Abstract
Insufficient lymph node harvest (< 12) may lead to incorrect classification of stage I and II disease. Many studies have indicated a poor prognosis with inadequate lymph node harvest in stages I to III, but few studies have demonstrated the relationship between low lymph node harvest and T4 disease. This study aimed to identify the influence of insufficient number of lymph nodes harvested on survival in T4N0 colorectal cancer. We enrolled patients with T4N0 colorectal cancer who underwent radical resection between 2010 and 2016. A total of 155 patients were divided into 2 groups; 142 patients had ≥ 12 harvested lymph nodes, and the other 13 had < 12 lymph nodes. All patients were followed up for at least 5 years. The primary outcome was the impact of the number of lymph nodes harvested on disease-free survival and overall survival, which were investigated using Kaplan-Meier survival techniques. There were no significant differences in recurrence rate, emergent or elective surgery, laparoscopic or open surgery, or chemotherapy between the 2 groups. Kaplan-Meier analyses showed no statistical differences in 5-year disease-free survival (P = .886) and 5-year overall survival (P = .832) between the groups. There were no significant differences in disease-free survival and overall survival between patients with adequate (≥ 12) and inadequate (< 12) lymph node harvest in T4N0 colorectal cancers.
Keywords: disease-free survival, lymph node harvest, overall survival, T4N0 colorectal cancer
1. Introduction
Radical resection of colorectal cancer (CRC) involves removal of the tumor with clear margins and harvesting of the regional lymph nodes (LNs). According to current guidelines, the difference between stage II and III disease is nodal positivity.[1,2] Adequate LN harvest (LNH) is critical for accurate nodal staging, which can determine whether it is appropriate to administer chemotherapy to CRC patients. Insufficient numbers of LNH may lead to incorrect classification of stage I and II disease. The American college of pathologists and the American joint committee on cancer recommend at least 12 LNH surgical specimens, which is the general consensus.[1]
However, insufficient numbers of LNH are common in the literature, ranging from 14.7% to 52%.[2–8] In most studies, low LNH was associated with poor survival outcomes in stage II and III CRC, and some studies also classified low LNH as a risk factor.[8,9] In the MOSAIC trial and other studies, the high-risk factors for recurrence of stage II CRC were T4 disease, poorly differentiated adenocarcinoma, inadequate LNH, lymphovascular invasion, and clinical presentation of bowel obstruction or perforation.[10,11]
However, few studies demonstrated a relationship between low LNH and T4 disease in CRC. A previous study also suggested that patients with multiple high-risk stage II CRC had worse survival than those with stage III disease, suggesting that chemotherapy should be administered in high-risk stage II disease.[12,13] Although adjuvant chemotherapy remains controversial in stage II CRC, most stage IIB and IIC diseases still require chemotherapy, whether oral or intravenous.[14,15]
Most studies indicated inadequate LNH (< 12) in stage II disease as a poor survival factor, and some studies discussed the optimal LNH number for stage II CRC[7,8,16]; however, few studies have demonstrated the impact of low LNH number on survival in stage IIB and IIC disease. This study aimed to identify the influence of insufficient number of LNH on survival among stage IIB and IIC CRC patients.
2. Methods
2.1. Study Population
This study was a retrospective review of 155 CRC patients who underwent radical surgical resection for primary pathological diagnosis of stage IIB (T4aN0M0) or IIC (T4bN0M0) in a would-be medical center in Kaohsiung, Taiwan from January 2010 to December 2016.
The evaluation of T category, number of LNs examined, and LN status was based on the pathological examination of surgical specimens, which followed the 7th edition of the American joint committee on cancer TNM staging system. Patients were divided into 2 groups according to the number of LNs examined: the 1st group comprised patients with harvested LNs < 12, and the other comprised patients with harvested LNs ≥ 12.
All patients underwent routine follow-up for at least 5 years with a series of carcinoembryonic antigen (CEA) tests, computed tomography of the chest, abdomen, and pelvis, and colonoscopy to evaluate local recurrence and distant metastasis.
In the data analyses, follow-up was defined as the time from the primary surgery to a patient event, such as disease recurrence, loss of follow-up, death, or follow-up until 60 months. After resection of the primary tumor, patients who developed local recurrence, peritoneal seeding, or distant metastasis were no longer considered disease-free in the analysis. The disease-free survival (DFS) was defined as the length of time after resection of primary tumor to cancer recurrence, second cancer, or death from any cause. Patients who were lost to follow-up and died at any time for any reason were regarded as deaths in overall survival (OS) in the analysis.
2.2. Ethics statement
The database included only de-identified data. No ethics approval was needed for retrospective data analyses with anonymous data.
2.3. Statistical analyses
In the present study, DFS and OS were the outcomes of interest. The impact of the number of harvested LNs on DFS and OS was investigated using survival techniques (Cox regression, Kaplan-Meier survival analysis, and log-rank tests). Subgroups were compared using an independent t test for continuous variables and Fisher’s exact test for categorical variables.
All statistical analyses were performed using IBM SPSS Statistics for Windows version 22.0. (IBM Corp., Armonk, NY). All p-values were calculated using 2-tailed tests, and statistical significance was defined as a 2-sided P value < 0.05.
3. Results
A total of 155 patients (94 men and 61 women) were included in the study. Their ages ranged from 26 to 93 years, with a median of 65 years. Amount 155 patients, 2 of the patients didn’t achieve R0 resection. Both 2 patients were pathology T4b, 1 invaded to bladder and the other invaded to pelvic wall. The R0 resection rate amount T4N0 in this study was 98.7%.
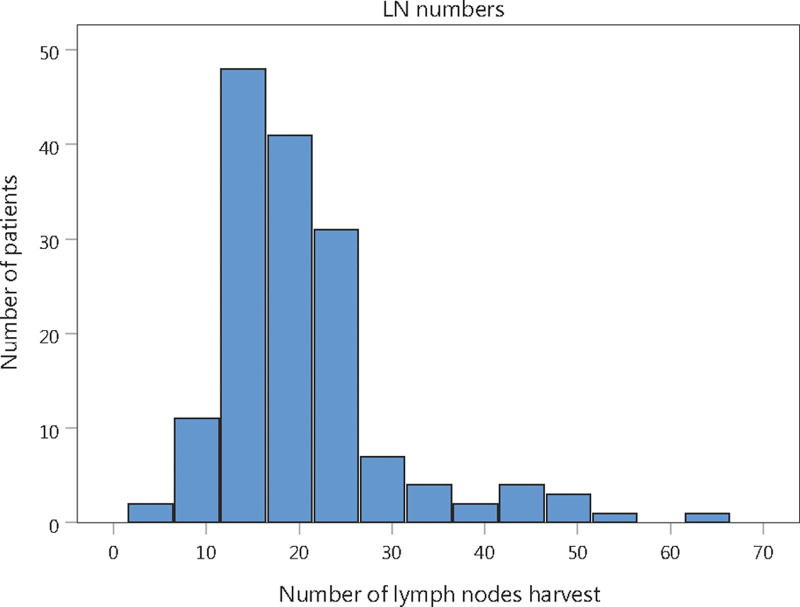
The number of harvested LNs ranged from 4 to 62, with a median of 18. (Fig. 1) Patients were categorized into 2 groups according to the number of LNH, with 142 (91.61%) patients having ≥ 12 LNH and 13 (8.39%) having < 12 LNH.
Figure 1.
Distribution of number of lymph nodes harvest.
The patients’ demographic and clinicopathologic characteristics, including age, sex, LNH number, operative urgency, preoperative CEA level, tumor location and size, pathological T stage, angiolymphatic invasion and perineural invasion, chemotherapy status, and tumor recurrence, are summarized in Table 1.
Table 1.
Patients’ demographic and clinicopathologic characteristics.
| Number of LNs harvest | |||
|---|---|---|---|
| Characteristics | ≥12 (n = 142)* | <12 (n = 13)* | P value |
| LNs number† | 21.22 (9.1) | 9.00 (2.1) | <.001 |
| Age† | 64.20 (13.8) | 62.62 (13.3) | .690 |
| Sex | .374 | ||
| Male | 88 (61.97) | 6 (46.15) | |
| Female | 54 (38.03) | 7 (53.85) | |
| T stage | .649 | ||
| T4a | 126 (88.73) | 11 (84.62) | |
| T4b | 16 (11.27) | 2 (15.38) | |
| Tumor location | .734 | ||
| Colon | 108 (76.06) | 11 (84.62) | |
| Rectum | 34 (23.94) | 2 (15.38) | |
| aTumor size† (mm) | 58.90 (23.8) | 53.69 (22.9) | .450 |
| Operative urgency | .512 | ||
| Emergency | 7 (4.93) | 1 (7.69) | |
| Elective | 135 (95.07) | 12 (92.31) | |
| Operative technique | >.999 | ||
| Laparoscopic surgery | 28(19.72) | 2(15.38) | |
| Open | 114(80.28) | 11(84.62) | |
| bPre-operative CEA level† (ng/mL) | 20.40 (55.9) | 28.28 (60.1) | .631 |
| aAngiolymphatic invasion | >.999 | ||
| Positive | 23 (16.31) | 2 (15.38) | |
| Negative | 118 (83.69) | 11 (84.62) | |
| aPerineural invasion | >.999 | ||
| Positive | 20 (14.18) | 1 (7.69) | |
| Negative | 121 (85.82) | 12 (92.31) | |
| Chemotherapy | .617 | ||
| Yes | 128 (90.14) | 13 (100) | |
| No | 14 (9.86) | 0 (0) | |
| Tumor recurrent | >.999 | ||
| Yes | 24 (16.9) | 2 (15.38) | |
| No | 118 (83.1) | 11 (84.62) | |
CEA = carcinoembryonic antigen, LNs = lymph nodes.
With percentages in parentheses unless indicated otherwise.
values are means (standard deviation).
One data point was not mentioned in the pathologic report in the group with LNs ≥ 12.
Sixteen patients in the group with LNs ≥ 12 lacked information on preoperative CEA level.
Of the 155 patients, 141 (90.97%) received chemotherapy; the remaining 14 (9.03%) patients did not receive chemotherapy due to patient and family refusal, high eastern cooperative oncology group score, or old age with multiple underlying diseases.
In the group with ≥ 12 LNH (n = 142), 24 (16.90%) had tumor recurrence, with 6 local recurrences and 18 distant metastases. Of the 13 patients with < 12 LNH, 2 had tumor recurrence, comprising 1 case of tumor seeding and 1 case of distant metastasis. Fisher’s exact test indicated no significant difference in the recurrence rate between the ≥ 12 LNH and < 12 LNH groups in stage IIB and IIC CRC.
Among the 2 groups, there were also no significant differences in age, sex, pathological T stage, tumor location, tumor size, preoperative CEA level, emergency surgery or elective surgery, laparoscopic or open surgery, angiolymphatic or perineural invasion, and receiving chemotherapy.
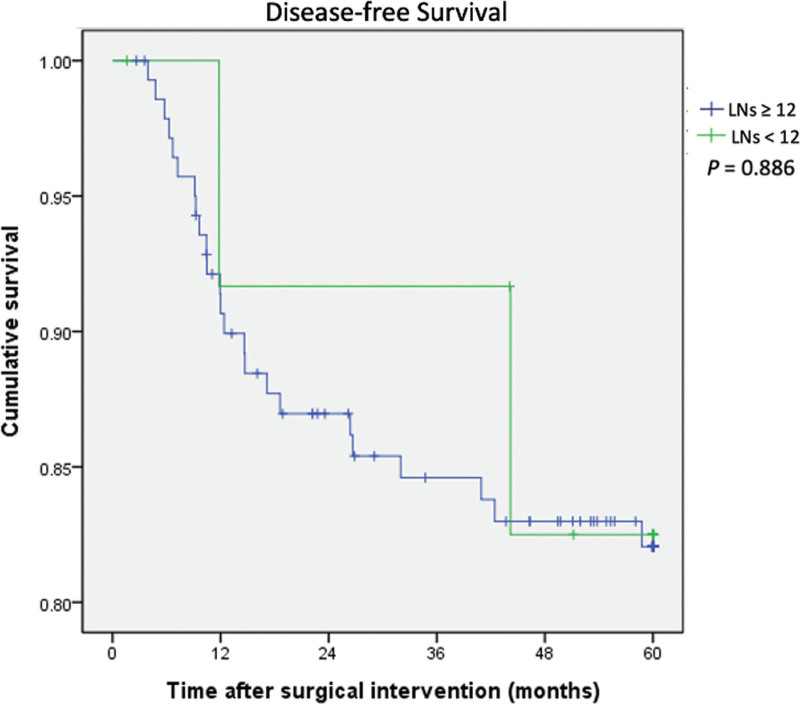
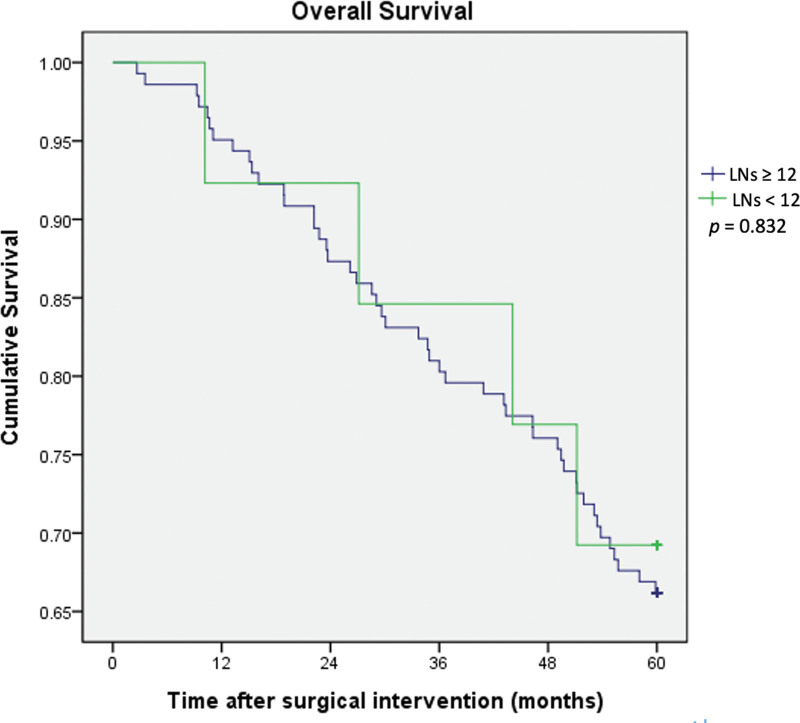
The DFS and OS rates between the 2 groups are shown in Figures 2 and 3, respectively. Kaplan-Meier analyses indicated that there were no statistical differences in 5-year DFS (P = .886) and 5-year OS (P = .832) between the adequate (LN ≥ 12) and inadequate (LN < 12) LNH groups. The mean follow-up time was 73.2 months, and the range of follow-up time was 2 to 141 months in the group of LNH ≥ 12. The mean follow-up time was 73.3 months, and the range of follow-up time was 10 to 118 months in the group of LNH < 12.
Figure 2.
Kaplan-Meier disease-free survival curves for colorectal cancer patients according to harvested lymph node number (lymph nodes number < 12 and ≥ 12).
Figure 3.
Kaplan-Meier overall survival curves for colorectal cancer patients according to harvested lymph node number (lymph nodes number < 12 and ≥ 12).
4. Discussion
Both inadequate LNH and T4 disease were risk factors for the recurrence of stage II CRC, and these 2 conditions were associated with a higher rate of receiving chemotherapy. Inadequate LNH may lead to inaccurate staging and different chemotherapy treatments.[15] In this study, we aimed to evaluate the impact of inadequate LNH numbers on stage IIB and IIC CRC.
Many studies have discussed the impact of inadequate LNH in stages I to III CRC, but few studies have demonstrated a relationship between inadequate LNH and T4 disease. In Taiwan, there were 2221 (16.87%) stage II colon cancers in a total of 13,169 patients, and 836 (12.92%) stage II rectal cancers in total of 6471 patients in 2019. In stage II CRC, there were 238 (10.72%) IIB and 141 (6.35%) IIC colon cancers and 55 (6.58%) IIB and 52 (6.22%) IIC rectal cancers.[17] In our study, we enrolled patients with stage IIB and IIC CRC between January 2010 and December 2016 in our hospital, comprising 119 colon cancers and 36 rectal cancers. Although we collected 7-year data, the total number of patients was still relatively small because stage IIB and IIC disease only accounted for a small proportion of patients with colon cancer.
Although there were no definite data of inadequate LNH in stage IIB and IIC in previous studies, insufficient numbers of LNH were not uncommon in the literature in stages I to III, ranging from 14.7% to 52%.3 to 8 In our study, the inadequate LNH rate was relatively low (8.39 %) because the number of LNH was 1 of the core indicators of CRC in our hospital. Furthermore, in our hospital, samples with insufficient LNH numbers would undergo a second examination, which can reduce the underestimation of stage III disease and the impact of inadequate LNH.
In the present study, the proportion of patients receiving emergent surgery was low in 1 (7.69%) of 13 patients and 7 (4.93%) of 142 patients in the LNH < 12 and LNH ≥ 12 groups, respectively. This was because some patients with obstruction who underwent diversion transverse colostomy or ileostomy 1st and had their primary tumor resected in the next operation were excluded from the emergency surgery group.
Previous published data have shown similar LNH numbers sampled between the surgical type of laparoscopic and open surgery,[18–20] and a similar result was also observed in our study. The mean LNH numbers were 20.38 and 19.40 in the open and laparoscopic surgery groups, respectively (P = .607). Klaver et al[21] concluded that laparoscopic surgery for T4a tumors might be safe but should be applied with caution for T4b colon cancer requiring multivisceral resection. Zhang et al[22] also suggested that laparoscopic multivisceral resection is safe and feasible for primary T4b colon cancer in select patients. In our present data, 30 of 155 patients underwent laparoscopic surgery, with 2 (15.38%) and 28 (19.72%) patients in the LNH < 12 and LNH ≥ 12 groups, respectively. All 30 patients had pathological T4a disease, and only 1 patient (LN ≥ 12) had recurrent lung metastasis.
Whether chemotherapy should be administered to patients with stage II CRC remains controversial. Although some studies suggest adjuvant chemotherapy for stage II disease with high-risk features,[15,23–25] there are still some opposing opinions.[26] In our study, approximately 90.97% of T4 disease patients received chemotherapy, with 90.14% and 100% in the adequate LNH and inadequate LNH groups, respectively (P = .617). A previous study presented benefits in the administration of adjuvant chemotherapy in T3N0 colon cancer with inadequate LNH,[25] but there is no such evidence for T4N0 CRC with inadequate LNH. In our study, there were no significant differences in OS and DFS (P = .832 and P = .886) in T4N0 patients between the adequate and inadequate LNH groups. We speculated that most T4 patients received adjuvant chemotherapy, which may decrease the influence of inadequate LNH.
In previous studies, the recurrence rate of stage II CRC has ranged from 13.3% to 25%.[27–29] Some studies have reported recurrence rates of T4N0 CRC ranging from 28% to 30.6%.[24,30] In our study, the recurrence rate of T4N0 disease was 16.77%, which was lower than that reported in the recent literature, and we speculate that the high adjuvant chemotherapy rate may have reduced the recurrence rate.
5. Limitations
Our study has several limitations. First, this was a single-hospital, retrospective study. Second, the sample size was relatively small. Although we enrolled patients with stage IIB and IIC CRC who underwent surgical resection between 2010 and 2016, only 155 patients were included. Furthermore, we had a low rate of inadequate LNH, which led to few patients in the LN < 12 subgroup. Third, the patients were not examined by a single operator, and the specimens were not examined by a single pathologist. Evans et al[31] concluded that LNH varied according to the reporting pathologist but not the operating surgeon. In addition, this study did not consider other possible risk factors, such as synchronous tumors, differentiation grade, comorbidities, specimen length, and pathologic assessment technique.
6. Conclusion
Despite these limitations, the results of this study revealed no significant differences in DFS and OS between patients with adequate (≥ 12) LNH and inadequate (< 12) LNH in T4N0 CRC patients.
Acknowledgments
The authors would like to thank Editage (www.editage.com.tw) for the English language review.
Author contributions
Conceptualization: Yi-Kai Kao, Hsin-Pao Chen, Chih-I Chen.
Data curation: Yi-Kai Kao.
Formal analysis: Yi-Kai Kao, Chih-I Chen.
Investigation: Ling-Chiao Song, Yi-Chieh Chen, Yu-Chun Lin.
Methodology: Yi-Kai Kao, Chih-I Chen.
Supervision: Hsin-Pao Chen, Kuang-Wen Liu.
Validation: Ling-Chiao Song, Yi-Chieh Chen, Yu-Chun Lin.
Visualization: Yi-Kai Kao, Chih-I Chen.
Writing – original draft: Yi-Kai Kao, Chih-I Chen.
Writing – review & editing: Yi-Kai Kao, Hsin-Pao Chen, Kuang-Wen Liu, Ling-Chiao Song, Yi-Chieh Chen, Yu-Chun Lin, Chih-I Chen.
Abbreviations:
- AJCC =
- American joint committee on cancer
- CEA =
- carcinoembryonic antigen
- CRC =
- colorectal cancer
- DFS =
- disease-free survival
- LN =
- Lymph node
- LNH =
- LN harvest
- OS =
- Overall survival
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Kao Y-K, Chen H-P, Liu K-W, Song L-C, Chen Y-C, Lin Y-C, Chen C-I. Impact on inadequate lymph node harvest on survival in T4N0 colorectal cancer: A would-be medical center experience in Taiwan. Medicine 2022;101:52(e32497).
Contributor Information
Yi-Kai Kao, Email: kevin.2512@hotmail.com.
Hsin-Pao Chen, Email: jimmyee0901@gmail.com.
Kuang-Wen Liu, Email: lkw1095@gmail.com.
Ling-Chiao Song, Email: kulairumi@gmail.com.
Yi-Chieh Chen, Email: jimmyee0901@gmail.com.
Yu-Chun Lin, Email: imklic5566@gmail.com.
References
- [1].Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [2].Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi64–72. [DOI] [PubMed] [Google Scholar]
- [3].Lee CHA, Wilkins S, Oliva K, et al. Role of lymph node yield and lymph node ratio in predicting outcomes in non-metastatic colorectal cancer. BJS Open. 2019;3:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu Q, Zhang Z, Chen Y, et al. Impact of inadequate number of lymph nodes examined on survival in stage II colon cancer. Front Oncol. 2021;11:736678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nathan H, Shore AD, Anders RA, et al. Variation in lymph node assessment after colon cancer resection: patient, surgeon, pathologist, or hospital? J Gastrointest Surg. 2011;15:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ahmadi O, Stringer MD, Black MA, et al. Clinico-pathological factors influencing lymph node yield in colorectal cancer and impact on survival: analysis of New Zealand Cancer Registry data. J Surg Oncol. 2015;111:451–8. [DOI] [PubMed] [Google Scholar]
- [7].Cai Y, Cheng G, Lu X, et al. The re-evaluation of optimal lymph node yield in stage II right-sided colon cancer: is a minimum of 12 lymph nodes adequate? Int J Colorectal Dis. 2020;35:623–31. [DOI] [PubMed] [Google Scholar]
- [8].Foo CC, Ku C, Wei R, et al. How does lymph node yield affect survival outcomes of stage I and II colon cancer? World J Surg Oncol. 2020;18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shinto E, Oki E, Shimokawa M, et al. A validation study for recurrence risk stratification of stage II colon cancer using the 55-gene classifier. Oncology. 2020;98:534–41. [DOI] [PubMed] [Google Scholar]
- [10].Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–16. [DOI] [PubMed] [Google Scholar]
- [11].Yamazaki K, Yamanaka T, Shiozawa M, et al. Oxaliplatin-based adjuvant chemotherapy duration (3 versus 6 months) for high-risk stage II colon cancer: the randomized phase III ACHIEVE-2 trial. Ann Oncol. 2021;32:77–84. [DOI] [PubMed] [Google Scholar]
- [12].Hajirawala LN, Yi Y, Herritt BC, et al. Multiple high-risk features for stage II colon carcinoma portends worse survival than stage III disease. Dis Colon Rectum. 2022:10.1097/DCR.0000000000002425. [DOI] [PubMed] [Google Scholar]
- [13].Kim HS, Kim KM, Lee SB, et al. Clinicopathological and biomolecular characteristics of stage IIB/IIC and stage IIIA colon cancer: Insight into the survival paradox. J Surg Oncol. 2019;120:423–30. [DOI] [PubMed] [Google Scholar]
- [14].Birkett RT, Chamely E, Concors SJ, et al. Overuse and limited benefit of chemotherapy for stage II colon cancer in young patients. Clin Colorectal Cancer. 2019;18:292–300. [DOI] [PubMed] [Google Scholar]
- [15].Zhang C, Yin S, Tan Y, et al. Patient selection for adjuvant chemotherapy in high-risk stage II colon cancer: a systematic review and meta-analysis. Am J Clin Oncol. 2020;43:279–87. [DOI] [PubMed] [Google Scholar]
- [16].Choi HK, Law WL, Poon JT. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer. 2010;10:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Cancer registry annual report. 2019. Taiwan. Available at: https://www.hpa.gov.tw/File/Attach/14913/File_18302.pdf. [access date December, 2021]. [Google Scholar]
- [18].Nelson H, Sargent DJ. Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–9. [DOI] [PubMed] [Google Scholar]
- [19].Tonini V, Birindelli A, Bianchini S, et al. Factors affecting the number of lymph nodes retrieved after colo-rectal cancer surgery: a prospective single-centre study. Surgeon. 2020;18:31–6. [DOI] [PubMed] [Google Scholar]
- [20].Ortega PM, Cienfuegos JA, Baixauli J, et al. Prognostic significance of lymph node count in high-risk node-negative colon carcinoma. Rev Esp Enferm Dig. 2020;112:609–14. [DOI] [PubMed] [Google Scholar]
- [21].Klaver CEL, Kappen TM, Borstlap WAA, et al. Laparoscopic surgery for T4 colon cancer: a systematic review and meta-analysis. Surg Endosc. 2017;31:4902–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang X, Wu Q, Gu C, et al. Comparison of short and long-time outcomes between laparoscopic and conventional open multivisceral resection for primary T4b colorectal cancer. Asian J Surg. 2019;42:401–8. [DOI] [PubMed] [Google Scholar]
- [23].Teufel A, Gerken M, Hartl J, et al. Benefit of adjuvant chemotherapy in patients with T4 UICC II colon cancer. BMC Cancer. 2015;15:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eom T, Lee Y, Kim J, et al. Prognostic factors affecting disease-free survival and overall survival in T4 colon cancer. Ann Coloproctol. 2021;37:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wells KO, Hawkins AT, Krishnamurthy DM, et al. Omission of adjuvant chemotherapy is associated with increased mortality in patients with T3N0 colon cancer with inadequate lymph node harvest. Dis Colon Rectum. 2017;60:15–21. [DOI] [PubMed] [Google Scholar]
- [26].Ejaz A, Casadaban L, Maker AV. Utilization and impact of adjuvant chemotherapy among patients with resected stage II colon cancer: a multi-institutional analysis. J Surg Res. 2017;215:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Watanabe T, Muro K, Ajioka Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sato H, Maeda K, Sugihara K, et al. High-risk stage II colon cancer after curative resection. J Surg Oncol. 2011;104:45–52. [DOI] [PubMed] [Google Scholar]
- [29].Fang SH, Efron JE, Berho ME, et al. Dilemma of stage II colon cancer and decision making for adjuvant chemotherapy. J Am Coll Surg. 2014;219:1056–69. [DOI] [PubMed] [Google Scholar]
- [30].Macari D, Kawak S, Raofi V, et al. Recurrence pattern and outcomes in T4 colon cancer: a single institution analysis. J Surg Oncol. 2020;121:337–41. [DOI] [PubMed] [Google Scholar]
- [31].Evans MD, Barton K, Rees A, et al. The impact of surgeon and pathologist on lymph node retrieval in colorectal cancer and its impact on survival for patients with Dukes’ stage B disease. Colorectal Dis. 2008;10:157–64. [DOI] [PubMed] [Google Scholar]