Background:
Although there are new treatments for non-small cell lung cancer with malignant pleural effusion, these therapies are prone to recurrent pleural effusion and poor in efficacy. And recombinant human endostatin is a new type of anti-tumor angiogenesis drug independently developed in my country. It has the effect of inhibiting tumor angiogenesis, inhibiting tumor proliferation and differentiation, and effectively inhibiting the formation and recurrence of malignant pleural effusion. Therefore, this study is aim to systematically review the efficacy and safety of intrapleural injection of endostar combined with cisplatin in the treatment of non-small cell lung cancer (NSCLC) with malignant pleural effusion.
Methods:
Databases including Cochrane Library, PubMed, CBM, Embase, CNKI, and WanFang Data were searched to collect randomized controlled trials about endostar combined with cisplatin for NSCLC with malignant pleural effusion from inception to April 2022. Two reviewers independently screened the literature, extracted data, and assessed the risk of bias in included studies. Finally, the meta-analysis was made by using RevMan 5.4.1 software.
Results:
A total of 11 randomized controlled trials involving 814 patients were finally included. The results of the meta-analysis showed that: The overall response rate and the improvement rate of quality of life in the endostar combined with cisplatin group were higher than that of the cisplatin alone group (relative risk = 1.58, 95% confidence interval = 1.42–1.76, P < .00001; relative risk = 1.63, 95% confidence interval = 1.38–1.93, P < .00001, respectively). Meanwhile, there were no significant differences between the 2 groups in the incidence of gastrointestinal reaction, the incidence of leucopenia, the incidence of thrombocytopenia, and the incidence of hypodynamia (all P values > .05).
Conclusion:
Compared with cisplatin, intrapleural injection of endostar combined with cisplatin could improve the overall response rate and the quality of life of NSCLC patients with malignant pleural effusion. Due to the limited quality and quantity of included studies, more high-quality studies are needed to verify the above conclusion.
Keywords: cisplatin, endostar, malignant pleural effusion, meta-analysis, non-small cell lung cancer, randomized controlled trial
1. Introduction
Lung cancer has now become the malignant tumor with the highest incidence in the world, and its mortality rate ranks first among malignant tumors.[1] Among them, non-small cell lung cancer (NSCLC) accounts for more than 80%. Because of its difficulty in early diagnosis, more than 2/3 of patients are diagnosed at an advanced stage, and the prognosis is poor.[2] Malignant pleural effusion is one of the common complications of advanced NSCLC, and it grows rapidly. Patients may suffer from symptoms such as chest tightness, shortness of breath, and dyspnea, which may lead to a decline in their quality of life. In severe cases, respiratory and circulatory failure may endanger the life.[3] Therefore, the effective control of pleural effusion is the main measure which can improve the quality of life of patients with advanced NSCLC. In recent years, the treatment of malignant pleural effusion mainly involves an intrapleural injection of immunosuppressive agents and chemotherapy drugs. However, this therapy is prone to recurrent pleural effusion and poor in efficacy. Endostatin (recombinant human endostatin) is a new type of anti-tumor angiogenesis drug independently developed in my country. It has the effect of inhibiting tumor angiogenesis, inhibiting tumor proliferation and differentiation, and effectively inhibiting the formation and recurrence of malignant pleural effusion.[3] Numerous studies have shown that the combined use of recombinant human endostatin and cisplatin injected into the pleural cavity for the treatment of NSCLC complicated with malignant pleural effusion has a higher overall response rate than cisplatin and other chemotherapy drugs alone, and the quality of life of patients is more elevated. It can be improved a lot, and the patients are well tolerated, and there is no statistical difference in adverse reactions between the two. However, there are generally problems of insufficient sample size and inconsistent quality in individual studies. Accordingly, this study adopted the methods of systematic review and meta-analysis in order to comprehensively evaluate recombinant human endostatin combined with intrapleural injection of cisplatin in the treatment of NSCLC complicated with malignant tumors. The therapeutic efficacy and prognosis of pleural effusion provide more evidence-based medical evidence for the clinical application of recombinant human endostatin.
2. Materials and methods
2.1. Search strategy
Cochrane Library, PubMed, Embase, CBM, CNKI, and WanFang Data databases were searched by computer to collect relevant randomized controlled trials (RCTs) comparing recombinant human endostatin combined with cisplatin and cisplatin alone in the treatment of NSCLC combined with malignant pleural effusion. The retrieval time limit is from the establishment of the database to April 2022. The search is carried out by combining subject words and free words. Chinese search terms included: endostar, recombinant human endostatin, non-small cell lung cancer, and malignant pleural effusion. English search terms had: endostar, non-small cell lung cancer, NSCLC, and malignant pleural effusion. Taking Cochrane Library as an example, Table 1 is showing specific search strategies.
Table 1.
Search strategy of Cochrane Library.
| Search name: Cochrane | |
|---|---|
| Last saved: 21/4/2022 20:49 | |
| Comment: Carcinoma, Non Small Cell Lung | |
| #1 | (endostar):ti,ab,kw (Word variations have been searched) |
| #2 | (endostatin,N-terminal-MGGSHHHHH):ti,ab,kw (Word variations have been searched) |
| #3 | (recombinant human endostatin protein):ti,ab,kw (Word variations have been search) |
| #4 | (endostar protein):ti,ab,kw (Word variations have been searched) |
| #5 | #1 OR #2 OR #3 OR #4 |
| #6 | MeSH descriptor: [Carcinoma, Non-Small-Cell Lung] explode all trees |
| #7 | (Carcinoma, Non Small Cell Lung):ti,ab,kw (Word variations have been searched) |
| #8 | (Carcinomas, Non-Small-Cell Lung):ti,ab,kw (Word variations have been searched) |
| #9 | (Lung Carcinoma, Non-Small-Cell):ti,ab,kw (Word variations have been searched) |
| #10 | (Lung Carcinomas, Non-Small-Cell):ti,ab,kw (Word variations have been searched) |
| #11 | (Non-Small-Cell Lung Carcinomas):ti,ab,kw (Word variations have been searched) |
| #12 | (Non-Small-Cell Lung Carcinoma):ti,ab,kw (Word variations have been searched) |
| #13 | (Non Small Cell Lung Carcinoma):ti,ab,kw (Word variations have been searched) |
| #14 | (Carcinoma, Non-Small Cell Lung):ti,ab,kw (Word variations have been searched) |
| #15 | (Non-Small Cell Lung Carcinoma):ti,ab,kw (Word variations have been searched) |
| #16 | (Non-Small Cell Lung Cancer):ti,ab,kw (Word variations have been searched) |
| #17 | (Nonsmall Cell Lung Cancer):ti,ab,kw (Word variations have been searched) |
| #18 | #6 OR #7 OR #8 OR #9 OR #10 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 |
| #19 | MeSH descriptor: [Pleural Effusion, Malignant] explode all trees |
| #20 | (Effusion, Malignant Pleural):ti,ab,kw (Word variations have been searched) |
| #21 | (Effusions, Malignant Pleural):ti,ab,kw (Word variations have been searched) |
| #22 | (Malignant Pleural Effusion):ti,ab,kw (Word variations have been searched) |
| #23 | (Malignant Pleural Effusions):ti,ab,kw (Word variations have been searched) |
| #24 | (Pleural Effusions, Malignant):ti,ab,kw (Word variations have been searched) |
| #25 | #19 OR #20 OR #21 OR #22 OR #23 OR #24 |
| #26 | #5 AND #18 AND #25 |
2.2. Inclusion criteria
2.2.1. Type of study.
Randomized controlled trials.
2.2.2. Research object.
For NSCLC patients diagnosed by pathology or cytology, malignant cells were found in the pleural effusion, and the pleural effusion was moderate or above (judged by thoracic B-ultrasound or chest X-ray).
2.2.3. Intervention measures.
The experimental group received the intrapleural injection of recombinant human endostatin and cisplatin
2.2.4. Control measures.
The control group received the intrapleural injection of cisplatin alone.
2.3. Exclusion criteria
Intrapleural injection of antitumor drugs, biological agents, or other sclerosing agents within the past 1 month;
Research on unique treatments such as pleural hyperthermia and thoracic radiofrequency hyperthermia;
Malignant pleural effusion caused by NSCLC;
Non-Chinese and English literature;
Graduation thesis, dissertation or review;
Case reports and animal experiments.
2.4. Outcomes
2.4.1. The overall response rate.
According to the WHO evaluation criteria for the treatment of pleural effusion[4]: complete remission, partial remission, stable, and progressive, complete remission + partial remission is regarded as effective, and the overall response rate is calculated accordingly.
2.4.2. Quality of life improvement rate.
After chemotherapy, an increase in KPS is Karnofsky's (Karnofsky, KPS, percentile) functional status scoring standard. The higher the score, the better the health, the more able to tolerate the side effects of treatment, and therefore the possibility of receiving thorough treatment. It is generally considered that Karnofsky's score of 80 or more is independent level, that is, independent living level. 50~70 is divided into semi-independent level, that is, semi-self-care in life. A score below 50 is dependent, that is, life needs help from others. Those with more than 80 points are in better postoperative condition and have a longer survival period of more than 10 points indicates improvement of the disease, a decrease in KPS of more than 10 points suggests a deterioration of the disease, and between the 2 is considered stable.
2.4.3. The incidence of major adverse reactions.
Gastrointestinal reactions such as nausea and vomiting, leukopenia, thrombocytopenia, and hypodynamia.
2.5. Literature screening and data extraction
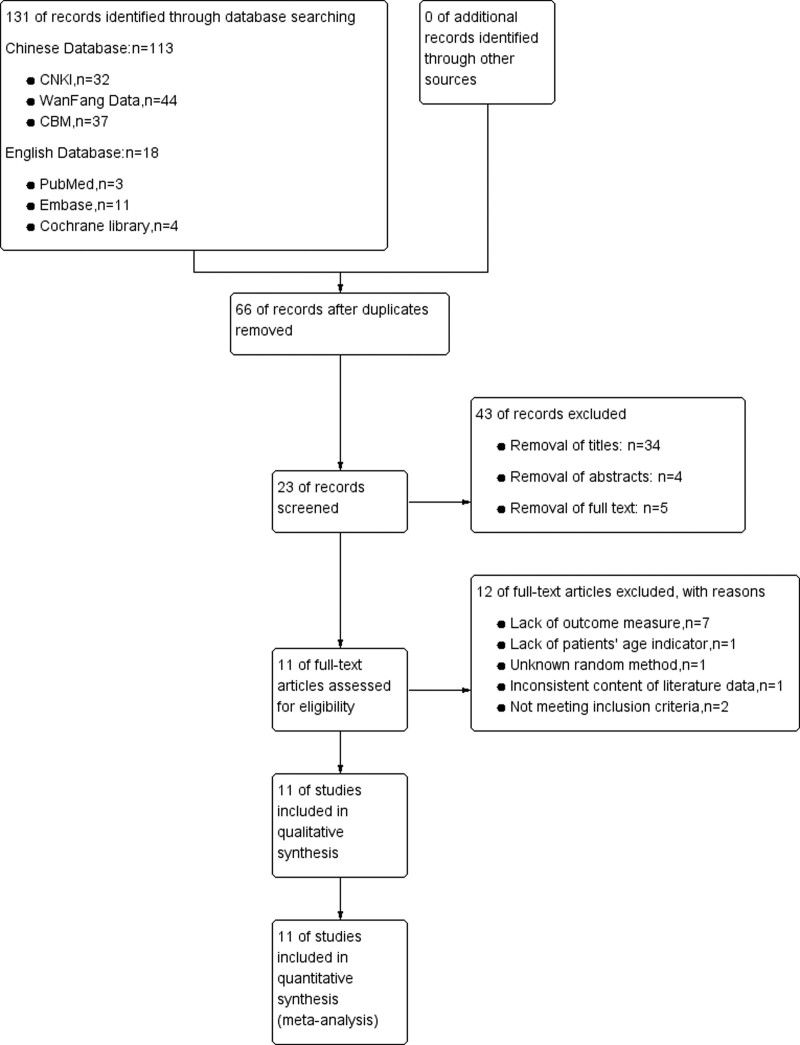
Two researchers independently screened the literature, extracted data, and cross-checked them. In case of disagreement, we would discuss and resolve, or the opinion of a third researcher was sought. The lack of data should be supplemented by contacting the authors as much as possible. During literature screening, the title and abstract were first read, and after excluding obviously irrelevant literature, the full text was further read to determine whether it was finally included. After screening by 2 researchers in this paper, we found 12 references in English databases: Cochrane Library, PubMed, and Embase after removing duplicate literature. After careful comparison, the available initial research materials are all in Chinese. In addition, the relevant English literature is mainly about (VEGF) vascular endothelial growth factor, also known as vascular permeability factor,VPF, is a highly specific pro-vascular endothelial cell growth factor, which has the effects of promoting increased vascular permeability, extracellular matrix degeneration, vascular endothelial cell migration, proliferation and vascular formation, the research on cancer progression and other factors did not meet the inclusion criteria of this systematic review. The original Chinese research data have been extracted from the Chinese database, so this systematic review did not use English literature; Chinese databases CBM, CNKI, and WanFang Data removed duplicate literature, then how to evaluate the following literature was shown according to the screening process in Figure 1. The reasons for removing the research data were that they did not meet the inclusion criteria of this systematic review, the outcome indicators were incomplete, or their research data was missing. The data extraction used self-made data extraction, the extracted data mainly includes the basic information of the included studies, and the baseline characteristics of the research subjects, intervention measures and outcome indicators, etc., shown in Table 2.
Figure 1.
PRISMA flow chart of selection process to identify studies eligible for pooling.
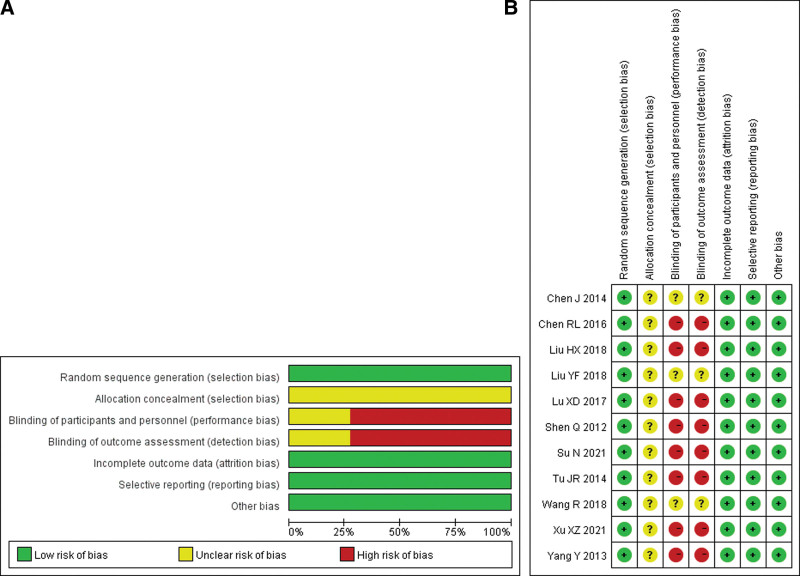
2.6. Risk of bias assessment
Risk of bias assessment of included studies was assessed using the Cochrane Handbook 5.1.0.[5] Risk of Bias Assessment Tool for RCTs. Seven areas were included: random sequence generation; allocation concealment; participant and performer blinding; outcome assessment blinding; incomplete outcome reporting; selective reporting; and other sources of bias. Then make 3 evaluations of “low risk,” “high risk,” and “unclear.”
2.7. Data analysis
Statistical and meta-analysis was performed using RevMan 5.4.1 software (NY). For enumeration data, relative risk (RR) was used as the effective index, and for measurement data, mean difference was used as the effective index. Each effect size was given its point estimate and 95% confidence interval (CI). The heterogeneity among the results of the included studies was analyzed by the χ2 test (the test level was α = 0.1), and the size of the heterogeneity was quantitatively judged by I2. If there is no statistical heterogeneity among the study results, a fixed-effect model is used for Meta-analysis; if there is statistical heterogeneity among the study results, the source of heterogeneity is further analyzed to exclude the influence of apparent clinical heterogeneity. Afterward, a random effects model was used for meta-analysis. Significant clinical heterogeneity was handled using methods such as subgroup analysis or sensitivity analysis, or only descriptive analysis.
2.8. Ethical approval
Since this study is based on published articles and does not involve patients, ethical approval and informed consent of patients are not required.
3. Results
3.1. Literature search results
3.2. Basic characteristics and risk of bias assessment results of included studies
Sixty-five related references were initially detected, and after the layer-by-layer screening, 11[6–16] RCTs were finally included, all in Chinese, with a total of 814 patients, including 407 in the experimental group and 407 in the control group. The literature search process and results are shown in Figure 1. The basic characteristics of the included studies are shown in Table 1, and the results of the risk of bias assessment are shown in Table 2. The ratio of risk of bias and quality assessment summary graphs of the 11 studies[6–16] are shown in Figure 2.
Table 2.
Characteristics of included 11 randomized controlled trials (A and B).
| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Number of cases | Gender proportion (male/female) | Age | Interventions | Outcomes | |||
| Control group | Experimental group | Control group | Experimental group | |||||
| Control group | Experimental group | |||||||
| Su N 2021[6] | 30 | 30 | 37/23 | 62.05 ± 6.29 | 61.43 ± 6.45 | Cisplatin 40–50 mg/time, d1, d4 | Endostar 60 mg/time, d1, d4 Cisplatin 40–50 mg/time, d2, d5 | ①③④ |
| ⑤ ⑥ | ||||||||
| Yang Y 2013[7] | 21 | 21 | 27/15 | 37–80 | Cisplatin 40 mg once a week | Endostar 30 mg twice a week Cisplatin 40 mg once a week | ①②③ | |
| ④⑤⑥ | ||||||||
| Liu YF 2018[8] | 34 | 34 | 38/30 | 65.55 ± 5.28 | 63.19 ± 4.73 | Cisplatin 60 mg + 0.9% sodium chloride solution 20 mg, twice a week | Cisplatin 60 mg + Endostar 60 mg + 0.9% sodium chloride solution 20 mg, twice a week | ① ② |
| ③ ④ | ||||||||
| Chen J 2014[9] | 30 | 30 | 44/16 | 55.6 ± 4.5 | 54.3 ± 5.6 | Cisplatin 40 mg twice a week | Cisplatin 40 mg + Endostar 45 mg twice a week | ①③④ |
| ⑤ ⑥ | ||||||||
| Tu JR 2014[10] | 45 | 45 | 48/42 | 47.5 ± 10.5 | 46.5 ± 11.5 | Cisplatin 40 mg/m2 + 0.9% sodium chloride solution 40 mL twice a week for 3 wk | Endostar 45 mg, concurrently injected with cisplatin 40 mg/m2 + 0.9% sodium chloride solution 40 mL, twice a week for 3 consecutive weeks | ①②③ |
| ④ ⑤ | ||||||||
| Lu XD 2017[11] | 31 | 31 | 35/27 | 45.7 ± 11.3 | 46.3 ± 10.6 | Inject cisplatin 40 mg/m2 + 0.9% sodium chloride solution 40 mL, twice a week for 3 consecutive weeks | Recombinant human Endostarin 45 mg + cisplatin 40 mg/m2 + 0.9% sodium chloride solution 40 mL, 2 times a week for 3 consecutive weeks | ①②③ |
| ④ ⑤ | ||||||||
| Xu XZ 2021[12] | 75 | 75 | 79/71 | 63.87 ± 5.38 | 63.65 ± 5.11 | Cisplatin injection 10 mg: 2 mL intrapleural injection, once a week for 3 wk | Cisplatin injection 10 mg: 2 mL intrapleural injection, once a week, for 3 weeks, recombinant human Endostarin injection 15 mg: 3 mL intrapleural infusion, 45 mg each time, once a week, 3 weeks of continuous use | ①③⑤ |
| Wang R 2018[13] | 30 | 30 | 35/25 | 60.54 ± 5.65 | 61.28 ± 6.32 | Cisplatin 40 mg/m2 twice a week for 3 wk | Cisplatin 40 mg/m2, 2 times/wk, for 3 wk Recombinant human Endostarin 45 mg/time, 2 times/wk, for 3 wk | ①③⑤ |
| Shen Q 2012[14] | 40 | 40 | 42/38 | 37–79 | Cisplatin 40 mg once a week | Cisplatin 40 mg once a week + Endostar 30 mg twice a week | ①②③ | |
| ④⑤⑥ | ||||||||
| Liu HX 2018[15] | 26 | 26 | 23/29 | 39–75 | 41–75 | Cisplatin 30 mg + 29mL 0.9% sodium chloride solution, twice a week, for 2 to 3 weeks, once a week after the pleural effusion is stable | Endostar 45mg + cisplatin 30 mg + 20 mL 0.9% sodium chloride solution, twice a week, for 2 to 3 wk, and once a week after the pleural effusion stabilizes | ①③ |
| ④⑤ | ||||||||
| Chen RL 2016[16] | 45 | 45 | 53/37 | 60.8 ± 7.5 | 60.6 ± 7.2 | Cisplatin injection 40 mg/m2 + 0.9% sodium chloride solution 40, 2 times/wk | Cisplatin injection 40 mg/m2 + 0.9% sodium chloride solution 40, 2 times/wk; Recombinant human Endostarin injection 45 mg, 2 times/wk | ①②③ |
| ④ ⑥ | ||||||||
| (B) | ||||||||
| Author | Random method | Allocation hidden | Blind | Selective reporting of research findings | Integrity of the resulting data | Other sources of bias | ||
| Su N 2021[6] | Random number table | Unclear | Unblinded | Not | Not | Not | ||
| Yang Y 2013[7] | Random number table | Unclear | Unblinded | Not | Not | Not | ||
| Liu YF 2018[8] | Unclear | Unclear | Unclear | Not | Not | Not | ||
| Chen J 2014[9] | Unclear | Unclear | Unclear | Not | Not | Not | ||
| Tu JR 2014[10] | Random number table | Unclear | Unblinded | Not | Not | Not | ||
| Lu XD 2017[11] | Random number table | Unclear | Unblinded | Not | Not | Not | ||
| Xu XZ 2021[12] | Random number table | Unclear | Unblinded | Not | Not | Not | ||
| Wang R 2018[13] | Random number table | Unclear | Unclear | Not | Not | Not | ||
| Shen Q 2012[14] | Random number table | Unclear | Unblinded | Not | Not | Not | ||
| Liu HX 2018[15] | Random number table | Unclear | Unblinded | Not | Not | Not | ||
| Chen RL 2016[16] | Unclear | Unclear | Unblinded | Not | Not | Not | ||
① = the overall response rate, ② = quality of life improvement rate, ③ = incidence of gastrointestinal reactions, ④ = the incidence of leukopenia, ⑤ = the incidence of thrombocytopenia, ⑥ = the incidence of hypodynamia.
Figure 2.
Risk of bias of the included studies—risk of bias graph (A) and summary (B).
3.3. Meta-analysis results
3.3.1. The overall response rate.
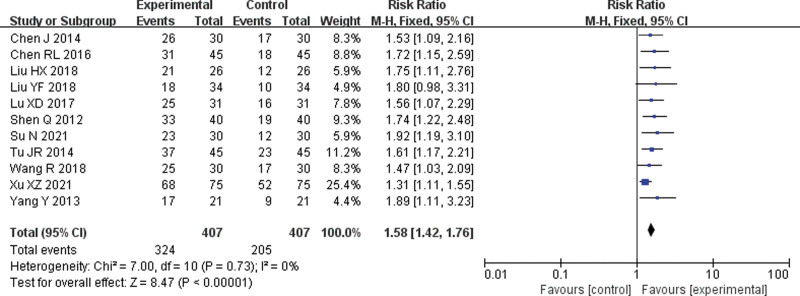
Eleven[6–16] RCTs were included. The Meta-analysis results of the fixed-effect model showed that the overall response rate of the endostar combined with cisplatin group was significantly higher than that of the single-agent cisplatin group, and the difference was statistically significant (RR = 1.58, 95% CI = 1.42–1.76, P < .001) (Fig. 3).
Figure 3.
Forest plot of meta-analysis of the overall response rate. CI = confidence interval, M-H = mantel-haenszel.
3.3.2. Quality of life improvement rate.
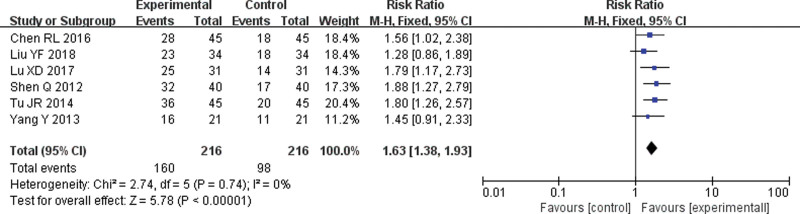
Six[7,8,10,11,14,16] RCTs were included. The meta-analysis results of the fixed-effect model showed that the improvement rate of quality of life in the endostar combined with cisplatin group was significantly higher than that in the single-agent cisplatin group, and the difference was statistically significant (RR = 1.63, 95% CI = 1.38–1.93, P < .001) (Fig. 4).
Figure 4.
Forest plot of meta-analysis of quality of life improvement rate. CI = confidence interval, M-H = mantel-haenszel.
3.3.3. The incidence of adverse reactions.
3.3.3.1. Incidence of gastrointestinal reactions.
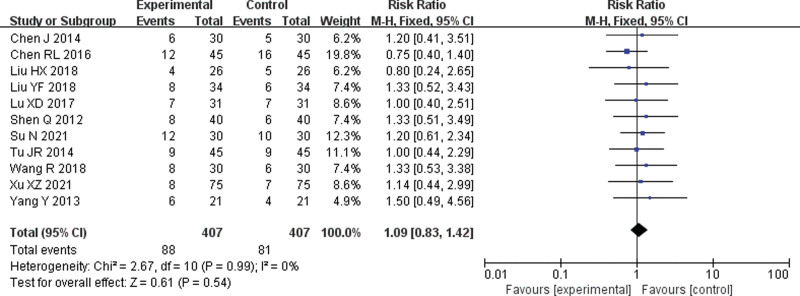
Eleven[6–16] RCTs were included. The meta-analysis results of the fixed-effect model showed no significant difference in the incidence of gastrointestinal reactions between the 2 groups (RR = 1.09, 95% CI = 0.83–1.42, P = .54) (Fig. 5)
Figure 5.
Forest plot of meta-analysis of the incidence of gastrointestinal reactions. CI = confidence interval, M-H = mantel-haenszel.
3.3.3.2. The incidence of leukopenia.
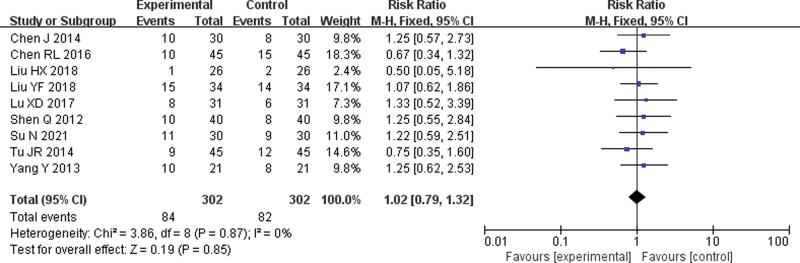
Nine[6–11,14–16] RCTs were included. The meta-analysis of the fixed-effect model showed no significant difference in the incidence of leukopenia between the 2 groups (RR = 1.02, 95% CI = 0.79–1.32, P = .85) (Fig. 6).
Figure 6.
Forest plot of meta-analysis of the incidence of leukopenia. CI = confidence interval, M-H = mantel-haenszel.
3.3.3.3. The incidence of thrombocytopenia.
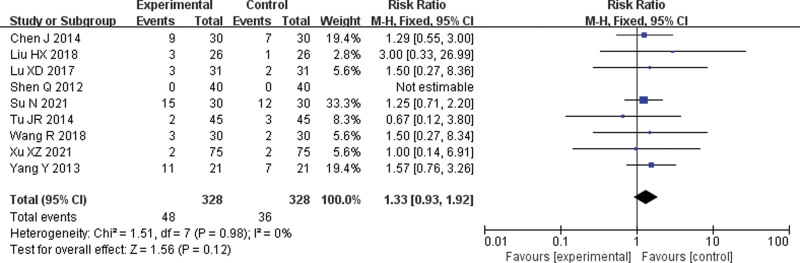
Nine[6,7,9–15] RCTs were included. The meta-analysis of the fixed effect model showed no significant difference in the incidence of thrombocytopenia between the 2 groups (RR = 1.33, 95% CI = 0.93–1.92, P = .12) (Fig. 7).
Figure 7.
Forest plot of meta-analysis of the incidence of thrombocytopenia. CI = confidence interval, M-H = mantel-haenszel.
3.3.3.4. The incidence of hypodynamia.
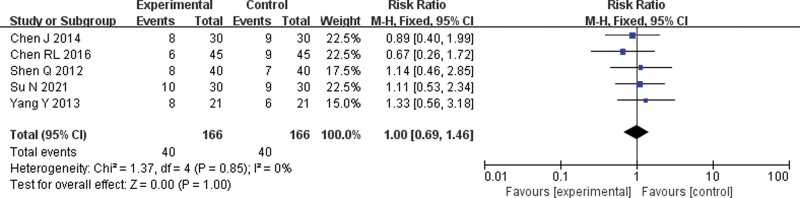
Five[6,7,9,14,16] RCTs were included. The fixed-effects model meta-analysis showed no significant difference in the incidence of hypodynamia between the 2 groups (RR = 1.00, 95% CI = 0.69–1.46, P = 1.00) (Fig. 8).
Figure 8.
Forest plot of meta-analysis of the incidence of hypodynamia. CI = confidence interval, M-H = mantel-haenszel.
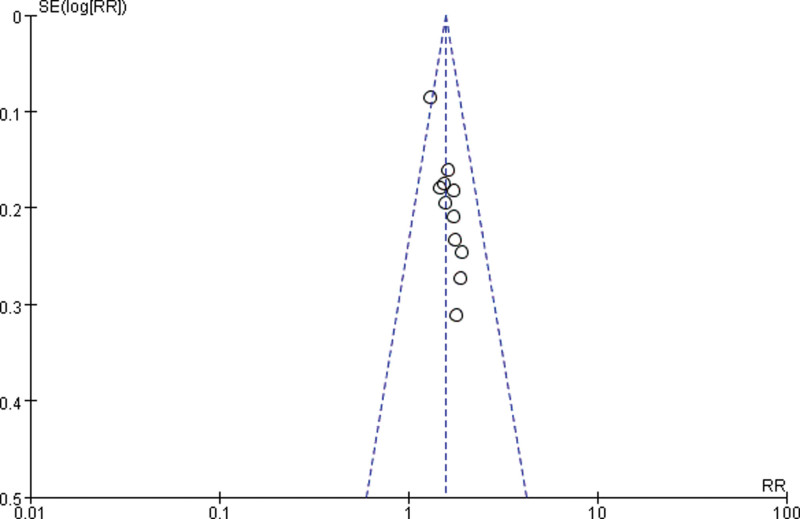
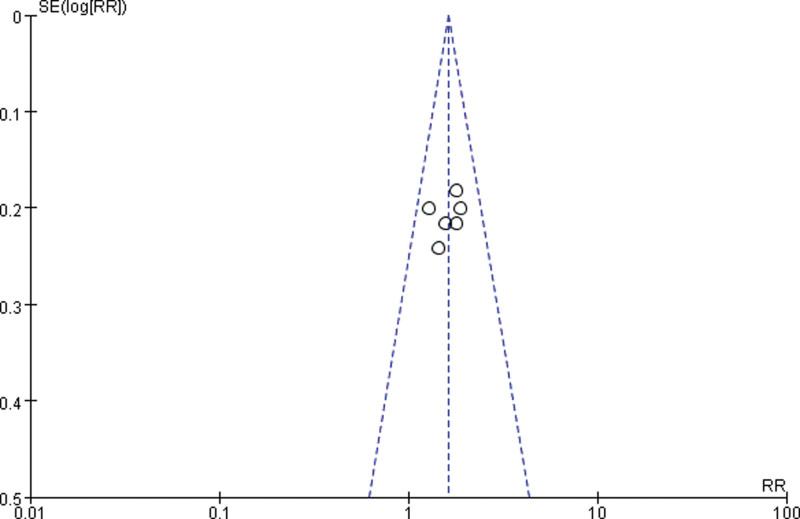
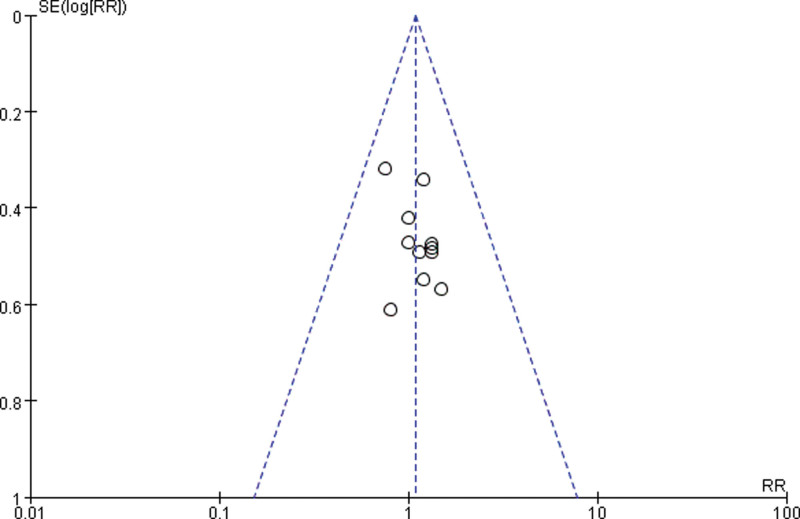
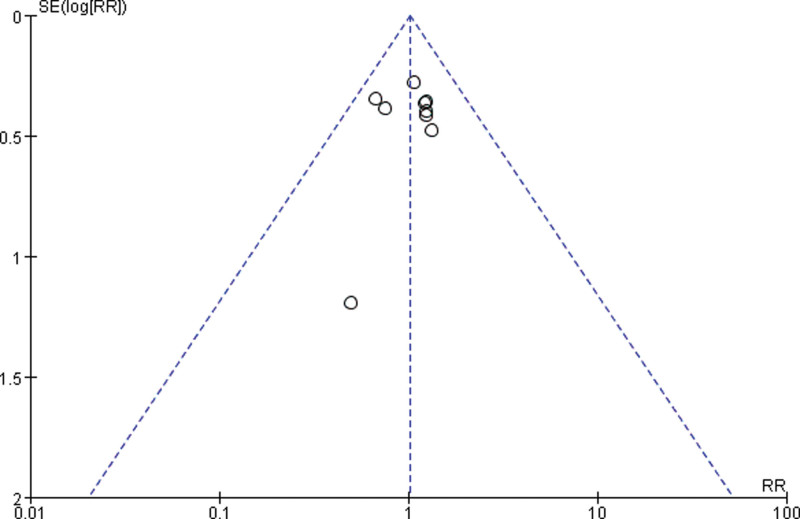
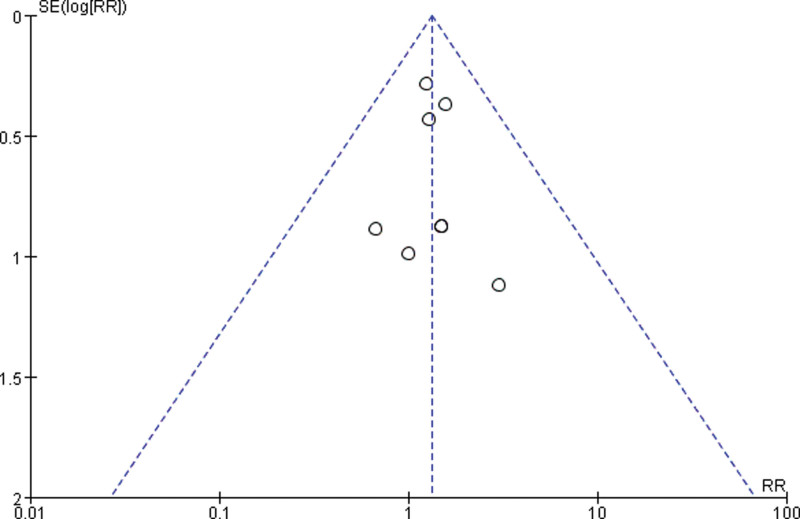
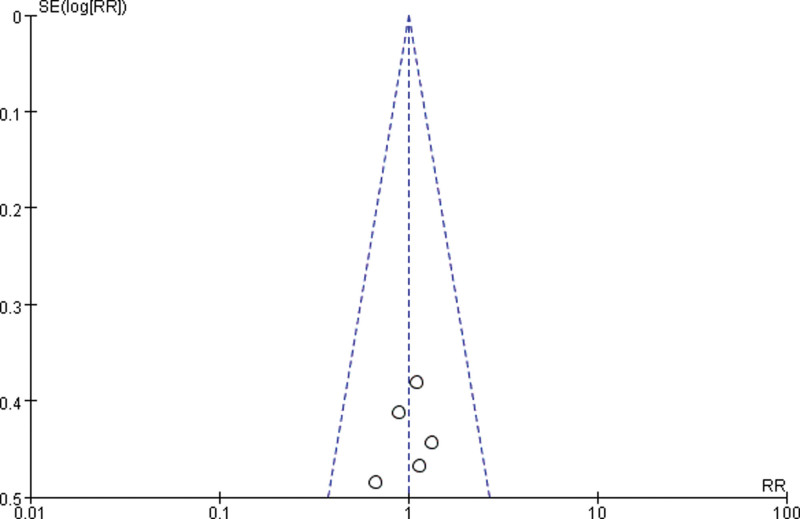
3.3.4. Publication bias.
Draw a funnel plot with the sample size content as the ordinate and the effect size as the abscissa. The funnel plot of the overall response rate was asymmetric among the 11 included studies (Fig. 9), suggesting publication bias. The funnel plot of the rate of improvement in quality of life among the 6 included studies showed asymmetry (Fig. 10), with publication bias. The funnel plot of the incidence of gastrointestinal reactions among the 11 included studies showed asymmetry (Fig. 11), with publication bias. The funnel plot of the incidence of leukopenia among the 9 included studies showed asymmetry (Fig. 12), with publication bias. The funnel plot of the incidence of thrombocytopenia among the 9 included studies showed asymmetry (Fig. 13), with publication bias. The funnel plot of the incidence of hypodynamia among the 5 included studies showed asymmetry (Fig. 14), with publication bias.
Figure 9.
The funnel plot of overall response rate. RR = relative risk, SE = standard error.
Figure 10.
The funnel plot of the rate of improvement in quality of life. RR = relative risk, SE = standard error.
Figure 11.
The funnel plot of the incidence of gastrointestinal reactions. RR = relative risk, SE = standard error.
Figure 12.
The funnel plot of the incidence of leukopenia. RR = relative risk, SE = standard error.
Figure 13.
The funnel plot of the incidence of thrombocytopenia. RR = relative risk, SE = standard error.
Figure 14.
The funnel plot of the incidence of hypodynamia. RR = relative risk, SE = standard error.
4. Discussion
In most cases of NSCLC complicated with pleural effusion, it indicates that lung cancer has developed to the middle and advanced stage, and most cases have lost the opportunity to achieve the purpose of radical cure by surgical means. At this time, turn the direction of treatment to the effective control of pleural effusion, which will help prolong the survival of patients and improve the quality of life.[16] The main treatment measures for malignant pleural effusion are pleural aspiration and pleural injection. The drugs mainly include chemotherapy drugs, biological response modifiers, and anti-tumor Chinese patent medicines.[17] Local chemotherapy in the thoracic cavity is used to directly kill tumor cells and cause pleural adhesions by reducing the generation of pleural effusion. However, this method leads to extensive adhesion and fibrosis of the pleura, and some patients may also develop certain resistance to chemotherapy drugs.[18] Currently, the combination of chemotherapy drugs is mostly used for local treatment of malignant pleural effusion in clinical practice. Commonly used chemotherapeutic drugs for pleural perfusion include cisplatin, fluorouracil, bleomycin, etc. Among them, cisplatin as a cell cycle nonspecific drug destroys its structure and function by forming interchain or intrachain binding with the DNA of tumor cells, thereby inhibiting the formation of tumor cells. It has the characteristics of a broad anti-tumor spectrum and effective on anaerobic cells. Local intracavitary medication can also lead to pleural hyperplasia and fibrosis and reduce the exudation of pleural effusion. In order to improve the therapeutic effect, different types of drugs are often combined with intrathoracic perfusion therapy, such as cisplatin combined with biological response modifiers or traditional Chinese medicine preparations. Despite the continuous enrichment and progress of treatment methods, the overall efficacy of malignant pleural effusion of patients is still not ideal, and the average survival time of patients after diagnosis is less than 6 months.[19]
Some studies[20] have shown that VEGF can promote the formation of malignant pleural effusion by stimulating angiogenesis, increasing vascular permeability, and promoting intraluminal fluid return. (EGFR) epidermal growth factor receptor,It is a member of the epidermal growth factor receptor HER family. EGFR is widely distributed on the surface of mammalian epithelial cells, fibroblasts, glial cells, keratinocytes and other cells, and EGFR signaling pathways play an important role in physiological processes such as cell growth, proliferation and differentiation can promote the secretion of VEGF by stimulating epithelial cell differentiation and inducing the expression of tumor proteins such as c-fos and c-myc. The formation of malignant pleural effusion is closely related to the increased level of VEGF, increased vascular permeability and tumor angiogenesis after tumor metastasis or infiltration into the pleura.[18] Endostar can play a role in multiple steps of tumor cell angiogenesis: block VEGF receptor, the effect of vascular endothelial growth factor on endothelial cells is inhibited; inhibit the migration of neovascular endothelial cells, induce endothelial cell apoptosis and Antagonize angiogenesis; restricted expression of VEGF in cancer cells and active proteolytic enzymes, which can cause tumor dormancy or degeneration; antagonize the attachment of new vascular endothelial cells to matrix proteins, leading to apoptosis of vascular endothelial cells and inhibiting tumor proliferation; the efficiency of cancer metastasis is reduced.[21] In addition, according to current research, endostar can target the neovascular endothelium to produce anti-angiogenesis and promote tumor dormancy or regression. VEGF is a pro-angiogenic factor that can promote the growth of new tumor blood vessels accelerates tumor metastasis and infiltration. The higher the level, the higher the degree of malignancy of NSCLC; HIF-1α is a DNA-binding protein that can regulate cell transcription, participate in tumor angiogenesis, and affect the expression of tumor factors. Based on this, the research of Feng[22] suggested that the levels of VEGF and HIF-1α were lower than those before treatment, and the levels of VEGF and HIF-1α after intrapleural injection of endostar combined with cisplatin were lower than those of cisplatin monotherapy, suggesting that combined treatment with endostar and cisplatin can more significantly reduce the level of VEGF and inhibit the expression of HIF-1α,thereby inhibiting tumor angiogenesis, accelerating tumor cell apoptosis, and reducing the probability of thoracic tissue being invaded by tumor cells. The results showed that endostar combined with cisplatin had a significant effect on the treatment of NSCLC complicated with malignant pleural effusion, which could reduce the influence on the platelet parameters, reduce the level of VEGF, inhibit the expression of HIF-1α, and improve the effusion condition. Li et al[23] and others studied different doses of endostar combined with cisplatin intracavitary infusion in the treatment of NSCLC complicated with malignant pleural effusion and found that the dose of the drug was positively correlated with the efficacy during the treatment of patients. The disease remission rate (76.67%) and disease control rate (90.00%) of patients with NSCLC and malignant pleural effusion in the experimental group treated with platinum intracavitary infusion were significantly higher than those in the control group treated with low-dose endostar combined with cisplatin for intrapleural infusion (50.00%), disease control rate (66.67%), P < .05; the occurrence of adverse reactions in the experimental group was less different from that in the control group, P > .05.[24–27]
5. Conclusion
The results showed that the combined administration of endostar and Cisplatin could inhibit tumor activity more effectively. The above studies provide a reliable theoretical basis for the feasibility, effectiveness, and prognosis of recombinant human endostatin combined with cisplatin in the treatment of NSCLC complicated with malignant pleural effusion. This meta-analysis showed that, compared with the control group, recombinant human endostatin combined with cisplatin intrapleural injection in the treatment of NSCLC complicated with malignant pleural effusion did not increase the gastrointestinal reaction, leukopenia, thrombocytopenia and hypodynamia of patients. Among the adverse reactions that occurred in the included studies, most of them were mild, and there were no serious adverse reactions, which indicated that the combined administration scheme had higher safety. Compared with the meta-analysis study conducted by Liu et al,[28] this article screened the retrieved literature in detail, and compared the relevant literature mentioned in that meta-analysis and found that Liu Xin which was included in his meta-analysis study lacked complete outcome indicators, and the whereabouts of the lack of indicators are not explained. In the literature of Huang Li et al, which was also included in his meta-analysis study, only a summary of randomization is described. They were both the low-quality literature, so this meta-analysis was not used. In conclusion, recombinant human endostatin combined with intrapleural injection of cisplatin in the treatment of patients with NSCLC complicated with malignant pleural effusion can improve the overall response rate, improve the quality of life of patients, and the adverse effects such as gastrointestinal reactions, leukopenia, thrombocytopenia, and hypodynamia did not increase significantly. Recombinant human endostatin combined with cisplatin for local treatment of NSCLC patients with malignant pleural effusion showed good efficacy and safety. In view of the limitations of this study, it is necessary to conduct large-scale and high-quality randomized controlled studies to guide clinical medication.
6. Limitations
Limitations of this study: ① of the included studies did not describe the randomization method, 3 did not describe the implementation of blinding, and all did not describe the hidden grouping and other sources of bias; ② the included studies provided limited data and did not study patient survival metrics; ③ most of the included studies have small sample sizes, and the results may slightly deviate from the actual results; ④ there are differences in the dosage, course of treatment, medication order and medication frequency of recombinant human endostatin and cisplatin, and the experimental results will also exist; ⑤ Su et al[6] used the efficacy index of quality of life improvement rate KPS in the study, but the statistical measurement data of the index could not be graded, and the specific improvement and the number of other indicators could not be judged, so this index was not used quality of life improvement rate indicators in that literature.
Author contributions
All authors read and approved the final draft. Luo Miao is the guarantor of this review.
Data management: Hu Yongqi, Zhou Zhenke.
Methodology: Luo Miao.
Research concept: Luo Miao.
Sources: Hu Yongqi, Zhou Zhenke.
Supervision: Luo Miao.
Writing – original draft: Hu Yongqi, Zhou Zhenke.
Writing – review and editing: Luo Miao.
Abbreviations:
- CI =
- confidence interval
- EGFR =
- epidermal growth factor receptor
- NSCLC =
- non-small cell lung cancer
- RCT =
- randomized controlled trial
- RR =
- relative risk
- VEGF =
- vascular endothelial growth factor
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Hu Y, Zhou Z, Luo M. Efficacy and safety of endostar combined with cisplatin in treatment of non-small cell lung cancer with malignant pleural effusion: A meta-analysis. Medicine 2022;101:52(e32207).
Contributor Information
Yongqi Hu, Email: Hyq17776466027@163.com.
Zhenke Zhou, Email: 1830795385@qq.com.
References
- [1].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- [2].Shi SB, Ma TH, Li CH, et al. Effect of maintenance therapy with dendritic cells: cytokine-induced killer cells in patients with advanced non-small cell lung cancer. Tumori. 2012;98:314–9. [DOI] [PubMed] [Google Scholar]
- [3].Duan CX, Liang XG, Zhang ZQ. Efficacy analysis of endorphin combined with cisplatin in the treatment of malignant pleural effusion in non-small cell lung cancer. J Baotou Med College. 2015;31:45–6. (恩度联合顺铂治疗非小细胞肺癌恶性胸腔积液的疗效分析). [Google Scholar]
- [4].Zheng QH, Hu W, Liao XF, et al. A comparision between intrapleural injection of cisplatin combined with endostar and cisplatin alone in the treatment for malignant pleural effusion. J Chin Oncol. 2013;19:386–9. (顺铂序贯恩度与单纯顺铂胸腔内灌注治疗恶性胸腔积液的临床对照研究). [Google Scholar]
- [5].Higgins JPT, Green S. (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org. [Google Scholar]
- [6].Su N, Fan LP, Qin LL, et al. Efficacy of ENDU combined with cisplatin intrapleural perfusion in the treatment of non-small cell lung cancer with malignant pleural effusion. J Med Inf. 2021;34:155–7. (恩度联合顺铂胸腔内灌注治疗非小细胞肺癌恶性胸腔积液的疗效). [Google Scholar]
- [7].Yang Y, Lin RY, Cao GM. Short-term and long-term efficacy of endostar combined with Cis-diaminedichloroplatinum in treating malignant pleural effusion of non-small cell lung cancer. China Pharma. 2013;22:21–2. (恩度联合顺铂治疗非小细胞肺癌恶性胸腔积液近远期疗效). [Google Scholar]
- [8].Liu YF, Huang M, Yao WR. Clinical analysis of recombinant human endostatin combined with intracavitary cisplatin chemotherapy in the treatment of non-small cell lung cancer complicated with malignant pleural effusion. J Hunan Univ Chin Med. 2018;38:159–60. (重组人血管内皮抑制素联合顺铂腔内化疗治疗非小细胞肺癌合并恶性胸腔积液的临床分析). [Google Scholar]
- [9].Chen J, Gou SP, Luan WG. Study on the efficacy of Endostar combined with cisplatin in treatment of non-small cell lung cancer with malignant pleural effusion and influence on tumor markers VEGF and HIF-1α. J Clin Exp Med. 2014;13:1778–80. (重组人血管内皮抑制素联合顺铂治疗非小细胞肺癌恶性胸腔积液的疗效及对VEGF、HIF-1α、肿瘤标志物的影响). [Google Scholar]
- [10].Tu JR, Huang SJ, Wang MJ. Clinical efficacy of pleural perfusion with recombinant human endostatin combined with cisdiammi dichloride platinum for advanced non-small cell lung cancer patients with malignant pleural effusion. Pract J Cancer. 2014;29:1592–4. (重组人血管内皮抑制素联合顺铂胸腔灌注治疗晚期非小细胞肺癌恶性胸腔积液的临床疗效). [Google Scholar]
- [11].Lu XD, Zhang TR. Clinical efficacy of pleural perfusion with recombinant human endostatin and cisplatin in advanced non-small cell lung cancer patients with malignant pleural effusion. Jiangsu Med J. 2017;43:1023–5. (重组人血管内皮抑制素联合顺铂胸腔内灌注治疗晚期非小细胞肺癌恶性胸腔积液的疗效). [Google Scholar]
- [12].Xu XZ, Liu PP, Zhang XB, et al. Efficacy and safety of recombinant human endostatin combined with cisplatin in the treatment of malignant pleural effusion in non-small cell lung cancer. Clin Res. 2021;29:69–71. (重组人血管内皮抑制素联合顺铂治疗非小细胞肺癌恶性胸腔积液的疗效及安全性观察). [Google Scholar]
- [13].Wang R. Clinical efficacy of recombinant human endostatin combined with cisplatin in the treatment of malignant pleural effusion in non-small cell lung cancer. China Pract Med. 2018;13:96–7. (重组人血管内皮抑制素联合顺铂治疗非小细胞肺癌恶性胸腔积液的临床疗效). [Google Scholar]
- [14].Shen Q, Gu AQ, Wu JY, et al. Therapeutic observation of endostar combined with cisdiammi dichloride platinum on non—small cell lung cancer with malignant pleural effusion. J Clin Med Pract. 2012;16:29–31. (重组人血管内皮抑制素联合顺铂治疗非小细胞肺癌恶性胸腔积液疗效观察). [Google Scholar]
- [15].Liu HX, Tan W. Recombinant vascular endostatin therapy for malignant pleural effusion. Acta Acad Med Weifang. 2018;40:217–9. (重组人血管内皮抑制素治疗恶性胸腔积液临床观察). [Google Scholar]
- [16].Chen RL, Zhang CC, Wu H, et al. Clinical effect of pleural perfusion of human recombinant endostatin injection combined with cisplatin injection on advanced non-small cell lung cancer complicated with malignant pleural effusion. Pract J Cardiac Cerebral Pneumal Vasc Dis. 2016;24:118–20. (重组人血管内皮抑制素注射液联合顺铂注射液胸腔灌注治疗晚期非小细胞肺癌恶性胸腔积液的临床疗效观察). [Google Scholar]
- [17].Xu JP, Zhou L, Yang WB. Research progress in the treatment of non-small cell lung cancer with malignant pleural effusion by endostar combined with cisplatin infusion. J Dalian Med Univ. 2015;37:89–92. (恩度联合顺铂胸腔内灌注治疗非小细胞肺癌恶性胸腔积液研究进展). [Google Scholar]
- [18].Wu K, Lu HY, Duan DJ. Clinical observation of intrapleural injection of endostar combined with cisplatin(DDP) in treatment of non-small cell lung cancer with malignant pleural effusion. J Clin Pulmonary Med. 2012;17:107–8. (重组人血管内皮抑制素注射液联合顺铂注射液胸腔灌注治疗晚期非小细胞肺癌恶性胸腔积液的临床疗效观察). [Google Scholar]
- [19].Yang MJ, He W, Wang F, et al. Recombinant human endostatin combined with cisplatin perfusion chemotherapy for malignant pleural effusions: a meta-analysis. Chin Clin Oncol. 2015;20:1117–23. (重组人血管内皮抑制素联合顺铂胸腔灌注化疗治疗恶性胸腔积液的Meta分析). [Google Scholar]
- [20].Mohammed KA, Nasreen N, Hardwick J, et al. Bacterial induction of pleural mesothelial monolayer barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;281:L119–25. [DOI] [PubMed] [Google Scholar]
- [21].Zhao WY, Chen DY, Chen JH, et al. Effects of intracavitary administration of Endostar combined with cisplatin in malignant pleural effusion and ascites. Cell Biochem Biophys. 2014;70:623–8. [DOI] [PubMed] [Google Scholar]
- [22].Feng ZY. Effects of Endostat combined with cisplatin on platelet parameters and levels of VEGF and HIF-1α in patients with non-small cell lung cancer complicated with malignant pleural effusion. Henan Med Res. 2017;26:4454–5. (恩度联合顺铂对非小细胞肺癌合并恶性胸腔积液患者血小板参数及VEGF、HIF-1α水平的影响). [Google Scholar]
- [23].Li M, Hu GY, Mei Q, et al. The effect and adverse reaction of non-small cell lung cancer with malignant pleural effusion patients treated with different dose endostar combined cisplatin intracavity perfusion. Chin J Med Guide. 2017;19:791–3. (不同剂量恩度联合顺铂腔内灌注治疗非小细胞肺癌恶性胸腔积液患者疗效及不良反应的差异). [Google Scholar]
- [24].Man L, Dong YY, Li ZH, et al. Clinical efficacy of bevacizumab in treatment of malignant pleural and peritoneal effusion. China Modern Doctor. 2013;51:158–60. (贝伐单抗治疗恶性胸腹腔积液l临床疗效). [Google Scholar]
- [25].Tian Y, Tian Z, Wu K, et al. A Meta analysis of endostar (rh-endostain, YH-16) plus chemotherapy containing platinum in the treatment of advanced non-small cell lung cancer. J Chongqing Med Univ. 2012;37:151–7. (恩度联合含铂类化疗药物治疗晚期非小细胞肺癌 的疗效及安全性 的 Meta分析). [Google Scholar]
- [26].Qu B, Jiang W, Zhou ZM. Clinical research of intrapleural combination therapy with bevacizumab and cisplatin for non-small cell lung cancer mediated malignant pleural effusion. J China Med Univ. 2015;44:648–52. (贝伐单抗联合顺铂治疗非小细胞肺癌恶性胸腔积液的l临床研究). [Google Scholar]
- [27].Jin S, Liang B, Jin F, et al. Effect of voriconazole combined with Kang’ai injection in the treatment of pulmonary fungal infections in patients with lung cancer and analysis of the prognosis. Chin J Nosocomiol. 2015;25:4670–2. (伏立康唑联合康艾注射液治疗肺癌患者肺部真菌感染的效果评价). [Google Scholar]
- [28].Liu ZL, Huang LN, Wang B, et al. Efficacy and safety of endostar combined with cisplatin in treatment of non-small cell lung cancer with malignant pleural effusion: a meta-analysis. Chin J Evid-based Med. 2016;16:557–63. (恩度联合顺铂治疗非小细胞肺癌合并恶性胸腔积液的Meta分析). [Google Scholar]