Abstract
One of the salient features of periodontitis and gingivitis is the increase in the levels of bacterial and host-derived proteolytic enzymes in oral inflammatory exudates. This study evaluated the potential of histatin 5, a 24-residue histidine-rich salivary antimicrobial protein, to inhibit these enzymes. Using biotinylated gelatin as a substrate, histatin 5 was found to inhibit the activity of the host matrix metalloproteinases MMP-2 and MMP-9 with 50% inhibitory concentrations (IC50s) of 0.57 and 0.25 μM, respectively. To localize the domain responsible for this inhibition, three peptides containing different regions of histatin 5 were synthesized and tested as inhibitors of MMP-9. Peptides comprising residues 1 to 14 and residues 4 to 15 of histatin 5 showed much lower inhibitory activities (IC50, 21.4 and 20.5 μM, respectively), while a peptide comprising residues 9 to 22 showed identical activity to histatin 5 against MMP-9. These results point to a functional domain localized in the C-terminal part of histatin 5. To evaluate the effect of histatin 5 on bacterial proteases, a detailed characterization of histatin 5 inhibition of gingipains from Porphyromonas gingivalis was carried out using purified Arg- and Lys-specific enzymes. Kinetic analysis of the inhibition of the Arg-gingipain revealed that histatin 5 is a competitive inhibitor, affecting only the Km with a Ki of 15 μM. In contrast, inhibition of Lys-gingipain affected both the Km and Vmax, suggesting that both competitive and noncompetitive competitive processes underlie this inhibition. The inhibitory activity of histatin 5 against host and bacterial proteases at physiological concentrations points to a new potential biological function of histatin in the oral cavity.
Histatin 5 is a member of a family of low-molecular-weight salivary proteins secreted by parotid, submandibular, and sublingual glands (32). Like other salivary proteins, histatin 5 appears to be multifunctional, and its major function is its antifungal activity against the opportunistic yeast Candida albicans (34, 48). Besides fungicidal and fungistatic properties, antibacterial properties have been attributed to histatins based on their killing and growth-inhibitory activity against several species of oral bacteria (24, 49). Only a few reports exist on the inhibitory effects of histatins on bacterial proteases (18, 30).
Periodontal disease is a chronic inflammatory disorder characterized by bone resorption, loss of tooth attachment, and formation of periodontal pockets populated with a flora composed of specific spectrum of bacteria. Many studies have shown that gingivitis and periodontitis lead to increased levels of both host and bacterial proteolytic enzymes in oral inflammatory exudates, which can enter the oral cavity as gingival crevicular fluid and become constituents of whole saliva (11, 25, 27, 29, 40). Among these proteinases, host-derived matrix metalloproteinases (MMPs) are considered key initiators of extracellular matrix degradation associated with periodontal and other oral diseases (39). These enzymes comprise a family of structurally and functionally related zinc-dependent enzymes capable of degrading extracellular matrix proteins, such as different types of collagen, gelatin, fibronectin, laminin, and elastin (2). MMPs are involved in the normal turnover of the extracellular matrix, which is an integral part of development, morphogenesis, and tissue remodeling. Besides participating in many normal physiologic processes, the unregulated activity of MMPs has been implicated in numerous disease conditions including arthritis, tumor cell metastasis, and periodontitis. Interestingly, the levels of at least two of these enzymes, MMP-2 and MMP-9, are elevated in the saliva of patients with periodontal disease (8, 11).
Inhibition of MMPs is a promising approach for treatment of diseases associated with these enzymes, and the structures of MMPs and the structural features of complexes of MMPs and their naturally occurring tissue inhibitors provide templates for the rational design of inhibitors (4). However, most of the attention in this area of research has been given to chelating agents that bind to zinc at the active site and inactivate the enzymes (6, 38, 47). We have recently demonstrated that histatin 5 forms complexes with metal cations including zinc (9). This property, together with the abundant presence of histatins in saliva, makes these peptides potential candidates as inhibitors of MMP activity in the oral cavity.
In addition to host enzymes, tissue destruction during the course of periodontal disease can result from bacterial enzymes. Porphyromonas gingivalis is an anaerobic, gram-negative bacterium which is present in the microflora of subgingival plaque and has been strongly implicated in the etiology of periodontal disease. This is principally because this microorganism shows many virulence features, such as the release of toxic products of metabolism and outer membrane vesicles containing numerous enzymes involved in invasion and tissue destruction, the elaboration of fimbriae and lipopolysaccharide, the utilization of lectin-type adhesions, and the promotion of hemagglutination and hemolysis (42). Several physiologically important proteins, including collagen (3, 18), fibrin and fibrinogen (21), fibronectin (44), plasma protease inhibitors (5), immunoglobulins (41), and complement factors (46), are degraded by proteases from P. gingivalis. Some of these proteolytic activities were previously attributed to trypsin-like proteases, but their isolation and characterization revealed that two distinct cysteine proteinase types occur with strict specificities for cleavage at either arginine (Arg-gingipains, RgpA and RgpB) or lysine (Lys-gingipain, Kgp) residues (33). These enzymes are now considered potential targets for testing and development of specific inhibitors (43). Histatin 5 inhibits a trypsin-like enzyme from P. gingivalis (30). However, at the time this work was done, it was not known that two enzymes are actually responsible for this activity, and therefore the specific effect of histatin 5 on the activity of Arg-gingipain and Lys-gingipain has not yet been determined.
The aim of the present study was to investigate the potential of histatin 5 to act as an inhibitor of host and bacterial enzymes involved in the development of periodontal disease. First, we evaluated whether histatin 5 and histatin 5-derived fragments were able to inhibit the proteolytic activity of the host-derived enzymes MMP-2 and MMP-9. Second, we studied in detail the inhibition of purified Arg-gingipain and Lys-gingipain by histatin 5 and elucidated the nature of this inhibition.
MATERIALS AND METHODS
Chemicals.
The materials used in this study were purchased from commercial sources as follows: leupeptin, l-cysteine, N-benzoyl-dl-arginine p-nitroaniline (BAPNA), trypsin inhibitor, aprotinin, biotinyl-N-hydroxysuccinimide ester, N,N-dimethylformamide, gelatin (type I from swine skin), p-nitrophenyl phosphate disodium (pNPP), APMA (p-aminophenylmercuric acetate) and EDTA were obtained from Sigma (St. Louis, Mo.); CaCl2 · 2H2O was purchased from Fisher (Pittsburgh, Pa.); and H-Val-Leu-Lys-pNA (Lys-pNA) was obtained from Bachem (Torrance, Calif.).
Enzymes and peptides.
Pro-MMP-2 and pro-MMP-9 were purchased from Boehringer Mannheim (Indianapolis, Ind.). The purity of both pro-MMP-2 and pro-MMP-9 was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Both enzyme preparations revealed only a single protein band, in the 72- and 92-kDa regions, respectively. Arg-gingipains (RgpA and RgpB) and Lys-gingipain (Kgp) were purified as described previously (33). Trypsin, chymotrypsin, aprotinin, and trypsin inhibitor were obtained from Sigma. Synthetic histatin 5 (DSHAKRHHGYKRKFHEKHHSHRGY; molecular weight [MW], 3,037) was obtained from American Peptide Co. (Sunnyvale, Calif.). Synthetic histatin 5-derived peptides, designated peptide 1 (DSHAKRHHGYKRKF; MW, 1,767), peptide 2 (GYKRKFHEKHHSHR; MW, 1,847), and peptide 3 (AKRHHGYKRKFH; MW, 1,563) were obtained from commercial sources.
Preparation of biotinylated gelatin.
Biotinylated gelatin was prepared in our laboratory by labeling type I gelatin with biotin as described previously (20). Gelatin (1 mg/ml) was dissolved in 0.2 M sodium carbonate (pH 8.8) containing 0.15 M NaCl. To this solution, 100 μl of biotinyl-N-hydroxysuccinimide ester was added, and the reaction was allowed to proceed for 15 min at room temperature. The reaction was terminated by addition of 75 μl of 1 M ammonium chloride (pH 6.0). This preparation was dialyzed for 3 days against 20 liters of water with two changes per day. The protein concentration of the final solution was determined using the bicinchoninic acid assay (Pierce, Rockford, Ill.). Subsequently, the biotinylated protein was divided into aliquots and stored at −20°C until use.
Biotinylated gelatin was diluted to 5 μg/ml in 50 mM bicarbonate buffer (pH 9.6), and 50 μl was applied to each well of a 96-well microtiter plate. The plates were incubated at 4°C for 24 h, and the unbound biotinylated gelatin was removed by washing the plates with phosphate-buffered saline (PBS). After that, the plates were blocked at 37°C for 30 min with 50 μl of 1% (wt/vol) gelatin solution dissolved in PBS. After three washes with PBS followed by one wash with water, the plates were used for MMP activity assays.
MMP-2 and MMP-9 activity assays.
MMP-2 and MMP-9 were tested using biotinylated gelatin-coated microtiter plates as a substrate. In this assay, estimation of enzyme activity is based on the loss of bound biotin resulting from proteolytic activity against the gelatin-biotin complex adsorbed to the wells of microtiter plates. A stock solution of 5.4 μM MMP-9 was diluted to 10.8 nM in enzyme buffer consisting of 50 mM Tris-HCl (pH 7.5) containing 0.5 M NaCl and 5 mM CaCl2. The diluted enzyme was activated by adding 1 mM 4-aminophenylmercuric acetate and was further incubated at room temperature for 30 min. Histatin 5 at concentrations ranging from 0.005 to 100 μM was incubated with activated enzyme for 10 min before being added to the microtiter plates. The same procedure was carried out with peptide 1, peptide 2, and peptide 3. As a positive control, EDTA was used at 25 mM. After incubation of the appropriate inhibitor with the enzyme, the wells of a microtiter plate were filled with 50 μl of this mixture and the plate was incubated at 37°C for 2 h. Wells containing enzyme without inhibitor were used to determine maximal activity (100%). Wells containing substrate and buffer alone were used as controls, representing no activity (0%). To stop the reactions, the plate was washed three times with 200 μl of PBS containing 1% Tween 20. Subsequently, 50 μl of streptavidin-alkaline phosphatase (1:2, 500 dilution in water) was added to each well, and the plate was incubated for 15 min at 37°C. The plate was then washed four times with 200 μl of PBS-Tween, and 200 μl of pNPP dissolved in diethanolamine buffer (1 mg of pNPP per ml of buffer) was added for 20 min at 37°C. The absorbance was recorded at 405 nm using a microtiter plate reader (Molecular Devices, Sunnyvale, Calif.).
MMP-2 was assayed essentially by the method previously described for MMP-9, using biotinylated gelatin. A 4.1 μM MMP-2 stock solution was dissolved in 50 mM Tris-HCl (pH 7.5) containing 0.5 M NaCl and 5 mM CaCl2, to result in a final concentration of 41 nM MMP-2. Subsequently, MMP-2 was activated by the addition of 1 mM APMA and was incubated in a water bath for 30 min at 37°C. All subsequent procedures were the same as described for the MMP-9.
Arg-gingipain and Lys-gingipain enzyme activity assays.
Arg-gingipain and Lys-gingipain activities were determined using a spectophotometric assay as described previously (36) with some modifications. Both Arg-gingipain (RgpB) and Lys-gingipain were dissolved in 0.2 M Tris-HCl–0.1 M NaCl–5 mM CaCl2–10 mM l-cysteine (pH 7.6). Arg-gingipain (3.3 nM) activity was measured with 80 μM BAPNA, while Lys-gingipain (4.0 nM) activity was measured with 80 μM Lys-pNA. Cleavage of the substrate was assessed in the presence and absence of concentrations of histatin 5 ranging from 5 to 100 μM. Arg-gingipain or Lys-gingipain was incubated with histatin 5 for 5 min prior to addition of this mixture to 600 μl of enzyme buffer (0.2 M Tris-HCl, 0.1 M NaCl, 5 mM CaCl2, 10 mM l-cysteine [pH 7.6]) containing the appropriate substrate. Reactions were carried out in cuvettes with a 1-cm light path at 25°C, and the formation of product (p-nitroaniline) was monitored by measuring the increase in absorbance at 410 nm using a Spectronic 1201 spectrometer (Milton Roy, Londonderry, N.H.). Reaction velocities were obtained from the initial slope of plots of absorbance at 410 nm versus time. Values for maximal enzyme activity were determined in the absence of inhibitor.
For the determination of the concentration required to inhibit 50% of the proteolytic activity (IC50), a series of concentrations of histatin 5 were prepared to yield activities in the range of 25 to 75%. The IC50 was determined graphically from the inhibition curves by plotting enzyme activity against inhibitor concentrations.
Kinetic studies.
The Vmax, Km, kcat, and kcat/Km were determined at 25°C using substrates at concentrations ranging from 30 to 160 μM, with final enzyme concentrations of 3.3 nM (Arg-gingipain, RgpB) and 4.0 nM (Lys-gingipain) either in the absence or in the presence of two different concentrations of histatin 5. The initial turnover rate at six different concentrations of substrate was calculated, and the type of inhibition and Michaelis-Menten parameters were determined from Lineaweaver-Burk plots (37) by using the equations derived from the linear-regression analysis of each curve. The kcat value was calculated by applying the equation kcat = Vmax/[E], where [E] is the enzyme concentration in the assay (26).
The Ki values were estimated by using a Dixon plot for reversible and competitive inhibition (10, 37), with two different concentrations of substrate (60 and 106 μM) in the presence or in the absence of increasing concentrations of histatin 5.
RESULTS
Inhibition of MMP-2 and MMP-9 by histatin 5.
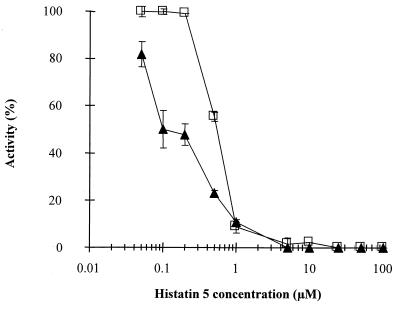
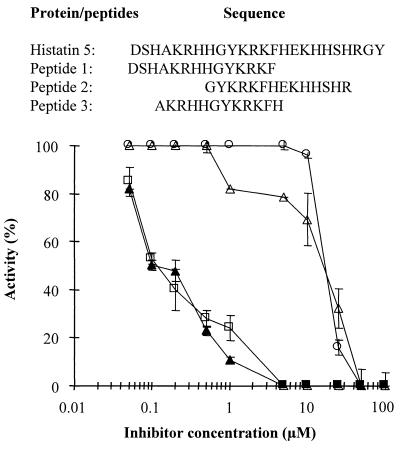
Both MMP-2 and MMP-9 activities were tested using biotinylated gelatin as a substrate. EDTA, a well-established inhibitor of metalloproteinases, was used in control experiments at a concentration of 25 mM, resulting in 99% inhibition (data not shown). Both MMP-2 and MMP-9 activities were measured in the presence of 0.05 to 100 μM histatin 5 (Fig. 1). Histatin 5 was found to inhibit the gelatinolytic activity of both MMPs tested (Fig. 1). Almost complete inhibition of MMP-2 and MMP-9 was observed when histatin 5 was used at concentrations higher than 1 μM (Fig. 1). The IC50s obtained for MMP-2 and MMP-9 were 0.57 and 0.25 μM, respectively (Table 1). The difference in the inhibition of MMP-2 and MMP-9 was statistically significant (Student's t test, p < 0.0005). Because histatin 5 demonstrated higher inhibitory properties against MMP-9, this enzyme was selected to test the inhibitory activity of histatin 5-derived peptides. These experiments were carried out to localize the region within histatin 5 responsible for the MMP-9 inhibition. Figure 2 shows the inhibitory activities of peptide 1, peptide 2, and peptide 3 against MMP-9. Different patterns of inhibition were observed. All three peptides completely inhibited MMP-9 at concentrations exceeding 50 μM. Peptide 1 and peptide 3 are both located in the N-terminal region of histatin 5 and showed less inhibitory activity than the intact protein (IC50s of 21.4 ± 0.5 and 20.5 ± 0.8 μM, respectively). In contrast, peptide 2, which contains the sequence located toward the C terminus, showed identical inhibitory activity to histatin 5 (IC50s of 0.25 ± 0.01 and 0.25 ± 0.03 μM, respectively).
FIG. 1.
Inhibition of MMP-2 and MMP-9 by histatin 5. Enzyme activity was measured using biotinylated gelatin coated in microtiter plates as substrate. Histatin 5 at final concentrations ranging from 0.005 to 100 μM was incubated with either 41 nM MMP-2 (□) or 10.8 nM MMP-9 (▴) for 2 h at 37°C.
TABLE 1.
Inhibition of host and bacterial proteases by histatin 5
| Enzyme class | Enzyme | IC50 (μM)a |
|---|---|---|
| Metalloproteinase | MMP-2 | 0.57 ± 0.02 |
| MMP-9 | 0.25 ± 0.01 | |
| Serine proteinase | Chymotrypsin | >50 |
| Trypsin | >50 | |
| Cysteine proteinase | Arg-gingipain | 22.0 ± 2.2 |
| Lys-gingipain | 13.8 ± 1.5 |
Mean and standard deviation of three separate experiments.
FIG. 2.
Effect of histatin 5 and derived peptides on MMP-9 activity. The upper panel shows the sequences of intact histatin 5 and derived peptides. The lower panel shows the gelatinolytic activity of 10.8 nM MMP-9, assessed at 37°C for 2 h with increasing concentrations of histatin 5 (▴), peptide 1 (○), peptide 2 (□), and peptide 3 (▵).
Members of another important class of enzymes, the serine proteinases chymotrypsin and trypsin, were also tested using the same method described for the MMPs. Histatin 5 did not inhibit either trypsin or chymotrypsin since the IC50s were higher than 50 μM for both enzymes (Table 1).
Inhibition of Arg-gingipain and Lys-gingipain by histatin 5.
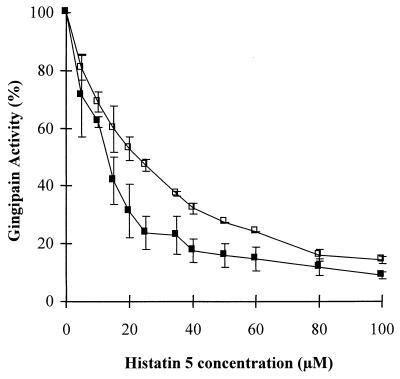
Inhibition of P. gingivalis-derived Arg-gingipain and Lys-gingipain by histatin 5 was investigated using the synthetic substrates BAPNA and Lys-pNA, respectively. Figure 3 shows the activities of both Arg or Lys gingipains in the presence of increasing concentrations of histatin 5. Histatin 5 inhibited both gingipains in a concentration-dependent manner, with IC50s of 22.0 ± 2.2 and 13.8 ± 1.5 μM, respectively (Table 1). Interestingly, histatin 5 was a stronger inhibitor of Lys-gingipain (Student's t test, p = 0.01).
FIG. 3.
Inhibition of Arg-gingipain and Lys-gingipain by histatin 5. Histatin 5 was added to either Arg-gingipain (3.3 nM) (□) or Lys-gingipain (4.0 nM) (■) at concentrations ranging from 5 to 100 μM. Cleavage of the synthetic substrate BAPNA or Ly-pNA (each at a concentration of 80 μM) was measured spectophotometrically at 410 nm. Activity was estimated from control experiments performed with enzyme only.
Kinetics of Arg-gingipain inhibition by histatin 5.
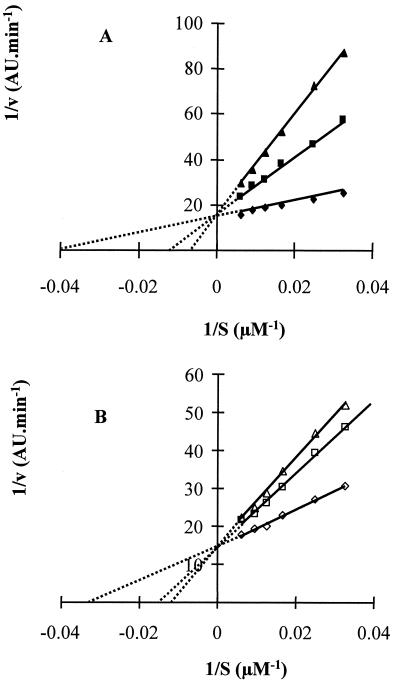
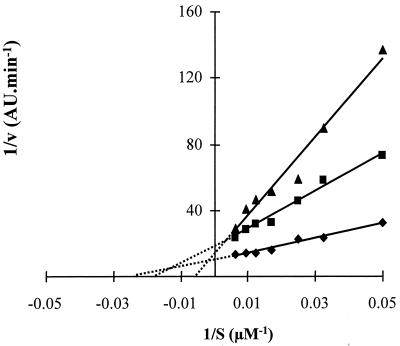
To determine the nature of the inhibition of Arg-gingipain by histatin 5, experiments were carried out using enzyme (3.3 nM RgpB) in the presence or absence of histatin 5 (20 or 40 μM) with six different concentrations of BAPNA (30.7, 40, 60, 80, 106, and 160 μM). The velocity (v) of the reaction was calculated for each substrate concentration (S), and the data were presented in a Lineweaver-Burk plot (1/v versus 1/S) to determine the kinetic parameters (Fig. 4A). The three lines obtained with enzyme only, histatin 5 at 20 μM, and histatin 5 at 40 μM intercept at the same position on the y axis, indicating that Vmax is the same in all cases (Fig. 4A; Table 2). Furthermore, the intercept of the lines on the x axis demonstrates that the Km value increase when the histatin 5 concentration increases. This is characteristic of a competitive inhibition. The mean and standard deviation of kinetic parameters from three separate experiments are given in Table 2. Kinetic parameters for Arg-gingipain without inhibitor are consistent with values described in the literature (36). While Vmax and kcat values obtained for Arg-gingipain were unchanged in the presence of histatin 5, clear changes were observed in the Km and kcat/Km values. These results further verify that the inhibition by histatin 5 is competitive, indicating that in the presence of histatin 5, Arg-gingipain is less effective and therefore has a lower specific activity. The same kind of kinetic studies were carried out with RgpA, and histatin 5 was found to competitively inhibit this enzyme as well (data not shown).
FIG. 4.
Lineweaver-Burk plots of the inhibition of Arg-gingipain by histatin 5 (A) and leupeptin (B). Enzyme assays were carried out with 3.3 nM Arg-gingipain (⧫, ◊) in the presence of either histatin 5 at 20 μM (■) and 40 μM (▴) or leupeptin at 0.16 μM (□) and 0.3 μM (▵).
TABLE 2.
Kinetic parameters of Arg-gingipain (RgpB) and Lys-gingipain in the presence and absence of histatin 5a
| Enzyme (histatin 5 concn) | Vmax (AU/min) | Km (μM) | kcat (S−1) | kcat/Km (106 S−1 M−1) |
|---|---|---|---|---|
| Arg-gingipain (0 μM) | 0.072 ± 0.007b | 22.3 ± 1.4 | 21.8 ± 1.53 | 0.9 ± 0.07 |
| Arg-gingipain (20 μM) | 0.058 ± 0.007b | 82.1 ± 6.9 | 17.5 ± 2.12 | 0.2 ± 0.03 |
| Arg-gingipain (40 μM) | 0.064 ± 0.002b | 161.3 ± 15.3 | 19.3 ± 0.46 | 0.1 ± 0.01 |
| Lys-gingipain (0 μM) | 0.117 ± 0.016 | 59.7 ± 15.3b | 29.2 ± 4.0 | 0.50 ± 0.06 |
| Lys-gingipain (10 μM) | 0.0565 ± 0.008 | 62.8 ± 12.6b | 14.1 ± 2.1 | 0.23 ± 0.03 |
| Lys-gingipain (20 μM) | 0.0613 ± 0.014 | 143.5 ± 36.8 | 15.3 ± 3.5 | 0.11 ± 0.003 |
Parameters were calculated using the equations obtained from the Lineweaver-Burk plots. Mean and standard deviation are given for three separated experiments.
Difference not statistically significant by analysis of variance (t test, P > 0.05).
Similar results were obtained with the positive control leupeptin (Fig. 4B). Inhibition assays were carried out with leupeptin, and it was found that concentrations of 0.16 and 0.3 μM leupeptin inhibited the activity of Arg-gingipain by 27 and 42%, respectively (data not shown). These concentrations were found to be suitable for the kinetic studies, and the same experiments described for histatin 5 were carried out with leupeptin. Data were then presented in a Lineawever-Burk plot (Fig. 4B). It was found that Km increased whereas Vmax was constant in the presence of leupeptin, indicating that this peptide is also a competitive inhibitor of Arg-gingipain.
Determination of the inhibition constant of histatin 5 against Arg-gingipain.
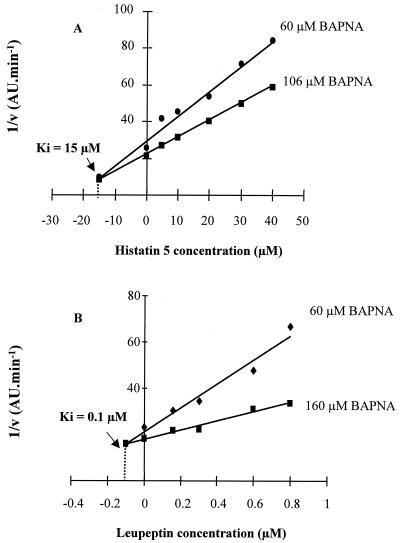
For the determination of the Ki of histatin 5 against Arg-gingipain, the velocity of cleavage of BAPNA (at 60 and 106 μM) was measured at histatin 5 concentrations ranging from 5 to 40 μM. In this plot, the reciprocals of velocity were plotted against inhibitor concentration at different concentrations of substrate. A series of straight lines was obtained, converging to the same point, and that value represents −Ki. It was found that the Ki for histatin 5 is 15 μM (Fig. 5A). When the same experiments were performed with leupeptin, a Ki of 0.1 μM was found (Fig. 5B).
FIG. 5.
Dixon plot for the determination of the inhibition constant (Ki) of either histatin 5 (A) or leupetin (B) against Arg-gingipain. Enzyme assays were carried out with 3.3 nM gingipain in the presence of increasing concentrations of histatin 5 at two different concentrations of BAPNA (60 and 106 μM). The reciprocals of velocity were plotted against the histatin 5 concentration, and the Ki value was obtained from the intercepts of two lines at two concentrations of substrate.
Kinetics of Lys-gingipain inhibition by histatin 5.
With the purpose of investigating the type of inhibition of Lys-gingipain by histatin 5, kinetic experiments were carried out using 4 nM enzyme in the presence or absence of histatin 5. The concentrations of histatin 5 used were 10 and 20 μM. Figure 6 shows the Lineweaver-Burk plot of Lys-gingipain inhibition by histatin 5. At the lower concentration of histatin 5 used (10 μM), the Vmax was altered, whereas the Km was the same as that when the enzyme only was used (Table 1; Fig. 6). This is typical of noncompetitive inhibition. However, when the histatin 5 concentration was increased to 20 μM a different effect on the kinetic parameters was observed. At this concentration of histatin 5, the Lineweaver-Burk plot shows that both Vmax and Km values were changed. This phenomenon was highly reproducible and therefore indicates that the inhibition of Lys-gingipain by histatin 5 is more complex than the inhibition of Arg-gingipain.
FIG. 6.
Lineweaver-Burk plot for the inhibition of Lys-gingipain by histatin 5. Enzyme assays were performed with 4 nM Lys-gingipain (⧫) in the presence of 10 μM (■) or 20 μM (▴) histatin 5 at various concentrations of substrate.
DISCUSSION
Characteristics of periodontal disease such as inflammation and attachment loss can result from the activity of an array of proteolytic enzymes secreted by both host cells and colonizing bacteria. Inhibition or at least regulation of the activity of these enzymes is required to control the processes that can lead not only to periodontal disease but also to other disorders in which these enzymes are involved. The present investigation was undertaken to evaluate the inhibitory effect of the naturally occurring salivary component histatin 5 on the proteolytic activity of host and bacterial proteases associated with connective tissue destruction. Histatin 5 exhibited strong inhibitory activity against the host enzymes MMP-2 and MMP-9. Structure-function analysis using three peptides derived from the histatin 5 sequence revealed that the inhibitory domain was located in a sequence comprising residues 9 to 22 (peptide 2). Two other peptides, comprising residues 1 to 14 (peptide 1) and residues 4 to 15 (peptide 3), both exhibited a significantly less potent inhibitory activity than peptide 2.
It is important to note that zinc is essential for the enzymatic activity of MMPs and that metal chelators such as EDTA and 1,10-phenanthroline are potent inhibitors of these enzymes. We have recently discovered that histatin 5 binds metal ions, including zinc (9), and have suggested that this significant chelating action of histatin 5 may be important in the inhibition of the MMPs. This hypothesis is supported by the fact that histatin 5 contains the sequence HEXXH, which is recognized as a zinc-binding motif in many proteins (17, 45). This consensus sequence occurs at residues 15 to 19 of histatin 5 (28) and therefore is absent in peptide 1 and peptide 3. Thus, the strongly reduced activity observed with peptides 1 and 3 can be explained by the absence of the zinc-binding motif in these peptides. The findings of this study highlight a novel property of histatin 5 as an inhibitor of zinc-dependent enzymes. Conceptually, the chelating capacity of histatins makes it feasible that histatins could functionally interfere with proteins or enzymes which require metals as cofactors or as an integral part of their structure.
Several studies have focused on the inhibition of MMPs by synthetic compounds, which include not only chelating agents but also substrate analogue peptides (6, 38, 47). The most potent of these have Kis in the low nanomolar range (38). However, only a few studies have evaluated the effect of natural products on the MMP activity. Some components of green tea, such as eppigallocatechin gallate, strongly inhibit both MMP-2 and MMP-9, with IC50s of 6 and 0.8 μM, respectively (7). In the present study, we found even lower IC50s for the inhibition of MMP-2 and MMP-9 by histatin 5, a naturally occurring salivary protein. It is possible that inhibition of these enzymes takes place in the oral cavity since the concentration of histatin 5 in salivary secretions has been reported to be almost 2 orders of magnitude higher than the IC50s found in this study (1, 15, 16).
Since the development and progression of periodontal disease involve independent and cooperative actions of both host and bacterial proteolytic enzymes, this study also evaluated the effect of histatin 5 on the activity of bacterial proteases, such as the cysteine proteases derived from P. gingivalis, Arg- and Lys-gingipains. In the present study we used purified Arg and Lys-gingipains and found that histatin 5 inhibited both enzymes at concentrations comparable to those occurring in oral secretions. A more detailed characterization of the inhibition of each enzyme was accomplished by determining the nature of this inhibition as well as the effect of histatin 5 on the enzyme kinetics. We found that histatin 5 is a competitive inhibitor of the Arg-gingipains, with a Ki of 15 μM against RgpB. A previous study, however, suggested that histatin 5 is not an inhibitor of but a substrate for the gingipains (31). The reasons for this discrepancy are not clear. One reason for the difference may be related to the fact that O'Brien-Simpson et al. (31) used fivefold-higher enzyme concentrations and used mixtures of Arg- and Lys-gingipains in their enzymatic analyses. Furthermore, if histatin 5 is indeed susceptible to proteolysis by gingipains, this salivary protein could serve as an alternative substrate and thereby act as a competitive inhibitor by binding to the same active site (37). Another important aspect to be considered is that histatins are almost continuously being secreted into the oral cavity, allowing histatin 5 to constantly inhibit gingipains under physiologic conditions even though some cleavage of this protein may occur. A similar phenomenon is known to occur with cystatins, which are members of another class of competitive inhibitors of cysteine proteases present in saliva (13, 14). These inhibitors are susceptible to proteolytic digestion, especially in the N-terminal region (12, 22, 23, 35).
In contrast to Arg-gingipain, analysis of the inhibition of Lys-gingipain by histatin 5 revealed that at lower concentrations histatin 5 acts as a noncompetitive inhibitor, since the Km values were not changed in the presence of histatin 5. A noncompetitive inhibition occurs when a molecule can bind to a site on an enzyme surface which is different from the catalytic site. However, at a higher concentration of histatin 5, the values of both the kinetic parameters Vmax and Km were altered. The increase in the Km value suggests that at increasing concentrations of histatin 5, additional lower-affinity associations of histatin 5 occur at the active site. An alternate explanation for the mixed type of inhibition observed at higher histatin 5 concentrations could be that binding outside the catalytic site could induce a conformational change at the catalytic site, resulting in a lowered affinity for the substrate. A more specific analysis of this interaction is required to elucidate the precise interaction of histatin 5 with the different sites on the enzyme.
In summary, histatin 5 was found to be an inhibitor of host and bacterial enzymes involved in the destruction of the periodontium. These findings support the idea that histatin 5 exerts an important function in the protection of oral tissues, thereby participating in the innate host defense system in the oral cavity. The inhibition of host enzymes, MMP-2 and MMP-9, which are participants in tumor invasion and metastasis (19) suggests that the role of histatins in the oral cavity may go beyond protecting oral tissues against connective tissue breakdown. It is speculated that histatins may be used as a template for the design of analogs aimed at the prevention or treatment of diseases in which these enzymes are involved.
ACKNOWLEDGMENTS
We gratefully acknowledge H. Kagan and P. Trackman for valuable discussions during the course of this investigation.
This work was supported in part by NIH/NIDCR grants DE05672 and DE07652.
REFERENCES
- 1.Atkinson J C, Yeh C, Oppenheim F G, Bermudez D, Baum B J, Fox P C. Elevation of salivary antimicrobial proteins following HIV-1 infection. J Acquired Immune Defic Syndr. 1990;3:41–48. [PubMed] [Google Scholar]
- 2.Birkedal-Hansen H, Moore W G I, Bodden M K, Windsor L J, Birkedal-Hansen B, DeCarlo A, Engler J A. Matrix Metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 3.Birkedal-Hansen H, Taylor R E, Zambon J J, Barwa P K, Neiders M E. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J Periodontal Res. 1988;23:258–264. doi: 10.1111/j.1600-0765.1988.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 4.Bode W, Fernandez-Catalan C, Grams F, Gomis-Ruth F X, Nagase H, Tschesche H, Maskos K. Insights into MMP-TIMP interactions. Ann N Y Acad Sci. 1999;878:73–91. doi: 10.1111/j.1749-6632.1999.tb07675.x. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson J, Herrmann B F, Hofling J F, Sundqvist G K. Degradation of the human proteinase inhibitors alpha-1-antitrypsin and alpha-2-macroglobulin by Bacteroides gingivalis. Infect Immun. 1984;43:644–648. doi: 10.1128/iai.43.2.644-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De B, Natchus M G, Cheng M, Pikul S, Almstead N G, Taiwo Y O, Snider C E, Chen L, Barnett B, Gu F, Dowty M. The next generation of MMP inhibitors. Design and synthesis. Ann N Y Acad Sci. 1999;878:40–60. doi: 10.1111/j.1749-6632.1999.tb07673.x. [DOI] [PubMed] [Google Scholar]
- 7.Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 2000;1478:51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 8.Ding Y, Liede K, Leppa S, Ingman T, Sepper R, Konttinen Y T, Sorsa T. Gingival crevicular fluid and salivary matrix metalloproteinases of heavy smokers as indicators of periodontal health. Ann N Y Acad Sci. 1994;732:453–455. doi: 10.1111/j.1749-6632.1994.tb24783.x. [DOI] [PubMed] [Google Scholar]
- 9.Gusman, H., U. Lendenmann, J. Grogan, R. F. Troxler, and F. G. Oppenheim. Is salivary histatin 5 a metallopeptide? Biochim. Biophys. Acta, in press. [DOI] [PubMed]
- 10.Hammes G G. Enzyme catalysis and regulation. Orlando, Fla: Academic Press, Inc.; 1982. pp. 38–60. [Google Scholar]
- 11.Ingman T, Sorsa T, Lindy O, Koski H, Konttinen Y T. Multiple forms of gelatinase/type IV collagenase in saliva and gingival crevicular fluid of periodontitis patients. J Clin Periodontol. 1994;21:26–31. doi: 10.1111/j.1600-051x.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 12.Isemura S, Saitoh E. Inhibitory activities of partially degraded salivary cystatins. Int J Biochem. 1994;26:825–831. doi: 10.1016/0020-711x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 13.Isemura S, Saitoh E, Sanada K. Characterization and amino acid sequence of a new acidic cysteine proteinase inhibitor (cystatin SA) structurally closely related to cystatin S, from human whole saliva. J Biochem. 1987;102:693–704. doi: 10.1093/oxfordjournals.jbchem.a122107. [DOI] [PubMed] [Google Scholar]
- 14.Isemura S, Saitoh E, Sanada K, Minakata K. Identification of full-sized forms of salivary (S-type) cystatins (cystatin SN, cystatin SA, cystatin S, and two phosphorylated forms of cystatin S) in human whole saliva and determination of phosphorylation sites of cystatin S. J Biochem. 1991;110:648–654. doi: 10.1093/oxfordjournals.jbchem.a123634. [DOI] [PubMed] [Google Scholar]
- 15.Jainkittivong A, Johnson D A, Yeh C-K. The relationship between salivary histatin levels and oral yeast carriage. Oral Microbiol Immunol. 1998;13:181–187. doi: 10.1111/j.1399-302x.1998.tb00730.x. [DOI] [PubMed] [Google Scholar]
- 16.Jensen J L, Xu T, Lamkin M S, Brodin P, Aars H, Berg T, Oppenheim F G. Physiological regulation of the secretion of histatins and statherins in human parotid saliva. J Dent Res. 1994;73:1811–1817. doi: 10.1177/00220345940730120401. [DOI] [PubMed] [Google Scholar]
- 17.Jongeneel C V, Bouvier J, Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989;242:211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- 18.Kato T, Takahashi N, Kuramitsu H K. Sequence analysis and characterization of the Porphyromonas gingivalis prtC gene, which expresses a novel collagenase activity. J Bacteriol. 1992;174:3889–3895. doi: 10.1128/jb.174.12.3889-3895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleiner D E, Stetler-Stevenson W G. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 20.Koritsas V M, Atkinson H J. An assay for detecting nanogram levels of proteolytic enzymes. Anal Biochem. 1995;227:22–26. doi: 10.1006/abio.1995.1247. [DOI] [PubMed] [Google Scholar]
- 21.Lantz M S, Allen R D, Duck L W, Blume J L, Switalski L M, Höök M. Bacteroides gingivalis and Bacteroides intermedius recognize different sites on human fibrinogen. J Bacteriol. 1990;172:716–726. doi: 10.1128/jb.172.2.716-726.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenarcic B, Kos J, Dolenc I, Lucovnick P, Krizaj I, Turk V. Cathepsin D inactivates cysteine proteinase inhibitors cystatins. Biochem Biophys Res Commun. 1988;154:765–772. doi: 10.1016/0006-291x(88)90206-9. [DOI] [PubMed] [Google Scholar]
- 23.Lenarcic B, Krasovec M, Ritonja A, Olafsson I, Turk V. Inactivation of human cystatin C and kininogen by human cathepsin D. FEBS Lett. 1991;280:211–215. doi: 10.1016/0014-5793(91)80295-e. [DOI] [PubMed] [Google Scholar]
- 24.MacKay B J, Denpitiya L, Iocono V J, Krost S P, Pollock J J. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect Immun. 1984;44:695–701. doi: 10.1128/iai.44.3.695-701.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makela M, Salo T, Uitto V J, Larjava H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status. J Dent Res. 1994;73:1397–1406. doi: 10.1177/00220345940730080201. [DOI] [PubMed] [Google Scholar]
- 26.Mathews C K, Van Holde K E. Biochemistry. 2nd ed. Menlo Park, Calif: The Benjamin/Cummings Publishing Co.; 1996. [Google Scholar]
- 27.McCulloch C A G. Collagenolytic enzymes in gingival crevicular fluid as diagnostic indicators of periodontitis. Ann NY Acad Sci. 1994;732:152–164. doi: 10.1111/j.1749-6632.1994.tb24732.x. [DOI] [PubMed] [Google Scholar]
- 28.Melino S, Rufini S, Sette M, Morero R, Grottesi A, Paci M, Petruzzelli R. Zn2+ ions seletively induce antimicrobial salivary peptide histatin 5 to fuse negatively charged vesicles. Identification and characterization of a zinc-binding motif present in the function domain. Biochemistry. 1999;38:9626–9633. doi: 10.1021/bi990212c. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura M, Slots J. Salivary enzymes. Origin and relationship to periodontal disease. J Periodontal Res. 1983;18:559–569. doi: 10.1111/j.1600-0765.1983.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 30.Nishikata M, Kanehira M, Takashi O H, Tani H, Tazaki M, Kuboki Y. Salivary histatin as an inhibitor of a protease produced by the oral bacterium Bacteroides gingivalis. Biochem Biophys Res Commun. 1991;174:625–630. doi: 10.1016/0006-291x(91)91463-m. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien-Simpson N M, Dashper S G, Reynolds E C. Histatin 5 is a substrate and not an inhibitor of the Arg- and Lys-specific proteinases of Porphyromonas gingivalis. Biochem Biophys Res Commun. 1998;250:474–478. doi: 10.1006/bbrc.1998.9318. [DOI] [PubMed] [Google Scholar]
- 32.Oppenheim F G, Xu T, McMillian F M, Levitz S M, Diamond R D, Offner G D, Troxler R F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure and fungistatic effects on Candida albicans. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- 33.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 34.Pollock J J, Denepitiya L, MacKay B J, Iacono V J. Fungistatic and fungicidal activity of human parotid saliva histidine-rich polypeptides on Candida albicans. Infect Immun. 1984;44:702–707. doi: 10.1128/iai.44.3.702-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popovic T, Cimerman N, Dolenc I, Ritonja A, Brzin J. Cathepsin L is capable of truncating cystatin C of 11 N-terminal amino acids. FEBS Lett. 1999;455:92–96. doi: 10.1016/s0014-5793(99)00824-8. [DOI] [PubMed] [Google Scholar]
- 36.Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thogersen I B, Enghild J J, Travis J. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- 37.Segel I H. Enzyme kinetics behavior and analysis of rapid equilibrium and steady-state enzyme systems. 2nd ed. New York, N.Y: Wiley-Interscience; 1975. [Google Scholar]
- 38.Skotnicki J S, Zask A, Nelson F C, Albright J D, Levin J I. Design and synthetic considerations of matrix metalloproteinase inhibitors. Ann NY Acad Sci. 1999;878:61–72. doi: 10.1111/j.1749-6632.1999.tb07674.x. [DOI] [PubMed] [Google Scholar]
- 39.Sorsa T, Suomalainen K, Uitto V J. The role of gingival crevicular fluid and salivary interstitial collagenases in human periodontal diseases. Arch Oral Biol. 1990;35:193S–196S. doi: 10.1016/0003-9969(90)90156-5. [DOI] [PubMed] [Google Scholar]
- 40.Sorsa T, Uitto V J, Suomalainen K. Characteristics of human salivary collagenase and its relationship to periodontal diseases. Matrix Suppl. 1992;1:406–407. [PubMed] [Google Scholar]
- 41.Sundqvist G, Carlsson J, Herrmann B, Tarnvik A. Degradation of human immunoglobulins G and M and complement factors C3 and C5 by black-pigmented Bacteroides. J Med Microbiol. 1985;19:85–94. doi: 10.1099/00222615-19-1-85. [DOI] [PubMed] [Google Scholar]
- 42.Travis J, Pike R, Imamura T, Potempa J. Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J Periodontal Res. 1997;32:120–125. doi: 10.1111/j.1600-0765.1997.tb01392.x. [DOI] [PubMed] [Google Scholar]
- 43.Travis J, Potempa J. Bacterial proteinases as targets for the development of second-generation antibiotics. Biochim Biophys Acta. 2000;1477:35–50. doi: 10.1016/s0167-4838(99)00278-2. [DOI] [PubMed] [Google Scholar]
- 44.Uitto V J, Larjava H, Heino J, Sorsa T. A protease of Bacteroides gingivalis degrades cell surface and matrix glycoproteins of cultured gingival fibroblasts and induces secretion of collagenase and plasminogen activator. Infect Immun. 1989;57:213–218. doi: 10.1128/iai.57.1.213-218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallee B L, Coleman J E, Auld D S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc Natl Acad Sci USA. 1991;88:999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wingrove J A, DiScipio R G, Chen Z, Potempa J, Travis J, Hugli T E. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;267:18902–18907. [PubMed] [Google Scholar]
- 47.Woessner J F., Jr Matrix metalloproteinase inhibition. From the Jurassic to the third millennium. Ann NY Acad Sci. 1999;878:388–403. doi: 10.1111/j.1749-6632.1999.tb07697.x. [DOI] [PubMed] [Google Scholar]
- 48.Xu T, Levitz S M, Diamond R D, Oppenheim F G. Anticandidal activity of major human salivary histatins. Infect Immun. 1991;59:2549–2554. doi: 10.1128/iai.59.8.2549-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu T, Oppenheim F G. Salivary antimicrobials: where are we? In: Bowen W H, Tabak L A, editors. Cariology for the nineties. Rochester, N.Y: University of Rochester Press; 1993. pp. 117–131. [Google Scholar]