Abstract
In the pathophysiology of acute coronary syndrome (ACS), platelet (PLT) and neutrophil (Neu) crosstalk may be important for activating coagulation and inflammation. It has been speculated that PLTs and Neu may affect each other’s cell counts; however, few studies have investigated this hypothesis. In this study, we measured changes in blood cell counts in 245 patients with ACS during treatment and investigated the mutual effects of each blood cell type. Path diagrams were drawn using structural equation modeling, and temporal changes in the count of each blood cell type and the relevance of these changes were analyzed. Throughout the treatment period, the numbers of all blood cell types (red blood cells [RBCs], leukocytes, and PLTs) were associated with each other before and after treatment. A detailed examination of the different cell types revealed that the PLT count at admission had a significant positive effect on the leukocyte (especially Neu) count after treatment. Conversely, the leukocyte (especially Neu) count at admission had a significant positive effect on the PLT count after treatment. During ACS, PLTs and leukocytes, especially Neu, stimulate each other to increase their numbers. The formation of a PLT-leukocyte complex may increase coagulation activity and inflammation, which can lead to a further increase in the counts of both blood cell types.
1. Introduction
Acute thrombus formation in the coronary arteries causes acute coronary syndrome (ACS). Increased coagulability is a systemic problem, not just a problem in the heart.[1] Platelet (PLT) function is generally considered to be central to thrombus formation. However, it is difficult to accurately determine PLT function in clinical practice because the index obtained in routine clinical practice is the number of PLTs. Interestingly, recent reports suggest that the PLT count alone may partially reflect the pathology of ACS.[2,3] In addition, it appears that the thrombotic condition of ACS can be assessed by the PLT-to-lymphocyte ratio (PLR).[4,5] Importantly, both the PLT count and leukocyte count have been shown to be involved in the pathology of ACS.[6–8] The neutrophil-to-lymphocyte ratio (NLR) is closely associated with the pathology of ACS.[9,10] Thus, it is likely that both PLTs and Neu are deeply involved in the thrombotic activity of ACS.
As mentioned above, the number of PLTs and Neus in the blood and the pathophysiology of ACS are thought to be closely related. However, it is unclear whether there is an association between the counts of each blood cell type. To the best of our knowledge, no research has been conducted on this topic in the ACS field. Perhaps activated PLTs and Neus affect each other and increase each other’s counts, increasing thrombotic activity and promoting myocardial ischemia.
Therefore, in this study, we investigated time-dependent changes in blood cell counts, particularly changes in PLTs and Neus, and their relationship during the acute and posttreatment stages of ACS. The degree of interaction was analyzed in detail using appropriate statistical analyses.
2. Materials and Methods
2.1. Patient population
This study included patients with ACS who required emergency admission to Jikei University Hospital between September 2014 and July 2019. ACS was defined as myocardial infarction (MI) and unstable angina pectoris, as described in detail previously.[11] Two of the following 3 criteria were needed for an MI diagnosis: a history of cardiac chest pain lasting at least 30 minutes; typical electrocardiographic changes; and an increase in the serum creatine kinase level. unstable angina pectoris was diagnosed according to the criteria of the Braunwald classification, without an increase in serum creatine kinase levels. During this period, 301 patients were treated for ACS. The following cases were excluded to determine the natural history of ACS due to organic stenosis or thrombi: Death during hospitalization (1 patient), Coronary spastic angina without significant stenosis or thrombosis (42 patients), Patients who underwent blood transfusion, which might have affected the results of the present study (4 patients), and Patients who underwent coronary artery bypass grafting, which can cause inflammatory reactions due to strong external stimulation (9 patients). Based on these selection criteria, 245 consecutive patients were enrolled in the current study. Of the 245 patients studied, 4 had a history of hematologic disease, but none had active disease. One patient had a history of inflammatory bowel disease that was not active during the study period. Five patients had a history of connective tissue disease and were taking steroids; however, there was no change in the prescription during hospitalization. The Ethics Committee of the Jikei University School of Medicine approved the study protocol (24–355[7121]), and we complied with the routine ethical regulations of our institution. This was a retrospective study, and informed consent was not obtained from any of the patients. Instead of obtaining informed consent from each patient, we posted a notice regarding the study design and contact information in a public location at our institution.
2.2. Data collection
Clinical characteristics and biochemical data were collected retrospectively from hospital medical records. Hematological data, including red blood cell (RBC), leukocyte, and PLT counts, were obtained at admission and after treatment. Subpopulations of leukocytes (neutrophil, lymphocyte [Lym], monocyte [Mon], eosinophil [Eos], and basophil [Bas] counts) were also assessed. Blood samples were collected at the time of emergency catheter sheath insertion for all but 1 patient, from whom blood was collected at the end of catheter insertion. When patients were admitted for ACS, they were first given intravenous heparin (100 U/kg) in the emergency room. Aspirin (200 mg) plus prasugrel (20 mg) or clopidogrel (300 mg) were also administered orally according to the appropriate guidelines.[12] The patient was then promptly transferred to the catheterization room, and after sheath insertion, admission blood tests were performed, the activated coagulation time (ACT) was measured, and ACT was measured every 30 minutes to 1 hour. Heparin was administered for 250 to 400 seconds to control ACT. Body mass index was calculated as body weight (kg) divided by height (m2). Hypertension, diabetes mellitus, and dyslipidemia were defined as described previously.[13]
2.3. Statistical analysis
Continuous variables are expressed as the mean ± standard deviation. The correlations between admission PLT and leukocyte counts and discharge PLT and leukocyte counts were examined using separate linear regression analysis. The Wilcoxon signed-rank test was used to compare the degree of inflammation and body temperature at admission and at discharge. All data were statistically analyzed using SPSS software (version 25.0; SPSS Inc., Chicago, IL). Statistical significance was set at a P value < .05, indicating statistical significance. A path model based on structural equation modeling was used to investigate the relationships between clinical factors in the study population and to survey the probable relationships among RBC, leukocyte subpopulation (neutrophils, Lym, Mon, Eos, and Bas), and PLT counts. Path analysis was performed using IBM SPSS AMOS software (version 25, Amos Development Corporation, Meadville, PA). We previously described how to write a path model.[14] For every regression, the total variance in the dependent variable is theorized to be caused by either independent variables that are included in the model or by extraneous variables (e).[15,16] The structural equation models that were obtained were tested and confirmed; a P value < .05 indicated statistical significance. In addition, we applied Bayesian estimation to structural equation modeling using a program embedded in the IBM SPSS AMOS software program (version 25.0; Amos Development Corporation). The frequency polygon is described using the marginal posterior distributions of the estimates. A selected 2-dimensional contour line was used in this study because it could be easily visualized. The credible region (CI) is conceptually similar to a bivariate confidence region that is familiar to most data analysts acquainted with classic statistical inference methods.
3. Results
3.1. Characteristics of the study participants
The clinical characteristics of the 245 patients are presented in Tables 1 and 2. At discharge, none of the patients received a heparin drip infusion. The percentage of patients taking antiplatelet medications was 100% for aspirin, 38.8% for clopidogrel, and 58.3% for prasugrel, and dual antiplatelet therapy was administered to 97.1% of patients. To compare the degree of inflammation, we compared body temperatures at admission and at discharge. The average body temperatures at admission and at discharge were 36.3 ± 0.8 and 36.3°C ± 0.4°C (median ± interquartile range), and no statistically significant difference was observed. (P = .89, Wilcoxon signed-rank test).
Table 1.
Clinical characteristics.
| Characteristic (N = 245) | Mean ± SD, number (%) |
|---|---|
| Age | 61.7 ± 12.1 |
| Male sex (%) | 218 (89.0) |
| Height (cm) | 168.2 ± 8.0 |
| BMI (kg/m2) | 25.2 ± 4.0 |
| Underlying disease | |
| Hypertension (%) | 163 (66.5) |
| Dyslipidemia (%) | 183 (74.7) |
| Diabetes mellitus (%) | 81 (33.1) |
| Atrial fibrillation (%) | 9 (3.7) |
| Prior MI (%) | 26 (10.6) |
| Prior PCI (%) | 33 (13.5) |
| Prior CABG (%) | 4 (1.6) |
| History of heart failure (%) | 6 (2.4) |
| History of stroke (%) | 13 (5.3) |
| Smoking history | |
| Current smoker (%) | 82 (33.4) |
| Past smoker (%) | 89 (36.3) |
| Never smoked (%) | 74 (30.2) |
| Medication | |
| Calcium channel blockers (%) | 76 (31.0) |
| Beta blockers (%) | 39 (16.0) |
| ACE inhibitors (%) | 13 (5.3) |
| ARBs (%) | 57 (23.3) |
| Nitrates (%) | 14 (5.7) |
| Nicorandil (%) | 10 (4.0) |
| Statins (%) | 58 (23.7) |
| Oral antidiabetic agents (%) | 46 (18.8) |
| Insulin (%) | 7 (2.9) |
| Aspirin (%) | 53 (21.6) |
| Clopidogrel (%) | 16 (6.5) |
| Prasugrel (%) | 6 (2.4) |
| Cilostazol (%) | 6 (2.4) |
| Ticlopidine (%) | 1 (0.4) |
| SAPT (%) | 40 (16.3) |
| DAPT (%) | 19 (7.8) |
| Warfarin (%) | 1 (0.4) |
| Rivaroxaban (%) | 2 (0.8) |
| Apixaban (%) | 1 (0.4) |
| Edoxaban (%) | 1 (0.4) |
| Predonine (%) | 5 (2.0) |
| Diagnosis | |
| STEMI (%) | 149 (60.8) |
| NSTEMI (%) | 48 (19.6) |
| UA (%) | 48 (19.6) |
| Emergent PCI (%) | 236 (96.3) |
| Received PCI (%) | 243 (99.1) |
| Length of hospital stay (d) | 9.8 ± 6.4 |
| Length of hospital stay for STEMI (d) | 12.4 ± 6.1 |
| Length of hospital stay for NSTEMI (d) | 7.4 ± 4.8 |
| Length of hospital stay for UA (d) | 4.0 ± 3.0 |
ACE = angiotensin converting enzyme, ARBs = angiotensin II type I receptor blockers, BMI = body mass index, CABG = coronary artery bypass grafting, DAPT = dual antiplatelet therapy, MI = myocardial infarction, NSTEMI = non-ST elevation myocardial infarction, PCI = percutaneous coronary intervention, SAPT = single antiplatelet therapy, STEMI = ST elevation myocardial infarction, UA = unstable angina.
Table 2.
Clinical characteristics.
| Characteristic (N = 245) | Mean ± SD (%) | Mean ± SD (%) |
|---|---|---|
| Admission | Discharge | |
| BNP (pg/mL) | 95.1 ± 176.1 | 118.9 ± 162.0 |
| Cr (mg/dL) | 0.96 ± 1.1 | 1.0 ± 1.2 |
| RBC (×103/μL) | 4.6 ± 0.5 | 4.4 ± 0.6 |
| Leukocyte (×103/μL) | 9.1 ± 3.1 | 7.0 ± 2.0 |
| Neu (×103/μl) | 6.6 ± 3.0 | 4.6 ± 1.8 |
| Lym (×103/μL) | 1.9 ± 0.9 | 1.7 ± 0.6 |
| Mon (×103/μL) | 0.4 ± 0.2 | 0.5 ± 0.2 |
| Eos (×103/μL) | 0.1 ± 0.1 | 0.3 ± 0.2 |
| Bas (×103/μL) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| PLT (×103/μL) | 218.7 ± 51.4 | 259.2 ± 79.5 |
| PLR | 138.2 ± 67.4 | 167.3 ± 76.3 |
| NLR | 4.4 ± 3.1 | 3.0 ± 1.8 |
Bas = basophil, BNP = B-type natriuretic peptide, Cr = creatinine, Eos = eosinophil, Lym = lymphocyte, Mon = monocyte, Neu = neutrophil, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, PLT = platelet, RBC = red blood cell.
3.2. Univariate analysis
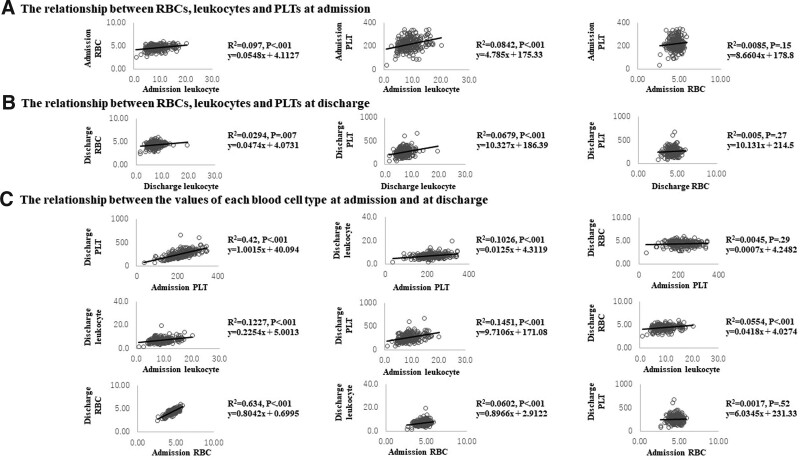
A simple regression analysis revealed the following (Fig. 1). First, at admission, leukocyte and RBC counts (P < .001) and leukocyte and PLT counts were correlated (P < .001), but RBC and PLT counts were not (P = .15). The results obtained at the time of discharge were similar. That is, there was a correlation between leukocyte and RBC counts (P = .007) and between leukocyte and PLT counts (P < .001) but not between RBC and PLT counts (P = .27). In a comparison between admission and discharge. The same blood cell types were correlated at both admission and at discharge (P < .001). Regarding the relationship with other blood cells, the PLT counts at admission were correlated with the leukocyte counts at discharge (P < .001), and the leukocyte counts at admission were correlated with the PLT counts at discharge (P < .001) and with the RBC counts at discharge (P < .001). The RBC counts at admission were correlated with the leukocyte counts at discharge (P < .001) but not with the PLT counts at discharge (P = .52).
Figure 1.
Univariate analysis. (a) The relationships between RBC, leukocyte and PLT counts at admission (3 figures). (b) The relationships between RBC, leukocyte and PLT counts at discharge (3 figures). (c) The relationships between the counts of each blood cell type at admission and at discharge (9 figures). PLT = platelet, RBC = red blood cell.
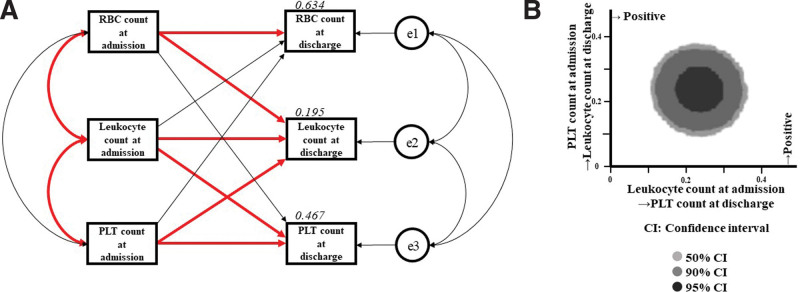
3.3. Concept of proposed path model (A)
To eliminate confounding biases and clarify the contributions of a RBC counts, leukocyte counts, and PLT counts at admission to RBC counts, leukocyte counts, and PLT counts at discharge more directly, path models based on structural equation modeling were proposed. A theoretical path model was created by positioning the RBC count, leukocyte count, and PLT count at admission in parallel considering the correlations among these 3 factors. The association between these 2 factors is indicated by the 2-way arrow. The paths between variables are drawn from independent to dependent variables, with directional arrows for each regression model.
3.4. Results of path model (A)
The precise results of path model (A) are shown in Table 3 and Figure 2a. There was a significant association between leukocyte counts and RBC counts at admission (correlation coefficient, β:0.331, P < .001) and a significant association between leukocyte counts and PLT counts at admission (correlation coefficient, β = 0.290, P < .001); however, there were no associations among the 3 at discharge. There were associations between RBC counts (standardized regression coefficient, β:0.801, P < .001), leukocyte counts (standardized regression coefficient, β:0.234, P < .001), and PLT counts (standardized regression coefficient, β:0.587, P < .001) at admission and discharge. Additionally, there were significant positive correlations between PLT counts at admission and leukocyte counts at discharge (standardized regression coefficient, β:0.239, P < .001) and between leukocyte counts at admission and PLT counts at discharge (standardized regression coefficient, β = 0.238, P < .001). Furthermore, there was a significant positive correlation between the RBC count at admission and leukocyte count at discharge. However, this relationship was weak (standardized regression coefficient, β = 0.15, P = .013).
Table 3.
The results of path model (A) based on structural equation modeling.
| Clinical factor | Estimate | Standard error | Test statistic | P value | Standard regression coefficient |
||
|---|---|---|---|---|---|---|---|
| RBC count at admission | → | RBC count at discharge | 0.809 | 0.041 | 19.648 | <.001 | 0.801 |
| → | Leukocyte count at discharge | 0.549 | 0.221 | 2.486 | .013 | 0.150 | |
| → | PLT count at discharge | −12.541 | 7.121 | −1.761 | .078 | −0.087 | |
| Leukocyte count at admission | → | RBC count at discharge | −0.002 | 0.008 | −0.309 | .76 | −0.013 |
| → | Leukocyte count at discharge | 0.151 | 0.040 | 3.727 | <.001 | 0.234 | |
| → | PLT count at discharge | 6.056 | 1.304 | 4.645 | <.001 | 0.238 | |
| PLT count at admission | → | RBC count at discharge | 0.000 | 0.000 | −0.071 | .94 | −0.003 |
| → | Leukocyte count at discharge | 0.009 | 0.002 | 3.975 | <.001 | 0.239 | |
| → | PLT count at discharge | 0.907 | 0.075 | 12.026 | <.001 | 0.587 | |
| Covariance | Correlation coefficient |
||||||
| RBC count at admission | ⇄ | Leukocyte count at admission | 0.530 | 0.114 | 4.645 | <.001 | 0.311 |
| ⇄ | PLT count at admission | 2.596 | 1.806 | 1.437 | .15 | 0.092 | |
| Leukocyte count at admission | ⇄ | PLT count at admission | 46.307 | 10.639 | 4.353 | <.001 | 0.290 |
| e1 | ⇄ | e2 | −0.021 | 0.038 | −0.559 | .58 | −0.036 |
| ⇄ | e3 | 1.936 | 1.245 | 1.554 | .12 | 0.100 | |
| e2 | ⇄ | e3 | 1.673 | 6.651 | 0.252 | .80 | 0.016 |
PLT = platelet, RBC = red blood cell.
Figure 2.
a (Left): Path model [A] based on structural equation modeling. An explanatory drawing of the possible cascade from RBC, leukocyte and PLT counts at admission to RBC, leukocyte and PLT counts at discharge. Each path has a coefficient showing the standardized coefficient of regressing an independent variable on a dependent variable in the relevant path. These variables indicate standardized regression coefficients (direct effect), correlations among exogenous variables [red bold typeface indicates significant values] and squared multiple correlations [narrow italics]. b (Right): Bayesian estimation in structural equation modeling. Bivariate marginal posterior distributions are shown to help visualize the relationships among pairs of estimands. PLT = platelet, RBC = red blood cell.
3.5. Results of Bayesian estimation in structural equation modeling
The SEM data were analyzed using Bayesian estimation. Bivariate marginal posterior distributions are shown in Figure 2b. This figure shows the influence of leukocyte number at admission on PLT number at discharge (x-axis) and the influence of PLT number at admission on leukocyte count at discharge (y-axis). All plots are far from the line relative to zero, and the Bayesian estimation clearly shows the effects of both.
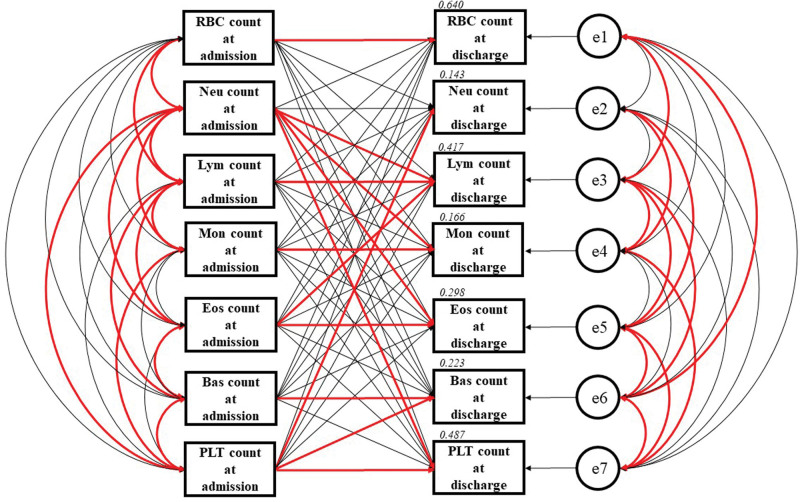
3.6. Concept of proposed path model (B)
To eliminate confounding biases and clarify the contributions of the RBC counts, neutrophil counts, Lym counts, Mon counts, Eos counts, Bas counts and PLT counts at admission to the RBC counts, neutrophil counts, Lym counts, Mon counts, Eos counts, Bas counts, and PLT counts at discharge more directly, path models based on structural equation modeling were proposed. A theoretical path model was created by positioning the RBC count, neutrophil count, Lym count, Mon count, Eos count, Bas count, and PLT count at admission in parallel considering the correlations between these 7 factors. The association between these 2 factors is indicated by a 2-way arrow. The paths between variables are drawn from independent to dependent variables, with directional arrows for each regression model.
3.7. Results of path model (B)
The precise results of path model (B) are presented in Table 4 and Figure 3. In the acute phase of ACS, neutrophil counts at admission were positively associated with RBC counts (correlation coefficient, β:0.269, P < .001) and PLT counts (correlation coefficient, β:0.248, P < .001) at admission but negatively associated with Lym counts at admission (correlation coefficient, β: -0.168, P = .009). However, these relationships were not observed after ACS treatment. Neutrophil count at discharge was not associated with RBC count (P = .27), PLT count (P = .77) or Lym count (P = .19) at discharge. The pre- and posttreatment counts of all blood cells, other than neutrophils (P = .13), were associated with each other (P < .001). Regarding the relationships between different blood cell counts, PLT counts at admission had a significant positive effect on neutrophil counts at discharge (standardized regression coefficient, β = 0.178, P = .006). Conversely, neutrophil count at admission had a significant positive effect on PLT count at discharge (standardized regression coefficient, β = 0.199, P = .001). In addition, the correlation between RBC counts at admission and leukocyte counts at discharge seen in path model (A) disappeared in this analysis in which leukocyte count was considered.
Table 4.
The results of path model (B) based on structural equation modeling.
| Clinical factor | Estimate | Standard error | Test statistic | P value | Standard regression coefficient |
||
|---|---|---|---|---|---|---|---|
| RBC count at admission | → | RBC count at discharge | 0.810 | 0.042 | 19.449 | <.001 | 0.802 |
| → | Neu count at discharge | 0.395 | 0.205 | 1.928 | .05 | 0.123 | |
| → | Lym count at discharge | 0.041 | 0.054 | 0.766 | .44 | 0.040 | |
| → | Mon count at discharge | 0.030 | 0.021 | 1.434 | .15 | 0.090 | |
| → | Eos count at discharge | −.011 | 0.019 | −.565 | .57 | −0.033 | |
| → | Bas count at discharge | −0.008 | 0.004 | −1.882 | .06 | −0.114 | |
| → | PLT count at discharge | −9.397 | 7.126 | −1.319 | .19 | −0.065 | |
| Neu count at admission | → | RBC count at discharge | −0.001 | 0.009 | −0.148 | .88 | −0.008 |
| → | Neu count at discharge | 0.072 | 0.047 | 1.533 | .13 | 0.123 | |
| → | Lym count at discharge | 0.050 | 0.012 | 4.091 | <.001 | 0.271 | |
| → | Mon count at discharge | −0.009 | 0.005 | −2.000 | .05 | −0.159 | |
| → | Eos count at discharge | 0.015 | 0.004 | 3.478 | <.001 | 0.253 | |
| → | Bas count at discharge | 0.001 | 0.001 | 1.200 | .23 | 0.092 | |
| → | PLT count at discharge | 5.199 | 1.623 | 3.204 | .001 | 0.199 | |
| Lym count at admission | → | RBC count at discharge | −0.026 | 0.029 | −.902 | .37 | −0.041 |
| → | Neu count at discharge | −0.072 | 0.144 | −0.500 | .62 | −0.035 | |
| → | Lym count at discharge | 0.424 | 0.038 | 11.260 | <.001 | 0.645 | |
| → | Mon count at discharge | −0.013 | 0.015 | −0.871 | .38 | −0.060 | |
| → | Eos count at discharge | −0.014 | 0.013 | −1.043 | .30 | −0.066 | |
| → | Bas count at discharge | −0.002 | 0.003 | −0.713 | .48 | −0.047 | |
| → | PLT count at discharge | −7.368 | 5.016 | −1.469 | .14 | −0.079 | |
| Mon count at admission | → | RBC count at discharge | 0.057 | 0.118 | 0.479 | .63 | 0.023 |
| → | Neu count at discharge | 0.946 | 0.583 | 1.623 | .10 | 0.118 | |
| → | Lym count at discharge | −0.201 | 0.152 | −1.320 | .19 | −0.079 | |
| → | Mon count at discharge | 0.343 | 0.059 | 5.828 | <.001 | 0.417 | |
| → | Eos count at discharge | −0.017 | 0.054 | −0.319 | .75 | −0.021 | |
| → | Bas count at discharge | 0.010 | 0.013 | 0.792 | .43 | 0.055 | |
| → | PLT count at discharge | 30.828 | 20.242 | 1.523 | .13 | 0.085 | |
| Eos count at admission | → | RBC count at discharge | 0.289 | 0.184 | 1.570 | .12 | 0.073 |
| → | Neu count at discharge | 0.907 | 0.906 | 1.002 | .32 | 0.072 | |
| → | Lym count at discharge | −0.627 | 0.236 | −2.652 | .008 | −0.157 | |
| → | Mon count at discharge | −0.022 | 0.091 | −0.240 | .81 | −0.017 | |
| → | Eos count at discharge | 0.748 | 0.084 | 8.948 | <.001 | 0.582 | |
| → | Bas count at discharge | −0.024 | 0.020 | −1.205 | .23 | −0.082 | |
| → | PLT count at discharge | 0.316 | 31.459 | 0.10 | .99 | 0.01 | |
| Bas count at admission | → | RBC count at discharge | −0.735 | 0.628 | −1.170 | .24 | −0.051 |
| → | Neu count at discharge | 4.571 | 3.092 | 1.478 | .14 | 0.099 | |
| → | Lym count at discharge | −0.022 | 0.807 | −0.028 | .98 | −0.002 | |
| → | Mon count at discharge | 0.612 | 0.312 | 1.958 | .05 | 0.130 | |
| → | Eos count at discharge | 0.150 | 0.286 | 0.526 | .60 | 0.032 | |
| → | Bas count at discharge | 0.437 | 0.067 | 6.546 | <.001 | 0.419 | |
| → | PLT count at discharge | −39.487 | 107.409 | −0.368 | .71 | −0.019 | |
| PLT count at admission | → | RBC count at discharge | 0.000 | 0.000 | −0.073 | .94 | 0.003 |
| → | Neu count at discharge | 0.006 | 0.002 | 2.768 | .006 | 0.178 | |
| → | Lym count at discharge | 0.001 | 0.001 | 1.724 | .09 | 0.092 | |
| → | Mon count at discharge | 0.000 | 0.000 | 1.377 | .17 | 0.088 | |
| → | Eos count at discharge | 0.000 | 0.000 | 0.531 | .60 | 0.031 | |
| → | Bas count at discharge | 0.000 | 0.000 | 2.600 | .009 | 0.160 | |
| → | PLT count at discharge | 0.937 | 0.077 | 12.156 | <.001 | 0.606 | |
| Covariance | Correlation coefficient |
||||||
| PLT count at admission | ⇄ | Bas count at admission | 0.501 | 0.130 | 3.851 | <.001 | 0.254 |
| ⇄ | Eos count at admission | 0.635 | 0.462 | 1.373 | .17 | 0.088 | |
| ⇄ | Mon count at admission | 1.684 | 0.730 | 2.308 | .02 | 0.149 | |
| ⇄ | Lym count at admission | 5.159 | 2.807 | 1.838 | .07 | 0.118 | |
| ⇄ | Neu count at admission | 38.596 | 10.282 | 3.754 | <.001 | 0.248 | |
| ⇄ | RBC count at admission | 2.596 | 1.806 | 1.437 | .15 | 0.092 | |
| Bas count at admission | ⇄ | Eos count at admission | 0.002 | 0.000 | 5.708 | <.001 | 0.393 |
| ⇄ | Mon count at admission | 0.000 | 0.001 | -0.647 | .52 | −0.041 | |
| ⇄ | Lym count at admission | 0.007 | 0.002 | 3.286 | .001 | 0.215 | |
| ⇄ | Neu count at admission | −0.011 | 0.008 | −1.401 | .16 | −0.090 | |
| ⇄ | RBC count at admission | 0.003 | 0.001 | 1.848 | .07 | 0.119 | |
| Eos count at admission | ⇄ | Mon count at admission | 0.001 | 0.002 | 0.326 | .74 | 0.021 |
| ⇄ | Lym count at admission | 0.042 | 0.008 | 5.174 | <.001 | 0.351 | |
| ⇄ | Neu count at admission | −0.145 | 0.029 | −5.022 | <.001 | −0.340 | |
| ⇄ | RBC count at admission | 0.005 | 0.005 | 1.014 | .31 | 0.065 | |
| Mon count at admission | ⇄ | Lym count at admission | 0.050 | 0.012 | 4.065 | <.001 | 0.270 |
| ⇄ | Neu count at admission | 0.291 | 0.047 | 6.236 | <.001 | 0.435 | |
| ⇄ | RBC count at admission | 0.014 | 0.008 | 1.811 | .07 | 0.117 | |
| Lym count at admission | ⇄ | Neu count at admission | −0.434 | 0.167 | −2.595 | .009 | −0.168 |
| ⇄ | RBC count at admission | 0.063 | 0.030 | 2.106 | .04 | 0.136 | |
| Neu count at admission | ⇄ | RBC count at admission | 0.447 | 0.110 | 4.053 | <.001 | 0.269 |
| e1 | ⇄ | e2 | −0.038 | 0.035 | −1.094 | .27 | −0.070 |
| ⇄ | e3 | 0.019 | 0.009 | 2.026 | .04 | 0.131 | |
| ⇄ | e4 | −0.005 | 0.004 | −1.368 | .17 | −0.088 | |
| ⇄ | e5 | 0.004 | 0.003 | 1.306 | .19 | 0.084 | |
| ⇄ | e6 | 0.002 | 0.001 | 2.353 | .02 | 0.152 | |
| ⇄ | e7 | 1.753 | 1.212 | 1.447 | .15 | 0.093 | |
| e2 | ⇄ | e3 | −0.058 | 0.045 | −1.299 | .19 | −0.083 |
| ⇄ | e4 | 0.153 | 0.020 | 7.711 | <.001 | 0.568 | |
| ⇄ | e5 | −0.039 | 0.016 | −2.435 | .02 | −0.158 | |
| ⇄ | e6 | −0.006 | 0.004 | −1.565 | .12 | −0.101 | |
| ⇄ | e7 | 1.746 | 5.942 | 0.294 | .77 | 0.019 | |
| e3 | ⇄ | e4 | 0.010 | 0.005 | 2.144 | .03 | 0.139 |
| ⇄ | e5 | 0.011 | 0.004 | 2.676 | .007 | 0.174 | |
| ⇄ | e6 | 0.003 | 0.001 | 3.105 | .002 | 0.203 | |
| ⇄ | e7 | 2.732 | 1.560 | 1.751 | .08 | 0.113 | |
| e4 | ⇄ | e5 | 0.000 | 0.002 | −0.117 | .91 | −0.007 |
| ⇄ | e6 | 0.000 | 0.000 | −0.688 | .49 | −0.044 | |
| ⇄ | e7 | −1.530 | 0.608 | −2.517 | .01 | −0.163 | |
| e5 | ⇄ | e6 | 0.001 | 0.000 | 2.616 | .009 | 0.170 |
| ⇄ | e7 | 1.440 | 0.556 | 2.589 | .01 | 0.168 | |
| e6 | ⇄ | e7 | 0.317 | 0.130 | 2.444 | .02 | 0.158 |
Bas = basophil, Eos = eosinophil, Lym = lymphocyte, Mon = monocyte, Neu = neutrophil, PLT = platelet, RBC = red blood cell.
Figure 3.
Path model [B] based on structural equation modeling. An explanatory drawing of the possible cascade from RBC, Neu, Lym, Mon, Eos, Bas and PLT counts at admission to RBC, Neu, Lym, Mon, Eos, Bas and PLT counts at discharge. Each path has a coefficient showing the standardized coefficient of regressing an independent variable on a dependent variable in the relevant path. These variables indicate standardized regression coefficients (direct effect), correlations among exogenous variables [red bold typeface indicates significant values] and squared multiple correlations [narrow italics]. Bas = basophil, Eos = eosinophil, Lym = lymphocyte, Mon = monocyte, Neu = neutrophil, PLT = platelet, RBC = red blood cell.
3.8. PLR and NLR at admission and at discharge
We calculated the PLR and NLR and determined that the PLR was 138.2 ± 67.4 at admission and 167.3 ± 76.3 at discharge, and the NLR was 4.4 ± 3.1 at admission and 3.0 ± 1.8 at discharge.
4. Discussion
In this study, structural equation modeling was used to investigate the direct relationships between the factors described above by eliminating the conjugates between the factors as much as possible.
As a result, it became clear that the numbers of leukocytes, especially neutrophils, and PLTs were significantly influenced by each other during ACS.
In this study, we examined blood cell counts; however, these numbers may also be associated with blood cell activation. Although it is difficult to prove this phenomenon directly, many studies suggest that these cell count ratios (PLR and NLR) are prognostic indicators of ACS.[4,5,9,10] Receiver operating characteristic curve analysis also revealed that high values of these blood cell types correlated with a PLR above 128 and an NLR above 2.6.[17] In fact, the PLRs and NLRs obtained in the current study tended to be higher than these values. Again, the increased numbers of PLTs and neutrophils are meaningful and may be associated with thrombotic activity.
The PLT-leukocyte complex has been well studied.[18–21] In brief, when vascular endothelial cells are damaged, receptors for cell adhesion factors (glycoprotein GPIbα, GPIIb/IIIa, etc) are expressed on the PLT cell membrane. Tissue factor is important for the interaction between PLTs and leukocytes.[20,22] P-selectins are also important in promoting tissue factor expression[23,24] and fibrin formation.[25,26] Subsequently, neutrophil extracellular traps are involved in thrombosis by promoting fibrin deposition and fibrin network formation.[27,28]
As mentioned above, PLTs and leukocytes can affect each other and increase cell numbers during ACS, which is an interesting finding. However, it is unclear why this occurs. It is possible that the PLT-leukocyte complex is also involved. Inflammation is caused by the formation of this complex. Inflammation increases PLT and leukocyte counts.[29–32] In other words, during ACS, the PLT count affects the subsequent leukocyte count, which affects the subsequent PLT count. Crosstalk between PLT and leukocyte counts is a phenomenon that is observed only in ACS and not in non-ACS patients. Figure 4 shows a conceptual diagram of the possibilities derived from this result. At this point, the precise mechanism of crosstalk between PLT and leukocyte numbers remains unclear and is an interesting topic for future research.
Figure 4.
Conceptual diagram. This figure shows the possibilities that can be derived from this result. ACS = acute coronary syndrome, PLT = platelet.
Incidentally, the importance of PLT and leukocyte activation has also been highlighted in other conditions, such as stress,[33] HIV,[34] sepsis,[35] ulcerative colitis,[36] and rheumatoid arthritis.[37] Interestingly, these diseases are associated with arteritis and arteriosclerosis, and it is easy to speculate that these conditions may lead to ischemic heart disease. Presumably, the induction of thrombosis and vasculitis by COVID-19 may also be partly related to leukocyte and PLT functions.[38,39]
Notably, associations between other types of blood cells were also observed. In this study, Mon were associated with Lym; Bas were associated with Lym, Eos and PLTs; and Eos were associated with Lym. However, the meaning of these relationships remains unclear, and the relationship between the counts and activities of each blood cell type should be investigated in the future.
4.1. Study limitations
The limitations of this study are as follows: The number of cases was small. Various drugs other than antiplatelet drugs were used, and the effects could not be eliminated. Regarding structural equation modeling, a path diagram was devised for analysis based on the rich experience of the analysts involved. In general, a path diagram should be as simple as possible and widely accepted. Therefore, in this study, we devised a diagram that considers path symmetry. We also attempted to make the diagram highly intuitive and understandable. However, structural equation modeling is not a method that can be used to examine true causality. These results only show the relationship between blood cell counts, which represents the degree of influence. Further validation using different paths and other statistical methods is required.
5. Conclusions
During ACS, PLTs and leukocytes, especially neutrophils, stimulate each other to increase their numbers. The formation of a PLT-leukocyte complex may increase coagulation activity and increases inflammation, which can lead to a further increase in the counts of both types of blood cells.
Acknowledgments
We thank all trial physicians and nurses at all participating hospitals for their important contributions to this study. We also thank Kumiko Nishiyama for providing assistance with data collection. We thank American Journal Experts (www.aje.com) for English language editing.
Author contributions
Conceptualization: Keisuke Shirasaki, Takayuki Ogawa.
Data curation: Kosuke Minai, Makoto Kawai, Toshikazu D. Tanaka, Michihiro Yoshimura.
Formal analysis: Kosuke Minai, Makoto Kawai.
Investigation: Keisuke Shirasaki, Kosuke Minai, Makoto Kawai, Kazuo Ogawa, Michihiro Yoshimura.
Methodology: Keisuke Shirasaki.
Project administration: Michihiro Yoshimura.
Supervision: Michihiro Yoshimura.
Validation: Keisuke Shirasaki, Yasunori Inoue, Satoshi Morimoto, Takayuki Ogawa, Kimiaki Komukai, Michihiro Yoshimura.
Visualization: Keisuke Shirasaki, Tomohisa Nagoshi, Michihiro Yoshimura.
Writing – original draft: Keisuke Shirasaki.
Writing – review & editing: Kosuke Minai, Makoto Kawai, Toshikazu D. Tanaka, Kazuo Ogawa, Yasunori Inoue, Satoshi Morimoto, Tomohisa Nagoshi, Takayuki Ogawa, Kimiaki Komukai, Michihiro Yoshimura.
Abbreviations:
- ACS =
- acute coronary syndrome
- ACT =
- activated coagulation time
- Bas =
- basophil
- Eos =
- eosinophil
- Lym =
- lymphocyte
- MI =
- myocardial infarction
- Mon =
- monocyte
- Neu =
- neutrophil
- NLR =
- neutrophil-to-lymphocyte ratio
- PLR =
- PLT-to-lymphocyte ratio
- PLT =
- platelet
- RBC =
- red blood cell
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Shirasaki K, Minai K, Kawai M, Tanaka TD, Ogawa K, Inoue Y, Morimoto S, Nagoshi T, Ogawa T, Komukai K, Yoshimura M. Unique crosstalk between platelet and leukocyte counts during treatment for acute coronary syndrome: A retrospective observational study. Medicine 2022;101:52(e32439).
Contributor Information
Keisuke Shirasaki, Email: shirasaki1818@gmail.com.
Makoto Kawai, Email: cadmk@jikei.ac.jp.
Toshikazu D. Tanaka, Email: jjuichi@gmail.com.
Kazuo Ogawa, Email: takaog39@jikei.ac.jp.
Yasunori Inoue, Email: yasunori.inoue27@gmail.com.
Satoshi Morimoto, Email: morisato@jikei.ac.jp.
Tomohisa Nagoshi, Email: tomohisa754@gmail.com.
Takayuki Ogawa, Email: takaog39@jikei.ac.jp.
Kimiaki Komukai, Email: komu@jikei.ac.jp.
Michihiro Yoshimura, Email: m.yoshimura@jikei.ac.jp.
References
- [1].Undas A, Szuldrzynski K, Brummel-Ziedins KE, et al. Systemic blood coagulation activation in acute coronary syndromes. Blood. 2009;113:2070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ly HQ, Kirtane AJ, Murphy SA, et al. Association of platelet counts on presentation and clinical outcomes in ST-elevation myocardial infarction (from the TIMI Trials). Am J Cardiol. 2006;98:1–5. [DOI] [PubMed] [Google Scholar]
- [3].Iijima R, Ndrepepa G, Mehilli J, et al. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb Haemost. 2007;98:852–7. [PubMed] [Google Scholar]
- [4].Oylumlu M, Yildiz A, Oylumlu M, et al. Platelet-to-lymphocyte ratio is a predictor of in-hospital mortality patients with acute coronary syndrome. Anatol J Cardiol. 2015;15:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li W, Liu Q, Tang Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta-analysis. Sci Rep. 2017;7:40426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cerletti C, de Gaetano G, Lorenzet R. Platelet - leukocyte interactions: multiple links between inflammation, blood coagulation and vascular risk. Mediterr J Hematol Infect Dis. 2010;2:e2010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ghasemzadeh M, Hosseini E. Platelet-leukocyte crosstalk: linking proinflammatory responses to procoagulant state. Thromb Res. 2013;131:191–7. [DOI] [PubMed] [Google Scholar]
- [8].Teague HL, Varghese NJ, Tsoi LC, et al. Neutrophil subsets, platelets, and vascular disease in psoriasis. JACC Basic Transl Sci. 2019;4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573–7. [DOI] [PubMed] [Google Scholar]
- [10].Zhang S, Diao J, Qi C, et al. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. BMC Cardiovasc Disord. 2018;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sekiyama H, Nagoshi T, Komukai K, et al. Transient decrease in serum potassium level during ischemic attack of acute coronary syndrome: paradoxical contribution of plasma glucose level and glycohemoglobin. Cardiovasc Diabetol. 2013;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kimura K, Kimura T, Ishihara M, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J. 2019;83:1085–196. [DOI] [PubMed] [Google Scholar]
- [13].Komukai K, Minai K, Arase S, et al. Impact of body mass index on clinical outcome in patients hospitalized with congestive heart failure. Circ J. 2012;76:145–51. [DOI] [PubMed] [Google Scholar]
- [14].Kinoshita K, Kawai M, Minai K, et al. Potent influence of obesity on suppression of plasma B-type natriuretic peptide levels in patients with acute heart failure: an approach using covariance structure analysis. Int J Cardiol. 2016;215:283–90. [DOI] [PubMed] [Google Scholar]
- [15].Uno G, Nagoshi T, Yoshii A, et al. Collaborative activities of noradrenaline and natriuretic peptide for glucose utilization in patients with acute coronary syndrome. Sci Rep. 2019;9:7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Itakura R, Inoue Y, Ogawa K, et al. A highly-sensitized response of B-type natriuretic peptide to cardiac ischaemia quantified by intracoronary pressure measurements. Sci Rep. 2020;10:2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cho KI, Ann SH, Singh GB, et al. Combined usefulness of the platelet-to-lymphocyte ratio and the neutrophil-to-lymphocyte ratio in predicting the long-term adverse events in patients who have undergone percutaneous coronary intervention with a drug-eluting stent. PLoS One. 2015;10:e0133934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Faraday N, Braunstein JB, Heldman AW, et al. Prospective evaluation of the relationship between platelet-leukocyte conjugate formation and recurrent myocardial ischemia in patients with acute coronary syndromes. Platelets. 2004;15:9–14. [DOI] [PubMed] [Google Scholar]
- [19].Klinkhardt U, Harder S. Flow cytometric measurement of platelet-leukocyte aggregates: a possible target to monitor platelet function? Semin Thromb Hemost. 2005;31:400–3. [DOI] [PubMed] [Google Scholar]
- [20].Brambilla M, Camera M, Colnago D, et al. Tissue factor in patients with acute coronary syndromes: expression in platelets, leukocytes, and platelet-leukocyte aggregates. Arterioscler Thromb Vasc Biol. 2008;28:947–53. [DOI] [PubMed] [Google Scholar]
- [21].Ren F, Mu N, Zhang X, et al. Increased platelet-leukocyte aggregates are associated with myocardial no-reflow in patients with ST elevation myocardial infarction. Am J Med Sci. 2016;352:261–6. [DOI] [PubMed] [Google Scholar]
- [22].Monroe DM, Roberts HR, Hoffman M. Platelet procoagulant complex assembly in a tissue factor-initiated system. Br J Haematol. 1994;88:364–71. [DOI] [PubMed] [Google Scholar]
- [23].Christersson C, Johnell M, Siegbahn A. Tissue factor and IL8 production by P-selectin-dependent platelet-monocyte aggregates in whole blood involves phosphorylation of Lyn and is inhibited by IL10. J Thromb Haemost. 2008;6:986–94. [DOI] [PubMed] [Google Scholar]
- [24].Halvorsen H, Olsen JO, Osterud B. Granulocytes enhance LPS-induced tissue factor activity in monocytes via an interaction with platelets. J Leukoc Biol. 1993;54:275–82. [DOI] [PubMed] [Google Scholar]
- [25].Palabrica T, Lobb R, Furie BC, et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–51. [DOI] [PubMed] [Google Scholar]
- [26].Celi A, Pellegrini G, Lorenzet R, et al. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci USA. 1994;91:8767–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stakos DA, Kambas K, Konstantinidis T, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lisman T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018;371:567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kornerup KN, Salmon GP, Pitchford SC, et al. Circulating platelet-neutrophil complexes are important for subsequent neutrophil activation and migration. J Appl Physiol. 2010;109:758–67. [DOI] [PubMed] [Google Scholar]
- [31].Couldwell G, Machlus KR. Modulation of megakaryopoiesis and platelet production during inflammation. Thromb Res. 2019;179:114–20. [DOI] [PubMed] [Google Scholar]
- [32].Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. [DOI] [PubMed] [Google Scholar]
- [33].Strike PC, Magid K, Whitehead DL, et al. Pathophysiological processes underlying emotional triggering of acute cardiac events. Proc Natl Acad Sci USA. 2006;103:4322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nkambule BB, Davison G, Ipp H. Platelet leukocyte aggregates and markers of platelet aggregation, immune activation and disease progression in HIV infected treatment naive asymptomatic individuals. J Thromb Thrombolysis. 2015;40:458–67. [DOI] [PubMed] [Google Scholar]
- [35].Claushuis TA, de Stoppelaar SF, Stroo I, et al. Thrombin contributes to protective immunity in pneumonia-derived sepsis via fibrin polymerization and platelet-neutrophil interactions. J Thromb Haemost. 2017;15:744–57. [DOI] [PubMed] [Google Scholar]
- [36].Pamuk GE, Vural O, Turgut B, et al. Increased circulating platelet-neutrophil, platelet-monocyte complexes, and platelet activation in patients with ulcerative colitis: a comparative study. Am J Hematol. 2006;81:753–9. [DOI] [PubMed] [Google Scholar]
- [37].Pamuk GE, Vural O, Turgut B, et al. Increased platelet activation markers in rheumatoid arthritis: are they related with subclinical atherosclerosis? Platelets. 2008;19:146–54. [DOI] [PubMed] [Google Scholar]
- [38].Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Skendros P, Mitsios A, Chrysanthopoulou A, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]