Abstract
Herein, we demonstrate that modification of TiO2 nanotubes with graphene–strontium and cobalt molybdate perovskite can turn them into active electrocatalysts for hydrogen evolution reaction (HER). For this purpose, a simple method of hydrothermal synthesis of perovskites was developed directly on the TiO2 nanotubes substrate. Moreover, the obtained hybrids were also decorated with graphene oxide (GO) during one-step hydrothermal synthesis. The obtained materials were characterized by scanning electron microscopy with energy dispersive X-ray analysis, Raman spectroscopy, and X-ray diffraction analysis. Catalytic properties were verified by electrochemical methods (linear voltammetry, chronopotentiometry). The obtained hybrids were characterized by much better catalytic properties towards hydrogen evolution reaction compared to TiO2 and slightly worse than platinum. The optimized hybrid catalyst (decorated by GO) can drive a cathodic current density of 10 mA cm−2 at an overpotential of 121 mV for HER with a small Tafel slope of 90 mV dec−1 in 0.2 M H2SO4.
Subject terms: Energy science and technology, Materials science
Introduction
Hydrogen evolution reaction (HER) is a crucial reaction in water splitting. It is still required to synthesize inexpensive HER electrocatalysts in a way that is effective, straightforward, and ecologically benign. The design of the appropriate core–shell structure of the catalyst, and controlling thereof shape affect the activity and durability of the catalyst1. A three-dimensional structure of the catalyst is beneficial for exposing the surface area, and furnishing the active sites, as well as is propitious to the diffusion and adsorption of hydrogen molecules, expediting the HER process2, 3. Furthermore, the three-dimensional structure of the catalyst influences oxygen evolution reaction performance4. A core–shell hierarchical nanostructure of a catalyst composed of many conductive interconnected networks, provides multiple channels for ionic or electron delivery5. The plasma-treated sponge-like nanoalloy exhibits high HER performance due to the exposure of more active and edge sites6. Besides the structure of the catalyst, another factor affecting the properties of the catalyst is its doping with, for example, nitrogen heteroatoms7. Compare to the well-known and expensive platinum catalyst the TiO2 is widely explored as an alternative catalyst. However, to enhance the electrocatalytic activity of TiO2 different modifications are needed. In some papers, TiO2 has been coupled with metals like nickel8, ruthenium9, 10, gold11, cobalt12, metal oxides like Co3O413, BiVO414, or metal–organic frameworks15. Very promising materials designed with TiO2 to catalyze the HER are metal sulfides, e.g., MoS216–19, CoS220, or WS221. Also, hybrid composites with polymers like truxene-based porous organic polymer22 or poly(aniline)23, poly(o-phenylenediamine), poly(thiophene), or poly(pyrrole)24 have proved to be desired candidates to enhance photocatalytic activity of TiO2. Another way to modify the properties of electrocatalyst is doping with non-metals. As described in many papers sulfur-doping, nitrogen-doping, or carbon-doping reduce bandgap of TiO225–27. Another way to improve properties was described by Pandey et al. where in theoretical predictions a hybrid structure of the ionic liquid 1-ethyl-3-methylimidazolium trifluoromethanesulfonate and (TiO2)n nanoclusters (with n = 2−12) has been investigated in the pursuit of new catalyst materials for effective HER28. Furthermore, hybrids of TiO2 and carbon materials like graphene oxide13, 29 and multi-wall carbon nanotubes30. Nanocomposites of graphene oxide with metal–organic framework described in the literature, were also efficient electrocatalyst for hydrogen production via Volmer and Heyrovsky mechanisms31. The disadvantage of many solutions is still doping with expensive metals like silver, platinum29. Therefore, it is important to find new solutions but without expensive metals and easy to preparation.
In this paper, a novel hybrid materials of CoMoO4, SrMoO4, or their composition SrMoO4 and CoMoO4 deposited on TiO2 nanotubes surface and supported with graphene oxide (GO) were achieved by hydrothermal method. Here, uniform TiO2 nanotubes were used as support to growth CoMoO4 or SrMoO4. The morphology was investigated by scanning electron microscopy (SEM) with energy dispersive X-ray analysis (EDX), Raman spectroscopy, and X-ray diffraction analysis (XRD). These hybrids were subjected as active catalysts for hydrogen evolution reaction in acidic medium. Compared with pristine TiO2 and commercial Pt, the optimal hybrid could attain a current density 10 mA cm−2 at a low overpotential of 121 mV with a Tafel slope of 90 mV dec−1. In addition, the hybrid displayed better cycling stability and durability.
Results and discussions
Physicochemical characterization
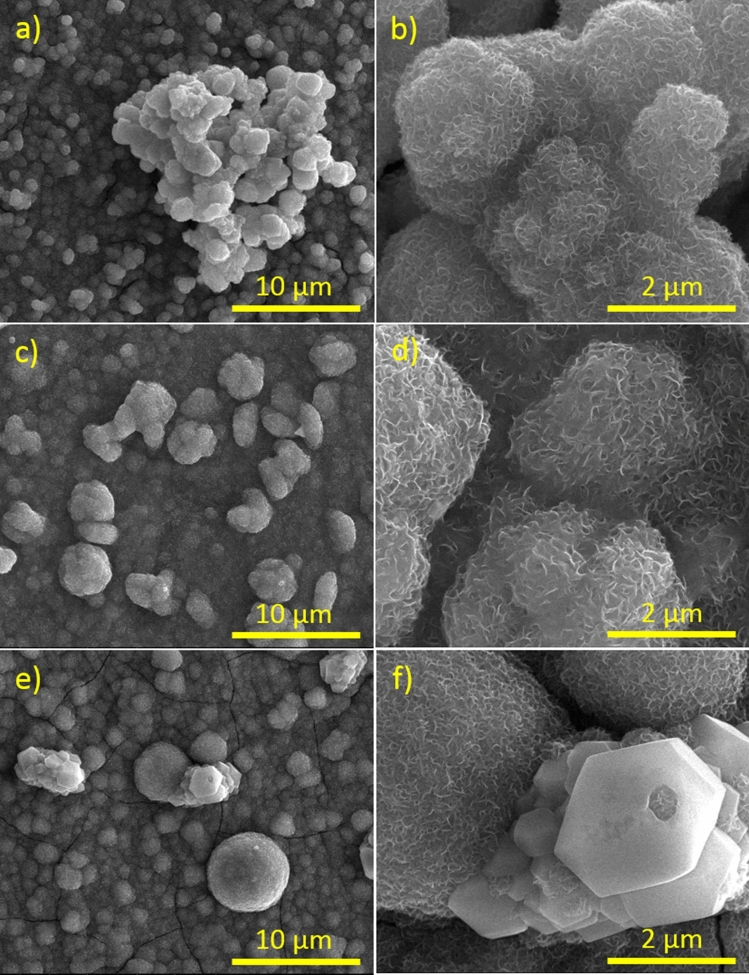
The morphology of the as-prepared samples was characterized by scanning electron microscopy. As shown in Fig. 1a, on the surface of sample TiO2/CoMoO4 the TiO2 nanotubes are not visible (pure TiO2 nanotubes are presented in Figure S1) because during the hydrothermal process the CoMoO4 particles densely covered TiO2 surface. Intriguingly, each CoMoO4 microspheres (Fig. 1b) is actually a three dimensionally interconnected porous structure and is assembled from numerous nanospheres. For TiO2/CoMoO4 sample the diameter of CoMoO4 particles ranges from 1 to 2 µm. It can be observed that the particles agglomerate. The surface of TiO2/SrMoO4 sample (Fig. 1c) clearly demonstrated that the product considered of a large amount of spherical structures covered TiO2 surface and shows the similar morphology to TiO2/CoMoO4 sample. The diameters of SrMoO4 spheres are about 2 µm. A higher-magnification SEM image (Fig. 1d) reveals that an individual sphere is composed of tens of similar nanosheets. These nanosheets are connected with each other to form a sphere with random orientation. Figure 1e,f illustrate the SEM images for TiO2/SrMoO4/CoMoO4 sample with different magnifications. Figure 1f shows the high magnification from which it can be seen that particles deposited on the TiO2 surface do not possess a uniform size and shape. The mean diameters of three-dimensional microspheres are greater and fluctuate between 2 and 4 µm. These nanosheets are connected with each other to form nanosheets-based microstructures with random orientation. Furthermore, the surface of some of the crystals is very smooth. In addition, the energy dispersive X-ray analysis also confirms the SrMoO4 and CoMoO4 structure (Fig. S2).
Figure 1.
SEM images of samples: (a,b) TiO2/CoMoO4, (c,d) TiO2/SrMoO4, (e,f) TiO2/SrMoO4/CoMoO4.
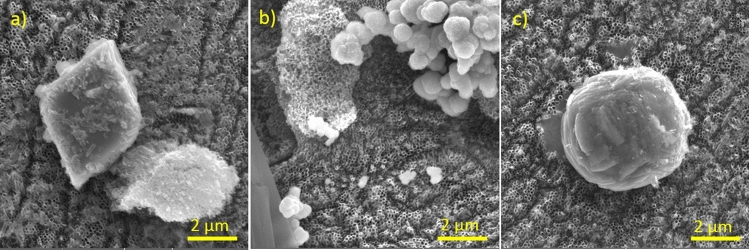
The morphologies and microstructures of the hybrid materials include of TiO2, SrMoO4 CoMoO4, and GO are present in Fig. 2. These images confirm that applying hydrothermal technology can prepare in one sample three-dimensional structures with different morphology. Figure 2a for TiO2/SrMoO4/GO sample indicates the SrMoO4 are randomly distributed on the TiO2 surface. Figure 2b displays that the as-prepared sample TiO2/CoMoO4 has microstructure with CoMoO4 agglomerated on the surface. It can also be found that these microspheres are consisted of a large number of nanosheets.
Figure 2.
SEM images of samples: (a) TiO2/SrMoO4/GO, (b) TiO2/CoMoO4/GO, (c) TiO2/SrMoO4/CoMoO4/GO.
The surface morphology of CoMoO4 with GO is comparable to sample without GO. The agglomeration of particles impacts on their size distribution and the average diameter is 1 µm. For sample TiO2/SrMoO4/CoMoO4/GO (Fig. 2c) addition of GO caused to stronger agglomeration of SrMoO4 and CoMoO4 to spherical structures. To visualize the distribution of Co, Mo, Sr, Ti, O, S, and C, energy dispersive X-ray spectroscopy mapping was performed (Fig. S3).
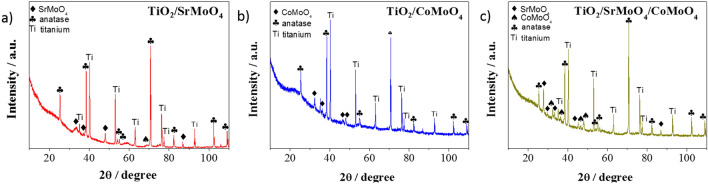
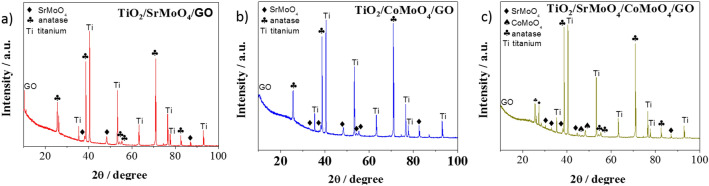
The obtained materials were also structurally characterized by XRD. Figures 3 and 4 shows the XRD pattern of TiO2/SrMoO4, TiO2/CoMoO4, TiO2/SrMoO4/CoMoO4, TiO2/SrMoO4/GO, TiO2/CoMoO4/GO, TiO2/SrMoO4/CoMoO4/GO. Some of the SrMoO4 and CoMoO4 peaks overlap with the intense anatase peaks. However, several characteristic peaks from these compounds can be observed, as indicated by the corresponding symbols in Figs. 3 and 4. However, the low intensity of these peaks proves the low crystallinity of SrMoO4 and CoMoO4. Nonetheless, the signature peaks of SrMoO4 at 33.7, 37.1, 38.5, 48.1, 76.3, 77.4, 82.3, 86.832–34 and of CoMoO4 at 25.4, 32.4, 36.3, 38.5, 40.2, 45.2, 63.035–37 are very prominent in all samples38, 39. The remaining crystallite phases were indexed as characteristic peaks of anatase and the titanium (Ti) phase acting as TiO2 NT support40. The ornamental GO structure peak was observed at 10.5 plane for only a TiO2/SrMoO4/GO hybrid. However, the presence of GO can be better characterized by Raman spectroscopy.
Figure 3.
X-ray diffraction spectra of samples: (a) TiO2/SrMoO4, (b) TiO2/CoMoO4, (c) TiO2/SrMoO4/CoMoO4.
Figure 4.
X-ray diffraction spectra of samples: (a) TiO2/SrMoO4/GO, (b) TiO2/CoMoO4/GO, (c) TiO2/SrMoO4/CoMoO4/GO.
Raman spectra of TiO2 nanotubes, TiO2/SrMoO4/CoMoO4, and TiO2/SrMoO4/CoMoO4/GO within the frequency range 100–3300 cm−1 are shown in Fig. 5. Several bands characteristic for the pure anatase crystalline form were identified in samples of pristine TiO2 and the hybrid materials. However, in the case of a material with added carbon, the anatase peaks are not that pronounced. The bands located at 143, 398, 516, and 640 cm−1 are attributed to Eg(1), B1g, A1g, and Eg(3) active anatase modes, respectively41, 42. The band at around 326 cm−1 is attributed to the symmetric stretching of the Co–O–Mo bond43. The peak of around to 300 cm−1 corresponds to Sr–O–Mo bond44. The band located at 802 cm−1 is associated with asymmetric stretching modes of O–Mo–O bond while the band at 904 cm−1 corresponds to the symmetric stretching mode of Mo–O bond45. On analyzing the Raman bands of modified material by GO, two distinct bands, namely, D and G bands, were obtained at 1347 cm−1 and 1580 cm−1. The D band is corresponding to disorder carbon while the G band is attributed to sp2 hybridized carbon46, 47. This confirmed that the graphene component is maintained during the hydrothermal process. Moreover, the second order of zone boundary phonons or 2D band which is related to the stacking nature of graphene layers was observed at 2706 cm−1 for GO48.
Figure 5.
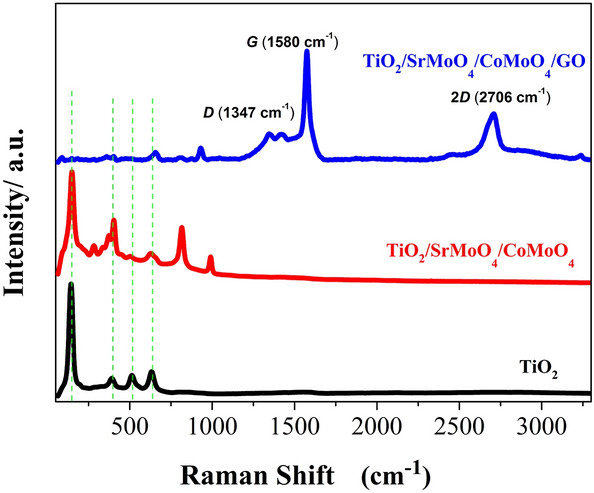
Raman spectra of TiO2 nanotubes, TiO2/SrMoO4/CoMoO4, and TiO2/SrMoO4/CoMoO4/GO.
Electrocatalytic activities for hydrogen evolution
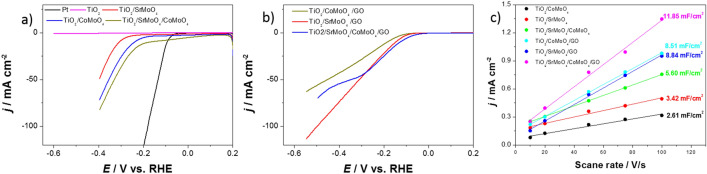
The catalytic activity of obtained hybrids for hydrogen evolution reaction (HER) was measured in 0.2 M H2SO4 using three-electrode configuration with a scan rate of 5 mV s−1. For comparison, bare TiO2 nanotubes and Pt were also tested. Figure 6a,b give the HER polarization curves of commercial Pt disc, pure TiO2 nanotubes, and the obtained hybrids without and with the addition of carbon.
Figure 6.
LSV curves of obtained catalysts (a) without and (b) with the addition of carbon. (c) Calculated double-layer capacitance (CDL) used to estimate the electrochemically active surface area of the obtained catalysts.
According to linear sweep voltammograms (LSVs) in Fig. 6a,b, the overpotential values for all electrodes at jHER = 10 mA cm−2 are summarized in Table 1. The obtained values indicate that a small amount of carbon has a large share in the HER performance of the obtained hybrids. It is worth noting that the η value of TiO2/SrMoO4/CoMoO4/GO is only 29 mV larger than that of pure Pt and comparable to or smaller than those of various transition metal-based electrocatalyst (Table S1).
Table 1.
The HER parameters, Tafel slopes and estimated electrochemically active surface area for the obtained catalysts.
| Electrode | Overpotential (mV vs. RHE) to achieve a current density of −10 mA cm−2 | Onset potential (mV vs. RHE) | b (mV dec−1) | Estimated ECSA (cm2) (0.04 mF cm−2) |
|---|---|---|---|---|
| Pt | 92 | −75 | 38 | – |
| TiO2 | Does not achieve | – | 330 | – |
| TiO2/SrMoO4 | 313 | −275 | 152 | 85.5 |
| TiO2/CoMoO4 | 242 | −219 | 168 | 65.25 |
| TiO2/SrMoO4/CoMoO4 | 167 | −209 | 91 | 140 |
| TiO2/SrMoO4/GO | 146 | −96 | 62 | 221 |
| TiO2/CoMoO4/GO | 184 | −121 | 75 | 212.75 |
| TiO2/SrMoO4/CoMoO4/GO | 121 | −60 | 90 | 296.25 |
The electrochemically active surface area (ECSA) was determined to elucidate the effect of composition on HER. The ECSA was estimated using the CV method's double-layer capacitance. For example, Fig. S4 shows the CV for TiO2/SrMoO4/CoMoO4 that was performed in the non-Faradaic region at different scanning rates from 0.35 to 0.45 V vs. Ag/AgCl/3 M KCl. For the all obtained electrodes, the charging current at the different scan rates was plotted (Fig. 6c) to obtain the double-layer capacitance from the slope. Can be observed a difference in the double-layer capacitance (CDL) between the catalysts depending on the composition. The catalysts modified with graphene oxide have higher CDL values. The ECSA was estimated using the commonly used specific capacity of 0.04 mF cm−249. Results are summarized in Table 1. The electrochemically active surface area is higher for materials with added carbon, which may impact better catalytic properties toward hydrogen evolution.
Figure S5 shows the electrochemical impedance spectra recorded for obtained catalysts at an open circuit potential. A slightly increasing slope of the curves, in the case of materials modified by carbon, indicates an improving capacitive behavior. Moreover, the small diameter or the absence of a semicircle, in the case of a material with the addition of graphene oxide, indicates the small charge transfer resistance (Rct)50. The Rct is associated with the electrocatalytic kinetics at the catalyst/electrolyte interface and gives information about the reaction rate of HER51. In summary, the lower the charge transfer resistance, the better the HER kinetics.
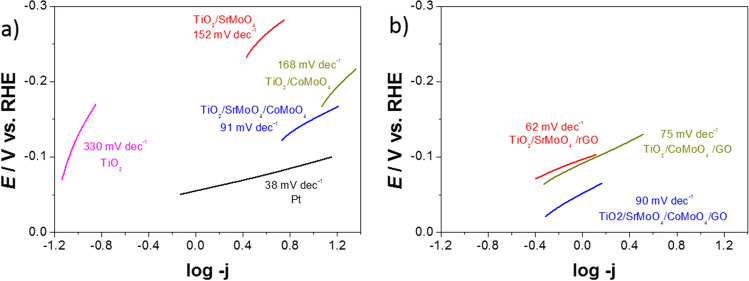
Figure 7 presents the Tafel plots. At high overpotentials the HER on the electrodes is kinetically controlled, which can be given by the Tafel equation52:
where η (V) means overpotential, a (V) is the cathodic intercept related to the exchange current density, b (V dec−1) means the cathodic Tafel slope, and j (A cm−2) means catalytic current density. Tafel slope values calculated from the linear portion of potential vs. logarithmic value of current density deliver useful kinetic metrics of the catalyst38. The Tafel slope values were 38, 330, 152, 168, 91, 62, 75, and 90 for Pt, TiO2 nanotubes, TiO2/SrMoO4, TiO2/CoMoO4, TiO2/SrMoO4/CoMoO4, TiO2/SrMoO4/GO, TiO2/CoMoO4/GO, TiO2/SrMoO4/CoMoO4/GO, respectively. Concerning as-synthesized electrocatalysts, TiO2/SrMoO4/GO showed the lowest Tafel slope value. In this case, the positive effect of the presence of carbon in all hybrids was also observed. The data of Tafel plots along are also tabulated in Table 1.
Figure 7.
The Tafel plots calculated from the corresponding LSV plots for obtained catalysts (a) without and (b) with the addition of carbon.
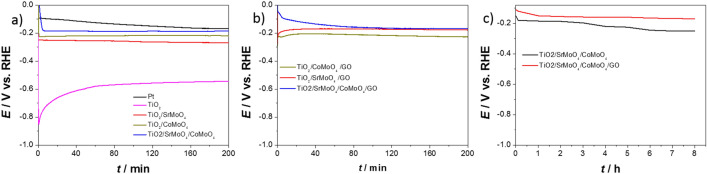
To examine the long-term stability of obtained electrodes, chronopotentiometric tests were also performed at the fixed current density of 10 mA cm−2 (Fig. 8a,b). As can be seen, almost all electrodes show good stability of overpotential with a polarization current of -10 mA cm−2. However, in the case of pure TiO2 nanotubes the overpotential had shifted from 842 to 541 mV by the end of 200 min. In other cases, the overpotential oscillates between 261 and 140 mV.
Figure 8.
Part variations of overvoltage value at a constant current density of 10 mA cm−2 with the processing time for (a) without and (b) with the addition of carbon. (c) Chronopotentiometry measurement with the current density of 10 mA cm−2 for 8 h for TiO2/SrMoO4/CoMoO4 and TiO2/SrMoO4/CoMoO4/GO.
In order to verify the validity of the modification with graphene oxide, an even longer stability test (8 h) was performed for the best materials without and with the addition of carbon. As shown in Fig. 8c, the overpotential of TiO2/SrMoO4/CoMoO4 decreased from −152 to −251 mV during 8 h, indicating lower stability compared with the same material but with the addition of carbon. The results demonstrate that adding GO during synthesis can greatly enhance its stability and electrocatalytic performance for hydrogen evolution in an acidic solution. According to the literature53–55, in carbon–metal hybrid materials, transition metals exhibit remarkable catalytic abilities, while the carbon material provides better conductivity, stability and greater surface area. In addition, interactions between carbon and metallic materials can change the properties of the whole hybrid, contributing to the formation of new sites with increased catalytic activity.
Materials and methods
TiO2 nanotubes preparation
TiO2 nanotubes were prepared by one-step anodization of titanium film according to a previously optimized procedure56. First, the titanium sheets, placed in a mixture of acetone and isopropanol (1:1 ratio), were cleaned using ultrasound for 20 min. Anodization was carried out in a two-electrode system in which both the anode and cathode were titanium sheets. Electrodes were placed in 50 cm3 water:glycol mixture (volume ratio 1:19) about 2 cm separated from each other. The electrolyte contained 0.27 M ammonium fluoride and 1 M phosphoric acid (V). The anodization process was carried out for 2 h at a constant voltage of 40 V. The nanotubes obtained on the titanium foil were washed with a solution of 100 μl HF in 50 cm3 water (to remove inorganic impurities), followed by distilled water. In the last step, the obtained material was calcined at 450 °C for 2 h. As a result, TiO2 NT were transformed from amorphous to crystalline form.
Synthesis of hybrids
The layer of SrMnO4, CoMoO4 and SrMoO4/CoMoO4, respectively, was deposited on TiO2 nanotubes by hydrothermal method. Appropriate amounts of each component of the reaction mixture (Fig. 9) were dissolved in 15 mL of deionized water to form a clear solution. Hybrids were made with or without 20 mg of GO added to the reaction mixture.
Figure 9.
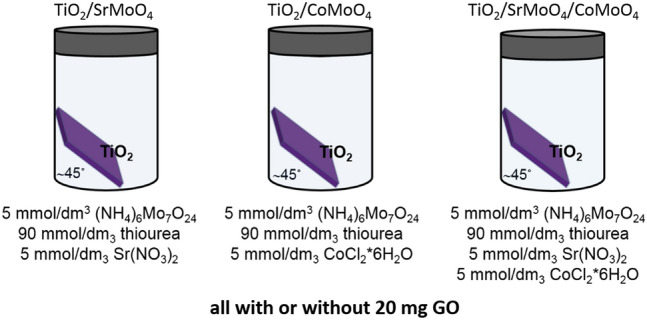
Reagents used in the hydrothermal method and their concentration.
After that, the as-prepared fresh solution and TiO2 nanotubes plate were transferred into a Teflon-lined stainless autoclave (30 mL), which was then tightly sealed and left in an oven at 220 °C for 7 h. After the autoclave cooled down to room temperature, the TiO2 nanotube plate with black layer was taken out and washed with deionized water several times to remove unstable products, which was then dried in a vacuum oven at 60 °C.
Solid state physics techniques
A MIRA3 scanning electron microscope (SEM) (Tescan, Czech Republic) was used to assess the surface. The crystalline phases were characterized by X-ray diffractometer (Philips X”Pert with detector X’Celerator Scientific). Raman spectra were recorded with a Raman spectrometer (Senterra, Bruker Optik) with a green laser (532 nm) used as the excitation source.
Electrochemical characterization
A three-electrode cell consisting of a working electrode, Pt as a counter electrode and Ag/AgCl (in 3 M KCl) as a reference electrode has been employed. 0.2 M H2SO4 solution has been used as electrolyte. All the electrochemical measurements have been performed using potentiostat/galvanostat (BioLogic VSP 2078) at room temperature. Unless otherwise specified, all potentials measured were referenced to the reversible hydrogen electrode (RHE) using the following equation: E (RHE) = E (Ag/AgCl) + 0.21 + 0.059 pH. Linear sweep voltammograms were obtained with a scan rate of 1 mV s−1. Tafel plots were derived from the corresponding LSV curves. The long-term stability was tested by a chronopotentiometric technique at a −10 mA cm−2 current density. The electrochemically active surface area (ECSA) was estimated through the cyclic voltammetry method. Under a non-Faradaic region, a series of CV scans were performed at different scan rates (10, 20, 50, 75, 100 mV s−1). The double-layer capacitance (CDL) was found by obtaining the slope of the linear fit. The ECSA of obtained electrodes was determined on the basis of the equation: ECSA = , where Cs is the specific capacitance. The Cs used for our calculations was 0.040 mF cm−2—value commonly used for metals49, 57. Moreover, EIS was performed for two of the best materials in a frequency range between 20 kHz and 1 Hz with a voltage amplitude of 10 mV at an open circuit potential.
Conclusions
An efficient hybrid catalyst in the form of TiO2 nanotubes with CoMoO4, SrMoO4, and graphene oxide on their surface was proposed in this research. The strategy of creating active centers by modification of TiO2 with carbon and perovskite-type metal oxide in one synthesis step may pave the way toward the synthesis of cheap, efficient, and stable catalysts. As a result, TiO2/SrMoO4/CoMoO4/GO hybrid was proved to be an efficient and durable HER electrocatalyst in acidic medium. The optimized catalyst only required a low overpotential of 120 mV at 10 mA cm−2 with a Tafel slope of 90 mV dec−1, superior to results of pristine TiO2. The high HER activity and excellent durability of TiO2/SrMoO4/CoMoO4/GO hybrid make it a promising alternative to commercial Pt-based HER catalyst.
Supplementary Information
Acknowledgements
The research leading to these results has received funding from the Norway Grants 2014-2021 via the National Centre for Research and Development. This work was carried out as a result of the research project no. NOR/SGS/IL-HYDROGEN/0202/2020-00.
Author contributions
M.Sz.: Conceptualization, Methodology, Investigation, Formal analysis, Visualization, Writing—original draf, Writing—review & editing, Supervision. A.I.: Methodology, Investigation, Formal analysis, Visualization, Writing—original draf, Writing—review & editing, Project administration, Funding acquisition. M.Sk.: Investigation, Formal analysis. M.W.: Investigation, Formal analysis, Technical help. J.P.L.: Conceptualization, Writing—review & editing.
Data availability
The datasets generated and/or analysed during the current study are available in the BRIDGE OF KNOWLEDGE repository (https://mostwiedzy.pl/en/open-research-data/x-ray-diffraction-spectra-of-modification-of-tio2-nanotubes-by-graphene-strontium-and-cobalt-molybda,1128104009119681-0).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-27143-5.
References
- 1.Chuyen Phan T, Nguyen V-T, Choi H-S, Kim H. Highly efficient and durable electrochemical hydrogen evolution reaction based on composition/shape controlled CuTi@Pt core-shell nanotubes in acidic media. Appl. Surface Sci. 2022;605:154331. doi: 10.1016/j.apsusc.2022.154331. [DOI] [Google Scholar]
- 2.Zhang X, et al. Defect-rich MnxOy complex Fe–Ni sulfide heterogeneous electrocatalyst for a highly efficient hydrogen evolution reaction. J. Power Sources. 2022;540:231664. doi: 10.1016/j.jpowsour.2022.231664. [DOI] [Google Scholar]
- 3.Nguyen V-T, et al. In situ engineering of Pd nanosponge armored with graphene dots using Br– toward high-performance and stable electrocatalyst for the hydrogen evolution reaction. ACS Appl. Mater. Interfaces. 2020;12:15500–15506. doi: 10.1021/acsami.9b13735. [DOI] [PubMed] [Google Scholar]
- 4.Yan Q, et al. In situ formed edge-rich Ni3S2–NiOOH heterojunctions for oxygen evolution reaction. J. Electrochem. Soc. 2022;169:054532. doi: 10.1149/1945-7111/ac7083. [DOI] [Google Scholar]
- 5.Nguyen V-T, Nguyen V-C, Phan TC, Choi H-S, Kim H. Boosting electrocatalytic oxygen evolution activity by in-situ growth of hierarchical vertically-erected Ni(OH)2 nanosheets on Ag nanowires. Int. J. Hydrogen Energy. 2022;47:31614–31623. doi: 10.1016/j.ijhydene.2022.06.017. [DOI] [Google Scholar]
- 6.Nguyen N-A, et al. Plasma-treated sponge-like NiAu nanoalloy for enhancing electrocatalytic performance in hydrogen evolution reaction. Catal. Today. 2019;337:90–96. doi: 10.1016/j.cattod.2019.03.019. [DOI] [Google Scholar]
- 7.Chai L, et al. Bottom-up synthesis of MOF-derived hollow N-doped carbon materials for enhanced ORR performance. Carbon. 2019;146:248–256. doi: 10.1016/j.carbon.2019.02.006. [DOI] [Google Scholar]
- 8.Franceschini EA, Gomez MJ, Lacconi GI. One step synthesis of high efficiency nickel/mesoporous TiO2 hybrid catalyst for hydrogen evolution reaction. J. Energy Chem. 2019;29:79–87. doi: 10.1016/j.jechem.2018.02.005. [DOI] [Google Scholar]
- 9.Wei Z, et al. Oxygen-deficient TiO2 and carbon coupling synergistically boost the activity of Ru nanoparticles for the alkaline hydrogen evolution reaction. J. Mater. Chem. A. 2021;9:10160–10168. doi: 10.1039/D0TA11986E. [DOI] [Google Scholar]
- 10.Wang K, et al. Crystalline Ru0.33Se nanoparticles-decorated TiO2 nanotube arrays for enhanced hydrogen evolution reaction. Small. 2018;14:1802132. doi: 10.1002/smll.201802132. [DOI] [PubMed] [Google Scholar]
- 11.Zabelin D, et al. Design of hybrid Au grating/TiO2 structure for NIR enhanced photo-electrochemical water splitting. Chem. Eng. J. 2022;443:136440. doi: 10.1016/j.cej.2022.136440. [DOI] [Google Scholar]
- 12.Feng JX, et al. Efficient hydrogen evolution electrocatalysis using cobalt nanotubes decorated with titanium dioxide nanodots. Angew. Chem. Int. Ed. 2017;56:2960–2964. doi: 10.1002/anie.201611767. [DOI] [PubMed] [Google Scholar]
- 13.Noor S, Sajjad S, Leghari SAK, Flox C, Kallio T. Efficient electrochemical hydrogen evolution reaction and solar activity via bi-functional GO/Co3O4–TiO2 nano hybrid structure. Int. J. Hydrogen Energy. 2020;45:17410–17421. doi: 10.1016/j.ijhydene.2020.04.240. [DOI] [Google Scholar]
- 14.Sadeghzadeh-Attar A. Boosting the photocatalytic ability of hybrid BiVO4–TiO2 heterostructure nanocomposites for H2 production by reduced graphene oxide (rGO) J. Taiwan Inst. Chem. Eng. 2020;111:325–336. doi: 10.1016/j.jtice.2020.04.001. [DOI] [Google Scholar]
- 15.Yan B, Liu D, Feng X, Shao M, Zhang Y. Ru species supported on MOF-derived N-doped TiO2/C hybrids as efficient electrocatalytic/photocatalytic hydrogen evolution reaction catalysts. Adv. Funct. Mater. 2020;30:2003007. doi: 10.1002/adfm.202003007. [DOI] [Google Scholar]
- 16.Song X, Chen G, Guan L, Zhang H, Tao J. Interfacial engineering of MoS2/TiO2 hybrids for enhanced electrocatalytic hydrogen evolution reaction. Appl. Phys. Exp. 2016;9:095801. doi: 10.7567/APEX.9.095801. [DOI] [Google Scholar]
- 17.Tao J, et al. Well-patterned Au nanodots on MoS2/TiO2 hybrids for enhanced hydrogen evolution activity. Electrochim. Acta. 2018;283:419–427. doi: 10.1016/j.electacta.2018.06.194. [DOI] [Google Scholar]
- 18.Wang W, et al. Edge-enriched ultrathin MoS2 embedded yolk-shell TiO2 with boosted charge transfer for superior photocatalytic H2 evolution. Adv. Funct. Mater. 2019;29:1901958. doi: 10.1002/adfm.201901958. [DOI] [Google Scholar]
- 19.Cheng C, et al. Enhanced visible light catalytic activity of MoS2/TiO2/Ti photocathode by hybrid-junction. Appl. Catal. B. 2018;237:416–423. doi: 10.1016/j.apcatb.2018.06.012. [DOI] [Google Scholar]
- 20.Ganesan P, Sivanantham A, Shanmugam S. CoS2–TiO2 hybrid nanostructures: Efficient and durable bifunctional electrocatalysts for alkaline electrolyte membrane water electrolyzers. J. Mater. Chem. A. 2018;6:1075–1085. doi: 10.1039/C7TA09096J. [DOI] [Google Scholar]
- 21.Liu S, et al. Ultrathin WS2 nanosheets vertically aligned on TiO2 nanobelts as efficient alkaline hydrogen evolution electrocatalyst. Int. J. Hydrogen Energy. 2020;45:1697–1705. doi: 10.1016/j.ijhydene.2019.11.018. [DOI] [Google Scholar]
- 22.Valverde-González A, et al. Understanding charge transfer mechanism on effective truxene-based porous polymers–TiO2 hybrid photocatalysts for hydrogen evolution. ACS Appl. Energy Mater. 2020;3:4411–4420. doi: 10.1021/acsaem.0c00118. [DOI] [Google Scholar]
- 23.Chen B, et al. Enhanced light-driven hydrogen-production activity induced by accelerated interfacial charge transfer in donor–acceptor conjugated polymers/TiO2 hybrid. Chem. A. Eur. J. 2019;25:3362–3368. doi: 10.1002/chem.201805740. [DOI] [PubMed] [Google Scholar]
- 24.Reddy KR, Hassan M, Gomes VG. Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl. Catal. A. 2015;489:1–16. doi: 10.1016/j.apcata.2014.10.001. [DOI] [Google Scholar]
- 25.Yuan W, et al. Laminated hybrid junction of sulfur-doped TiO2 and a carbon substrate derived from Ti3C2 MXenes: Toward highly visible light-driven photocatalytic hydrogen evolution. Adv. Sci. 2018;5:1700870. doi: 10.1002/advs.201700870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basavarajappa PS, et al. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy. 2020;45:7764–7778. doi: 10.1016/j.ijhydene.2019.07.241. [DOI] [Google Scholar]
- 27.Liu, M. et al. Regulation of hydrogen evolution performance of titanium oxide–carbon composites at high current density with a Ti–O hybrid orbital. Carbon Energy (2022).
- 28.Pandey DK, Kagdada HL, Materny A, Singh DK. Hybrid structure of ionic liquid and TiO2 nanoclusters for efficient hydrogen evolution reaction. J. Phys. Chem. A. 2021;125:2653–2665. doi: 10.1021/acs.jpca.0c10912. [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Han L, Zhu C, Zhai Y, Dong S. Aqueous-phase synthesis of Ag-TiO2-reduced graphene oxide and Pt-TiO2-reduced graphene oxide hybrid nanostructures and their catalytic properties. Nano Res. 2011;4:1153–1162. doi: 10.1007/s12274-011-0165-2. [DOI] [Google Scholar]
- 30.Tian L, Ye L, Deng K, Zan L. TiO2/carbon nanotube hybrid nanostructures: Solvothermal synthesis and their visible light photocatalytic activity. J. Solid State Chem. 2011;184:1465–1471. doi: 10.1016/j.jssc.2011.04.014. [DOI] [Google Scholar]
- 31.Makhafola MD, et al. Palladinized graphene oxide-MOF induced coupling of Volmer and Heyrovsky mechanisms, for the amplification of the electrocatalytic efficiency of hydrogen evolution reaction. Sci. Rep. 2021;11:17219. doi: 10.1038/s41598-021-96536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseinpour-Mashkani SM, Sobhani-Nasab A, Mehrzad M. Controlling the synthesis SrMoO4 nanostructures and investigation its photocatalyst application. J. Mater. Sci. Mater. Electron. 2016;27:5758–5763. doi: 10.1007/s10854-016-4489-2. [DOI] [Google Scholar]
- 33.Liu Y-F, Dai S-H, Lu Y-N, Min H-H. Microwave heating synthesis and luminescence property of Eu3+ doped SrMoO4 micro-octahedrons. Powder Technol. 2012;221:412–418. doi: 10.1016/j.powtec.2012.01.039. [DOI] [Google Scholar]
- 34.Chun F, et al. Preparation and luminescent properties of self-organized broccoli-like SrMoO4: Pr3+ superparticles. J. Lumin. 2017;190:69–75. doi: 10.1016/j.jlumin.2017.05.028. [DOI] [Google Scholar]
- 35.Mai L-Q, et al. Hierarchical MnMoO4/CoMoO4 heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011;2:1–5. doi: 10.1038/ncomms1387. [DOI] [PubMed] [Google Scholar]
- 36.Nti F, Anang DA, Han JI. Facilely synthesized NiMoO4/CoMoO4 nanorods as electrode material for high performance supercapacitor. J. Alloy Compd. 2018;742:342–350. doi: 10.1016/j.jallcom.2018.01.289. [DOI] [Google Scholar]
- 37.Ramkumar R, Minakshi M. Fabrication of ultrathin CoMoO4 nanosheets modified with chitosan and their improved performance in energy storage device. Dalton Trans. 2015;44:6158–6168. doi: 10.1039/C5DT00622H. [DOI] [PubMed] [Google Scholar]
- 38.Kareem A, Kunhiraman AK, Maiyalagan T. Hydrogen evolution reaction catalyzed by microstructured SrMoO4 decorated on three-dimensional nanostructured rGO/f-MWCNT in acidic medium. Ionics. 2020;26:5055–5064. doi: 10.1007/s11581-020-03634-1. [DOI] [Google Scholar]
- 39.Zhang Y, et al. New NiMoO4/CoMoO4 composite electrodes for enhanced performance supercapacitors. Ionics. 2020;26:3579–3590. doi: 10.1007/s11581-020-03470-3. [DOI] [Google Scholar]
- 40.Li H, Tang N, Yang H, Leng X, Zou J. Interface feature characterization and Schottky interfacial layer confirmation of TiO2 nanotube array film. Appl. Surf. Sci. 2015;355:849–860. doi: 10.1016/j.apsusc.2015.07.088. [DOI] [Google Scholar]
- 41.Gupta SM, Tripathi M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011;56:1639–1657. doi: 10.1007/s11434-011-4476-1. [DOI] [Google Scholar]
- 42.Trzciński K, Szkoda M, Siuzdak K, Sawczak M, Lisowska-Oleksiak A. Electrochemical and photoelectrochemical characterization of photoanodes based on titania nanotubes modified by a BiVO4 thin film and gold nanoparticles. Electrochim. Acta. 2016;222:421–428. doi: 10.1016/j.electacta.2016.10.194. [DOI] [Google Scholar]
- 43.Ahmed J, Ubaidullah M, Ahmad T, Alhokbany N, Alshehri SM. Synthesis of graphite oxide/cobalt molybdenum oxide hybrid nanosheets for enhanced electrochemical performance in supercapacitors and the oxygen evolution reaction. ChemElectroChem. 2019;6:2524–2530. doi: 10.1002/celc.201900055. [DOI] [Google Scholar]
- 44.Muralidharan M, Anbarasu V, Elaya Perumal A, Sivakumar K. Enhanced ferromagnetism in Cr doped SrMoO4 scheelite structured compounds. J. Mater. Sci. Mater. Electron. 2016;27:2545–2556. doi: 10.1007/s10854-015-4057-1. [DOI] [Google Scholar]
- 45.Liu F, et al. Multi-dimensional CuO nanorods supported CoMoO4 nanosheets heterostructure as binder free and high stable electrode for supercapacitor. J. Mater. Sci. Mater. Electron. 2018;29:10353–10361. doi: 10.1007/s10854-018-9092-2. [DOI] [Google Scholar]
- 46.Zhou G, et al. Oxygen bridges between NiO nanosheets and graphene for improvement of lithium storage. ACS Nano. 2012;6:3214–3223. doi: 10.1021/nn300098m. [DOI] [PubMed] [Google Scholar]
- 47.Li N, et al. Battery performance and photocatalytic activity of mesoporous anatase TiO2 nanospheres/graphene composites by template-free self-assembly. Adv. Funct. Mater. 2011;21:1717–1722. doi: 10.1002/adfm.201002295. [DOI] [Google Scholar]
- 48.How GTS, Pandikumar A, Ming HN, Ngee LH. Highly exposed 001 facets of titanium dioxide modified with reduced graphene oxide for dopamine sensing. Sci. Rep. 2014;4:1–8. doi: 10.1038/srep05044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connor P, Schuch J, Kaiser B, Jaegermann W. The determination of electrochemical active surface area and specific capacity revisited for the system MnOx as an oxygen evolution catalyst. Z. Phys. Chem. 2020;234:979–994. doi: 10.1515/zpch-2019-1514. [DOI] [Google Scholar]
- 50.Lu C, et al. Nitrogen-doped Ti3C2 MXene: mechanism investigation and electrochemical analysis. Adv. Funct. Mater. 2020;30:2000852. doi: 10.1002/adfm.202000852. [DOI] [Google Scholar]
- 51.Li R, et al. Nitrogen doped MoS2 nanosheets synthesized via a low-temperature process as electrocatalysts with enhanced activity for hydrogen evolution reaction. J. Power Sources. 2017;356:133–139. doi: 10.1016/j.jpowsour.2017.04.060. [DOI] [Google Scholar]
- 52.Herraiz-Cardona I, Ortega E, Vázquez-Gómez L, Pérez-Herranz V. Double-template fabrication of three-dimensional porous nickel electrodes for hydrogen evolution reaction. Int. J. Hydrogen Energy. 2012;37:2147–2156. doi: 10.1016/j.ijhydene.2011.09.155. [DOI] [Google Scholar]
- 53.Zhang, W. et al. Recent development of transition metal doped carbon materials derived from biomass for hydrogen evolution reaction. Int. J. Hydrogen Energy (2022).
- 54.Murthy AP, Madhavan J, Murugan K. Recent advances in hydrogen evolution reaction catalysts on carbon/carbon-based supports in acid media. J. Power Sources. 2018;398:9–26. doi: 10.1016/j.jpowsour.2018.07.040. [DOI] [Google Scholar]
- 55.Zhou W, et al. Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy. 2016;28:29–43. doi: 10.1016/j.nanoen.2016.08.027. [DOI] [Google Scholar]
- 56.Szkoda M, et al. Electrodes consisting of PEDOT modified by Prussian Blue analogues deposited onto titania nanotubes—Their highly improved capacitance. Solid State Ionics. 2017;302:197–201. doi: 10.1016/j.ssi.2016.12.025. [DOI] [Google Scholar]
- 57.McCrory CC, et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015;137:4347–4357. doi: 10.1021/ja510442p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the BRIDGE OF KNOWLEDGE repository (https://mostwiedzy.pl/en/open-research-data/x-ray-diffraction-spectra-of-modification-of-tio2-nanotubes-by-graphene-strontium-and-cobalt-molybda,1128104009119681-0).