| Right middle lobe | 8/78 (10.2) |
| Right lower lobe | 9/78 (11.5) |
| Left upper lobe | 22/78 (28.2) |
| Left lower lobe | 7/78 (8.9) |
| Pathologic diagnosis | |
| Malignant | 61/78 (78.2) |
| Nonmalignant | 7/78 (8.9) |
| Nondiagnostic | 10/78 (12.8) |
| Sedation type | |
| General anesthesia | 16/72 (22.2) |
| Moderate sedation | 56/72 (77.8) |
| Complications | |
| Pneumothorax | 2/78 (2.6) |
| Size of nodule, cm | |
| Overall median size | 2 |
| Range | 0.8-4 |
| <1 | 1/78 (1.3) |
| 1-1.9 | 22/78 (28.2) |
| 2-3 | 54/78 (69.2) |
| >3 | 1/78 (1.2) |
| Distance from the pleura, mm | |
| Overall distance (median) | 1 |
| Range | 1-15 |
| 1 | 45/78 (58) |
| 2 | 11/78 (14) |
| 3 | 10/78 (13) |
| 4 | 6/78 (8) |
| 5 | 5/78 (5) |
| >5 | 1/78 (1) |
| Target correction, mm | |
| Average | 15.4 |
| Minimum-maximum | 0.4-29.8 |
| Suspected clinical stage | |
| Stage I | 25/72 (34.7) |
| Stage II | 17/72 (23.6) |
| Stage III | 8/72 (11.1) |
| Stage IV | 22/72 (30.5) |
Percentages are listed out of 78 total number of nodules and 72 total patients. Suspected clinical stage I to IV before bronchoscopy (based of CT scan review and 8th edition of TNM staging of lung cancer).
CT indicates computed tomography; N, number of pulmonary nodules or patients; TNM, primary tumor, regional lymph nodes, distant metastasis.
Procedure Methods
There were 2 types of sedation methods utilized: general anesthesia (GA) and moderate sedation. Patients receiving GA underwent orotracheal intubation and were placed on a mechanical ventilator in the operating room for the procedure. Anesthesia was administered by the anesthesiologist through inhalation and intravenous sedation and muscle paralytics. Patients receiving moderate sedation were sedated using a combination of intravenous midazolam, fentanyl or propofol and received supplemental oxygen at various concentrations through nasal cannula to ensure adequate oxygenation during the procedure. Moderate sedation medications were administered by registered nurses and were supervised by bronchoscopist performing the procedure.
All patients underwent ENB initial registration in the exact same manner as the S-ENB system (Fig. 2) that has been previously reported.4,11 A 180-degree curved extended working channel/locatable guide (EWC/LG) (Medtronic) was utilized for each case. For patients receiving GA, a breath hold (8 to 30 s) was started at max inspiration, while no breath hold was done for patients receiving moderate sedation. A continuous smooth fluoroscopic left to right “sweep” (of 12 to 15 s) was performed from a left anterior oblique position at 25 degrees to a right anterior oblique position of −25 degrees. At the end of the fluoroscopic “sweep,” ventilation was resumed for the GA group. The operator then marked the tip of the LG and registered the target lesion in 2 positions on the newly generated digital tomosynthesis image (Fig. 1), which corrected for CTBD and allowed for sampling of the lesion more accurately (Figs. 1 and 2). At this time, local registration was completed, and if a significant correction was done, the operator renavigated to the corrected target lesion. Once navigation was complete and target lesion deemed in an appropriate position for biopsy, the LG was removed from the EWC and continuous navigation guidance (CG) was initiated and provided a continuous ENB image of the target lesion (Fig. 2). A radial EBUS probe was placed through the EWC to confirm adequate positioning for a biopsy (Fig. 1). Samples were then taken under direct fluoroscopic and CG. Each patient had 7 fine needle aspirations with the Arcpoint 18-gauge needle (Medtronic) and 7 forceps samples collected with the SuperDimension biopsy forceps (Medtronic). Rapid on-site cytologic evaluation by a cytopathologist was utilized to confirm adequate tissue collection and if a preliminary diagnosis of malignancy was noted, coiled SuperLock fiducial markers (Medtronic) were placed around the target lesion utilizing the ENB system in patients with stage I or II disease who were not deemed surgical candidates.
Primary and Secondary Outcomes
The primary outcome of this study was the diagnostic yield of the samples collected with the new F-ENB system utilizing fluoroscopy tomosynthesis advanced visualization, intraprocedural correction, and CG. A sample was considered diagnostic when the tissue collected from the nodule was determined to be consistent with a definitive diagnosis (ie, cancer, granuloma or + culture infection).
For samples deemed to have a nonspecific diagnosis, or pathologic finding of acute inflammation was identified and repeat CT scan of the chest demonstrated a resolved abnormality, or had fluoroscopy navigation failure, these biopsies were calculated as nondiagnostic. Secondary outcomes for this study included the impact of nodule size, location, sedation method used on the overall diagnostic yield of the PPN and number of complications (Table 3).
TABLE 3.
Secondary Outcomes for Diagnostic Yield by Location, Nodule Size, and Sedation Type
| Secondary Outcomes | Diagnostic Yield, N (%) | P |
|---|---|---|
| Nodule location | 0.231 | |
| Right upper lobe | 30/32 (94) | |
| Right middle lobe | 6/8 (75) | |
| Right lower lobe | 9/9 (100) | |
| Left upper lobe | 17/22 (77) | |
| Left lower lobe | 6/7 (85.7) | |
| Nodule size | 0.338 | |
| <1 cm | 1/1 (100) | |
| 1-1.9 cm | 16/21 (76.2) | |
| 2-3 cm | 50/55 (90.1) | |
| >3 cm | 1/1 (100) | |
| Sedation type | 0.112 | |
| Moderate sedation | 52/62 (84) | |
| General anesthesia | 16/16 (100) |
Pearson χ2 test statistic and exact P-value.
Total of 78 pulmonary nodules and 72 patients. Includes secondary outcomes and statistics using the Pearson χ2 test statistic model and exact P-value for nodules biopsied that resulted in a diagnostic yield based on location, size, and sedation type. Diagnostic biopsy results were 68/78 (87.2): resulting in a definitive diagnosis including cancer, granuloma, or infection as noted in Table 2.
Statistical Analysis
Quantitative variables are reported as median or mean along with minimum and maximum values. Categorical variables are reported as frequency and percentage. The exact Pearson χ2 tests were used to test associations between diagnostic yield and potential predictive factors. The exact Pearson χ2 test was chosen because some counts were 0 or otherwise small. The 95% Clopper Pearson confidence interval was calculated for pneumothorax rate. IBM SPSS Statistics 27 was used for the statistical analyses.
RESULTS
Data was collected over 3 months in 72 patients with 78 pulmonary nodules (Table 1). There were 48 men and 24 women with the median age of 69 years (37 to 96). Sedation was performed using either moderate sedation in the bronchoscopy suite or in the operating room under GA. Most of our patients 78% (56/72) received moderate sedation and a minority of the patients 22% (16/72) were taken to the operating room and were sedated using GA. The type of sedation and location of the procedure was only determined based on procedure room availability. The overall diagnostic yield using fluoroscopic tomosynthesis-assisted ENB (F-ENB) with continuous navigational guidance was 87.2% (68/78). The nondiagnostic group was 12.8% (10/78) and all occurred in the moderate sedation cohort. The majority (78%) of the PPN had definitive diagnosis of malignancy (61/78) with 31% showing adenocarcinoma (31/78) and 19% showing squamous cell carcinoma (15/78) of the lung and the remainder of the results are listed in Table 2. Eight percent (6/78) of the pulmonary nodules biopsied from either transbronchial lung biopsy or fine needle aspiration were diagnosed with infection from a positive culture. Infections including mycobacterium avium complex, Staphylococcus, Moraxella catarrhalis, and actinomyces and were considered diagnostic. Four percent (3/78) showed a pathologic finding of acute inflammation, which were negative for malignancy and resolved on subsequent imaging and one patient (1/78) had fluoroscopy navigation failure occur (samples were taken but no diagnosis obtained). Both acute inflammation and the navigation failure were considered nondiagnostic (Table 2), using a more conservative definition of diagnostic yield and more consistent with recent ENB publications.8 The majority of the PPN were between 2 and 3 cm (69%) and 28% were 1 and 1.9 cm. Only 1% (1/72) were <1 cm and 1% (1/78) were >3 cm. In comparison, our previous study had 40% of lesions that were >3 cm.4 Regarding distance from the pleura, 85% (66/78) were located 3 mm or less from the pleura, and 69% (54/78) were in the upper lobes (Table 1). Seventy eight percent (56/72) of patients had the procedure performed under moderate sedation, while the remaining 22% (16/72) underwent GA. There was an 84% (52/62) diagnostic yield using moderate sedation and 100% (16/16) diagnostic yield using GA. Two patients (2.6%) in the moderate sedation group developed a pneumothorax, which did not require a chest tube intervention, with 95% confidence interval (0.3%, 9.0%). No other complications were noted. The distance of target correction was recorded for 77 PPN (98.7%) with only one nodule that fluoroscopic navigation failed to register. The average distance corrected after fluoroscopic navigation was 15.4 mm (range: 0.4 to 28.9 mm) (Table 1). The diagnostic yield was not significantly influenced by the location of the PPN (P=0.231), size of the PPN (P=0.338), or choice of sedation method utilized (P=0.112) (Table 3).
TABLE 2.
Histopathologic Diagnosis
| Pathologic Diagnosis | N (%) |
|---|---|
| Diagnostic results | 68/78 (87.2) |
| Adenocarcinoma | 31/78 (39.7) |
| Squamous cell carcinoma | 15/78 (19.2) |
| Nonsmall-cell carcinoma NOS | 10/78 (12.8) |
| Small cell carcinoma | 3/78 (3.8) |
| Carcinoid | 1/78 (1.3) |
| Renal cell carcinoma | 1/78 (1.3) |
| Granuloma | 1/78 (1.3) |
| Infection | 6/78 (7.7) |
| Nondiagnostic results* | 10/78 (12.8) |
Percentages are listed out of 78 total number of biopsy specimens, out of 72 total patients. Two groups including: 1: diagnostic biopsy results were 68/78 (87.2): resulting in a definitive diagnosis including cancer, granuloma, or infection. Infection was seen in 6/78 (77) of the nodules biopsied (positive cultures for infections included Mycobacterium avium complex, Staphylococcus, Moraxella catarrhalis, Actinomyces.
2: Nondiagnostic biopsy results were 10/78 (12.8) not resulting in a definitive diagnosis and occurred in the moderate sedation cohort, including three nodules showing acute inflammation but were negative for definitive diagnosis and resolved on subsequent imaging and one patient that had fluoroscopy navigation failure.
N indicates diagnostic yield by pathologic diagnosis; NOS, not otherwise specified.
DISCUSSION
We report our first use of this new system which utilizes fluoroscopic tomosynthesis advanced visualization (F-ENB), intraprocedural positional correction, with CG for the evaluation of PPN. Our data shows an overall diagnostic yield of 87% (68/78) with only 2 complications (2.6%) and only one failure of the fluoroscopic navigational technology (1.3%), both occurring in the moderate sedation group. There was 100% (16/16) diagnostic yield using GA and 84% (52/62) diagnostic yield using moderate sedation for total of 72 patients. This difference could partially be because of the ability to perform a breath hold during local registration and improve target location with GA and/or due the small sample size and limited numbers of patients receiving GA (16 patients) versus moderate sedation (52 patients). Although our prior ENB study in 2015, Bowling et al,4 showed an overall diagnostic yield of 73.6%, there was a more even patient distribution and found no difference in diagnostic yield for GA (70%) and moderate sedation (78%) (P=0.38).
Although we did not prospectively compare the new F-ENB with continuous navigational guidance versus older S-ENB systems, we have noticed some intriguing and noteworthy observations when compared with our previously reported data from 2015 using the S-ENB platform.4,7 First, there was a 13% increase in overall diagnostic yield utilizing the new F-ENB system compared with our previously reported diagnostic yield of 74% (67/91).4 In the NAVIGATE trial the diagnostic yield was 73%.13 In our 2015 study utilizing S-ENB we had a diagnostic yield of 74%4 with the same bronchoscopy operator as in this study showing a diagnostic yield of 87%. Between study years, the operator has had extensive experience with ENB and performs over 300 ENB procedures a year. This experience alone may have had significant impact on the diagnostic yield seen in this study. Both Lamprecht et al14 and Bansal et al15 reported an increase in diagnostic yield with ENB guided biopsies of PPN with increasing operator experience. However, this is not consistent with the data by Aboudara et al7 which supports that the correction of CTBD using fluoroscopy guidance tomosynthesis appeared to have the most significant impact on diagnostic yield. It may be that the new system using fluoroscopy guided tomosynthesis with continuous guidance could be more impactful in those users that have average to lower diagnostic yields with the S-ENB system as was demonstrated by Aboudara et al.7 Direct comparisons need to be performed to evaluate the actual impact of the new F-ENB system on diagnostic yield compared with S-ENB systems and user experience.
The impact of nodule size on diagnostic yield when utilizing ENB guided biopsy remains unclear.4,16,17 It has been speculated that after removal of the LG and radial probe, the EWC may drift out of the appropriate sampling alignment from the target lesion during or in between tissue collection when using the S-ENB systems thus leading to a nondiagnostic sample.4 This may have a distinct impact on smaller PPN since there is a smaller surface area and a slight change in alignment can make a significant impact in diagnostic success.4 There was no significant increase in diagnostic yield with enlarging nodule size when using the new F-ENB system with continuous guidance system (P=0.338) (Table 3). This effect may be because of the combination of the fluoroscopic navigational technology, correcting for CTBD and the use of the continuous guidance feature, allowing the ability to align the target during sampling. It is unclear which feature may have had the most impact among our cohort. Although there is some speculation, insight can be gained by evaluating the data presented by Aboudara et al7 and Pritchett et al,6 that may help in answering this question concerning nodule size and continuous guidance impact on diagnostic yield. A recent retrospective single center study by Katsis et al8 using F-ENB found there was an overall improvement in diagnostic yield to 77.4% at 6 months as compared with S-ENB of 70% and increased from their prior reported diagnostic yield of 54% and their pneumothorax rate was 2.5%. Similar to our study, they chose a more conservative definition of diagnostic yield and did not include inflammation or benign lesion material. When they included the more traditional definition of diagnostic yield, which includes nonmalignant histology with resolution or stability at 6 months, their overall diagnostic yield was 87.9%, and in their study, they described this definition as flawed.8
The Aboudara et al7 review reported a 25% increase in diagnostic yield using F-ENB (79%) when compared with S-ENB (54%) system without fluoroscopic navigational technology. Interestingly, this study had an average nodule size of 1.5 cm versus 2 cm in our study. It appears clear that the fluoroscopic navigational tomosynthesis (F-ENB) technology had an impressive impact on the diagnostic yield, but given the smaller lesion size in their cohort, the addition of the continuous guidance feature may improve the diagnostic accuracy even further. Pritchett et al6 utilized the SuperDimension V7.2 (Medtronic) F-ENB system in a feasibility study evaluating the ability of this platform to correct CTBD. Although not a primary endpoint, the malignancy rate was 61% and the majority of PPN were <2 cm.6 It is possible that the addition of the continuous guidance feature may improve the diagnostic yield with these smaller lesions. In our study the average correction distance of the target lesion using continuous guidance was 15.0 mm and is consistent with the accumulated divergence data (13.0 mm) reported by Aboudara.7 The fluoroscopic navigation technology appears clearly impactful, and the addition of the continuous guidance feature may further increase diagnostic yield for the elusive PPN. Further investigation is needed to assess the impact of this new technology as compared with the S-ENB platform.
As noted, the only complication that occurred in our cohort were 2 pneumothoraces (2.6%), with a 95% confidence interval (0.3%, 9.0%), which occurred in the moderate sedation cohort. The NAVIAGTE study reported a pneumothorax rate of 4.3%. Our pneumothorax rate is similar to the data presented by Aboudara and colleagues (2 pneumothoraces with the Fluoroscopic navigation system) and Pritchett and colleagues (1 pneumothorax).6,7,13 It may be that the correction of the target lesion with the fluoroscopic navigation technology can aid in lowering the risk of pneumothorax. In addition, the CG feature may have an impact on the risk for a pneumothorax since it allows the operator to always have accurate alignment of the target lesion while sampling. Given that the 2 pneumothoraces occurred in the moderate sedation patient group, using GA may lower this risk. The effect that the addition of continuous fluoroscopy guidance to fluoroscopic tomosynthesis-assisted system has on the risk of procedural complications with ENB guided biopsies for PPN needs further evaluation given the small sample size in our study.
Limitations
There are several limitations to our study. First, this is a retrospective investigation with relatively few patients that were enrolled and short duration of enrollment. Second, this was a single center study, and 1 very experienced operator performed all the procedures. The latter makes these findings less generalizable to various practices and users. Third, although, the practice of moderate sedation with the new ENB system was successful in our cohort, it may be that the most consistent performance of the fluoroscopic navigation tool is under GA where a breath hold can be performed during local registration to allow more accurate use of the fluoroscopy tomosynthesis to correct for CTBD, as noted by difference sedation types utilized (Table 3). Lastly, given that the majority of the pulmonary nodules biopsied were 2 to 3 cm in size, it may be too assertive to state a lack of difference in size-based yield and further studies are needed.
CONCLUSIONS
The new ILLUMISITE system using newer technology with tomosynthesis-fluoroscopy advanced visualization, intraprocedural positional correction for CTBD, and continuous navigational guidance allows for constant target alignment during tissue sampling and has demonstrated a reasonable diagnostic yield (87%) for the evaluation of PPN in our small single center, single operator retrospective study. This new technology and platform may provide more confidence for sampling PPN. Because of the straightforward operator format in combination with the improved target accuracy, this new ENB system may improve overall diagnostic yields approaching those of real time image guided sampling of PPN. To determine if there is a significant difference in terms of safety, performance, and operator usability, this new ENB system needs to be evaluated in a proper prospective trial where these endpoints can be compared with other diagnostic modalities in the evaluation of PPN.
Footnotes
Disclosure: M.R.B.: consultant for Medtronic. For the remaining there is no conflict of interest or other disclosures.
Contributor Information
Bryan K. Dunn, Email: dunnb20@ecu.edu.
Michael Blaj, Email: BLAJM16@ECU.EDU.
Jennifer Stahl, Email: Stahlj@ecu.edu.
James Speicher, Email: SPEICHERJ15@ECU.EDU.
Carlos Anciano, Email: ancianoc14@ecu.edu.
Suzanne Hudson, Email: hudsons@ecu.edu.
Emily A. Kragel, Email: kragele17@students.ecu.edu.
Mark R. Bowling, Email: bowlingm@ecu.edu.
REFERENCES
- 1.Mehta AC, Hood KL, Schwarz Y, et al. The evolutional history of electromagnetic navigation bronchoscopy. Chest. 2018;154:935–947. [DOI] [PubMed] [Google Scholar]
- 2.Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loo FL, Halligan AM, Port JL, et al. The emerging technique of electromagnetic navigation bronchoscopy-guided fine-needle aspiration of peripheral lung lesions: promising results in 50 lesions. Cancer Cytopathol. 2014;122:191–199. [DOI] [PubMed] [Google Scholar]
- 4.Bowling MR, Kohan MW, Walker P, et al. The effect of general anesthesia versus intravenous sedation on diagnostic yield and success in electromagnetic navigation bronchoscopy. J Bronchology Interv Pulmonol. 2015;22:5–13. [DOI] [PubMed] [Google Scholar]
- 5.Chen A, Pastis N, Furukawa B, et al. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest. 2015;147:1275–1281. [DOI] [PubMed] [Google Scholar]
- 6.Pritchett MA, Bhadra K, Mattingley JS. Electromagnetic navigation bronchoscopy with tomosynthesis-based visualization and positional correction: three-dimensional accuracy as confirmed by cone-beam computed tomography. J Bronchology Interv Pulmonol. 2021;28:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboudara M, Roller L, Rickman O, et al. Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: initial experience with a novel adjunct. Respirology. 2020;25:206–215. [DOI] [PubMed] [Google Scholar]
- 8.Katsis J, Roller L, Aboudara M, et al. Diagnostic yield of digital tomosynthesis-assisted navigational bronchoscopy for indeterminate lung nodules. J Bronchology Interv Pulmonol. 2021;28:255–261. [DOI] [PubMed] [Google Scholar]
- 9.Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronch ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142:1393–1400.e1. [DOI] [PubMed] [Google Scholar]
- 10.Pearlstein DP, Quinn CC, Burtis CC, et al. Electromagnetic navigation bronchoscopy performed by thoracic surgeons: one center’s early success. Ann Thorac Surg. 2012;93:944–949. [DOI] [PubMed] [Google Scholar]
- 11.Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest. 2008;133:887–891. [DOI] [PubMed] [Google Scholar]
- 12.Karnak D, Ciledag A, Ceyhan K, et al. Rapid on-site evaluation and low registration error enhance the success of electromagnetic navigation bronchoscopy. Ann Thorac Med. 2013;8:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol. 2019;14:445–458. [DOI] [PubMed] [Google Scholar]
- 14.Lamprecht B, Porsch P, Wegleitner B, et al. Electromagnetic navigation bronchoscopy (ENB): increasing diagnostic yield. Respir Med. 2012;106:345710–345715. [DOI] [PubMed] [Google Scholar]
- 15.Bansal S, Hale K, Seth S, et al. Electromagnetic navigational bronchoscopy: a learning curve analysis. Chest. 2007;132:514b. [Google Scholar]
- 16.Mohanasundaram U, Ho LA, Kuschner WG, et al. The diagnostic yield of navigational bronchoscopy performed with propofol deep sedation. Endoscopy. 2013;2013:1–5. [Google Scholar]
- 17.Jensen KW, Hsia DW, Seijo LM, et al. Multicenter experience with electromagnetic navigation bronchoscopy for the diagnosis of pulmonary nodules. J Bronchology Interv Pulmonol. 2012;19:195–199. [DOI] [PubMed] [Google Scholar]


