Abstract
The effective application of wastewater surveillance is dependent on testing capacity and sensitivity to obtain high spatial resolution testing results for a timely targeted public health response. To achieve this purpose, the development of rapid, high-throughput, and sensitive virus concentration methods is urgently needed. Various protocols have been developed and implemented in wastewater surveillance networks so far, however, most of them lack the ability to scale up testing capacity or cannot achieve sufficient sensitivity for detecting SARS-CoV-2 RNA at low prevalence. In the present study, using positive raw wastewater in Hong Kong, a PEG precipitation-based three-step centrifugation method was developed, including low-speed centrifugation for large particles removal and the recovery of viral nucleic acid, and medium-speed centrifugation for the concentration of viral nucleic acid. This method could process over 100 samples by two persons per day to reach the process limit of detection (PLoD) of 3286 copies/L wastewater. Additionally, it was found that the testing capacity could be further increased by decreasing incubation and centrifugation time without significantly influencing the method sensitivity. The entire procedure uses ubiquitous reagents and instruments found in most laboratories to obtain robust testing results. This high-throughput, cost-effective, and sensitive tool will promote the establishment of nearly real-time wastewater surveillance networks for valuable public health information.
Keywords: SARS-CoV-2, Wastewater surveillance, PEG precipitation, High-throughput
Graphical abstract
1. Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has led to several hundred million infections and several million death globally on August 13, 2022. Continuous pandemics affect the social economy and daily life in multiple aspects. To implement timely control measures and policies, regular large-scale testing is of great importance for screening the pandemic process. Wastewater Surveillance (WWS), or wastewater-based epidemiology (WBE), was considered as a non-invasive and independent surveillance tool for large-scale population testing that is not limited by the clinical testing capacity or individual health-seeking behaviors (WHO, 2022). More than 60 countries globally have started to monitor SARS-CoV-2 RNA from wastewater to trace infection dynamics and implement public health actions in response to the COVID-19 pandemic (Naughton et al., 2021), including the United States (Peccia et al., 2020; Wolfe et al., 2021; Wu et al., 2021), Netherlands (Medema et al., 2020), Australia (Ahmed et al., 2020a), Finland (Tiwari et al., 2022), Switzerland (Huisman et al., 2022), Spain (Carcereny et al., 2021), and Singapore (Lee et al., 2021), etc.
In Hong Kong, the wastewater surveillance network has been established for routine screening monitoring and has been proved to be a useful tool in providing valuable public health information, such as providing early warning signals of community outbreaks (Xu et al., 2021), monitoring pandemic dynamics (Zheng et al., 2022b), informing public health interventions (Deng et al., 2022b), and discovering infected individuals with variants of concern from communities (Deng et al., 2022a). Multiple analytical protocols have been developed during this process to address technical concerns (Xu et al., 2022a, 2022b; Zheng et al., 2022a), and practical experience has been compiled for data interpretation (Deng et al., 2022b; Zhang, 2022).
Owing to the rapid onset and transmission of the virus, it is necessary to obtain high-resolution wastewater surveillance results for near real-time testing and conduct a regular wastewater monitoring at a community-scale level (Wu et al., 2022). Correspondingly, the current testing protocol should be optimized to increase the wastewater testing capacity for large-scale applications. Based on our previous systematic evaluation, the ultracentrifugation method outperformed other methodologies in terms of method sensitivity and has been implemented in routine wastewater surveillance in Hong Kong (Zheng et al., 2022a). However, samples scalability is a limitation, and it is dependent on the expensive ultracentrifugation equipment. Therefore, in this study, we aimed to develop a high-throughput, rapid, and sensitive method for the large-scale application of wastewater surveillance.
For the whole analytical procedures, the bottleneck of increasing testing capacity is the virus concentration and RNA extraction of viral nucleic acids from wastewater samples. To date, limited high-throughput methods have been reported (Daigle et al., 2022; Karthikeyan et al., 2021; Mailepessov et al., 2022) and their method sensitivity needs to be optimized for the detection of SARS-CoV-2 RNA during the post-vaccination pandemic stage with fewer infections and/or more asymptomatic/mild infections. Furthermore, due to the variances in the matrix effect from different wastewater treatment plants (WWTPs), the applicability and feasibility of analytical methods should be adapted according to the local wastewater samples.
In the present study, we focused on precipitation-based approaches because of their potential scalability without the requirement of specialized equipment or intensive labor. The performance of analytical methods is evaluated using the following criteria: (1) method sensitivity, (2) robustness, (3) turnaround time, and (4) scalability for large-scale applications. To be more specific, this study focused on the evaluations of (1) different flocculants (i.e., PEG, MgCl2, AlCl3, FeCl3, skimmed milk); (2) different extraction methods (i.e., QIAamp Viral RNA Kit, MagMAX Microbiome Ultra Nucleic Acid Isolation Kit, Power Microbiome Kit); (3) different processing volumes (i.e., 1.6 mL, 40 mL); and (4) different processing time lengths (i.e. incubation times of 0, 0.5, 2, or 8 h, and centrifugation times of 15, 30, or 60 min).
2. Materials and methods
2.1. Samples collection
This study used raw wastewater samples collected from the manhole or WWTPs from March 1, 2022, to April 28, 2022, during the 5th COVID-19 pandemic outbreak in Hong Kong for method evaluation. During the sampling period, the daily incidence rates in Hong Kong were reported to be 5 to 1027 cases per 100,000 individuals, covering the descending stage of the 5th pandemic wave. The 3 h and 24 h composite samples were taken by the Drainage Services Department for manhole samples and WWTPs influent samples, respectively. The wastewater samples were delivered to the laboratory on ice in a separate container. To ensure laboratory safety, all wastewater samples were conducted heat-inactivation at 60 ℃ for 30 mins before processing.
2.2. Preanalytical methods
The preanalytical methods used in this study were summarized in Table S1.
Method 1 was the benchmark for PEG-precipitation method. Specifically, the wastewater samples were briefly centrifuged at 2000 × g for 2 min to remove large particles. After separating the supernatant (40 mL), 4 g PEG (10%, w/v) and 0.8 g NaCl (2%, w/v) were added for flocculation. The mixture was shaken in an orbital shaker at 25 ℃ for 2 h with the speed of 180 rpm for incubation. After that, a second centrifugation was performed at 4750 × g for 30 min to obtain approximately 1∼2 mL of precipitate for transferring into a new 2 mL microcentrifuge tube. The third centrifugation at 20,000 × g was conducted for 2 min, and the supernatant was discarded carefully without disturbing the pellet at the bottom using a pipette. Next, the pellet was used for RNA extraction using the QIAamp Viral RNA Kit (Qiagen, referred to as “QIAamp Viral” in the following description, Catalog number: 52904) in the QIAcube Connect System (Qiagen). The elution volume was 50 μL.
2.2.1. Comparison of the flocculants
Methods 2–9 began with the addition of different flocculants into the 40 mL supernatant after low-speed centrifugation at 2000 × g for 2 min, including single flocculants (MgCl2/AlCl3/FeCl3/skimmed milk) and combined flocculants (PEG + MgCl2/PEG + AlCl3/PEG + FeCl3/PEG + skimmed milk) as described in Table S1. These single flocculants were selected for testing because they have been reported for the application of the virus concentration method (Haramoto et al., 2020; Langenfeld et al., 2021; Philo et al., 2021; Randazzo et al., 2020), and the combination of multiple flocculants was used considering the possible synergistic effects on the flocculation process (Cui et al., 2020). The remaining steps were the same as those in Method 1.
2.2.2. Comparison of the extraction methods
Methods 10–11 used the MagMAX Magnetic-Bead Microbiome Ultra Nucleic Acid Isolation Kit (Thermo Fisher, referred to as “Magnetic Bead”, Catalog number: A42357, conducted manually in this study following the manufacturer's instructions), and RNeasy PowerMicrobiome Kit (Qiagen, referred to as “Power Microbiome” in the following description, Catalog number: 26000–50) in QIAcube Connect System for RNA extraction. These extraction kits were selected for evaluation because they are widely applicable in SARS-CoV-2 wastewater surveillance (Table S2) and in automated extraction platforms to minimize labor requirements. The final RNA elution volume for Magnetic Bead and Power Microbiome was 50 µL, the same as those for QIAamp Viral in the Method 1.
2.2.3. Comparison of the processing volumes
Method 12 proceeded 1.6 mL wastewater samples with 0.4 mL liquid mixture of PEG and NaCl in a 2 mL microcentrifuge tube (“PEG-small” method). In comparison to Method 1 (“PEG-large” method) of 40 mL wastewater samples, the startup volume of 1.6 mL in PEG-small could simplify the processing procedure, increase the testing capacity, and minimize the turnaround time. A liquid mixture of PEG (50%, w/v) and NaCl (10%, w/v) was prepared in advance by heating in a water bath at 60 ℃ for 30 min. The 2 mL microcentrifuge tube was then shaken at 4500 × g for 15 min and further centrifuged at 20,000 × g for 15 min to obtain the concentrated pellet for RNA extraction using QIAamp Viral.
2.2.4. Comparison with ultracentrifugation method
Method 13 is based on ultracentrifugation method (called “UC”) as described in our previous study (Zheng et al., 2022a) with slight modification. In details, wastewater samples were first centrifuged at 4750 × g for 10 min to separate into two subsamples, i.e. supernatant and pellet. Next, 30 mL supernatant was performed ultracentrifugation at 150,000 × g for 30 min to obtain a 200 μL concentrated sample for RNA extraction using QIAamp Viral.
2.2.5. Optimization of the method parameters
Methods 14–16 were modified versions of Method 1 using different incubation time periods, i.e., 0 h (no incubation), shaking for 0.5 h or 8 h (representing overnight in most published papers).
Methods 17–18 were modified versions of Method 1 that used different centrifugation time periods for the recovery of viral nucleic acid, i.e., centrifugation at 4750 × g for 15 min or 60 min.
Method 19 was modified version of Method 1 by adding PEG as water solution. The 32 mL supernatant was mixed with 8 mL PEG solution mentioned in Method 12 for flocculation, and the following steps were the same as in Method 1.
Although PEG solution has been used in the purification of exosomes for total protein and RNA extraction (Rider et al., 2016), it has rarely been used to enrich viral nucleic acids from wastewater samples. Considering the similarity in the size of exosomes (30–150 nm) (Doyle and Wang, 2019) and SARS-CoV-2 viral particles (70–90 nm) (Lee, 2020), the PEG solution was tested and evaluated for viral enrichment. To the best of our knowledge, this is the first trial in which SARS-CoV-2 was concentrated from wastewater samples using a PEG solution.
Method 20 used 200 μL raw wastewater for direct RNA extraction using QIAamp Viral.
2.3. RT-qPCR detection
The SARS-CoV-2 virus concentration in the wastewater samples was quantified by RT-qPCR using N1 primers and probe from the United States Center for Disease Control (US CDC) (CDC, 2020). The RT-qPCR was performed in a 20 μL reaction mixture containing 4 μL template RNA, 5 μL 4 × TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher), a forward primer concentration of 500 nM, a reverse primer concentration of 500 nM, a probe concentration of 250 nM, and DEPC-treated water to 20 μL. The thermal cycling conditions were conducted at 50 °C for 5 min, and 95 °C for 20 s, followed by 45 cycles of 95 °C for 5 s and 55 °C for 30 s on the Applied Biosystems ViiA7 qPCR system (Thermo Fisher). Quantification of SARS-CoV-2 in wastewater samples was performed with the standard curve generating from a synthetic plasmid containing N1 target (Beijing Genomics Institute, Hong Kong, China). The concentration of the plasmid was quantified using a Qubit dsDNA HS assay kit (Thermo Fisher, USA), and then the copy number of it was calculated based on the sequence length and Avogadro's number. For each 96-well plate, the plasmid was conducted 10-fold serial dilution by ddH2O to obtain the virus concentrations ranging from 107 to 1 copy/μL. The slope, Y-intercept, and R2 values of each standard curve were obtained using the linear regression. Each sample was run in duplicate and ddH2O was served as the negative control in each test batch. Wastewater samples with a Ct value less than 40 in one out of two reactions were considered to have signals of SARS-CoV-2. Each batch experiment was QA/QC checked according to the MIQE guidelines. The performance of standard curves and MIQE checklist were summarized in Table S3 and S4.
2.4. Experimental designs for the method comparison
To evaluate the performance of the different flocculants, each raw wastewater sample was randomly divided into nine split samples and processed independently using Method 1–9 as described in Section 2.2. Biological duplicates were performed with six different wastewater samples (n = 6), and technical duplicates were performed in the RT-qPCR step by running two reactions for each RNA sample (n = 2).
Similarly, 20 raw wastewater samples were used as biological duplicate for the evaluation of three extraction kits using Methods 1, 10, and 11 (n = 20), 46 raw wastewater samples were used for the comparison of processing volume using Methods 1 and 12 (n = 46), 14 raw wastewater samples were used for the comparison of incubation time using Methods 1, 14, and 16 (n = 14), 12 raw wastewater samples were used for the comparison with ultracentrifugation method using Methods 1 and 13 (n = 12), 16 raw wastewater samples were used for the comparison of centrifugation time using Methods 1, 17, and 18 (n = 16), and 18 raw wastewater samples were used for the comparison of PEG forms using Methods 1 and 19 (n = 18).
2.5. Serial dilution of raw wastewater
Raw wastewater samples were screened to determine whether it is positive or negative samples by the two-step ultracentrifugation method using the US CDC N1 and E assays in Hong Kong routine wastewater surveillance (Deng et al., 2022a). The positive raw wastewater with a concentration of approximately 107 copies/L was 10-fold diluted using the negative raw wastewater to obtain a SARS-CoV-2 virus concentration range of approximately 102 – 107 copies/L wastewater for the evaluation of method sensitivity. For each virus concentration, the wastewater samples were aliquoted into subsamples and processed by Methods 1, 12, and 13 as mentioned in Section 2.2. The wastewater samples at each virus concentration were proceeded in duplicate. To calculate the theoretical value of the virus concentration in raw wastewater, triplicate aliquots of 200 μL of positive raw wastewater (n = 3) was extracted directly for detection The entire serial dilution experiment was repeated in duplicate using positive wastewater samples at two different timepoints, i.e. ST-052 on March 10, 2022, and KT Site 2–1 on March 14, 2022. The process limit of detection (PLoD) was defined as the lowest wastewater concentration at which above 95% detection can be achieved throughout the entire processing procedure, from virus concentration, RNA extraction to the RT-qPCR detection (Ahmed et al., 2022b).
2.6. Calculation of recovery efficiency
The recovery efficiency (RE) of SARS-CoV-2 was calculated using the following equation.
Where,
: Detected SARS-CoV-2 virus concentration in wastewater samples using the evaluated methods, copies/L;
SARS-CoV-2 virus concentration in raw wastewater samples, which was detected by direct extraction of 200 μL raw wastewater samples for detection, copies/L.
This study calculated the relative recovery efficiency by using the raw positive wastewater samples as stated in another study of Mailepessov et al. (Mailepessov et al., 2022), which may lead to the difference of RE values obtained using the spiked wastewater.
2.7. Statistical analysis
The performance of the different preanalytical methods was evaluated using the following four criteria: (1) method sensitivity, (2) robustness, (3) turnaround time, and (4) scalability for large-scale applications. The method sensitivity was assessed by comparing Ct value, detected virus concentration, and recovery efficiency for the same wastewater samples. Lower Ct values in the same batch experiment was considered to present higher method sensitivity. Higher detected virus concentrations or higher recovery efficiency also indicated higher method sensitivity. In addition, the detection dynamic range and PLoD were compared in the serial dilution experiment to evaluate the method sensitivity. Robustness was assessed by the variation of Ct values among the technical replicates for the same wastewater samples. Turnaround time was assessed by comparing required time for processing the same number of wastewater samples. Scalability for large-scale applications was assessed based on the requirements of the instruments.
One-way analysis of variance (ANOVA) was firstly used to determine whether there were any statistically significant differences between the means of three or more independent groups. If there were significant differences, a post-hoc T-test was subsequently performed for pairwise comparisons between group means, and to determine differences between specific groups. When p value was less than 0.05, the difference was considered significant. All statistical tests and figures were performed using R Studio version 1.3.
3. Results and discussions
3.1. Selection of the flocculant
Nine precipitation-based virus concentration methods with different flocculants, either individual or combined, were evaluated at the same wastewater volume of 40 mL (n = 6) (Fig. 1 a and Fig. 1b). For individual flocculants, PEG showed significantly lower Ct values and higher virus concentrations than other flocculants, including MgCl2, AlCl3, FeCl3, and skimmed milk, which demonstrated the superior performance of PEG on virus enrichment and the highest recovery efficiency from wastewater samples. Furthermore, PEG combined with MgCl2 resulted in higher virus concentrations than PEG-precipitation alone, while the difference was insignificant. Additional wastewater samples (n = 8) were evaluated using PEG-precipitation, and PEG combined with MgCl2 of different doses (1%, v/v, and 10%, v/v). As shown in Fig. 1c and 1d, there was an insignificant difference in the detected Ct values and virus concentrations among the three evaluated methods. Therefore, PEG-precipitation was chosen as the benchmark method for subsequent comparisons owing to its feasibility.
Fig. 1.
Selection of flocculants on SARS-CoV-2 preanalytical methods. (a) Ct value and (b) detected SARS-CoV-2 virus concentration of single and combined flocculants from six different wastewater samples (n = 6). (c) Ct value and (d) detected SARS-CoV-2 virus concentration of single flocculants using PEG, and combined flocculants with different percentage (PEG + 1% MgCl2 and PEG+10% MgCl2) from eight different wastewater samples (n = 8). *: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001.
Similarly, PEG-precipitation presented higher recovery efficiency than AlCl3 flocculation (Perez-Cataluna et al., 2021), and skimmed milk flocculation (Pino et al., 2021) in other studies comparing virus concentration methods. However, this result disagrees with our previous study, which found that AlCl3 flocculation outperformed PEG-precipitation using SARS-CoV-2 spiked wastewater for method evaluation (Zheng et al., 2022a). The difference may be caused by different sample types for technical evaluation, such as spiked SARS-CoV-2 in the latter study versus positive raw wastewater samples in this investigation. For spiked wastewater, SARS-CoV-2 is inactivated before spiking into wastewater by heat-treatment or gamma-irradiation, which results in the disintegration of virion structure and the presence of free RNA or intact viral particles (Wurtzer et al., 2021). The exogenously spiked virus is different from the endogenous SARS-CoV-2 in wastewater in terms of partitioning behaviors and ultimately affects the affinity for different flocculants. Therefore, positive raw wastewater is closer to the targeted samples for wastewater surveillance and is recommended for method evaluation to identify an optimal protocol for applications.
In the current study, the synergistic effects on flocculation were not efficient in terms of virus concentration. It is probable that the inhibitory substances are co-enriched with viral nucleic acids together in the flocculation procedure and further interfere with the nucleic acid extraction and RT-qPCR detection. Significant inhibitory matrix effects have been reported in virus detection when processing large-volume wastewater samples for virus enrichment in Hong Kong (Xu et al., 2022b).
Additionally, the recovery efficiency of the PEG precipitation method ranged from 0.08% to 44% (Ahmed et al., 2020b; D'Aoust et al., 2021; LaTurner et al., 2021), which was attributed to the differences in processing sample volume, extraction method, and centrifugation time, etc., Thus, these key parameters for PEG precipitation were selected for evaluation in the subsequent analysis.
3.2. Selection of the extraction method
As shown in Fig. 2 , the highest SARS-CoV-2 virus concentration was observed in the QIAamp Viral, followed by the Magnetic Bead and finally in the Power Microbiome (p > 0.05). Using QIAamp Viral as the reference extraction method for comparison, 50% (10/20) of the samples obtained lower Ct values in Magnetic Bead, while only 20% (4/20) of the samples presented lower Ct values in the Power Microbiome (Table S5). In terms of method sensitivity, the three selected extraction methods are comparable to the extraction of SARS-CoV-2 RNA from wastewater samples without significant difference (p > 0.05).
Fig. 2.
Selection of extraction methods on SARS-CoV-2 preanalytical methods. (a) Ct value and (b) detected SARS-CoV-2 virus concentration of different extraction methods from twenty different wastewater samples (n = 20).
Meanwhile, wastewater surveillance aims to provide the early detection of pandemics in the community, putting greater emphasis on the turnaround time of reliable results. These three extraction methods are all applicable for automation, and the main factor contributing to turnaround time is the throughput of the automated extraction system, for example, 24 samples per run for the KingFisher Flex Purification System and 12 samples per run for the QIAcube Connect System. Thus, the turnaround time for extracting RNA from 24 samples was 2 h, 3 h and 1 h for the QIAamp Viral, Power Microbiome and Magnetic Bead, respectively. There could be different arrangements depending on the available resources, the supply chain and the surveillance purpose. In this study, the QIAamp Viral was selected as the extraction method in the combination with PEG-precipitation method, and it was adaptable to the QIAcube Connect System for automation.
3.3. Selection of the processing volume
Currently, most reported PEG-precipitation methods focus on 40 - 50 mL (Ahmed et al., 2020b; Wu et al., 2020), or 200 - 250 mL (La Rosa et al., 2021; LaTurner et al., 2021). The input volume of wastewater influences the instrument requirements, reproducibility, sensitivity, and variability (LaTurner et al., 2021). In this study, a small volume (1.6 mL) of wastewater (PEG-small) combined with PEG solution was developed and compared with a large volume (40 mL) PEG-precipitation method (PEG-large). PEG-small exhibited the same detected dynamic range as PEG-large in the serial dilution experiment, and obtained the same detection rates as PEG-large for 46 raw wastewater samples at different SARS-CoV-2 concentration levels (Fig. 3 , Table S6).
Fig. 3.
Selection of processing volume on SARS-CoV-2 preanalytical methods. (a) Ct value of in 10-fold serial dilution experiments. (b) Comparison of detected and theoretical SARS-CoV-2 virus concentration. (c) Ct value and (d) detected SARS-CoV-2 virus concentration from forty-six wastewater samples (n = 46).
In addition, we divided the evaluated wastewater samples into three groups according to the detected Ct values using the PEG-large method: high- concentration (Ct values of [26 – 30)), medium- concentration (Ct values of [30 – 32)), and low-concentration (Ct values of [32 – 34)). As shown in Table S6, the detection rate was the same for PEG-small and PEG-large, regardless of SARS-CoV-2 levels. The average ΔCT (PEG-small - PEG-large) values were 2.60, 1.81, and 0.28 for high-, medium-, and low-concentration, respectively. All values were lower than the theoretical Ct difference (4.64), with a volume factor of 25. These results imply that PEG-small is applicable for wastewater surveillance, and its recovery efficiency is higher than that of PEG-large.
Furthermore, when processing 48 samples/run, the required time for PEG-small (1.5 h) is half that of PEG-large (3 h), indicating the viability of generating timely data for high-resolution wastewater surveillance. However, smaller volumes have the disadvantage of larger testing biases, and may be more influenced by the heterogeneous nature of wastewater, which may compromise the robustness of the testing results. Therefore, this new method was suggested to be applied into smaller sewersheds with smaller population sizes and lower dilution effects, or during the peak period of the pandemic.
3.4. Performance of the method sensitivity
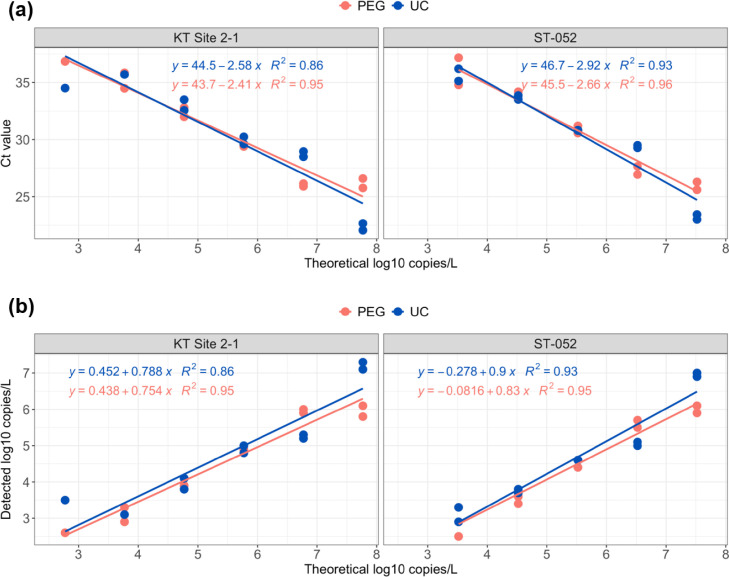
To evaluate the sensitivity of the PEG-precipitation method, it was compared with UC method, which is currently adopted in the Hong Kong wastewater surveillance network owing to its superior method sensitivity in previous evaluations (Zheng et al., 2022a). As shown in Fig. 4 , both methods were able to detect viral signal at the dynamic ranges of 2.52–7.77 log10 copies/L in serial diluted wastewater, and the PLoD was estimated to be < 3286 copies/L wastewater samples. The good linear regression for dilution (R2 > 0.85) revealed the reproducibility of quantification for these two methods. In addition, the same detection rate of 91.6% (11/12) was observed in raw wastewater for both the PEG and UC method, while the recovery efficiency of the PEG method (22.49 ± 15.66%) was 3-fold higher than that of the UC method (7.49 ± 6.83%) (Fig. 5 ). Meanwhile, both of them obtained reproducible results, with the standard deviation (SD) of Ct values between technical triplicate within 0.6 Ct among four wastewater samples (n = 4) (Table S7), suggesting their robustness in practical application. Taken together, PEG approach is equivalent to or ever better than the UC method in term of method sensitivity and reproducibility.
Fig. 4.
Comparison of method performances between PEG-precipitation (PEG) and ultracentrifugation (UC) methods in the serial dilution experiment. (a) Ct value. (b) Detected SARS-CoV-2 virus concentration.
Fig. 5.
Comparison of method performances between PEG-precipitation (PEG) and ultracentrifugation (UC) methods in the raw wastewater samples (n = 12). (a) Ct value. (b) Detected SARS-CoV-2 virus copies per reaction. (c) Detected SARS-CoV-2 virus concentration. (d) Recovery efficiency.
Calculation of RE and PLoD is an important process control for the comparisons among different protocols and the evaluation of genome loss during analytical procedures. Ideally, the RE and PLoD need to be measured by spiking live SARS-CoV-2 virus with an exact known virus amount into the residential toilets and then detecting the samples from downstream manhole or WWTPs. This evaluation experiment, however, is difficult to carry out owing to the substantial risks associated with handling live SARS-CoV-2. Alternatively, the negative raw wastewater was spiked with proxy viruses in the laboratories to estimate the RE and PLoD values, such as inactivated SARS-CoV-2 (Ahmed et al., 2022b; Perez-Cataluna et al., 2021), bovine coronavirus (BCoV) (LaTurner et al., 2021), Alphacoronavirus HCoV-229E (La Rosa et al., 2021), and murine hepatitis virus (MHV) (Ahmed et al., 2020b), etc.
However, there are several uncertainties associated with spiked wastewater. Firstly, the spike-in virus was treated with inactivation methods before spiking into the wastewater, leading to changes in virus forms, and the labile RNA came into contact with the highly RNase-containing wastewater matrix (Wurtzer et al., 2021). Secondly, spiking procedure was performed in a short time (0.5∼2 h), even though shaking completely by the shaker in our study, it is still difficult to stimulate the real behavior of viral particles in wastewater transportation. In this aspect, the difference between endogenous and exogenous viruses may result in differences in virus concentration methods owing to the differences in viral partition behavior in the supernatant or pellet. Thirdly, the wastewater samples matrix was associated with the physico-chemical characteristics of different wastewater treatment processes and varied among different WWTPs (Kantor et al., 2021). Thus, the wastewater selected for spiking may not be representative of the target wastewater for routine surveillance. Fourthly, the calculation of RE and PLoD values relies on the quantification procedures, including the quantification method for surrogate viruses, the quantification platform (qPCR or digital PCR), and the standard curves of PCR assays, etc. (Ahmed et al., 2022b; Kantor et al., 2021).
In contrast, the positive raw wastewater used in this study experienced transportation procedures and used matrix-relevant samples for quantification, which contributed to the selection of a more feasible method for practical application. However, the exact amount of viral nucleic acids in wastewater is unclear, although the aliquots of raw wastewater were quantified directly to obtain the relative true values in this study.
Considering the challenges and limitations with the calculation of RE and PLoD values, it is suggested that the comparison of different concentration protocols is conducted using the local wastewater and then evaluated by the measured Ct values or detected virus concentration in the same batch experiment without the calculation of the real recovery rates. In the future, more research is needed to standardize analytical procedures and establish environmental reference standard materials for virus quantification across studies, which are essential for longitudinal trend analysis and quantitative epidemiological models.
3.5. Optimization of the PEG-precipitation method
Turnaround time for a protocol should be considered to proceed a large number of samples and report testing results for a timely public health response. In this study, we evaluated different incubation and centrifugation times to examine the potential of PEG-precipitation method to decrease turnaround time. As shown in Fig. 6 , the detected SARS-CoV-2 RNA virus concentrations showed insignificant difference with the change in incubation or centrifugation time, implying that the turnaround time could be further shortened without a significant change in the method sensitivity. Interestingly, overnight incubation led to similar results as those obtained without the incubation step, suggesting that the incubation step in the conventional PEG-precipitation method could be skipped to simplify the procedure and increase sample capacity.
Fig. 6.
Evaluation of different parameters on the method performances. (a) Ct value. (b) Detected SARS-CoV-2 virus concentration.
Similarly, another study also found that overnight incubation did not increase the recovery efficiency of MHV using the PEG-precipitation procedure, and no incubation yielded a recovery efficiency comparable to that of a 2 h incubation time (Torii et al., 2022). In a prior study, the requirement of overnight incubation time hindered the choice of PEG-precipitation for practical application, even though PEG-precipitation had the highest recovery efficiency when compared to other analytical procedures (Perez-Cataluna et al., 2021). The present study overcomes this limitation with reliable experimental results to shorten turnaround time, which could increase the sample capacity from 100 samples per day to 150 samples per day by two persons.
Next, we evaluated the use of PEG solution instead of PEG powder to precipitate viral nucleic acid from wastewater because of its higher reaction surface, which may lead to the potential of increasing method sensitivity. Using 18 raw wastewater samples, it was found that the PEG solution yielded similar Ct values and higher detected virus concentrations than the PEG powder method, although the difference was not statistically significant (p > 0.05) (Fig. 6). These findings suggest that the PEG solution could also be used to concentrate viral nucleic acids from wastewater samples, although it is insignificant to increase method sensitivity. Regarding the practicality, the PEG solution of high concentration (>50%) used in the present study had high viscosity, leading to difficulty in dispensing the PEG solution into wastewater samples. Thus, the PEG solution is workable but may need further modification for high-throughput virus concentration.
3.6. Implications and limitations
In the present study, we developed a high-throughput protocol to process 100 samples within 6 h by two persons with a detection limit of 3286 copies/L in wastewater samples, which used ubiquitous reagents and instruments and could be applied by most laboratories in large SARS-CoV-2 wastewater monitoring schemes. This sensitive and rapid method promotes the establishment of a near real-time wastewater surveillance network for routine monitoring of viruses and other pathogens, and facilitates targeted sampling strategies at the communities/districts level. In addition, considering supply chain stability and consumable cost, the PEG precipitation method would be more practical for wastewater testing in resource-limited settings. In addition, the turnaround time could be shortened by decreasing centrifugation and incubation time, which simplify the processing procedures and is essential for timely public health responses. Moreover, we recommend the use of positive raw wastewater from local WWTPs or manholes for method evaluation to determine a suitable and tailored method for wastewater surveillance in practical.
There are some limitations of this study. Firstly, to maintain consistency for method comparison, different flocculants were evaluated under the same specific experimental conditions. The optimization of other flocculants under different experimental conditions (sample volume, pH, temperature, turbidity, etc.) may exhibit better performance than PEG precipitation. Secondly, current studies only focus on precipitation-based methods, whereas the ultrafiltration-based methods also have the potential scalability (Mailepessov et al., 2022) and Nanotrap beads-based method obtained high throughput performance (Ahmed et al., 2022a; Karthikeyan et al., 2021) in previous studies. Thirdly, the performances of the developed method to another virus like PMMoV or CrAssPhage remain unclear, which relied on the physico-chemical properties of specific virus, such as size of viral particles, surface affinity, and weight of viral particles, etc. Fourthly, the method sensitivity could be further improved by modifying detection process, for examples, RT-dPCR instead of RT-qPCR could be integrated into the established protocols for more accurate absolute quantification and higher sensitivity (Ahmed et al., 2022b; Graham et al., 2021). Overall, the selection of wastewater testing methods should be case-specific and decided according to the goals, required method sensitivity, required turnaround time, and available resources (funding, equipment, labor), etc.
4. Conclusions
-
•
A PEG-precipitation method was developed for rapid, high-throughput, and sensitive SARS-CoV-2 testing in wastewater, which could significantly increase the testing capacity and could be applied by most laboratories for large-scale surveillance to generate nearly real-time datasets.
-
•
This new method obtained a sensitivity comparable to that of the ultracentrifugation method with a process limit of detection as low as 3286 copies/L, which could detect virus signals even in a low prevalence community.
-
•
The turnaround time was optimized by reducing the centrifugation and incubation time without significantly affecting the method sensitivity, which is essential for obtaining timely results for public health responses.
-
•
Raw wastewater in Hong Kong was used for method evaluation, which could provide a reference basis for establishing practical methods in other regions.
CRediT authorship contribution statement
Xiawan Zheng: Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Mengying Wang: Methodology, Investigation. Yu Deng: Methodology. Xiaoqing Xu: Methodology. Danxi Lin: Investigation. Yulin Zhang: Writing – review & editing. Shuxian Li: Investigation. Jiahui Ding: Investigation. Xianghui Shi: Investigation. Chung In Yau: Investigation. Leo L.M. Poon: Resources. Tong Zhang: Conceptualization, Methodology, Formal analysis, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We appreciated the help from Environmental Protection Department (EPD) and Drainage Services Department (DSD) of Hong Kong SAR Government for the wastewater sample collection and delivery. Xiawan Zheng, Mengying Wang, Xiaoqing Xu, Yulin Zhang, Shuxian Li, Jiahui Ding, and Xianghui Shi would like to thank for The University of Hong Kong for the Postgraduate Studentship (PGS).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.watres.2022.119560.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bivins A., Korajkic A., Metcalfe S., Smith W.J.M., Simpson S.L. Comparative analysis of adsorption-extraction (AE) and nanotrap® magnetic virus particles (NMVP) workflows for the recovery of endogenous enveloped and non-enveloped viruses in wastewater. Sci. Total Environ. 2022;859 doi: 10.1016/j.scitotenv.2022.160072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bivins A., Metcalfe S., Smith W.J.M., Verbyla M.E., Symonds E.M., Simpson S.L. Evaluation of process limit of detection and quantification variation of SARS-CoV-2 RT-qPCR and RT-dPCR assays for wastewater surveillance. Water Res. 2022;213 doi: 10.1016/j.watres.2022.118132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcereny A., Martinez-Velazquez A., Bosch A., Allende A., Truchado P., Cascales J., Romalde J.L., Lois M., Polo D., Sanchez G., Perez-Cataluna A., Diaz-Reolid A., Anton A., Gregori J., Garcia-Cehic D., Quer J., Palau M., Ruano C.G., Pinto R.M., Guix S. Monitoring emergence of the SARS-CoV-2 B1.1.7 Variant through the Spanish National SARS-CoV-2 Wastewater Surveillance System (VATar COVID-19) Environ. Sci. Technol. 2021;55(17):11756–11766. doi: 10.1021/acs.est.1c03589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC 2020 2019-novel coronavirus (2019-nCoV) real-time RT-PCR primers and probes. [DOI] [PMC free article] [PubMed]
- Cui H., Huang X., Yu Z., Chen P., Cao X. Application progress of enhanced coagulation in water treatment. RSC Adv. 2020;10(34):20231–20244. doi: 10.1039/d0ra02979c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aoust P.M., Mercier E., Montpetit D., Jia J.J., Alexandrov I., Neault N., Baig A.T., Mayne J., Zhang X., Alain T., Langlois M.A., Servos M.R., MacKenzie M., Figeys D., MacKenzie A.E., Graber T.E., Delatolla R. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle J., Racher K., Hazenberg J., Yeoman A., Hannah H., Duong D., Mohammed U., Spreitzer D., Gregorchuk B.S.J., Head B.M., Meyers A.F.A., Sandstrom P.A., Nichani A., Brooks J.I., Mulvey M.R., Mangat C.S., Becker M.G. A sensitive and rapid wastewater test for SARS-COV-2 and its use for the early detection of a cluster of cases in a remote community. Appl. Environ. Microb. 2022;88(5) doi: 10.1128/aem.01740-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Xu X., Zheng X., Ding J., Li S., Chui H.K., Wong T.K., Poon L.L.M., Zhang T. Use of sewage surveillance for COVID-19 to guide public health response: a case study in Hong Kong. Sci. Total Environ. 2022;821 doi: 10.1016/j.scitotenv.2022.153250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Zheng X., Xu X., Chui H.K., Lai W.K., Li S., Tun H.M., Poon L.L.M., Ding J., Peiris M., Leung G.M., Zhang T. Use of sewage surveillance for COVID-19: a large-scale evidence-based program in Hong Kong. Environ. Health Perspect. 2022;130(5):57008. doi: 10.1289/EHP9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8(7) doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K.E., Loeb S.K., Wolfe M.K., Catoe D., Sinnott-Armstrong N., Kim S., Yamahara K.M., Sassoubre L.M., Mendoza Grijalva L.M., Roldan-Hernandez L., Langenfeld K., Wigginton K.R., Boehm A.B. SARS-CoV-2 RNA in wastewater settled solids is associated with COVID-19 cases in a large Urban Sewershed. Environ. Sci. Technol. 2021;55(1):488–498. doi: 10.1021/acs.est.0c06191. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman J.S., Scire J., Caduff L., Fernandez-Cassi X., Ganesanandamoorthy P., Kull A., Scheidegger A., Stachler E., Boehm A.B., Hughes B., Knudson A., Topol A., Wigginton K.R., Wolfe M.K., Kohn T., Ort C., Stadler T., Julian T.R. Wastewater-based estimation of the effective reproductive number of SARS-CoV-2. Environ. Health Perspect. 2022;130(5):57011. doi: 10.1289/EHP10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor R.S., Nelson K.L., Greenwald H.D., Kennedy L.C. Challenges in measuring the recovery of SARS-CoV-2 from wastewater. Environ. Sci. Technol. 2021;55(6):3514–3519. doi: 10.1021/acs.est.0c08210. [DOI] [PubMed] [Google Scholar]
- Karthikeyan S., Ronquillo N., Belda-Ferre P., Alvarado D., Javidi T., Longhurst C.A., Knight R. High-throughput wastewater SARS-CoV-2 detection enables forecasting of community infection dynamics in San Diego county. mSystems. 2021;6(2) doi: 10.1128/mSystems.00045-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Bonanno Ferraro G., Veneri C., Iaconelli M., Bonadonna L., Lucentini L., Suffredini E. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenfeld K., Chin K., Roy A., Wigginton K., Duhaime M.B. Comparison of ultrafiltration and iron chloride flocculation in the preparation of aquatic viromes from contrasting sample types. PeerJ. 2021;9:e11111. doi: 10.7717/peerj.11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaTurner Z.W., Zong D.M., Kalvapalle P., Gamas K.R., Terwilliger A., Crosby T., Ali P., Avadhanula V., Santos H.H., Weesner K., Hopkins L., Piedra P.A., Maresso A.W., Stadler L.B. Evaluating recovery, cost, and throughput of different concentration methods for SARS-CoV-2 wastewater-based epidemiology. Water Res. 2021;197 doi: 10.1016/j.watres.2021.117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.U. Minimum sizes of respiratory particles carrying SARS-CoV-2 and the possibility of aerosol generation. Int. J. Environ. Res. Public Health. 2020;17(19) doi: 10.3390/ijerph17196960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.L., Imakaev M., Armas F., McElroy K.A., Gu X., Duvallet C., Chandra F., Chen H., Leifels M., Mendola S., Floyd-O'Sullivan R., Powell M.M., Wilson S.T., Berge K.L.J., Lim C.Y.J., Wu F., Xiao A., Moniz K., Ghaeli N., Matus M., Thompson J., Alm E.J. Quantitative SARS-CoV-2 Alpha Variant B.1.1.7 tracking in wastewater by allele-specific RT-qPCR. Environ. Sci. Tech. Lett. 2021 [Google Scholar]
- Mailepessov D., Arivalan S., Kong M., Griffiths J., Low S.L., Chen H., Hapuarachchi H.C., Gu X., Lee W.L., Alm E.J., Thompson J., Wuertz S., Gin K., Ng L.C., Wong J.C.C. Development of an efficient wastewater testing protocol for high-throughput country-wide SARS-CoV-2 monitoring. Sci. Total Environ. 2022;826 doi: 10.1016/j.scitotenv.2022.154024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Tech. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Naughton C.C., Roman F.A., Alvarado A.G.F., Tariqi A.Q., Deeming M.A., Bibby K., Bivins A., Rose J.B., Medema G., Ahmed W., Katsivelis P., Allan V., Sinclair R., Zhang Y., Kinyua M.N. Show us the data: global covid-19 wastewater monitoring efforts, equity, and gaps. medRxiv. 2021 doi: 10.1101/2021.1103.1114.21253564. 2021.03.14.21253564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cataluna A., Cuevas-Ferrando E., Randazzo W., Falco I., Allende A., Sanchez G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo S.E., Keim E.K., Swanstrom R., Ong A.Q.W., Burnor E.A., Kossik A.L., Harrison J.C., Demeke B.A., Zhou N.A., Beck N.K., Shirai J.H., Meschke J.S. A comparison of SARS-CoV-2 wastewater concentration methods for environmental surveillance. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.144215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino N.J., Rodriguez D.C., Cano L.C., Rodriguez A. Detection of SARS-CoV-2 in wastewater is influenced by sampling time, concentration method, and target analyzed. J. Water Health. 2021;19(5):775–784. doi: 10.2166/wh.2021.133. [DOI] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider M.A., Hurwitz S.N., Meckes D.G. ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci. Rep. 2016;6:23978. doi: 10.1038/srep23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Lipponen A., Hokajarvi A.M., Luomala O., Sarekoski A., Rytkonen A., Osterlund P., Al-Hello H., Juutinen A., Miettinen I.T., Savolainen-Kopra C., Pitkanen T. Detection and quantification of SARS-CoV-2 RNA in wastewater influent in relation to reported COVID-19 incidence in Finland. Water Res. 2022;215 doi: 10.1016/j.watres.2022.118220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Oishi W., Zhu Y., Thakali O., Malla B., Yu Z., Zhao B., Arakawa C., Kitajima M., Hata A., Ihara M., Kyuwa S., Sano D., Haramoto E., Katayama H. Comparison of five polyethylene glycol precipitation procedures for the RT-qPCR based recovery of murine hepatitis virus, bacteriophage phi6, and pepper mild mottle virus as a surrogate for SARS-CoV-2 from wastewater. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, W.H.O. 2022. Environmental Surveillance For SARS-COV-2 to Complement Public Health Surveillance – Interim Guidance. [Google Scholar]
- Wolfe M.K., Archana A., Catoe D., Coffman M.M., Dorevich S., Graham K.E., Kim S., Grijalva L.M., Roldan-Hernandez L., Silverman A.I., Sinnott-Armstrong N., Vugia D.J., Yu A.T., Zambrana W., Wigginton K.R., Boehm A.B. Scaling of SARS-CoV-2 RNA in settled solids from multiple wastewater treatment plants to compare incidence rates of laboratory-confirmed COVID-19 in their sewersheds. Environ. Sci. Tech. Lett. 2021;8(5):398–404. doi: 10.1021/acs.estlett.1c00184. [DOI] [PubMed] [Google Scholar]
- Wu F., Lee W.L., Chen H., Gu X., Chandra F., Armas F., Xiao A., Leifels M., Rhode S.F., Wuertz S., Thompson J., Alm E.J. Making waves: wastewater surveillance of SARS-CoV-2 in an endemic future. Water Res. 2022;219 doi: 10.1016/j.watres.2022.118535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Jian b., Moniz K., Endo N., Armas F., Bushman M., Chai P.R., Duvallet C., Erickson T.B., Foppe K., Ghaeli N., Gu X., Hanage W.P., Huang K.H., Lee W.L., McElroy K.A., Rhode S.F., Matus M., Wuertz S., Thompson J., Alm E.J. Wastewater surveillance of SARS-CoV-2 across 40 U.S. states from February to June 2020. Water Res. 2021;202 doi: 10.1016/j.watres.2021.117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Erickson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5(4):e00614–e00620. doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Waldman P., Ferrier-Rembert A., Frenois-Veyrat G., Mouchel J.M., Boni M., Maday Y., consortium O., Marechal V., Moulin L. Several forms of SARS-CoV-2 RNA can be detected in wastewaters: implication for wastewater-based epidemiology and risk assessment. Water Res. 2021;198 doi: 10.1016/j.watres.2021.117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Deng Y., Ding J., Zheng X., Li S., Liu L., Chui H.K., Poon L.L.M., Zhang T. Real-time allelic assays of SARS-CoV-2 variants to enhance sewage surveillance. Water Res. 2022;220 doi: 10.1016/j.watres.2022.118686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Deng Y., Zheng X., Li S., Ding J., Yang Y., On H.Y., Yang R., Chui H.K., Yau C.I., Tun H.M., Chin A.W.H., Poon L.L.M., Peiris M., Leung G.M., Zhang T. Evaluation of RT-qPCR primer-probe sets to inform public health interventions based on COVID-19 sewage tests. Environ. Sci. Technol. 2022;56(12):8875–8884. doi: 10.1021/acs.est.2c00974. [DOI] [PubMed] [Google Scholar]
- Xu X., Zheng X., Li S., Lam N.S., Wang Y., Chu D.K.W., Poon L.L.M., Tun H.M., Peiris M., Deng Y., Leung G.M., Zhang T. The first case study of wastewater-based epidemiology of COVID-19 in Hong Kong. Sci. Total Environ. 2021;790 doi: 10.1016/j.scitotenv.2021.148000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T. Wastewater as an information source of COVID-19. Sci. Bull. 2022;67(11):1090–1092. doi: 10.1016/j.scib.2022.04.006. [DOI] [PubMed] [Google Scholar]
- Zheng X., Deng Y., Xu X., Li S., Zhang Y., Ding J., On H.Y., Lai J.C.C., In Yau C., Chin A.W.H., Poon L.L.M., Tun H.M., Zhang T. Comparison of virus concentration methods and RNA extraction methods for SARS-CoV-2 wastewater surveillance. Sci. Total Environ. 2022;824 doi: 10.1016/j.scitotenv.2022.153687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Li S., Deng Y., Xu X., Ding J., Lau F.T.K., In Yau C., Poon L.L.M., Tun H.M., Zhang T. Quantification of SARS-CoV-2 RNA in wastewater treatment plants mirrors the pandemic trend in Hong Kong. Sci. Total Environ. 2022;844 doi: 10.1016/j.scitotenv.2022.157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.