Abstract

Ashish Singh
Background Regarding gallbladder cancer (GBC) there is conflicting evidence in the literature whether retroperitoneal lymph nodal metastases (RLNM) should be considered as regional nodal metastasis or as distant metastasis (DM) and the jury is out on radical curative surgery in presence of RLNM. This is an analysis of GBC patients, to see the effect of RLNM on survival and to compare with that of patients with DMs.
Methods A retrospective analysis of a prospective database of patients of GBC with RLNM (interaortocaval and paraaortic) or DM on frozen section biopsy at surgery, between January 2013 and December 2018. Data was analyzed using the Statistical Package for the Social Sciences software (version 22.0). Survival in these two groups (RLNM and DM) was compared with log-rank test. A p -value of < 0.05 was considered significant.
Results A total of 235 patients with ostensibly resectable GBC underwent surgical exploration. The planned curative resection was abandoned in 91 (39%) patients because of RLNM ( n = 20, 9%) or DM ( n = 71, 30%) on frozen section biopsy. Demographic profile and blood parameters were similar. The median survival for RLNM and DM groups were 5 (range 2–26) and 6 (range 2–24) months, respectively, with no significant difference on log-rank test ( p = 0.64). There was no 3-year or longer survivor in either group.
Conclusion Due to similar poor survival in presence of RLNM or DM, RLNM should be considered as the equivalent of DM. This study strengthens evidence to avoid curative surgery in patients with RLNM. These lymph nodes should be sampled preoperatively, if suspicious on imaging, for fine-needle aspiration cytology and at surgery, as a routine for frozen section histological examination before initiating curative resection to avert a futile exercise.
Keywords: gallbladder cancer, retroperitoneal lymph node metastasis, interaortocaval lymph node metastasis, para-aortic lymph-node metastasis, distant metastasis
Introduction
In gallbladder cancer (GBC), there is conflicting evidence in the literature whether retroperitoneal lymph nodal metastases (RLNM) should be considered as regional nodal metastases or as distant metastases (DMs) and the jury is out on radical curative surgery in presence of RLNM. This is an analysis of GBC patients, to see the effect of RLNM on survival and to compare with that of patients with DMs, where curative surgery was abandoned due to RLNM or DM, found intraoperatively.
Material and Methods
This study was performed at the department of surgical gastroenterology at a tertiary care center in North India where GBC is rife. All patients of GBC, where curative resection was abandoned between January 2013 and December 2018, on account of positive retroperitoneal lymph node (RLN) or DM on frozen section biopsy, were studied for survival. The study design is depicted in Fig. 1 .
Fig. 1.
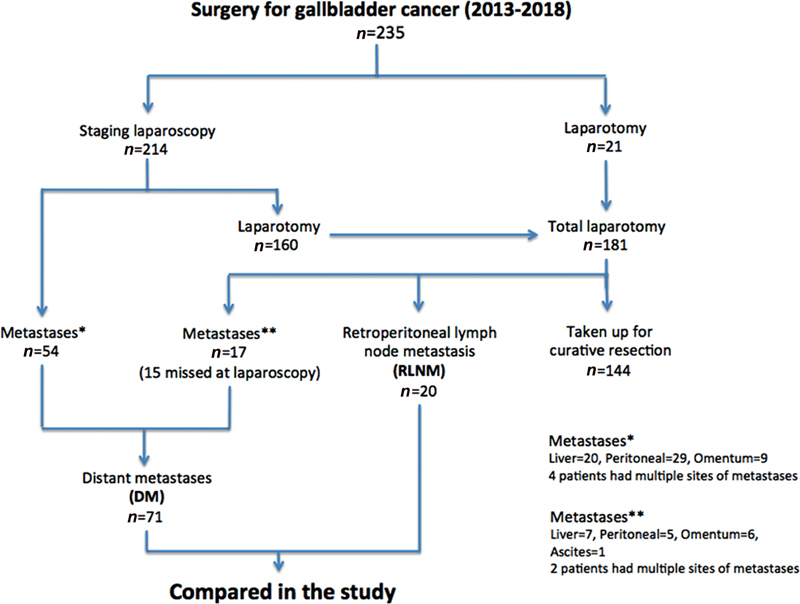
Study design.
All patients were staged with a triple-phase computed tomography (CT) scan. In case of locally advanced disease where major hepatectomy or hepaticopancreaticoduodenectomy was contemplated, neoadjuvant treatment was instituted after a staging laparoscopy (SL). Patients with obstructive jaundice underwent preoperative or preneoadjuvant treatment biliary drainage. Those who were nonmetastatic and possibly resectable on imaging were considered for curative surgery. Most of the patients underwent SL. In case a liver, peritoneal, or omental nodule was seen on SL, the lesion was biopsied and sent for frozen section examination. In the absence of dissemination (liver, peritoneal, omental nodule, or ascites) on SL, the findings were confirmed at laparotomy when the RLNs were sampled for frozen section biopsy. The planned curative resection was abandoned if the biopsy report suggested metastatic disease and the patients were offered palliative care. The study population was divided into two groups based on the site of metastatic disease—RLNM and DM, and outcome was compared. Demographic profile, preoperative blood parameters, neoadjuvant treatment, biliary drainage, and postoperative palliative treatment were recorded. Survival was calculated from the day of surgery. Patients were followed up through hospital visits and telephonically.
Data was analyzed using the Statistical Package for the Social Sciences software (version 22.0). Continuous variables were compared with independent t -test and categorical variables were compared with chi-square test. A p -value of < 0.05 was considered significant. In case of skewed variables, that is, bilirubin and survival, median and interquartile range (IQR) were used. Survival among both the groups (RLNM and DM) was compared with log-rank test.
Results
A total of 235 patients with ostensibly resectable GBC underwent surgical exploration between January 2013 and December 2018. The planned curative resection was executed in 144 (61%) patients and abandoned in 91 (39%) patients because of RLNM ( n = 20, 9%) or DM ( n = 71, 30%) on frozen section biopsy. Demographic profile including age, gender, comorbidities, preoperative clinical features, blood parameters (hemoglobin, bilirubin, albumin, and international normalized ratio), requirement of biliary drainage, or neoadjuvant treatment were comparable between the two groups as shown in Table 1 . SL was performed in 214 out of 235 patients (21 patients underwent laparotomy without SL for techno-logistical reasons). In the 71 patients, where the curative resection was aborted, metastases were detected on laparoscopy in 54 patients and at laparotomy in 17 patients (15 of these were missed at SL, while 2 were found in patients who did not undergo a prelaparotomy SL). Metastases missed at laparoscopy were peritoneal ( n = 6), omental ( n = 5), liver ( n = 3), and gastric serosal nodule ( n = 1). Overall, SL changed the management in 54/214 (25%) patients by averting a laparotomy and abandoning curative resection. The RLNM included interaortocaval nodes ( n = 18) and paraaortic nodes ( n = 2) in patients without any DM. The DM group consisted of liver nodules ( n = 27), peritoneal nodules ( n = 34), omental metastasis ( n = 15), and malignant ascites on fluid cytology ( n = 1). Six patients had polymetastatic disease.
Table 1. Comparison between retroperitoneal lymph node metastasis (RLNM) and distant metastasis (DM) groups.
| Parameters | RLNM ( n = 20) | DM ( n = 71) | p -Value |
|---|---|---|---|
| Age in years, mean ± SD [range] | 53.6 ± 8.5 [35–71] | 51.2 ± 10.8 [30–80] | 0.14 |
| Sex, M:F | 7:13 | 26:45 | 1.0 |
| BMI in kg/m 2 , mean ± SD [range] | 23.5 ± 3.9 [17.7–32.3] | 22.4 ± 3.6 [18.5–40.2] | 0.29 |
| Comorbidity, n (%) | 8 (40) | 20 (28.1) | 0.14 |
| Diabetes, n (%) | 6 (30) | 9 (12.6) | 0.08 |
| Hypertension, n (%) | 4 (20) | 10 (14) | 0.5 |
| Coronary artery disease (CAD), n (%) | 0 | 3 (4.2) | 1.0 |
| Jaundice, n (%) | 4 (20) | 20 (28.1) | 0.57 |
| LOA, n (%) | 11 (55) | 38 (53.5) | 0.55 |
| LOW, n (%) | 11 (55) | 39 (54.9) | 1.0 |
| Incidental, n (%) | 1 (5) | 12 (16.9) | 0.28 |
| Node involvement (N + ) on preoperative images (CECT/USG), n (%) | 17 (85) | 52 (73.2) | 0.38 |
| Hemoglobin in g/dL mean ± SD | 11.3 ± 1.4 | 11.5 ± 1.8 | 0.29 |
| Bilirubin in g/dL median (IQR) | 0.8 (0.45–1.4) | 0.8 (0.5–1.5) | 0.07 |
| Albumin in g/dL mean ± SD | 3.9 ± 0.57 | 3.9 ± 0.51 | 0.6 |
| INR mean ± SD | 1.01 ± 0.13 | 1.03 ± 0.11 | 0.74 |
| Preoperative biliary drainage, n (%) | 4 (20) | 11 (15.4) | 0.73 |
| Neoadjuvant treatment, n (%) | 2 (10) | 3 (4.2) | 0.3 |
| Palliative treatment, n (%) | 10 (50) | 16 (22.5) | 0.04 |
| Survival in months median (IQR) [range] | 5 (3–11) [2–26] | 6 (4–10) [2–24] | 0.08 |
| Six-month survival, n (%) | 9 (45) | 37 (52.1) | 0.62 |
| 1-year survival, n (%) | 4 (20) | 13 (18.3) | 1.0 |
| 2-year survival, n (%) | 2 (10) | 1 (1.4) | 0.12 |
Abbreviations: BMI, body mass index; CECT, contrast-enhanced computed tomography; INR, international normalized ratio; IQR, interquartile range; LOA, loss of appetite; LOW, loss of weight; SD, standard deviation; USG, ultrasonography.
Note that 50% patients in the RLNM group received palliative treatment as compared with 22% in the DM group; the difference was statistically significant ( p = 0.04). In majority of the cases, the patients opted against chemotherapy either due to logistic issues or the nihilism associated with disseminated disease. All patients were followed up. The median survival for RLNM and DM groups were 5 months (range 2–26; IQR 3–11) and 6 months (range 2–24; IQR 4–10), respectively, without any significant difference on log-rank test ( p -value = 0.64) ( Fig. 2 ).
Fig. 2.
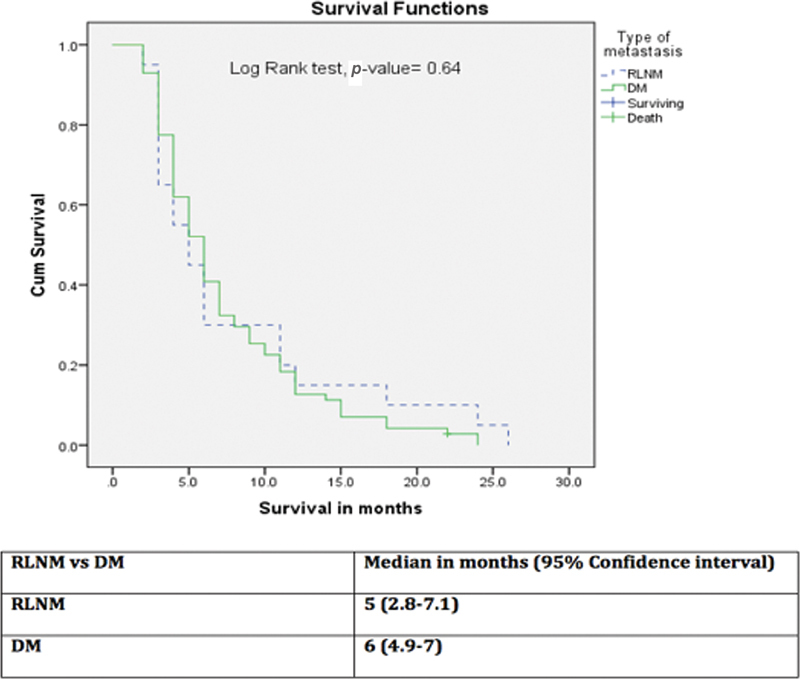
Comparison of survival between retroperitoneal lymph node metastasis (RLNM) and distant metastasis (DM) groups.
There was no 3-year survivor in either group. In the studied population, only one patient with liver metastasis and stable disease is still alive (18 months postsurgery), with no evidence of disease progression after 6 cycles of chemotherapy.
When survival was compared between the RLNM and DM groups receiving palliative chemotherapy, there was no significant survival difference (median 11 months in both the groups and on log-rank test p -value = 0.89) ( Fig. 3 ).
Fig. 3.
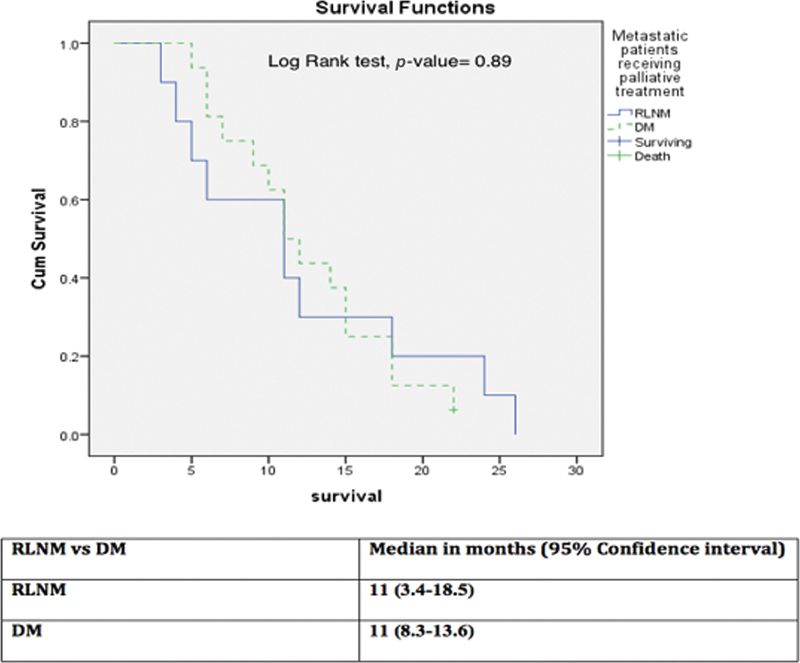
Survival comparison among retroperitoneal lymph node metastasis (RLNM) and distant metastasis (DM) groups who received palliative chemotherapy.
Discussion
GBC is the most common malignancy in the biliary tract. 1 In GBC lymph node involvement is associated with poor survival. 2 3 4 The extent of lymphadenectomy and the level of lymph node involvement which precludes curative surgery is still a matter of controversy. A study by Murakami et al on the survival of patients with paraaortic lymph node ( n = 17) metastasis in resected biliary carcinoma documented absence of any survival advantage. 5 Similarly, other authors have also documented poor outcomes in the presence of RLNM in GBC akin to DM. 3 4 6 7 8 9 On the contrary, Nishio et al have shown that in presence of RLN involvement, the survival is better after resection than nonoperated similar patients with RLNM ( p -value 0.014). 10 There are also few case reports showing anecdotal long-term survival in the presence of distant lymph node metastasis. 11 12 To the best of our knowledge, this report is the largest reported experience on survival of GBC patients with RLNM. In this population, there was no significant difference in survival among both the groups indicating that the prognosis of the RLNM group was as poor as of the DM group. All patients were counseled about the disease stage and prognosis and they were offered palliative chemotherapy. In the subgroup of patients receiving palliative chemotherapy again the survival was similar for patients with RLNM or DM. Our study validates poor prognosis of the RLNM, as with the DM groups.
Because of poor prognosis, RLNM should actively be sought for in the preoperative imaging, so as to avoid unnecessary laparotomy. Presently, imaging modalities are less accurate in evaluation of RLNM unless the nodes are large in size with obvious signs of involvement. CT criteria for evaluation for metastatic lymph node 13 have been found to have poor sensitivity (14.7%) and positive predictive value (33.3%), 14 similarly positron emission tomography scan also has limited role. 15 If an enlarged RLN is suspected to be involved at imaging, it should be target for fine-needle aspiration cytology (FNAC) under ultrasound, CT, or endoscopic ultrasound guidance. But the preoperative sampling has limitations because of difficult location and sampling error leading to a false negative rate of almost 30%. 14 Hence, in all cases of GBC, retroperitoneal (interaortocaval and paraaortic) lymph nodes should be sampled as a routine for frozen section histological examination before starting the curative resection to avert a futile extensive surgery.
Conclusion
GBC patients with RLNM have poor survival similar to DMs and should be considered as the equivalent of DM. This study strengthens evidence to avoid curative resectional surgery in patients with RLNM. These lymph nodes should be sampled, preoperatively if suspicious on imaging for FNAC and at surgery, as a routine for frozen section histological examination before initiating curative resection to avert a futile exercise.
Funding Statement
Funding No funds procured for this study.
Conflict of Interest All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Authors' Contributions
Conception and design: A.S., N.K.G., R.S., V.K.K.
Administrative support: V.K.K., R.S.
Provision of study materials or patients: R.S., V.K.K.
Collection and assembly of data: All authors.
Data analysis and interpretation: A.S., N.K.G., Rahul.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical Approval
It was a retrospective analysis of prospective database so no ethical clearance was sought.
References
- 1.American Cancer Society . Atlanta, GA: American Cancer Society; 2016. Cancer Facts & Figures. [Google Scholar]
- 2.Rückert J C, Rückert R I, Gellert K, Hecker K, Müller J M. Surgery for carcinoma of the gallbladder. Hepatogastroenterology. 1996;43(09):527–533. [PubMed] [Google Scholar]
- 3.Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer. 1997;79(05):892–899. doi: 10.1002/(sici)1097-0142(19970301)79:5<892::aid-cncr4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Chijiiwa K, Kai M, Nagano M, Hiyoshi M, Ohuchida J, Kondo K. Outcome of radical surgery for stage IV gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2007;14(04):345–350. doi: 10.1007/s00534-006-1186-1. [DOI] [PubMed] [Google Scholar]
- 5.Murakami Y, Uemura K, Sudo T. Is para-aortic lymph node metastasis a contraindication for radical resection in biliary carcinoma? World J Surg. 2011;35(05):1085–1093. doi: 10.1007/s00268-011-1036-4. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa T, Horimi T, Shima Y. Evaluation of aggressive surgical treatment for advanced carcinoma of the gallbladder. J Hepatobiliary Pancreat Surg. 2003;10(03):233–238. doi: 10.1007/s00534-003-0848-5. [DOI] [PubMed] [Google Scholar]
- 7.Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87(04):418–422. doi: 10.1046/j.1365-2168.2000.01384.x. [DOI] [PubMed] [Google Scholar]
- 8.Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Extensive surgery for carcinoma of the gallbladder. Br J Surg. 2002;89(02):179–184. doi: 10.1046/j.0007-1323.2001.02001.x. [DOI] [PubMed] [Google Scholar]
- 9.Kondo S, Nimura Y, Kamiya J. Five-year survivors after aggressive surgery for stage IV gallbladder cancer. J Hepatobiliary Pancreat Surg. 2001;8(06):511–517. doi: 10.1007/s005340100018. [DOI] [PubMed] [Google Scholar]
- 10.Nishio H, Nagino M, Ebata T, Yokoyama Y, Igami T, Nimura Y. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J Hepatobiliary Pancreat Surg. 2007;14(04):351–357. doi: 10.1007/s00534-006-1187-0. [DOI] [PubMed] [Google Scholar]
- 11.Amemiya T, Yokoyama Y, Oda K. A patient with gallbladder cancer with paraaortic lymph node and hepatic metastases who has survived for more than 13 years after the primary extended radical operation. J Hepatobiliary Pancreat Surg. 2008;15(06):648–651. doi: 10.1007/s00534-007-1316-4. [DOI] [PubMed] [Google Scholar]
- 12.Shinkai H, Kimura W, Sata N, Muto T, Nagai H. A case of gallbladder cancer with para-aortic lymph node metastasis who has survived more than seven years after the primary extended radical operation. Hepatogastroenterology. 1996;43(11):1370–1376. [PubMed] [Google Scholar]
- 13.Ohtani T, Shirai Y, Tsukada K, Muto T, Hatakeyama K. Spread of gallbladder carcinoma: CT evaluation with pathologic correlation. Abdom Imaging. 1996;21(03):195–201. doi: 10.1007/s002619900045. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal A K, Kalayarasan R, Javed A, Sakhuja P. Role of routine 16b1 lymph node biopsy in the management of gallbladder cancer: an analysis. HPB (Oxford) 2014;16(03):229–234. doi: 10.1111/hpb.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluge R, Schmidt F, Caca K. Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33(05):1029–1035. doi: 10.1053/jhep.2001.23912. [DOI] [PubMed] [Google Scholar]


