Abstract
Background
There is accumulating evidence that the lymphocyte-to-monocyte ratio (LMR) is related to the outcomes of cancer patients treated with immune checkpoint inhibitors (ICIs). However, the results remain controversial.
Method
Electronic databases were searched to retrieve the studies that explore the relationship between LMR and the efficacy of ICIs. The primary endpoints were overall survival (OS) and progression-free survival (PFS), evaluated by the hazard ratios (HRs) with 95% confidence intervals (CI), and the secondary endpoints included disease control rate (DCR) and immune-related adverse events (irAEs), assessed by the odd ratios (ORs) with 95% CI.
Results
A total of 27 studies involving 4,322 patients were eligible for analysis. The results indicated that increased LMR at baseline was associated with a superior OS (HR: 0.46, 95% CI: 0.39-0.56, p < 0.001), PFS (HR: 0.60, 95% CI: 0.49-0.74, p < 0.001), and DCR (OR: 3.16, 95% CI: 1.70-5.87, p < 0.001). Posttreatment LMR was linked to a better PFS (HR: 0.46, 95% CI: 0.29-0.71, p = 0.001), but failed to show this correlation in the analysis of OS and DCR. No correlation existed between LMR and irAEs regardless of the testing time (baseline or posttreatment). Subgroup analyses focusing on baseline LMR revealed that higher baseline LMR possessed a better OS in renal cell cancer (RCC) arm, nonsmall cell lung cancer (NSCLC) arm, multiple cancer arm, monotherapy arm, LMR <2 arm, LMR ≥2 arm, western countries arm, eastern countries arm, and anti-PD-1 arm. Higher baseline LMR correlated with better PFS in RCC arm, NSCLC arm, gastric cancer (GC) arm, multiple cancer arm, LMR <2 arm, LMR ≥2 arm, western countries arm, and eastern countries arm.
Conclusions
Higher LMR at baseline was positively correlated with a superior OS, PFS, and DCR for ICIs, but not with irAEs.
1. Introduction
Cancer immunotherapy has made great strides with the advancement of multiple forms of treatment, including immune checkpoint inhibitors (ICIs), oncolytic virus therapies, cancer vaccines, cytokine therapies, and adoptive cell transfer [1, 2]. Impressively, some incurable tumors with poor prognoses, such as metastatic melanoma and nonsmall cell lung cancer (NSCLC), have been recognized as sensitive to immunotherapy, and therefore have acquired a long-term maintenance of remission [3]. ICIs, which stimulate the host immune system to eliminate cancer cells by inhibiting the immune checkpoint pathway, are the most representative agents [4–6]. However, only a proportion of patients achieved a clinically desirable efficacy, and due to the high price and potential severe immune-related adverse events (irAEs) of ICIs, seeking for effective biomarkers to predict better respond to ICIs remains the current challenge in clinical practice [7–9].
Biomarkers identification is an important area in the diagnosis and management of malignant tumors. During the past decades, evidence-based meta-analyses have increased exponentially, which enrich our understanding of particular associations and trends in contemporary literature by improving statistical power and reducing outlier studies [10]. At the same time, there is a growing need to develop fast and easily accessible biospecimens, such as blood and urine, and corresponding biomarkers among clinical communities [11, 12]. So far, mismatch repair deficiency (MMR), programmed cell death-ligand 1 (PD-L1), tumor mutational burden (TMB), and gut microbiota (GM) features [8, 13–15] have been regarded as the best available biomarkers to predict the efficacy of ICIs, but they are confronted with some limitations, including high cost, obstacles in obtaining tissue samples, and lack of robust prognostic accuracy. Thus, there is an urgent need to identify novel biomarkers to precisely predict the therapeutic effects of ICIs.
Tumor associated inflammation is one of the hallmarks of cancer that enables tumorigenesis, angiogenesis, and tumor progression [16, 17]. Epidemiological researches have manifested that about a quarter of human cancers are associated with chronic inflammation [18]. Neutrophils involve in both innate and adaptive immune response and promote the tumor growth by secreting tumor growth factors that assist invasion and metastasis and promote angiogenesis [19, 20]. Monocytes participate in and prompt the process of inflammation by differentiating into either dendritic cells or tissue macrophages within tissue microenvironment [21]. T lymphocytes can recognize and kill tumor cells and correlate with a favorable clinical prognosis in several human tumors [22]. Thus, the blood-derived parameters, which can indicate systemic inflammatory responses, have proved to be related with the survival of cancer patients. Among these markers, neutrophil-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-lymphocyte ratio (PLR), and systemic immune-inflammatory (SII) are intensively investigated, and a wealth of studies have demonstrated the significant association between these biomarkers and survival in malignant tumors. For instance, higher NLR and PLR, and lower LMR indicate a poor prognosis in lung cancer, colorectal cancer, renal cell carcinoma, melanoma, and so on [23–29]. In addition, blood-derived parameters can be easily utilized in routine work. Therefore, study on whether there is association between peripheral blood biomarkers and clinical outcomes of ICIs is on the agenda.
Recently, several meta-analyses have been published focusing on the relationship between NLR or PLR and the efficacy of ICIs, but to our knowledge, only one on LMR, which recruited limited four studies in nonsmall cell lung cancer with endpoints of only overall survival (OS) and progression-free survival (PFS) [30]. Since previous studies yielded controversial conclusions regarding the association between LMR and the efficacy of ICIs, we conducted an updated and comprehensive investigation which recruited 27 studies reporting the endpoints of OS, PFS, disease control rate (DCR), and irAEs and performed detailed subgroup analyses based on the testing time of LMR (baseline or posttreatment), cancer types, combination medication, LMR cut-off, study region, and types of ICIs.
2. Materials and Methods
2.1. Search Strategy
This meta-analysis was designed and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist. PubMed, Cochrane Library, and EMBASE were searched for eligible studies up to September 4, 2022. The search strategy based on the following key words: “immune checkpoint inhibitor”, “ICIs”, “immune checkpoint blocker”, “PD-L1 inhibitor”, “PD 1 inhibitor”, “programmed cell death protein 1 inhibitor”, “programmed death ligand 1 inhibitor”, “cytotoxic T lymphocyte associated protein 4 inhibitor”, “CTLA-4 inhibitor”, “pembrolizumab”, “nivolumab”, “tremelimumab”, “avelumab”, “toripalimab”, “envafolimab”, “sintilimab”, “camrelizumab”, “cemiplimab”, “tislelizumab”, “cetrelimab”, “pidilizumab”, “triprizumab”, “atezolizumab”, “durvalumab”, “ipilimumab”, “monocyte”, “lymphocyte”, “monocyte to lymphocyte ratio (MLR)”, and “lymphocyte to monocyte ratio (LMR)”, and articles were limited to English-language publications. If the title and abstract failed to provide enough information, a full text evaluation was conducted. In addition, the references list of all related articles were manually reviewed to identify potential relevant studies. Reviews, meta-analysis, case reports, comments, and conference abstracts without original data were excluded.
2.2. Study Selection
Two independent investigators individually screened the titles and abstracts, and full-text articles were obtained and evaluated to acquire eligible researches. Inclusion criteria were as follows: (1) patients were pathologically diagnosed as solid malignant tumors; (2) ICI agents were administered alone or in combination; (3) therapeutic outcomes (OS, PFS, and DCR) were determined by RECIST criteria, or the association between LMR and irAEs were evaluated; (4) a hazard ratio (HR) and/or an odds ratio (OR) with 95% confidence interval (CI) could be extracted or calculated from the literature; (5) patients were assigned into high or low LMR groups by cutoff value; and (6) articles were published in full texts.
2.3. Data Extraction
The Newcastle-Ottawa Quality Assessment Scale (NOS) was adopted to evaluate the quality of researches, and those scoring five or more stars were considered of medium to high quality. Studies were screened and evaluated by two independent investigators according to inclusion criteria. Any disagreement were settled by consultation. Data extracted were the first author's name, publication year, country, study type, tumor type, sample size, line of therapy, type of ICIs, combined medication, the testing time, and cut-off of LMR, age, HRs with 95% CI of OS and PFS, ORs with 95% CI of DCR and irAEs.
2.4. Statistical Analysis
The primary endpoints were OS and PFS and the secondary endpoints were DCR and irAEs. The pooled HRs/ORs with 95% CI were evaluated to identify the association between LMR and the efficacy or adverse events of ICIs. Results relating to MLR was converted into the form of LMR. The median value of LMR was used as the cut-off value. Statistical analyses were conducted with Stata version 12. Heterogeneity among recruited studies was checked by I2 tests: I2 > 50% or P < 0.1 means substantial heterogeneity and a random-effects model was used; otherwise, a fixed-effects model was applied. A statistically significant difference was set as p <0.05. Funnel plot and Egger's test were performed to assess the publication bias. Sensitivity analyses were carried out by excluding one article each time to verify the reliability of our results.
3. Result
3.1. Study Characteristics
A total of 996 articles were retrieved from the PubMed, EMBASE, and Cochrane Library. After removing duplicates, 950 studies were left. By examining titles and abstracts, 887 were excluded due to non-ICIs or non-LMR studies, nonhuman studies, nonmalignant tumors, reviews, comments, case reports, meta-analyses, and conference abstracts without original data; consequently, 63 articles were identified for further study. Through full-text review of these literature, 36 were disregarded due to not meeting the inclusion criteria or low quality (NOS < 5), and 27 studies incorporating 4,322 patients were finally identified as eligible for this meta-analysis (Figure 1).
Figure 1.
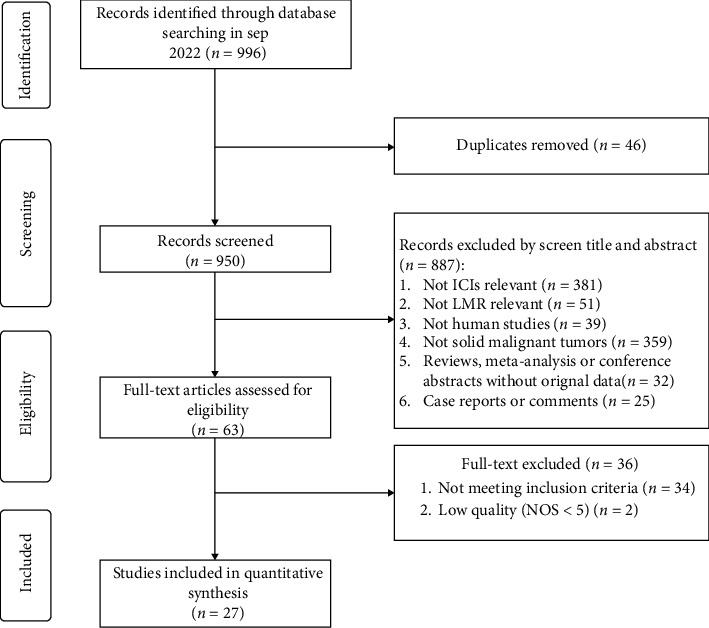
Flowchart of study selection procedure.
All studies were retrospective and were published between 2017 and 2022. Of these studies, 11 were conducted in China, 10 in Japan, 2 in Italy, 2 in USA, 1 in Korea, and 1 in Spain. 7 on NSCLC, 3 on gastric cancer (GC), 2 on hepatocellular carcinoma (HCC), 3 on renal cell cancer (RCC), 1 on small cell lung cancer (SCC), 1 on melanoma, 1 on esophageal cancer (EC), 1 on biliary tract cancer (BTC), 2 on lung cancer (LC), 1 on urothelial carcinoma (UC), and 5 on two or more types of solid tumors. Meanwhile, all the patients treated with ICIs: anti-PD-1/PD-L1 (Pembrolizumab, Nivolumab, Atezolizumab, Sintilimab, Camrelizumab, Triprizumab, and Toripalimab) or anti-CTLA-4; 24 studies measured the LMR at baseline and 5 studies measured LMR after treatment, with 2 evaluated LMR at both baseline and posttreatment; 21 trails had OS, 15 trails PFS, 8 trails DCR, and 6 trails irAEs. Characteristics of these studies enrolled are listed in Table 1.
Table 1.
The characteristics and further details of recruited studies in this meta-analysis.
(a).
| Author | Year | Country | Study type | Cancer type | Sample (H/L) | Male | ICIs agents | Line of therapy | Combined medication | Testing time | LMR cut-off | Outcome | Age | Quality evaluation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Man [64] | 2022 | China | R | HCC | 160 (61/99) | 129 | Pembro/Nivo/+Lenva; Sinti/Camre +lenva/Sora | 1st-line | Combined therapy | Baseline | 3.6 | OS, PFS | 58 (26-86) | 7 |
| Takashi [65] | 2022 | Japan | R | UC | 149 | 126 | Pembro | 2nd-line or later | NA | Baseline | 1.7 | OS | 72 (67–77) | 6 |
| Eisuke [66] | 2022 | Japan | R | Multiple | 61 (32/29) | 49 | Pembro/Nivo/Nivo followed by Pembro | 2nd-line or later | Monotherapy, combined therapy | Baseline | 1.8 or 2.6 | DCR | 71 (46-86) | 6 |
| Xueping [67] | 2022 | China | R | LC | 125 (69/56) | 90 | Nivo, Pembro, Atezo | NA | Monotherapy, combined therapy | Baseline | 2.3 | OS | 55 | 6 |
| Kosuke [68] | 2022 | Japan | R | RCC | 38 (25/13) | 30 | Nivo | 2nd-line or later | Monotherapy | Baseline | 3.3 | OS, PFS | 68 (44–78) | 5 |
| Chan [69] | 2022 | Korea | R | BTC | 68(29/39) | NA | Pembro | 2nd-line or later | Monotherapy | Baseline | 2.5 | OS | 66 | 6 |
| Hiroyuki [70] | 2022 | Japan | R | EC | 41(20/21) | 34 | Nivo | 2nd-line or later | Monotherapy | Baseline | 2.2 | OS, PFS, DCR, irAEs | 68 (51-81) | 6 |
| Rui [31] | 2022 | China | R | HCC | 110 (79/31) | 100 | Anti-PD-1 | NA | Combined therapy | Posttreatment | 1.8 | OS, PFS | 55 (31-84) | 6 |
| Xue [71] | 2022 | China | R | Multiple | 1,047 | 713 | Anti-PD-(L)1 | 1st-line or later | Monotherapy, combined therapy | Baseline | 3.4 | irAEs | 60 | 6 |
| Jia [72] | 2022 | China | R | NSCLC | 85(63/22) | 62 | Pembro/Nivo/Sinti/Toripa +/- chemo +/-antiangiogenesis | 1st-line or later | Monotherapy, combined therapy | Baseline | 1.8 | OS, DCR | 66 (47-80) | 7 |
| Zhenzhen [73] | 2022 | China | R | NSCLC, SCLC | 66 | 47 | Anti-PD-(L)1 | 1st-line or later | NA | Baseline | 2.1 | PFS | NA | 5 |
| Amparo [74] | 2021 | Spain | R | NSCLC | 51(27/24) | 37 | Pembro | 1st-line | Monotherapy | Baseline | 1.9 | OS, PFS | 66 (46–85) | 8 |
| Shigeo [75] | 2021 | Japan | R | GC | 51(25/26) | NA | Nivo | 2nd-line or later | Monotherapy | Posttreatment | 3.3 | OS, DCR | 69 (40–84) | 8 |
| Yang [76] | 2021 | China | R | GC | 139 (71/68) | 103 | Anti-PD-(L)1+ chemo/anti-VEGF/anti-HER/anti-CTLA-4 | 1st-line or later | Monotherapy, combined therapy | Baseline, posttreatment | 3.5 | OS, PFS, DCR | 60 (51–67) | 7 |
| Saeka [77] | 2021 | Japan | R | NSCLC | 171 | 113 | Nivo | 2nd-line or later | Monotherapy | Posttreatment | 2.2 | irAEs | 64 (56–69) | 6 |
| Sara [78] | 2021 | Italy | R | RCC | 571 | 402 | Nivo | 2nd-line or late | Monotherapy | Baseline | 2.6 | OS, PFS, DCR | 61 (49–73) | 8 |
| Despina [79] | 2021 | USA | R | Multiple | 390 | 275 | Anti-PD-(L)1, anti-CTLA-4 or combination | NA | Monotherapy, combined therapy | Baseline | 1.4 | OS, irAEs | 65 | 6 |
| Wei [80] | 2021 | China | R | SCLC | 53 | 34 | Atezo + chemo +/- trilaciclib | 1st-line | Combined therapy | Baseline | 2.7 | OS | NA | 6 |
| Saeka [81] | 2021 | Japan | R | NSCLC | 92 | 64 | Pembro | 1st-line or later | Monotherapy | Baseline | 1.5 | irAEs | 60 (34–85) | 6 |
| Xiaona [82] | 2021 | China | R | Multiple | 111 (61/50) | 56 | Anti-PD-1 | 1st-line or late | NA | Baseline | 3.2 | OS, PFS, DCR, irAEs | NA | 7 |
| Haiping [83] | 2021 | China | R | Multiple | 207 | 156 | Sinti+/- chemo | 1st-, 2nd-, or 3rd-line | Monotherapy, combined therapy | Baseline | 2.8 | OS, PFS | 56 (24-72) | 9 |
| DanYun [84] | 2021 | China | R | GC | 53(21/32) | NA | Toripa | 2nd-line or later | Monotherapy | Baseline | 2.8 | OS, PFS | 60 (52–66) | 6 |
| Yuki [85] | 2020 | Japan | R | NSCLC | 81 | 37 | Atezo | 2nd-line or later | Monotherapy | Baseline | 1.5 | OS, PFS | 71 (42–84) | 7 |
| Sabrina [86] | 2020 | Italy | R | NSCLC | 65(16/49) | 44 | Nivo | 2nd-line or later | NA | Baseline, posttreatment | 1.4 | OS | 68 (39-86) | 6 |
| Kazuki [87] | 2020 | Japan | R | NSCLC | 146 | NA | Nivo, Pembro | 1st-line or later | NA | Baseline | 2.1 | PFS | NA | 6 |
| Hiroki [88] | 2019 | Japan | R | RCC | 58(21/37) | 45 | Nivo | 2nd-line or later | NA | Baseline | 3.3 | OS, PFS, DCR | 34 | 6 |
| Jarrett [89] | 2017 | USA | R | Melanoma | 133 (98/35) | 87 | Pembro | NA | NA | Baseline | 1.7 | OS, PFS | 61 | 8 |
(b).
| Author | Year | Baseline | Posttreatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR for OS and 95% CI | HR for PFS and 95% CI | OR for DCR and 95% CI | OR for irAEs and 95% CI | HR for OS and 95% CI | HR for PFS and 95% CI | OR for DCR and 95% CI | OR for irAEs and 95% CI | ||||
| Man | 2022 | 0.90 (0.56-1.47) | 1.50 (0.98-2.30) | NA | NA | NA | NA | NA | NA | ||
| Takashi | 2022 | 0.49 (0.32-0.74) | NA | NA | NA | NA | NA | NA | NA | ||
| Eisuke | 2022 | NA | NA | 1.28 (0.38-4.26) | NA | NA | NA | NA | NA | ||
| Xueping | 2022 | 0.41(0.19-0.86) | NA | NA | NA | NA | NA | NA | NA | ||
| Kosuke | 2022 | 0.61 (0.20-1.89) | 0.58 (0.27-1.24) | NA | NA | NA | NA | NA | NA | ||
| Chan | 2022 | 0.33 (0.17-0.60) | NA | NA | NA | NA | NA | NA | NA | ||
| Hiroyuki | 2022 | 0.17 (0.06-0.60) | 0.22 (0.10-0.51) | 18.00 (3.68-88.00) | 11.33 (2.46-52.15) | NA | NA | NA | NA | ||
| Rui | 2022 | NA | NA | NA | NA | 0.61 (0.21-1.79) | 0.36 (0.13-1.06) | NA | NA | ||
| Xue | 2022 | NA | NA | NA | 0.59 (0.36–0.97) | NA | NA | NA | NA | ||
| Jia | 2022 | 0.32 (0.14-0.72) | NA | 3.84 (1.39-10.65) | NA | NA | NA | NA | NA | ||
| Zhenzhen | 2022 | NA | 0.45 (0.25-0.83) | NA | NA | NA | NA | NA | NA | ||
| Amparo | 2021 | 0.34 (0.15-0.76) | 0.60 (0.31-1.15) | NA | NA | NA | NA | NA | NA | ||
| Shigeo | 2021 | NA | NA | NA | NA | 2.17 (0.99-4.63) | NA | 0.19 (0.05-0.70) | NA | ||
| Yang | 2021 | 0.38(0.24-0.62) | 0.58 (0.38-0.90) | 2.87 (1.22-6.74) | NA | 0.52 (0.31-0.88) | 0.48 (0.29-0.78) | 2.06 (0.81-5.20) | NA | ||
| Saeka | 2021 | NA | NA | NA | NA | NA | NA | NA | 1.79 (0.90-3.56) | ||
| Sara | 2021 | 0.69 (0.53-0.91) | 0.81 (0.65-1.00) | 1.38 (0.94-2.03) | NA | NA | NA | NA | NA | ||
| Despina | 2021 | 0.43 (0.30-0.60) | NA | NA | 2.96 (1.56-5.60) | NA | NA | NA | NA | ||
| Wei | 2021 | 0.78 (0.29-2.07) | NA | NA | NA | NA | NA | NA | NA | ||
| Saeka | 2021 | NA | NA | NA | 0.12 (0.03-0.52) | NA | NA | NA | NA | ||
| Xiaona | 2021 | 0.46 (0.24-0.88) | 0.55 (0.33-0.93) | 4.08 (1.76-9.46) | 1.01 (0.44-2.34) | NA | NA | NA | NA | ||
| Haiping | 2021 | 0.48 (0.34-0.70) | 0.77 (0.57-1.04) | NA | NA | NA | NA | NA | NA | ||
| DanYun | 2021 | 2.14 (0.39-11.69) | 0.62 (0.34-1.13) | NA | NA | NA | NA | NA | NA | ||
| Yuki | 2020 | 0.30 (0.17-0.55) | 0.48 (0.30-0.79) | NA | NA | NA | NA | NA | NA | ||
| Sabrina | 2020 | 0.98 (0.27-3.49) | NA | NA | NA | 0.14 (0.01-1.66) | NA | NA | NA | ||
| Kazuki | 2020 | NA | 0.49 (0.34-0.71) | NA | NA | NA | NA | NA | NA | ||
| Hiroki | 2019 | 0.29 (0.09-1.31) | 0.38 (0.19-0.83) | 6.33 (1.60-25.20) | NA | NA | NA | NA | NA | ||
| Jarrett | 2017 | 0.29 (0.15-0.59) | 0.55 (0.34-0.92) | NA | NA | NA | NA | NA | NA | ||
NA, not available; R, retrospective; NSCLC, non-small cell lung cancer; GC, gastric cancer; RCC, renal cell carcinoma; SCC, small cell lung cancer; UC, urothelial carcinoma; LC, lung cancer; BTC, biliary tract cancer; EC, esophageal cancer; HCC, hepatocellular carcinoma; CRC, colorectal cancer; PLC, primary liver cancer; H/L, LMR high group/ low group; chemo, chemotherapy; ICIs, immune checkpoint inhibitors; Pembro, Pembrolizumab; Nivo, Nivolumab; Atezo, Atezolizumab; Sinti, Sintilimab; Camre, Camrelizumab; Tripri, Triprizumab; Toripa, Toripalimab; Lenva, lenvatinib; Sora, Sorafenib; Apa, apatinib; PD-(L)1, programmed death- (ligands) 1; CTLA-4, cytotoxic T lymphocyte antigen 4; OS, overall survival; PFS, progression free survival; ORR, objective response rate; DCR, disease control rate; irAEs, immune-related adverse events.
3.2. Quality Assessment
All the included 27 studies were rated as moderate or high quality with a score from five to eight based on the NOS criteria, which were eligible for meta-analysis (Table 1).
3.3. Main Results
3.3.1. LMR and OS
Twenty-one cohorts incorporating 2,739 individuals were included in our analysis of the association between LMR and OS, with 17 cohorts provided only baseline LMR values, 2 only posttreatment LMR values, and 2 both baseline and posttreatment LMR values. Polled analysis showed high LMR value was significantly associated with a better OS in cancer patients treated with ICIs (HR: 0.49, 95% CI: 0.41-0.60, p < 0.001, Figure 2(a)), but with an obvious heterogeneity (I2 = 52.4%, p = 0.002). Hence, a further analysis was performed according to the testing time of LMR. Results showed that high baseline LMR contributed to a better OS (HR: 0.46, 95% CI: 0.39-0.56, p < 0.001, Figure 2(a)) with significant heterogeneity (I2 = 42.5%, p = 0.027), but there was no relationship between posttreatment LMR and OS (HR: 0.74, 95% CI: 0.30-1.84, p = 0.51, Figure 2(a)).
Figure 2.
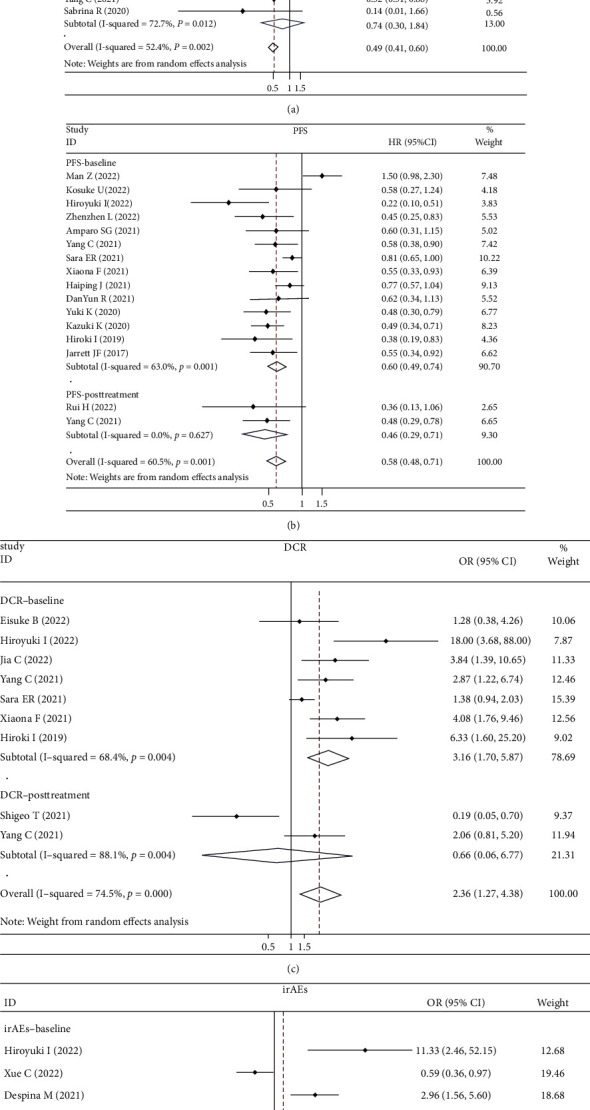
Forest plots for (a) overall survival (OS), (b) progression-free survival (PFS), (c) disease control rate (DCR), and (d) immune-related adverse event (irAEs).
Therefore, subgroup analyses focused only on baseline LMR, and high baseline LMR indicated a better OS in RCC arm (HR: 0.66, 95% CI: 0.51-0.86, p = 0.002, Figure 3(a)), NSCLC arm (HR: 0.35, 95% CI: 0.24-0.52, p < 0.001, Figure 3(a)), multiple cancer arm (HR: 0.45, 95% CI: 0.36-0.57, p < 0.001, Figure 3(a)), monotherapy arm (HR: 0.39, 95% CI: 0.25-0.62, p < 0.001, Figure 3(b)), LMR ≥2 arm (HR: 0.51, 95% CI: 0.40-0.66, p < 0.001, Figure 3(c)), LMR <2 arm (HR: 0.40, 95% CI: 0.33-0.50, p < 0.001, Figure 3(c)), eastern countries arm (HR: 0.45, 95% CI: 0.36-0.57, p < 0.001, Figure 3(d)), western countries arm (HR: 0.48, 95% CI: 0.33-0.70, p < 0.001, Figure 3(d)), and anti-PD-1 arm (HR: 0.49, 95% CI: 0.38-0.62, p < 0.001, Figure 3(e)). However, higher baseline LMR values indicated a better OS in GC group (HR: 0.74, 95% CI: 0.14-3.83, p = 0.718, Figure 3(a)), combination therapy group (HR: 0.70, 95% CI: 0.45-1.10, p = 0.12, Figure 3(b)), and anti-PD-L1 group (HR: 0.44, 95% CI: 0.18-1.12, p = 0.085, Figure 3(e)) without statistical significance.
Figure 3.
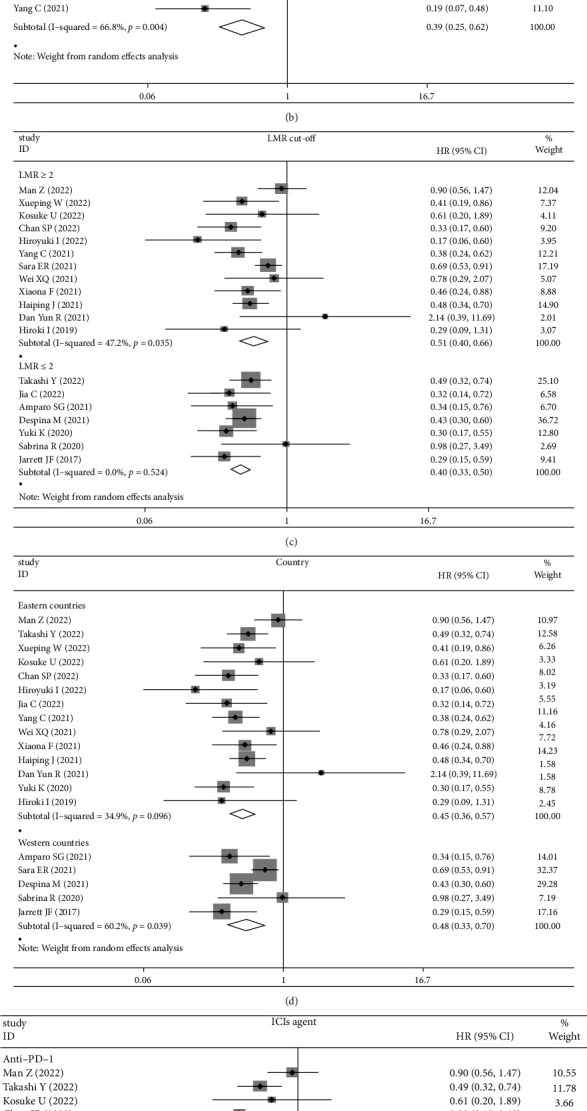
(a) The pooled HRs for overall survival (OS) by LMR at baseline stratified on tumor types (RCC, NSCLC, GC, and multiple); (b) whether monotherapy or combined therapy; (c) LMR cut-off (<2 and ≥2); (d) countries (western countries and eastern countries); (e) type of ICI agents (anti-PD-1 and anti-PD-L1); (f) funnel plot for the evaluation of publication bias considering the association between the LMR at baseline and OS (19 studies).
3.3.2. LMR and PFS
Fifteen cohorts provided the data of LMR and PFS, in which 13 cohorts displayed baseline LMR values, 1 cohort posttreatment LMR values, and 1cohort both baseline and posttreatment LMR values. As with the results of OS analyses, a higher LMR was also associated with a better PFS in cancer patients treated with ICIs (HR: 0.58, 95% CI: 0.48-0.71, p < 0.001, Figure 2(b)) in both baseline and posttreatment LMR studies (HR: 0.60, 95% CI: 0.49-0.74, p < 0.001; HR: 0.46, 95% CI: 0.29-0.71, p = 0.001, respectively, Figure 2(b)), but with an obvious heterogeneity (I2 = 60.5%, p = 0.001).
Because only 2 researches provided the data of posttreatment LMR, which were insufficient for subgroup analysis, and further stratified analyses were performed based on the baseline LMR studies. Results exhibited that high baseline LMR led to a better PFS in RCC arm (HR: 0.63, 95% CI: 0.40-0.99, p = 0.047, Figure 4(a)), NSCLC arm (HR: 0.50, 95% CI: 0.39-0.66, p < 0.001, Figure 4(a)), GC arm (HR: 0.59, 95% CI: 0.42-0.84, p = 0.003, Figure 4(a)), multiple cancer arm (HR: 0.70, 95% CI: 0.52-0.94, p = 0.019, Figure 4(a)), western countries arm (HR: 0.72, 95% CI: 0.57-0.92, p =0.008, Figure 4(b)), eastern countries arm (HR: 0.57, 95% CI: 0.44-0.75, p < 0.001, Figure 4(b)), LMR <2 arm (HR: 0.53, 95% CI: 0.39-0.72, p < 0.001, Figure 4(c)), and LMR ≥2 arm (HR: 0.61, 95% CI: 0.48-0.79, p < 0.001, Figure 4(c)).
Figure 4.
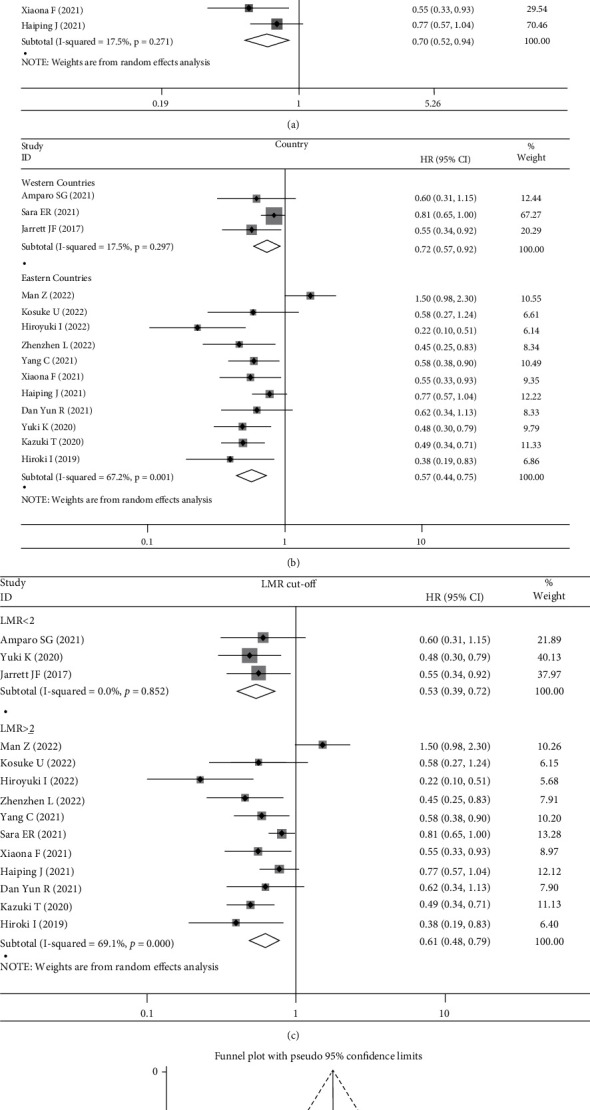
(a) The pooled HRs for progression-free survival (PFS) by LMR at baseline stratified on tumor types (RCC, NSCLC, GC, and multiple); (b) LMR cut-off (<2 and ≥2); (c) and countries (eastern countries and western countries); (d) funnel plot for the evaluation of publication bias considering the association between the LMR at baseline and PFS (14 studies).
3.3.3. LMR and DCR
Eight cohorts incorporating 1,117 cases provided the data of LMR and DCR, with 1 cohort displayed both baseline and posttreatment LMR, 6 baseline LMR, and 1 posttreatment LMR. Similarly, a higher LMR value was correlated with a better DCR (OR: 2.36, 95% CI: 1.27-4.38, p = 0.006, Figure 2(c)), but with significant heterogeneity (I2 = 74.5%, p < 0.001). Subgroup analysis were then conducted according to the testing time of LMR, which displayed a positive association between higher LMR at baseline and a better DCR (OR: 3.16, 95% CI: 1.70-5.87, p < 0.001, Figure 2(c)) but no obvious correlation between LMR at posttreatment and DCR (OR: 0.66, 95% CI: 0.06-6.77, p = 0.724, Figure 2(c)).
3.3.4. LMR and irAEs
Six studies with 1,852 patients were available in the analysis of the association between LMR and irAEs of any grade, with 5 displayed baseline LMR and 1 posttreatment LMR. Our pooled analysis showed that LMR did not exist a correlation with irAEs regardless of the testing time (OR: 1.26, 95% CI: 0.53-3.02, p = 0.599, Figure 2(d)).
3.4. Publication Bias
Among the above results, the analysis of the relationships of LMR at baseline with OS and PFS included enough articles (>10 studies) and funnel plot (Figure 3(f)) and Egger's test were conducted. The shape of the funnel plot suggested no publication bias for recruited studies on OS (Egger: p = 0.33) (Figure 3(f)), while there was a publication bias for PFS (Egger: p = 0.03) (Figure 4(d)). Meanwhile, because of the limited number of studies for meaningful assessment (<10 studies), the publication bias was not performed in other analyses.
3.5. The Sensitivity Analysis
We performed sensitivity analysis for baseline LMR due to their clinical significance by excluding one single study from the primary analysis, which proved that no individual study influenced the results on OS, PFS, and DCR, suggesting the results were relatively credible (Figure 5).
Figure 5.
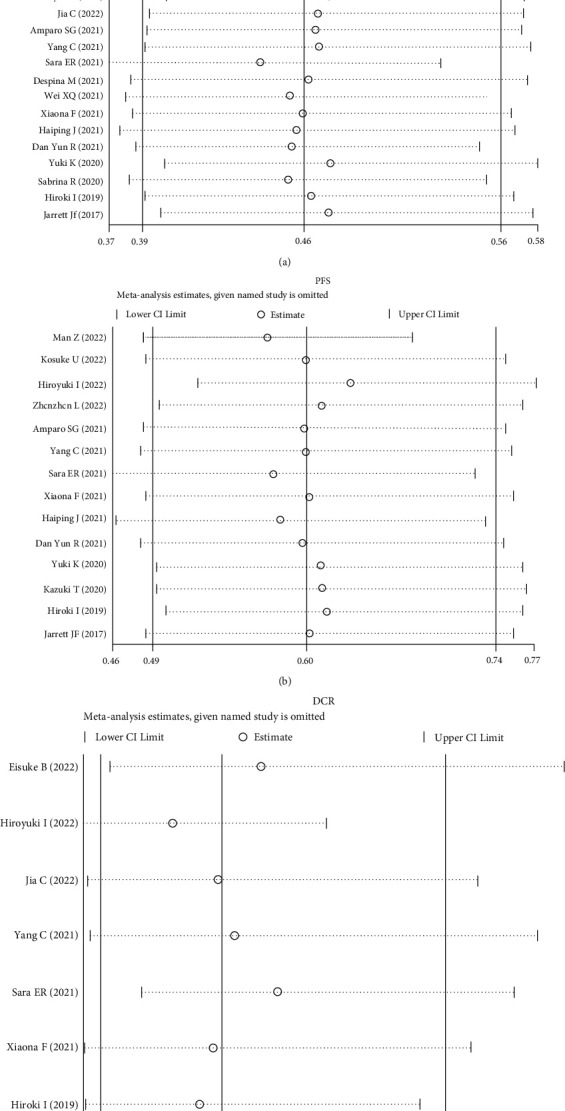
Sensitivity analysis of (a) overall survival (OS), (b) progression-free survival (PFS), and (c) disease control rate (DCR).
4. Discussion
The relationship between inflammation and neoplasm progression or metastasis has long been discussed. Blood-derived parameters, which are easily accessible and reproducible indicators of systemic inflammation, have already been used as objective biomarkers for predicting the prognoses of cancer patients [31, 32]. In light of this, increasing studies have explored whether some of them possess the ability to predict the efficacy of immunotherapy. However, among these markers, LMR is relatively less investigated. LMR was initially identified in hematological malignancies as a prognostic predictor, then a growing body of work demonstrated its positive association with better prognoses in many solid tumors, including lung cancer, gastric cancer, breast cancer, and melanoma [24–26, 33, 34]. For example, in patients of melanoma treated with ipilimumab, higher level of monocyte was found in cases that did not respond to this agent [35]. Similarly, higher baseline absolute lymphocyte count indicated an improved OS in patients treated with pembrolizumab [36]. In this meta-analysis, we investigated the association between LMR and the therapeutic effect of ICIs based on 27 studies incorporating 4,322 patients and multiple tumor types, and the results displayed that higher baseline LMR was positively correlated with a superior OS, PFS, and DCR for ICIs, indicating that higher LMR may be a signal for better efficacy for patients receiving ICIs treatment.
LMR, which is calculated by lymphocytes and monocytes, represents the antitumor immunity and tumor burden in human body [37]. On the one hand, tumor-infiltrating lymphocytes (TILs) are transformed from circulating lymphocytes in tumor microenvironments and well-known to contribute to antitumor immunity through their cytolytic activity. Therefore, insufficient numbers of lymphocyte are regarded as a contributing factor to the under-activation of the immunologic reaction to the tumor [38], which indicates poor clinical outcomes in multiple cancer types [39]. Previous studies showed that higher level of tumor infiltrating CD8+ T cell predicted better efficacy of ICIs in melanoma and clear cell renal cell carcinoma patients [40, 41]. In addition, B lymphocytes are also reported to be associated with good clinical response in cancer patients receiving anti–PD-1 therapy [42, 43]. On the other hand, monocytes infiltrate tumors and evolved into tumor-associated macrophages (TAMs) in response to chemokines, which involved in tumor proliferation, invasion, metastasis, and angiogenesis [44–46]. In gastric cancer, TAMs have been reported to suppress the function of cytotoxic T cells through the PD-1/PD-L1 pathway [47] and indicate poor prognoses [48, 49]. Consistently, in in vivo experiment, TAMs can lead to resistance of PD-1 inhibitors [50]. Therefore, LMR was thought to reflect host immune status and have the potential to serve as a predictor of therapeutic effect of ICIs treatment.
The differential responses to ICIs can be linked to the diversity of individual innate immune system and some other factors [7, 8], including the patient's specific GM. As accumulating evidence has demonstrated the critical role of GM in modulating the host's immune system [8, 51], GM manipulation is further proved to be a powerful therapeutic strategy to affect ICIs efficacy and irAEs [52, 53]. For instance, antibiotic administration could lead to a disrupted GM and therefore compromise the therapeutic effect of ICIs, while fecal microbiota transplantation (FMT) resulted in overcoming of anti-PD-1 therapy [53–55]. In addition, previous study shed light on the role of neutrophil-lymphocyte ratio (NLR) as a systemic inflammation marker to reflect the status of GM, and individuals with lower NLR showed increased diversity in their gut microbiota [56]. In turn, short-chain fatty acids (SCFAs), one of metabolites produced by microbes from components in the gut, can promote both the effector and regulatory effects of T cells and the antibody production, and may therefore enhance the host's immunity [57]. Taken together, these results revealed the interaction of microbiota, hematological inflammatory indicators, and ICIs efficacy, through which the inflammatory markers may predict the clinical outcomes of ICIs therapy.
This study displayed that baseline LMR is positively correlated with OS, PFS, and DCR, while posttreatment LMR failed to show this correlation in the analysis of OS and DCR. This may be ascribed to the limited number of studies reporting the results of posttreatment LMR and the inconsistencies of the testing time of LMR, which varies from 2 weeks after initial administration to 8 weeks. Previous research indicated that the least time of activated leukocytes “truly” mobilize into peripheral blood is 4 weeks [58], which may partly explain the discordant conclusion of articles reporting LMR at posttreatment. Hence, future studies may more specifically investigate whether the different testing time of posttreatment LMR could influence the outcomes and whether changes of LMR between pre- and post-ICIs correlate with the clinical efficacies.
At present, the precise mechanisms of the presentation of irAEs have not been fully elucidated. One explanation is that tumor cells and the affected tissue have shared antigens, and activated CD8-positive T-lymphocytes cannot distinguish between them and attack normal tissue cells unexpectedly [59]. Other potential mechanisms include subclinical autoimmune responses and microbiome [60, 61]. A number of studies have examined the relationships of peripheral blood biomarkers with the risk of irAEs, which yielded different conclusions [62, 63]. In this study, we observed that LMR had no relationship with irAEs both before or post ICIs treatment, but in consideration of the limited data, prospective studies with larger patient cohorts and more detailed patients' clinical information are needed.
This study encountered several limitations: first, all the recruited studies were retrospective, while no randomized controlled trial (RCT) was available, which may lead to potential confounders. Second, subgroup analysis based on specific ICIs agent were not able to conduct due to the less comprehensive data. Third, there was publication bias for pooled PFS in the analysis of LMR at baseline. Nonetheless, our results are still interesting because few meta-analyses focus on the relationship between LMR and the clinical outcomes of ICIs.
In conclusion, this study showed that the value of baseline LMR is positively associated with a better OS, PFS, and DCR in cancer patients undergoing ICI therapy, and subgroup analyses on tumor types, ICIs agents, combination therapy, cutoff value of LMR, and study regions exhibited similar results or trends, indicating the promising prognostic value of LMR on ICIs therapy in clinical practice.
Acknowledgments
We thank Mr. Ziyu Gao for his assistance in manuscript preparation. This study was supported by Joint Funds for the Innovation of Science and Technology, Fujian province [Grant number: 2021Y9148], and Startup Fund for Scientific Research of Fujian Medical University [Grant number: 2021QH1085].
Data Availability
Data extracted from included studies and used for all analyses are available in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
LYW and XHX are responsible for the study design. LYW, CLW, and SML are responsible for data extraction and analysis. LYW is responsible for writing the manuscript. SML and XHX are responsible for the revision of the draft. All authors approved the final draft.
References
- 1.Zhang Y., Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cellular & Molecular Immunology . 2020;17(8):807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christofi T., Baritaki S., Falzone L., Libra M., Zaravinos A. Current Perspectives in Cancer Immunotherapy. Cancers (Basel) . 2019;11(10) doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonardi G. C., Candido S., Falzone L., Spandidos D. A., Libra M. Cutaneous melanoma and the immunotherapy revolution (review) International Journal of Oncology . 2020;57(3):609–618. doi: 10.3892/ijo.2020.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotecha R., Miller J. A., Venur V. A., et al. Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. Journal of Neurosurgery . 2018;129(1):50–59. doi: 10.3171/2017.1.JNS162797. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A., Wolchok J. D. Cancer immunotherapy using checkpoint blockade. Science . 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assi H. I., Kamphorst A. O., Moukalled N. M., Ramalingam S. S. Immune checkpoint inhibitors in advanced non–small cell lung cancer. Cancer . 2018;124(2):248–261. doi: 10.1002/cncr.31105. [DOI] [PubMed] [Google Scholar]
- 7.Havel J. J., Chowell D., Chan T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nature Reviews. Cancer . 2019;19(3):133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivarelli S., Falzone L., Leonardi G., Salmeri M., Libra M. Novel insights on gut microbiota manipulation and immune checkpoint inhibition in cancer (review) International Journal of Oncology . 2021;59(3) doi: 10.3892/ijo.2021.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnabei A., Strigari L., Corsello A., et al. Immune checkpoint inhibitor-induced central diabetes insipidus: looking for the needle in the haystack or a very rare side-effect to promptly diagnose? Frontiers in Oncology . 2022;12, article 798517 doi: 10.3389/fonc.2022.798517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu V. M., Shah A. H., Eichberg D. G., et al. Utilizing systematic reviews and meta-analyses effectively to evaluate brain tumor biomarkers. Biomarkers in Medicine . 2020;14(10):817–820. doi: 10.2217/bmm-2020-0209. [DOI] [PubMed] [Google Scholar]
- 11.Godos J., Giampieri F., Micek A., et al. Effect of Brazil nuts on selenium status, blood lipids, and biomarkers of oxidative stress and inflammation: a systematic review and meta-analysis of randomized clinical trials. Antioxidants . 2022;11(2):p. 403. doi: 10.3390/antiox11020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laukhtina E., Shim S. R., Mori K., et al. Diagnostic accuracy of novel urinary biomarker tests in non-muscle-invasive bladder cancer: a systematic review and network meta-analysis. European Urology Oncology . 2021;4(6):927–942. doi: 10.1016/j.euo.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Lizardo D. Y., Kuang C., Hao S., Yu J., Huang Y., Zhang L. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: from bench to bedside. Biochimica Et Biophysica Acta. Reviews on Cancer . 2020;1874(2, article 188447) doi: 10.1016/j.bbcan.2020.188447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng X., Huang Z., Teng F., Xing L., Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treatment Reviews . 2015;41(10):868–876. doi: 10.1016/j.ctrv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Strickler J. H., Hanks B. A., Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clinical Cancer Research . 2021;27(5):1236–1241. doi: 10.1158/1078-0432.CCR-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D. S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature . 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 17.Leone P., Buonavoglia A., Fasano R., et al. Insights into the regulation of tumor angiogenesis by micro-RNAs. Journal of Clinical Medicine . 2019;8(12):p. 2030. doi: 10.3390/jcm8122030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain S. P., Harris C. C. Inflammation and cancer: an ancient link with novel potentials. International Journal of Cancer . 2007;121(11):2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 19.Gu X. B., Tian T., Tian X. J., Zhang X. J. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Scientific Reports . 2015;5(1):p. 12493. doi: 10.1038/srep12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Templeton A. J., McNamara M., Šeruga B., et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Journal of the National Cancer Institute . 2014;106(6):p. dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 21.Shi C., Pamer E. G. Monocyte recruitment during infection and inflammation. Nature Reviews. Immunology . 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diakos C. I., Charles K. A., McMillan D. C., Clarke S. J. Cancer-related inflammation and treatment effectiveness. The Lancet Oncology . 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 23.Guthrie G. J., Charles K. A., Roxburgh C. S. D., Horgan P. G., McMillan D. C., Clarke S. J. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Critical Reviews in Oncology/Hematology . 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Nishijima T. F., Muss H. B., Shachar S. S., Tamura K., Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treatment Reviews . 2015;41(10):971–978. doi: 10.1016/j.ctrv.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Gandini S., Ferrucci P. F., Botteri E., et al. Prognostic significance of hematological profiles in melanoma patients. International Journal of Cancer . 2016;139(7):1618–1625. doi: 10.1002/ijc.30215. [DOI] [PubMed] [Google Scholar]
- 26.Leontovich A. A., Dronca R. S., Nevala W. K., et al. Effect of the lymphocyte-to-monocyte ratio on the clinical outcome of chemotherapy administration in advanced melanoma patients. Melanoma Research . 2017;27(1):32–42. doi: 10.1097/CMR.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 27.Naszai M., Kurjan A., Maughan T. S. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: a systematic review and meta-analysis. Cancer Medicine . 2021;10(17):5983–5997. doi: 10.1002/cam4.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mjaess G., Chebel R., Karam A., et al. Prognostic role of neutrophil-to-lymphocyte ratio (NLR) in urological tumors: an umbrella review of evidence from systematic reviews and meta-analyses. Acta Oncologica . 2021;60(6):704–713. doi: 10.1080/0284186X.2021.1886323. [DOI] [PubMed] [Google Scholar]
- 29.Kumarasamy C., Tiwary V., Sunil K., et al. Prognostic utility of platelet-lymphocyte ratio, neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio in head and neck cancers: a detailed PRISMA compliant systematic review and meta-analysis. Cancers . 2021;13(16):p. 4166. doi: 10.3390/cancers13164166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu N., Mao J., Tao P., Chi H., Jia W., Dong C. The relationship between NLR/PLR/LMR levels and survival prognosis in patients with non-small cell lung carcinoma treated with immune checkpoint inhibitors. Medicine . 2022;101(3, article e28617) doi: 10.1097/MD.0000000000028617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang R., Zheng Y., Zou W., Liu C., Liu J., Yue J. Blood biomarkers predict survival outcomes in patients with hepatitis B virus-induced hepatocellular carcinoma treated with PD-1 inhibitors. Journal of Immunology Research . 2022;2022:9. doi: 10.1155/2022/3781109.3781109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinh H., Dzul S. P., Hyder J., et al. Prognostic value of changes in neutrophil-to-lymphocyte ratio (NLR), platelet- to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) for patients with cervical cancer undergoing definitive chemoradiotherapy (dCRT) Clinica Chimica Acta . 2020;510:711–716. doi: 10.1016/j.cca.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Li Z. M., Huang J. J., Xia Y., et al. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS One . 2012;7(7, article e41658) doi: 10.1371/journal.pone.0041658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porrata L. F., Ristow K., Colgan J. P., et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin's lymphoma. Haematologica . 2012;97(2):262–269. doi: 10.3324/haematol.2011.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gebhardt C., Sevko A., Jiang H., et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clinical Cancer Research . 2015;21(24):5453–5459. doi: 10.1158/1078-0432.CCR-15-0676. [DOI] [PubMed] [Google Scholar]
- 36.Weide B., Martens A., Hassel J. C., et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clinical Cancer Research . 2016;22(22):5487–5496. doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goto W., Kashiwagi S., Asano Y., et al. Predictive value of lymphocyte-to-monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer . 2018;18(1):p. 1137. doi: 10.1186/s12885-018-5051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stotz M., Pichler M., Absenger G., et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. British Journal of Cancer . 2014;110(2):435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Hu X., Xu W., Wang H., Huang Y., Che G. Prognostic value of a novel scoring system using inflammatory response biomarkers in non-small cell lung cancer: a retrospective study. Thorac Cancer . 2019;10(6):1402–1411. doi: 10.1111/1759-7714.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong P. F., Wei W., Smithy J. W., et al. Multiplex quantitative analysis of tumor-infiltrating lymphocytes and immunotherapy outcome in metastatic melanoma. Clinical Cancer Research . 2019;25(8):2442–2449. doi: 10.1158/1078-0432.CCR-18-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pignon J. C., Jegede O., Shukla S. A., et al. irRECIST for the evaluation of candidate biomarkers of response to nivolumab in metastatic clear cell renal cell carcinoma: analysis of a phase II prospective clinical trial. Clinical Cancer Research . 2019;25(7):2174–2184. doi: 10.1158/1078-0432.CCR-18-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petitprez F., de Reyniès A., Keung E. Z., et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature . 2020;577(7791):556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 43.Cabrita R., Lauss M., Sanna A., et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature . 2020;577(7791):561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 44.Olingy C. E., Dinh H. Q., Hedrick C. C. Monocyte heterogeneity and functions in cancer. Journal of Leukocyte Biology . 2019;106(2):309–322. doi: 10.1002/JLB.4RI0818-311R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou J., Tang Z., Gao S., Li C., Feng Y., Zhou X. Tumor-associated macrophages: recent insights and therapies. Frontiers in Oncology . 2020;10:p. 188. doi: 10.3389/fonc.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura K., Smyth M. J. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cellular & Molecular Immunology . 2020;17(1):1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang F., Li B., Wei Y., et al. Tumor-derived exosomes induce PD1+ macrophage population in human gastric cancer that promotes disease progression. Oncogene . 2018;7(5):p. 41. doi: 10.1038/s41389-018-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larionova I., Cherdyntseva N., Liu T., Patysheva M., Rakina M., Kzhyshkowska J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology . 2019;8(7):p. 1596004. doi: 10.1080/2162402X.2019.1596004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X., Xu D., Huang C., et al. Regulatory T cells and M2 macrophages present diverse prognostic value in gastric cancer patients with different clinicopathologic characteristics and chemotherapy strategies. Journal of Translational Medicine . 2019;17(1):p. 192. doi: 10.1186/s12967-019-1929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arlauckas S. P., Garris C. S., Kohler R. H., et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Science Translational Medicine . 2017;9(389) doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maynard C. L., Elson C. O., Hatton R. D., Weaver C. T. Reciprocal interactions of the intestinal microbiota and immune system. Nature . 2012;489(7415):231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vivarelli S., Falzone L., Salmeri M., Libra M. Role of Microbiota in Lung Cancer: Focus on Immune-Checkpoint Inhibition. Role of Microbiota in Lung Cancer: Focus on Immune-Checkpoint Inhibition. . 2021;8(1):11–25. doi: 10.1615/ForumImmunDisTher.2021038938. [DOI] [Google Scholar]
- 53.Wu J., Wang S., Zheng B., Qiu X., Wang H., Chen L. Modulation of gut microbiota to enhance effect of checkpoint inhibitor immunotherapy. Frontiers in Immunology . 2021;12, article 669150 doi: 10.3389/fimmu.2021.669150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierrard J., Seront E. Impact of the gut microbiome on immune checkpoint inhibitor efficacy-a systematic review. Current Oncology . 2019;26(6):395–403. doi: 10.3747/co.26.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davar D., Dzutsev A. K., McCulloch J. A., et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science . 2021;371(6529):595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoon H. Y., Kim H. N., Lee S. H., et al. Association between neutrophil-to-lymphocyte ratio and gut microbiota in a large population: a retrospective cross-sectional study. Scientific Reports . 2018;8(1):p. 16031. doi: 10.1038/s41598-018-34398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim C. H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cellular & Molecular Immunology . 2021;18(5):1161–1171. doi: 10.1038/s41423-020-00625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamphorst A. O., Pillai R. N., Yang S., et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proceedings of the National Academy of Sciences of the United States of America . 2017;114(19):4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berner F., Bomze D., Diem S., et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncology . 2019;5(7):1043–1047. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dubin K., Callahan M. K., Ren B., et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nature Communications . 2016;7(1):p. 10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwama S., de Remigis A., Callahan M. K., Slovin S. F., Wolchok J. D., Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Science Translational Medicine . 2014;6(230):p. 230ra45. doi: 10.1126/scitranslmed.3008002. [DOI] [PubMed] [Google Scholar]
- 62.Nakaya A., Kurata T., Yoshioka H., et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. International Journal of Clinical Oncology . 2018;23(4):634–640. doi: 10.1007/s10147-018-1250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukui T., Okuma Y., Nakahara Y., et al. Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: a prospective observational study. Clinical Lung Cancer . 2019;20(3):208–214.e2. doi: 10.1016/j.cllc.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 64.Zhao M., Duan X., Han X., et al. Sarcopenia and systemic inflammation response index predict response to systemic therapy for hepatocellular carcinoma and are associated with immune cells. Frontiers in Oncology . 2022;12, article 854096 doi: 10.3389/fonc.2022.854096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida T., Ohe C., Ito K., et al. Clinical and molecular correlates of response to immune checkpoint blockade in urothelial carcinoma with liver metastasis. Cancer Immunology, Immunotherapy . 2022;71(11):2815–2828. doi: 10.1007/s00262-022-03204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Booka E., Kikuchi H., Haneda R., et al. Neutrophil-to-lymphocyte ratio to predict the efficacy of immune checkpoint inhibitor in upper gastrointestinal cancer. Anticancer Research . 2022;42(6):2977–2987. doi: 10.21873/anticanres.15781. [DOI] [PubMed] [Google Scholar]
- 67.Wang X., He Z., Liu W., et al. Development of a clinically oriented model to predict antitumor effects after PD-1/PD-L1 inhibitor therapy. Journal of Oncology . 2022;2022:11. doi: 10.1155/2022/9030782.9030782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ueda K., Suekane S., Kurose H., et al. Absolute lymphocyte count is an independent predictor of survival in patients with metastatic renal cell carcinoma treated with nivolumab. Japanese Journal of Clinical Oncology . 2022;52(2):179–186. doi: 10.1093/jjco/hyab157. [DOI] [PubMed] [Google Scholar]
- 69.Park C. S., Sung M. J., Kim S. J., et al. Prognostic factors in patients treated with pembrolizumab as a second-line treatment for advanced biliary tract cancer. Cancers . 2022;14(17):p. 4323. doi: 10.3390/cancers14174323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inoue H., Shiozaki A., Fujiwara H., et al. Absolute lymphocyte count and C-reactive protein-albumin ratio can predict prognosis and adverse events in patients with recurrent esophageal cancer treated with nivolumab therapy. Oncology Letters . 2022;24(2):p. 257. doi: 10.3892/ol.2022.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen X., Jiang A., Zhang R., et al. Immune checkpoint inhibitor-associated cardiotoxicity in solid tumors: real-world incidence, risk factors, and prognostic analysis. Frontiers in Cardiovascular Medicine . 2022;9, article 882167 doi: 10.3389/fcvm.2022.882167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J., Wei S., Zhao T., Zhang X., Wang Y., Zhang X. Clinical significance of serum biomarkers in stage IV non-small-cell lung cancer treated with PD-1 inhibitors: LIPI score, NLR, dNLR, LMR, and PAB. Disease Markers . 2022;2022:25. doi: 10.1155/2022/7137357.7137357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z., Diao Y., Li X. Body mass index and serum markers associated with progression-free survival in lung cancer patients treated with immune checkpoint inhibitors. BMC Cancer . 2022;22(1):p. 824. doi: 10.1186/s12885-022-09744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sánchez-Gastaldo A., Muñoz-Fuentes M. A., Molina-Pinelo S., Alonso-García M., Boyero L., Bernabé-Caro R. Correlation of peripheral blood biomarkers with clinical outcomes in NSCLC patients with high PD-L1 expression treated with pembrolizumab. Translational Lung Cancer Research . 2021;10(6):2509–2522. doi: 10.21037/tlcr-21-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tokumaru S., Koizumi T., Sekino Y., et al. Lymphocyte-to-monocyte ratio is a predictive biomarker of response to treatment with nivolumab for gastric cancer. Oncology . 2021;99(10):632–640. doi: 10.1159/000517344. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y., Zhang C., Peng Z., et al. Association of lymphocyte-to-monocyte ratio with survival in advanced gastric cancer patients treated with immune checkpoint inhibitor. Frontiers in Oncology . 2021;11, article 589022 doi: 10.3389/fonc.2021.589022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Egami S., Kawazoe H., Hashimoto H., et al. Absolute lymphocyte count predicts immune-related adverse events in patients with non-small-cell lung cancer treated with nivolumab monotherapy: a multicenter retrospective study. Frontiers in Oncology . 2021;11, article 618570 doi: 10.3389/fonc.2021.618570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rebuzzi S. E., Signori A., Banna G. L., et al. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: the development of a novel prognostic score (meet-URO 15 study) Therapeutic advances in medical oncology . 2021;13 doi: 10.1177/17588359211019642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michailidou D., Khaki A. R., Morelli M. P., Diamantopoulos L., Singh N., Grivas P. Association of blood biomarkers and autoimmunity with immune related adverse events in patients with cancer treated with immune checkpoint inhibitors. Scientific Reports . 2021;11(1):p. 9029. doi: 10.1038/s41598-021-88307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qi W. X., Xiang Y., Zhao S., Chen J. Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with first-line chemotherapy and atezolizumab. Cancer Immunology, Immunotherapy . 2021;70(11):3199–3206. doi: 10.1007/s00262-021-02926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Egami S., Kawazoe H., Hashimoto H., et al. Peripheral blood biomarkers predict immune-related adverse events in non-small cell lung cancer patients treated with pembrolizumab: a multicenter retrospective study. Journal of Cancer . 2021;12(7):2105–2112. doi: 10.7150/jca.53242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fan X., Wang D., Zhang W., et al. Inflammatory markers predict survival in patients with advanced gastric and colorectal cancers receiving anti-PD-1 therapy. Frontiers in Cell and Development Biology . 2021;9, article 638312 doi: 10.3389/fcell.2021.638312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang H., Li N., Wang H., et al. Assessment of TMB, PD-L1, and lymphocyte to monocyte ratio as predictive potential in a phase Ib study of sintilimab in patients with advanced solid tumors. American Journal of Cancer Research . 2021;11(9):4259–4276. [PMC free article] [PubMed] [Google Scholar]
- 84.Ruan D. Y., Chen Y. X., Wei X. L., et al. Elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with an immunosuppressive tumour microenvironment and decreased benefit of PD-1 antibody in advanced gastric cancer. Gastroenterology report . 2021;9(6):560–570. doi: 10.1093/gastro/goab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katayama Y., Yamada T., Chihara Y., et al. Significance of inflammatory indexes in atezolizumab monotherapy outcomes in previously treated non-small-cell lung cancer patients. Scientific Reports . 2020;10(1):p. 17495. doi: 10.1038/s41598-020-74573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rossi S., Toschi L., Finocchiaro G., Santoro A. Neutrophil and lymphocyte blood count as potential predictive indicators of nivolumab efficacy in metastatic non-small-cell lung cancer. Immunotherapy . 2020;12(10):715–724. doi: 10.2217/imt-2019-0154. [DOI] [PubMed] [Google Scholar]
- 87.Takada K., Takamori S., Yoneshima Y., et al. Serum markers associated with treatment response and survival in non-small cell lung cancer patients treated with anti-PD-1 therapy. Lung Cancer . 2020;145:18–26. doi: 10.1016/j.lungcan.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 88.Ishihara H., Tachibana H., Takagi T., et al. Predictive impact of peripheral blood markers and C-reactive protein in nivolumab therapy for metastatic renal cell carcinoma. Targeted Oncology . 2019;14(4):453–463. doi: 10.1007/s11523-019-00660-6. [DOI] [PubMed] [Google Scholar]
- 89.Failing J. J., Yan Y., Porrata L. F., Markovic S. N. Lymphocyte-to-monocyte ratio is associated with survival in pembrolizumab-treated metastatic melanoma patients. Melanoma Research . 2017;27(6):596–600. doi: 10.1097/CMR.0000000000000404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data extracted from included studies and used for all analyses are available in this published article.


