Abstract
Dust particles (DPs) are one of the most important public health concerns in the urban environment. The presence of heavy metals (HMs) on the surface of DPs might increase the health risk of exposure to the DPs. Accordingly, The purpose of this study was to examine the content of HMs in the outdoor and indoor DPs in Neyshabur city and assess the cytotoxic effects of DPs exposure on lung, gastric, and skin cell lines. To this end, the city was divided into three areas, high-traffic, medium-traffic, and low-traffic (rural). The average concentration of the HMs in the indoor DPs were as follows, 655.5 μg g-1 for Zn, 114.6 μg g-1 for Cu, 77.7 μg g-1 for Cr, 108.6 μg g-1 for Ni, 52 μg g-1 for Pb, 12 μg g-1 for Co, and 3.3 μg g-1 for Cd, while the average concentration of Zn, Cu, Cr, Ni, Pb, Co, Cd in the outdoor DPs were 293.7 μg g-1, 200.6 μg g-1, 100.7 μg g-1, 68.4 μg g-1, 44.7 μg g-1, 18.6 μg g-1, 0.25 μg g-1, respectively. A higher concentration of HMs, as well as cytotoxicity, were revealed in the indoor samples compared to outdoor ones. The degree of cytotoxicity of DPs collected from high-traffic areas was higher than that of low and medium-traffic ones. In addition, treatment of AGS and L929 cells with indoor dust samples induced the expression level of inflammatory agents such as TNFα, IL6, and, CYP1A1 genes more than in outdoor dust samples (P < 0.05). Briefly, a higher level of HMs concentration and cytotoxicity effect on the given cell lines was observed in the samples taken from indoor environments and high-traffic areas.
Keywords: Heavy metals, Indoor dust, Outdoor dust, Cytotoxicity, Gene expression
Heavy metals, Indoor dust, Outdoor dust, Cytotoxicity, Gene expression.
1. Introduction
Heavy metals (HMs) are non-degradable, highly toxic, and cumulative carcinogenic pollutants. These contaminants are found in air, water, building materials, kitchen appliances, and even clothing. Based on our best knowledge, there is no mechanism through which HMs could be metabolized in the body. Accordingly, these metals accumulate in the tissues and cause several malfunctions and diseases [1, 2]. HMs enter the body in several ways, the most common way of entering these compounds is through digestion, then breathing, and finally, the skin [3] HMs in urban soils may endanger human and animal health by entering the food chain; thus, metals present in soils are harmful to human health [4, 5].
Today, human activities in urban areas increase the problem of HMs pollution in urban soils. Dust particles (DPs) and particulate matters (PMs) are the most critical parts of the urban environment in terms of public health, which are considered important carriers of HMs and other pollutants [6, 7]. Exposure to DPs and PMs is associated with many adverse health effects, including increased hospitalization, emergency visits, respiratory symptoms, exacerbation of chronic respiratory and cardiovascular diseases, decreased lung function, and premature death [8, 9]. In addition, scientists have suggested that exposure to high levels of particles may lead to various symptoms, including medium birth weight in infants, and possibly fetal and infant death [10]. The effects of particles on cellular metabolisms have been frequently assessed in earlier studies. For example, Kermani et al. (2021) Çakmak et al. (2019), found the genotoxic and cytotoxic effects of organic extract of subway particles on normal human cells [7, 11]. Oxidative stress introduces the majority of critical mechanisms of these harmful effects. On the other hand, HMs can cause both genotoxic and cytotoxic reactions in the human body, which could be accompanied by the destructive effects of particles [7, 11]. Consequently, the presence of compounds such as metals as exogenous inflammatory agents that induce both acute and inflammatory responses usually leads to the expression of cytokines and interferon. These responses are considered innate immunity due to their immediate and non-specific effects [12]. Accordingly, the present study aimed to evaluate the inflammatory responses and toxicity of HMs found in indoor and outdoor settled dust extracts on lung, gastric, and skin cell lines.
2. Materials and methods
2.1. Sampling procedure
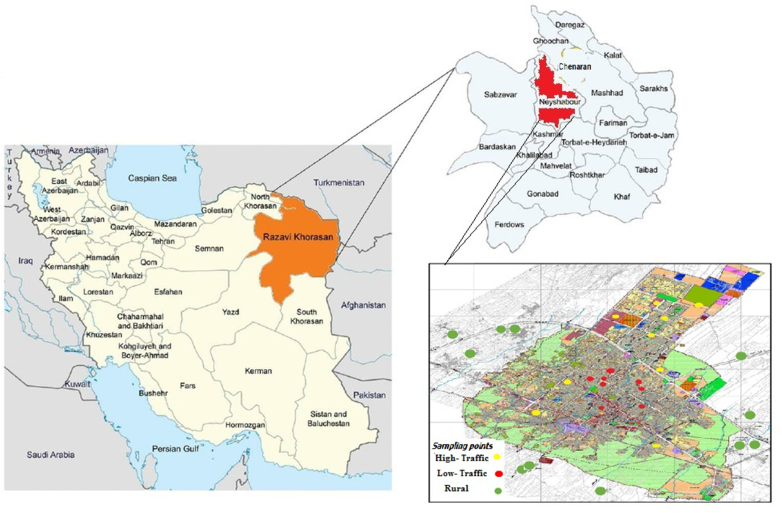
The present study was performed in Neyshabur city located in the northeast of Iran, in the range of 58° and 8′ to 59° and 20′ of longitude and 35° and 35′ to 36° and 52′ north latitude (Figure 1). Based on the urban traffic map, the city was divided into three areas, high-traffic (HT), medium-traffic (MT) and low-traffic or rural (LT). The sampling of deposited urban dust was performed from inside residential houses (indoor dust) and outside residential houses (outdoor dust). The indoor dust samples were collected from vacuum cleaners inside residential houses. Also, the outdoor dust samples were taken by brush from a specified area of 1 m. The DPs samples were sieved using mesh number 40 in order to remove plant particles or coarse particles. The samples were placed in zipper plastic bags and sent to the laboratory for analysis.
Figure 1.
Location of the study area and sampling points.
2.2. Analysis of metal compositions deposited in dust particles
To extract HMs from dust samples, the DPs samples were dried in an oven at 50 °C for 2 h and then placed in a desiccator to prevent moisture absorption. For digestion, 1 g of the sample and 3 ml of hydrofluoric acid (HF) were poured into a Teflon beaker and then heated at 50 °C until complete dryness. The beaker opening was covered with a watch glass. Then, 5 ml of concentrated nitric acid (HNO3) and 15 ml of concentrated hydrochloric acid (HCL) were added to each sample. The samples were placed at room temperature for 60 min and then heated on a heater at 105 °C for 2 h to complete digestion and obtain a clear liquid. After observing the precipitate, the samples were passed through filter paper and transferred to the final volume in a 25-ml volumetric flask. Finally, Inductively coupled plasma optical emission spectrometry (ICP-OES model Spectro Arcos- 76004555 plasma) was used to measure metallic elements.
Six high-purity standards were used to draw calibration curves. After each run, the calibration curve generated from the high purity standards was checked for linearity and replication. The blanks were used to check the analytical procedure before test sample analysis.
Heavy metal concentrations were calculated using the following Eq. (1) [13, 14, 15]:
| (1) |
Cs: Concentration of the desired element (in mg/L) in the solution sample read by the device
Vs: Volume of the main sample solution (in mL)
Ws: Weight of the main sample (in gr), the weight of the sample weighed for preparation
C: Concentration of the desired element in the main solid sample (in mg/kg)
2.3. Preparation of aqueous extracts
The water-soluble suspension was prepared as follows; 2 g of the DPs sample was placed in centrifuge tubes and filled with 10 ml of 18ῼ distilled water. It was subjected to ultrasonic waves for 20 min, and then the resulting mixture was placed on a shaker for 1 h. The resulting solution was passed through a 0.45 μm syringe filter. Finally, this solution was kept at -20 °C until cell treatment experiments [16, 17].
2.4. Preparation of the study cell lines
2.4.1. Cell culture
The cytotoxicity of DPs samples was determined using human lung carcinoma cell lines (A549), human gastric adenocarcinoma cell line (AGS), and Mouse normal fibroblasts cell line (L929) obtained from the National Cell Bank of Iran (Pasteur Institute of Iran, Tehran). After transferring the cells to the cell culture laboratory, the flasks containing the cells were placed under a microscope to check cell density and adhesion. The cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS), and 10% penicillin/streptomycin and incubated at 37 °C, 95% humidity, and 5% carbon dioxide.
2.4.2. Cell line treatment and cytotoxicity assessment (MTT assay)
The MTT (3-(4,5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) assay was utilized to examine the cytotoxicity of the water-soluble fraction in the DPs samples. Cell culture was performed on 96-well plates; a well consists of 5×104 cells. Each well was added with 20 μl of the water-soluble fraction of the DPs and incubated for 24, 48, and 72 h. After that time elapsed, 20 μl of MTT solution (0.5 mg/mL in PBS) was added to the medium, and the cells were incubated at 37 °C for 4 h. The produced formazan crystals in the viable cells were dissolved by adding 200 μl of DMSO solution after removing MTT containing medium. The optical density (OD) of each sample was read at 570 nm using Microplate Reader. The cell viability percentage was calculated using the following formula: Percentage of viable cells (%Viability) = (mean OD values of control cells/mean OD values of treated cells) × 100.
2.4.3. Total RNA extraction and qRT-PCR assay
The effects of mentioned treatments on mRNA expression of Cytochrome p450 1A1 (CYP1A1), Cytochrome p450 1A1 (CYP1B1), necrosis factor-alpha (TNF-α) and IL-6 interleukin (IL) -6 were determined using real-time PCR. Thus, total RNA (2 μg) was extracted from cells using the TRIzol reagent (Invitrogen, USA) and reverse-transcribed using the Excel RT Reverse-transcriptase kit (RP1300, SMOBIO, Hsinchu City, Taiwan). The cDNA samples were kept at -20 °C until the next testing. PCR Reactions were carried out in 96-well plates under the following conditions: denaturation at 95 °C for 15 min, annealing at 59 °C for 5 s, and elongation at 72 °C for 5 s. Also, GAPDH was used as the endogenous control gene. The gene expression alterations were calculated using the 2–ΔΔCt method according to the Ct of target and control genes in treated and non-related samples [18].
3. Results
The concentration of heavy in indoor and outdoor DPs samples is described in Table 1. Averagely, the highest level of Zn was observed in the indoor DPs samples of the HT area (982.3 μg g−1), while the lowest concentration was recorded in outdoor samples collected in the LT area (204.4 μg g−1); broadly speaking, Zn had a higher concentration in indoor DPs. The average level of Pb in indoor DPs was slightly lower (48.1 μg g−1) than that of outdoor DPs (57 μg g−1) only in the HT area, however, the average level of Pb in indoor DPs collected from MT and the LT areas had higher concentration than outdoor DPs. The average level of Ni in indoor DPs was higher than outdoor DPs in all of the given areas. However, the highest average of Ni was observed in the indoor samples collected from the LT area (154.9 μg g−1). The lowest average concentration of Ni was recorded for the outdoor samples of LT area (60.8 μg g−1). The Cd levels in indoor DPs in HT, MT, and LT areas were (7.1 μg g−1), (3.4 μg g−1), and (1 μg g−1), respectively, which were higher than outdoor measurements in HT (0.19 μg g−1), MT (0.34 μg g−1) and LT (0.21 μg g−1) areas. The average level of other elements including Cu, Cr, and Co was lower in indoor DPs in comparison to the outdoor samples.
Table 1.
Statistical heavy metal concentration in a different area of Neyshabur (μg g−1).
| Zn |
Pb |
Ni |
Cu |
Cr |
Co |
Cd |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | |
| High traffic | ||||||||||||||
| Average | 388.5 | 982.3 | 57.0 | 48.1 | 75.8 | 94.1 | 278.5 | 142.1 | 114.1 | 72.8 | 16.9 | 10.1 | 0.19 | 7.1 |
| Min | 239.5 | 973.4 | 41.3 | 40.1 | 71.2 | 51.6 | 121.9 | 129.8 | 91.6 | 36.3 | 16.8 | 1.3 | 0.12 | 1.3 |
| Max | 537.4 | 991.2 | 72.7 | 56.2 | 80.3 | 136.6 | 435.1 | 154.4 | 136.6 | 109.2 | 17.0 | 19.0 | 0.27 | 12.9 |
| SD | 210.6 | 12.6 | 22.2 | 11.4 | 6.4 | 60.0 | 221.4 | 17.4 | 31.8 | 51.6 | 0.1 | 12.5 | 0.10 | 8.2 |
| Medium traffic | ||||||||||||||
| Average | 288.2 | 726.3 | 50.2 | 66.5 | 68.5 | 76.9 | 206.5 | 137.1 | 99.6 | 45.7 | 15.9 | 9.5 | 0.34 | 1.9 |
| Min | 250.7 | 546.7 | 31.7 | 55.6 | 61.0 | 54.1 | 175.9 | 104.3 | 91.1 | 35.8 | 14.6 | 7.7 | 0.22 | 1.2 |
| Max | 342.0 | 905.9 | 61.1 | 77.5 | 78.8 | 99.8 | 224.5 | 169.9 | 106.6 | 55.7 | 17.2 | 11.2 | 0.51 | 2.7 |
| SD | 44.0 | 254.0 | 13.7 | 15.5 | 7.5 | 32.4 | 23.1 | 46.4 | 7.4 | 14.1 | 1.1 | 2.5 | 0.12 | 1.0 |
| Low traffic (Rural) | ||||||||||||||
| Average | 204.4 | 258.0 | 26.9 | 41.3 | 60.8 | 154.9 | 116.8 | 64.7 | 88.4 | 114.7 | 22.9 | 16.5 | 0.21 | 1.0 |
| Min | 170.1 | 165.0 | 26.5 | 23.7 | 57.7 | 143.8 | 88.2 | 43.8 | 86.3 | 95.8 | 15.5 | 13.5 | 0.08 | 0.9 |
| Max | 238.7 | 373.9 | 27.2 | 60.6 | 63.9 | 162.2 | 145.5 | 98.1 | 90.4 | 149.7 | 30.4 | 21.4 | 0.35 | 1.2 |
| SD | 48.5 | 106.3 | 0.5 | 18.6 | 4.4 | 9.8 | 40.5 | 29.2 | 2.9 | 30.3 | 10.5 | 4.3 | 0.19 | 0.1 |
3.1. The effect of indoor and outdoor dust on the viability of different cell lines
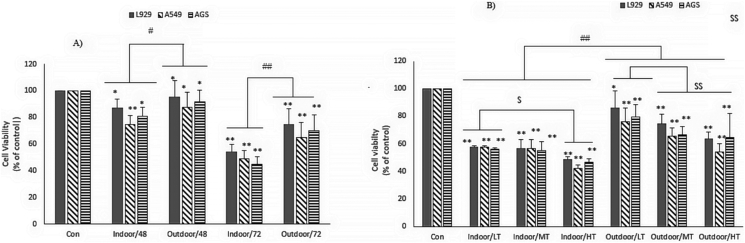
The toxicity of indoor and outdoor dust at 24, 48, and 72 h incubation time in three cell lines (AGS, A549, and L929) was determined by MTT assay. Indoor DPs showed a greater ability to decrease cell viability than the corresponding outdoor DPs.
Cell treatment at 24 h showed no significant effect on the viability of the three cell lines. As shown in Figure 2 A, in the 48-hour treatment of L929 cells with indoor samples of DPs, a smaller percentage of cells survived than in the treatment with outdoor ones (% Cell viability: 87.3 ± 6.4 for indoor DPs; 95.1 ± 12.3 for outdoor DPs; P ≤ 0.01). The toxicity effect of indoor and outdoor DPs on the AGS cell line was higher than that of the L929 cell line. In these cells, the effect of indoor DPs was greater than that of outdoor dust (%Cell viability: 81.3 ± 6.4 for indoor DPs, 92 ± 8.8 for outdoor DPs; P ≤ 0.01). The highest cytotoxicity of DPs was observed in the A549 cell line. In these cells, 87.8 ± 11 cells survived in the presence of the indoor dust, while 74.8 ± 6.4 cells survived in the presence of the indoor dust (P ≤ 0.01). In all three cell lines, the cytotoxicity was higher at 72 h of treatment compared to 48 h (P ≤ 0.01). The cytotoxicity of indoor DPs on the cell lines of L929 (% Cell viability: 54.26 ± 5.4 for indoor DPs, 74.58 ± 11.94 for outdoor DPs; P ≤ 0.01), AGS (% Cell viability: 44.87 ± 5.6 for indoor DPs, 72.22 ± 11.6 for outdoor DPs; P ≤ 0.01) and A549 (% Cell viability: 48.84 ± 6.1 for indoor DPs, 65.17 ± 11.33 for outdoor DPs; P ≤ 0.01) was higher than that of outdoor DPs. When the traffic zones are concerned, the collected samples were classified into three groups: LT, MT, and HT. The effect of exposure to DPs collected from the zones was investigated on the viability of three cell lines at 72 h. As shown in Figure 2B, the cytotoxicity of indoor DPs collected from HT areas in the A549 cells in the (%Cell viability: 42.29 ± 2.51) was higher than those of the MT (%Cell viability: 56.6 ± 6.3%) and LT (% Cell viability: 57.77 ± 0.74) areas. In addition, higher cytotoxicity was found for indoor DPs collected from HT areas compare to the samples taken from MT and LT areas in the AGS (% Cell viability: 46.5 ± 2.3 for HT; 55.03 ± 6.6 for MT; 56.2 ± 0.77 for LT; P ≤ 0.01) and L929 cells (% Cell viability: 48.4 ± 2.2 for HT; 56.6 ± 6.3 for MT; 57.7 ± 0.74 for LT; P ≤ 0.01). In all three cell lines, exposure to DPs collected from HT areas showed higher cytotoxicity than those of MT and LT areas.
Figure 2.
Effect of indoor and outdoor dusts on AGS, A549 and L929 cell viability. A) The cells were cultured in 10% FBS medium and treated with outdoor and indoor dusts for 48 and 72 h and cell viability was measured by MTT assay. B|) The indoor and outdoor dust samples were categorized according to traffic states (LT: low traffic, MT: medium traffic and HT: high traffic) and cell viability was measured after 72 h cell treatment with these. The percent of viable cells (%) was calculated in comparison to untreated cells. The number of cells in the control was taken as 100%. Values were expressed as mean ± SD: of three independent experiments, each performed in triplicate (∗P < 0.05 and ∗∗P < 0.01; #P < 0.05 and ##P < 0.01; $ P < 0.05 and $$ P < 0.01).
3.2. The effect of indoor and outdoor DPs on the gene expression of the cell lines
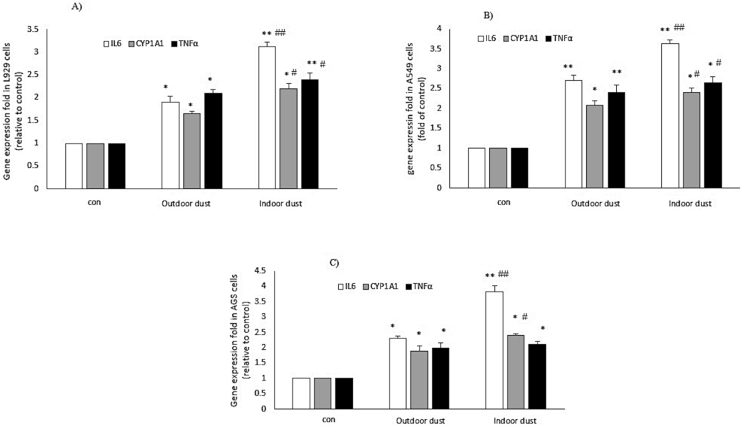
The effect of indoor and outdoor exposure to DPs on the expression level of TNFα, IL6, CYP1B1, and CYP1A1 genes was evaluated after 24 h of treatment in L929, AGS, and A549 cell lines (Table 2). As shown in Figure 3 (A-C), treatment of A549 cells (24 h) with indoor DPs significantly increased the gene expression level of TNFα (Fold to control; Indoor: 2.65 ± 0.16; outdoor: 1.8 ± 0.2; P < 0.05) and IL6 (Fold to control; Indoor: 3.63 ± 0.0.09; outdoor: 2.2 ± 0.13; P < 0.05) and CYP1A1 (Fold to control; Indoor: 2.4 ± 0.11; outdoor: 1.59 ± 0.11; P < 0.05) when compared with outdoor dust samples Figure 3B. Treatment of AGS cells with indoor dust samples increased the expression level of TNFα, IL6, and CYP1A1 genes more than in outdoor DPs (P < 0.05) Figure 3C. In the L929 cell line, the effect of indoor and outdoor DPs on the expression level of the CYP1A1 gene was not significant. However, cell treatment with indoor DPs increased the expression level of TNFα and IL6 genes more than outdoor ones (P ≥ 0.05). No significant change (P ≥ 0.05) in CYP1B1 gene expression was observed in all three cell lines (data not shown) Figure 3A.
Table 2.
Primer sequnces used for the analysis of expression in human (AGS and A549) and mouse (L929) cell lines.
| Gene | Forward (5′–3′) | reverse (5′–3′) |
|---|---|---|
| IL6 (Human) IL6 (Mouse) |
GTCCAGTTGCCTTCTCCCTG TACCACTTCACAAGTCGGAGGC |
ACCAGGCAAGTCTCCTCATTG CTGCAAGTGCATCATCGTTGTTC |
| TNF-α(Human) TNF-α(Mouse) |
AGCCCATGTTGTAGCAAACC GGTGCCTATGTCTCAGCCTCTT |
ACATTGGGTCCCCCAGGATA GCCATAGAACTGATGAGAGGGAG |
| CYP1A1 (Human) CYP1A1 (Mouse) |
ACCATCCCCCACAGGAAGCTA CATCACAGACAGCCTCATTGAGC |
GGCAGGATCCCTTAGGCTTG CTCCACGAGATAGCAGTTGTGAC |
| GAPDH (Human) GAPDH (Mouse) |
CTCCCGCTTCGCTCTCTG CATCACTGCCACCCAGAAGACTG |
TCCGTTGACTCCGACCTTC ATGCCAGTGAGCTTCCCGTTCAG |
Figure 3.
The effect of indoor and outdoor dusts on mRNA expression of inflammatory markers (IL6 and TNF-α) and CYP1A in human (AGS and A549) and mouse (L929) cell lines. The cells were treated with indoor and outdoor dusts for 24 h. RNA was extracted from treated cells, cDNA was synthesized, and gene expression changes were measured relative to non-treated (∗p < 0.05, ∗∗p < 0.01). # (p < 0.05) and ## (P < 0.01) represent the gene expression changes in presence of indoor vs outdoor samples.
4. Discussion
The present study was designed aiming the evaluation of the HMs content of DPs collected from indoor and outdoor environments in Neyshabur, a city with several industrial complexes such as a great steel industry and a power plan. We also evaluated the cytotoxicity and gene expression related to DPs exposure on normal, and cancer cell lines.
DPs are important routes for loading HMs into the human body [19]. The health risks posed by HMs in indoor and outdoor DPs have attracted particular attention [20, 21, 22]. https://journals.sagepub.com/action/doSearch?target=default&ContribAuthorStored=Naimabadi%2C+Abolfazl Previously, Naimabadi et al. reported the presence of high concentrations of HMs in indoor and outdoor DPs of Neyshabur [3, 23]. Similarly, the content of HMs in indoor and outdoor DPs was different; remarkably high concentrations of Zn and Ni in indoor DPs compared to that of outdoor ones were observed. These results were obtained due to the different origins of HMs in indoor and outdoor DPs. Electrical devices, smoking tobacco, and cigarette, traffic sources, old building materials, building paint colors as well as outdoor dust can be considered as important sources of HMs in indoor environments [24, 25]. Our results also indicated that the increased concentration of HMs in indoor or outdoor DPs is more likely in the crowded area with high traffic volume (Figure 1). In line with our results, the role of traffic (braking cars and tire friction) on the elevated concentration of some HMs such as Cu+2 and Zn+2 is well-established [26, 27]. In indoor samples, HMs are frequently used in electrical devices [28]. In the majority of the samples, the concentration of Pb+2 in indoor samples was higher than outdoor ones. The source of Pb+2 in indoor samples is the paints [25] and cement [25] used in the building. In addition, one of the most important sources of Pb+2 contamination in homes is lead-acid batteries, which are used for various household appliances [29].
As shown in Figure 2(A-B), in the current study, the indoor and outdoor dust severely reduced the cell viability of normal and cancerous cell lines. Numerous studies have been performed on the cytotoxic effects of dust in human cell lines [30, 31, 32, 33, 34]. Consistent with our study, Goodarzi et al. reported that increasing the concentration of HMs in dust samples collected from the city of Ahvaz (Iran) lead to increased toxicity in cell line A549 [35,36]. Also, the cytotoxic effects of dust particles collected from around Turin (Italy) on cell line A549 have been reported by Alessandria, L et al. [37]. Previous studies have focused on the cytotoxic effects of PM2.5 and PM10 dust particles. There are limited studies on the effects of indoor dust and its comparison with outdoor particles. For example, a study reported the cytotoxic effects of PM2.5 particles on human lung epithelial cells. This study showed that indoor and outdoor particles significantly reduced cell viability. The presence of HMs such as Ni, Cu, and Pb, as well as charged particles such as NO3-, SO42-, and NH4+ in these samples has been reported as the leading cause of toxicity in these samples [32]. Roy et al. reported a high percentage of cytotoxicity and a high load of pathogenic microbes in indoor samples [38]. In addition, our results showed that the cytotoxic effect of indoor DPs was higher than that of outdoor DPs. These results align with the results obtained from the analysis of HMs concentrations in indoor and outdoor samples. Therefore, the increase in cytotoxicity of indoor samples can be attributed to the higher concentration of HMs in these samples. A number of studies have reported the high toxicity of HMs. The role of HMs such as Mn, Cr, Ti, Fe, Cu, Zn, Ni, and Mo in the cytotoxicity of indoor and outdoor DPs has been reported by Spagnolo et al. [39]. Some HMs such as Fe, Cu+2, and Zn+2 at non-toxic concentrations are vital to physiological functions. However, their presence at high concentrations is toxic for cells [40]. HMs bind to DNA and RNA molecules in the cell and subsequently denature these vital molecules. They also destroy the vital molecules of cells by producing free radicals [41]. Binding to and thus inhibiting the glutathione molecule as an effective antioxidant is another mechanism that converts HMs into highly toxic molecules [41]. Consistent with the present study, Vicente et al. reported that PM10 particles in indoor samples were more toxic to A549 cell lines than in outdoor samples. In the study, the cytotoxicity of PM10 particles was attributed to HMs and organic compounds such as PAH [42]. In contrast with our results, Monn et al. have previously reported that PM10 particles from outdoor samples – but not indoor particles - have shown significant cytotoxic effects in lung cells. The cytotoxic effects observed in their study were associated with the presence of HMs [43].
Our results also showed that cell viability in the presence of particles collected from HT areas was significantly higher than in particles collected from LT areas. This result seems logical because similar to our findings, earlier studies reported a higher concentration of HMs in the HT area compared to LT one [44, 45]. In terms of cytotoxicity, the effect of exposure to DPs on cell viability of cancer cells such as A549 and AGS was higher than that of normal cells. The greater vulnerability of cancer cells can be attributed to the higher rate of cell growth and the rapid and abnormal metabolic processes in these cells, which increases the rate of nanoparticle uptake. On the other hand, cancer cells are more acidic than normal cells and HMs are more likely to be released more quickly in the acidic condition, resulting in a more death rate in cancer cells [46, 47].
Furthermore, the present study examined the effect of indoor and outdoor dust particles on the gene expression of inflammatory agents. The expression of genes involved in inflammation, such as IL-6 TNFα, is significantly increased in the presence of indoor and outdoor DPs. Increased inflammation is one of the most important results of cell exposure to DPs, which can lead to necrosis and apoptosis of cells and increase the cytotoxicity of DPs [48, 49]. The production of proinflammatory factors such as IL6 and TNFα in cells can be increased due to the presence of pathogenic microbes in the DPs or due to the high production of reactive oxygen species in the presence of HMs [38]. Previous studies have reported the production of IL6 and IL8 in the presence of dust particles in human cells [41, 43]. High concentrations of IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12, and TNF-α produced by lung epithelial cell lines have been reported following exposure to indoor and outdoor DPs [38]. Also, increased expression of inflammatory factors in cells can be attributed to the higher content of HMs in the DPs [50].
Our study also showed increased expression of the CYP1A1 gene, not CYP1B1, related to indoor and outdoor DPs exposure in both AGS and A549 cell lines. Enzymes of the cytochrome P450 family are involved in the metabolic response of xenobiotic compounds [51, 52]. Modulation of CYP1A1 gene expression and activation by metals such as As3+, Hg2+, Pb2+, Cd2+, Cr6+, Cu2+, and V5+ has been evaluated [53]. For example, Hg2+ can act at different levels on CYP1A1 modulation, and its effects depend on the cell line used for the study [54]. The increased level of CYP1A1 expression in the presence of Cd was also reported by Mohamed et al. [55]. Some studies demonstrated a reduction in CYP1A1 activity despite an increase in mRNA levels [53, 56]. Although in the current study, the effects of indoor or outdoor dust on CYP1A1 activity were not evaluated, almost all studies have demonstrated HMs as potent inhibitors of CYP1A1 activity [53]. Therefore, environmental exposure to indoor or outdoor DPs can influence drug metabolism and markedly change pharmacokinetics, response, or resistance to the drugs.
5. Conclusion
In the present study, it was shown that the majority of studied HMs had a higher concentration in indoor DPs compared to outdoor ones. In addition, HMs in samples collected from the HT zone were higher than those in the MT and LT zones. Statistical analysis of the cytotoxic effects showed significantly higher cytotoxicity of the indoor DPs than outdoor ones. Cytotoxicity effects of samples collected from the HT zone were also much higher than samples collected from MT and LT zones, which could be related to the higher concentration of HMs in indoor DPs.
The gene expression results show an increased expression of inflammatory markers (IL6 and TNF-α) as well as CYP1A1 in the presence of both indoor and outdoor samples. Due to the high content of HMs in indoor dust and their cytotoxic effects on normal and cancerous cell lines, the application of HMs should be limited as much as possible.
5.1. Limitation of study
Since normal cells cannot be cultured in the environment conditions, therefore we are reluctant to use cancer cells.
Declarations
Author contribution statement
Abolfazl Naimabadi; Mohsen Azimi-Nezhad: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Ahmad Ghasemi: Conceived and designed the experiments; Analyzed and interpreted the data.
Mahnaz Mohtashami; Jafar Saeidi: Performed the experiments; Analyzed and interpreted the data.
Mehdi Bakaeian: Contributed reagents, materials, analysis tools or data.
Aliakbar Haddad Mashadrizeh; Ali Akbar Mohammadi: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Neyshabur Noncommunicable Diseases Research Center, Neyshabur University of Medical Sciences [Project ID: 9804148] (Ethics Code: IR.NUMS.REC.1398.039).
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Contributor Information
Mohsen Azimi-Nezhad, Email: aziminm@nums.ac.ir.
Ali Akbar Mohammadi, Email: mohammadi.eng73@gmail.com.
References
- 1.Swaringen B.F., Gawlik E., Kamenov G.D., McTigue N.E., Cornwell D.A., Bonzongo J.-C.J. Children's exposure to environmental lead: a review of potential sources, blood levels, and methods used to reduce exposure. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112025. [DOI] [PubMed] [Google Scholar]
- 2.Wu X., Cobbina S.J., Mao G., Xu H., Zhang Z., Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Control Ser. 2016;23:8244–8259. doi: 10.1007/s11356-016-6333-x. [DOI] [PubMed] [Google Scholar]
- 3.Naimabadi A., Gholami A., Ramezani A.M. Determination of heavy metals and health risk assessment in indoor dust from different functional areas in Neyshabur, Iran. Indoor Built Environ. 2021;30:1781–1795. [Google Scholar]
- 4.Hu Z., Li J., Wang H., Ye Z., Wang X., Li Y., Liu D., Song Z. Soil contamination with heavy metals and its impact on food security in China. J. Geosci. Environ. Protect. 2019;7:168. [Google Scholar]
- 5.Kumar S., Prasad S., Yadav K.K., Shrivastava M., Gupta N., Nagar S., Bach Q.-V., Kamyab H., Khan S.A., Yadav S. Hazardous heavy metals contamination of vegetables and food chain: role of sustainable remediation approaches-A review. Environ. Res. 2019;179 doi: 10.1016/j.envres.2019.108792. [DOI] [PubMed] [Google Scholar]
- 6.Ageel H.K., Harrad S., Abdallah M.A.-E. Occurrence, human exposure, and risk of microplastics in the indoor environment. Environ. Sci. J. Integr. Environ. Res.: Process. Impacts. 2022;24 doi: 10.1039/d1em00301a. [DOI] [PubMed] [Google Scholar]
- 7.Kermani M., Rahmatinia T., Oskoei V., Norzaee S., Shahsavani A., Farzadkia M., Kazemi M.H. Potential cytotoxicity of trace elements and polycyclic aromatic hydrocarbons bounded to particulate matter: a review on in vitro studies on human lung epithelial cells. Environ. Sci. Pollut. Control Ser. 2021;28:55888–55904. doi: 10.1007/s11356-021-16306-y. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y., Sun Q., Li T., Ren X., Lin L., Sun M., Duan J., Sun Z. Adverse outcome pathway of fine particulate matter leading to increased cardiovascular morbidity and mortality: an integrated perspective from Toxicology and Epidemiology. J. Hazard Mater. 2022 doi: 10.1016/j.jhazmat.2022.128368. [DOI] [PubMed] [Google Scholar]
- 9.Brauer M., Amann M., Burnett R.T., Cohen A., Dentener F., Ezzati M., Henderson S.B., Krzyzanowski M., Martin R.V., Van Dingenen R. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environ. Sci. Technol. 2012;46:652–660. doi: 10.1021/es2025752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heo S., Fong K.C., Bell M.L. Risk of particulate matter on birth outcomes in relation to maternal socio-economic factors: a systematic review. Environ. Res. Lett. 2019;14 doi: 10.1088/1748-9326/ab4cd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Çakmak G., Arı P.E., Emerce E., Arı A., Odabaşı M., Schins R., Burgaz S., Gaga E.O. Investigation of spatial and temporal variation of particulate matter in vitro genotoxicity and cytotoxicity in relation to the elemental composition. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2019;842:22–34. doi: 10.1016/j.mrgentox.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., Zhao H., Wang Y., Shao Y., Zhang L., Xing M. Impacts of simultaneous exposure to arsenic (III) and copper (II) on inflammatory response, immune homeostasis, and heat shock response in chicken thymus. Int. Immunopharm. 2018;64:60–68. doi: 10.1016/j.intimp.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Gondal M., Aldakheel R., Almessiere M., Nasr M., Almusairii J., Gondal B. Determination of heavy metals in cancerous and healthy colon tissues using laser induced breakdown spectroscopy and its cross-validation with ICP-AES method. J. Pharmaceut. Biomed. Anal. 2020;183 doi: 10.1016/j.jpba.2020.113153. [DOI] [PubMed] [Google Scholar]
- 14.Naimabadi A., Ghadiri A., Idani E., Babaei A.A., Alavi N., Shirmardi M., Khodadadi A., Marzouni M.B., Ankali K.A., Rouhizadeh A. Chemical composition of PM10 and its in vitro toxicological impacts on lung cells during the Middle Eastern Dust (MED) storms in Ahvaz, Iran. Environ. Pollut. 2016;211:316–324. doi: 10.1016/j.envpol.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Zou Y., Wu Y., Wang Y., Li Y., Jin C. Physicochemical properties, in vitro cytotoxic and genotoxic effects of PM1. 0 and PM2. 5 from Shanghai, China. Environ. Sci. Pollut. Control Ser. 2017;24:19508–19516. doi: 10.1007/s11356-017-9626-9. [DOI] [PubMed] [Google Scholar]
- 16.Schilirò T., Bonetta S., Alessandria L., Gianotti V., Carraro E., Gilli G. PM10 in a background urban site: chemical characteristics and biological effects. Environ. Toxicol. Pharmacol. 2015;39:833–844. doi: 10.1016/j.etap.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Meng Z., Zhang Q. Damage effects of dust storm PM2. 5 on DNA in alveolar macrophages and lung cells of rats. Food Chem. Toxicol. 2007;45:1368–1374. doi: 10.1016/j.fct.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Mohtashami M., Mohamadi M., Azimi Nezhad M., Saeidi J., Nia F.F., Ghasemi A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2021;68:1421–1431. doi: 10.1002/bab.2064. [DOI] [PubMed] [Google Scholar]
- 19.Shamsi M., Zamani A., Khosravi Y., Parizanganeh A., Shamsi Z. Spatial variability and pollution status of lead and nickel the street Dust of Zanjan City, Iran. Journal of Human Environment and Health Promotion. 2020;6:11–18. [Google Scholar]
- 20.Cao S., Chen X., Zhang L., Xing X., Wen D., Wang B., Qin N., Wei F., Duan X. Quantificational exposure, sources, and health risks posed by heavy metals in indoor and outdoor household dust in a typical smelting area in China. Indoor Air. 2020;30:872–884. doi: 10.1111/ina.12683. [DOI] [PubMed] [Google Scholar]
- 21.Aguilera A., Bautista F., Goguitchaichvili A., Garcia-Oliva F. Health risk of heavy metals in street dust. Front. Biosci. 2021;1:327–345. doi: 10.2741/4896. [DOI] [PubMed] [Google Scholar]
- 22.Kaonga C.C., Kosamu I.B.M., Utembe W.R. A review of metal levels in urban dust, their methods of determination, and risk assessment. Atmosphere. 2021;12:891. [Google Scholar]
- 23.Soleymani S., Javan S., Naimabadi A. Heavy metal concentrations and health risk assessment in urban soils of Neyshabur. Iran, Environmental monitoring and assessment. 2022;194:218. doi: 10.1007/s10661-021-09724-5. [DOI] [PubMed] [Google Scholar]
- 24.Hashemi S.E., Fazlzadeh M., Ahmadi E., Parand M., Ramavandi B., Taghizadeh F., Arfaeinia H. Occurrence, potential sources, in vitro bioaccessibility and health risk assessment of heavy metal in indoor dust from different microenvironment of Bushehr, Iran. Environ. Geochem. Health. 2020;42:3641–3658. doi: 10.1007/s10653-020-00598-z. [DOI] [PubMed] [Google Scholar]
- 25.Dingle J.H., Kohl L., Khan N., Meng M., Shi Y.A., Pedroza-Brambila M., Chow C.-W., Chan A.W. Sources and composition of metals in indoor house dust in a mid-size Canadian city. Environ. Pollut. 2021;289 doi: 10.1016/j.envpol.2021.117867. [DOI] [PubMed] [Google Scholar]
- 26.Adachi K., Tainosho Y. Characterization of heavy metal particles embedded in tire dust. Environ. Int. 2004;30:1009–1017. doi: 10.1016/j.envint.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Councell T.B., Duckenfield K.U., Landa E.R., Callender E. Tire-wear particles as a source of zinc to the environment. Environ. Sci. Technol. 2004;38:4206–4214. doi: 10.1021/es034631f. [DOI] [PubMed] [Google Scholar]
- 28.Zhang T., Ruan J., Zhang B., Lu S., Gao C., Huang L., Bai X., Xie L., Gui M., Qiu R.-l. Heavy metals in human urine, foods and drinking water from an e-waste dismantling area: identification of exposure sources and metal-induced health risk. Ecotoxicol. Environ. Saf. 2019;169:707–713. doi: 10.1016/j.ecoenv.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 29.Van der Kuijp T.J., Huang L., Cherry C.R. Health hazards of China’s lead-acid battery industry: a review of its market drivers, production processes, and health impacts. Environ. Health. 2013;12:1–10. doi: 10.1186/1476-069X-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W., Wu F.-Y., Huang M.-J., Kang Y., Cheung K.C., Wong M.H. Size fraction effect on phthalate esters accumulation, bioaccessibility and in vitro cytotoxicity of indoor/outdoor dust, and risk assessment of human exposure. J. Hazard Mater. 2013;261:753–762. doi: 10.1016/j.jhazmat.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Wang W., Huang M.-J., Wu F.-Y., Kang Y., Wang H.-S., Cheung K.C., Wong M.H. Risk assessment of bioaccessible organochlorine pesticides exposure via indoor and outdoor dust. Atmos. Environ. 2013;77:525–533. [Google Scholar]
- 32.Niu X., Ho K.F., Hu T., Sun J., Duan J., Huang Y., Lui K.H., Cao J. Characterization of chemical components and cytotoxicity effects of indoor and outdoor fine particulate matter (PM2. 5) in Xi’an, China. Environ. Sci. Pollut. Control Ser. 2019;26:31913–31923. doi: 10.1007/s11356-019-06323-3. [DOI] [PubMed] [Google Scholar]
- 33.Happo M.S., Sippula O., Jalava P.I., Rintala H., Leskinen A., Komppula M., Kuuspalo K., Mikkonen S., Lehtinen K., Jokiniemi J. Role of microbial and chemical composition in toxicological properties of indoor and outdoor air particulate matter. Part. Fibre Toxicol. 2014;11:1–18. doi: 10.1186/s12989-014-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viegas S., Caetano L.A., Korkalainen M., Faria T., Pacifico C., Carolino E., Quintal Gomes A., Viegas C. Cytotoxic and inflammatory potential of air samples from occupational settings with exposure to organic dust. Toxics. 2017;5:8. doi: 10.3390/toxics5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goudarzi G., Shirmardi M., Naimabadi A., Ghadiri A., Sajedifar J. Chemical and organic characteristics of PM2. 5 particles and their in-vitro cytotoxic effects on lung cells: the Middle East dust storms in Ahvaz, Iran. Sci. Total Environ. 2019;655:434–445. doi: 10.1016/j.scitotenv.2018.11.153. [DOI] [PubMed] [Google Scholar]
- 36.Naimabadi A., Shirmardi M., Goudarzi G., Ghadiri A., Oskoei V., Mohammadi A.A., Conti G.O., Ferrante M. In vitro cytotoxicity effects of polycyclic aromatic hydrocarbons (PAHs) associated with PM10 during the Middle Eastern Dust (MED) storms in Ahvaz. Arabian J. Geosci. 2022;15:531. [Google Scholar]
- 37.Schilirò T., Alessandria L., Degan R., Traversi D., Gilli G. Chemical characterisation and cytotoxic effects in A549 cells of urban-air PM10 collected in Torino, Italy. Environ. Toxicol. Pharmacol. 2010;29:150–157. doi: 10.1016/j.etap.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Roy R., Jan R., Joshi U., Bhor R., Pai K., Satsangi P.G. Characterization, pro-inflammatory response and cytotoxic profile of bioaerosols from urban and rural residential settings in Pune, India. Environ. Pollut. 2020;264:114698. doi: 10.1016/j.envpol.2020.114698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spagnolo A.M., Ottria G., Perdelli F., Cristina M.L. Chemical characterisation of the coarse and fine particulate matter in the environment of an underground railway system: cytotoxic effects and oxidative stress—a preliminary study. Int. J. Environ. Res. Publ. Health. 2015;12:4031–4046. doi: 10.3390/ijerph120404031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014;7:60. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchetti S., Longhin E., Bengalli R., Avino P., Stabile L., Buonanno G., Colombo A., Camatini M., Mantecca P. In vitro lung toxicity of indoor PM10 from a stove fueled with different biomasses. Sci. Total Environ. 2019;649:1422–1433. doi: 10.1016/j.scitotenv.2018.08.249. [DOI] [PubMed] [Google Scholar]
- 42.Vicente E.D., Figueiredo D., Goncalves C., Lopes I., Oliveira H., Kovats N., Pinheiro T., Alves C.A. In vitro toxicity of indoor and outdoor PM10 from residential wood combustion. Sci. Total Environ. 2021;782:146820. doi: 10.1016/j.scitotenv.2021.146820. [DOI] [PubMed] [Google Scholar]
- 43.Monn C., Becker S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2. 5) and coarse particles (PM10–2.5) in outdoor and indoor air. Toxicol. Appl. Pharmacol. 1999;155:245–252. doi: 10.1006/taap.1998.8591. [DOI] [PubMed] [Google Scholar]
- 44.Miazgowicz A., Krennhuber K., Lanzerstorfer C. Metals concentrations in road dust from high traffic and low traffic area: a size dependent comparison. Int. J. Environ. Sci. Technol. 2020;17:3365–3372. [Google Scholar]
- 45.Mehdipour A., Zaeimdar M., Sekhavatjou M.S., Jozi S.A. Heavy metal concentrations in the outdoor and indoor air of high-traffic areas in Tehran, Iran. J. Adv. Environ. Health Res. 2020;8:25–37. [Google Scholar]
- 46.Khorrami S., Zarrabi A., Khaleghi M., Danaei M., Mozafari M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018;13:8013. doi: 10.2147/IJN.S189295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saeidi J., Dolatabadi S., Esfahani M.B., Saeidi M., Mohtashami M., Mokhtari K., Ghasemi A. Anticancer potential of doxorubicin in combination with green-synthesized silver nanoparticle and its cytotoxicity effects on cardio-myoblast normal cells, anti-cancer agents in medicinal chemistry. Form. Curr. Med. Chem. Anti-Canc. Agent. 2021;21:1842–1849. doi: 10.2174/1871520621666201207093913. [DOI] [PubMed] [Google Scholar]
- 48.Nemmar A., Holme J.A., Rosas I., Schwarze P.E., Alfaro-Moreno E. Recent advances in particulate matter and nanoparticle toxicology: a review of the in vivo and in vitro studies. BioMed Res. Int. 2013:2013. doi: 10.1155/2013/279371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallach D., Kang T.-B., Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat. Rev. Immunol. 2014;14:51–59. doi: 10.1038/nri3561. [DOI] [PubMed] [Google Scholar]
- 50.Perrone M.G., Gualtieri M., Ferrero L., Porto C.L., Udisti R., Bolzacchini E., Camatini M. Seasonal variations in chemical composition and in vitro biological effects of fine PM from Milan. Chemosphere. 2010;78:1368–1377. doi: 10.1016/j.chemosphere.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 51.Dilger M., Orasche J., Zimmermann R., Paur H.-R., Diabaté S., Weiss C. Toxicity of wood smoke particles in human A549 lung epithelial cells: the role of PAHs, soot and zinc. Arch. Toxicol. 2016;90:3029–3044. doi: 10.1007/s00204-016-1659-1. [DOI] [PubMed] [Google Scholar]
- 52.Rossner P., Strapacova S., Stolcpartova J., Schmuczerova J., Milcova A., Neca J., Vlkova V., Brzicova T., Machala M., Topinka J. Toxic effects of the major components of diesel exhaust in human alveolar basal epithelial cells (A549) Int. J. Mol. Sci. 2016;17:1393. doi: 10.3390/ijms17091393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anwar-Mohamed A., Elbekai R.H., El-Kadi A.O. Regulation of CYP1A1 by heavy metals and consequences for drug metabolism. Expet Opin. Drug Metabol. Toxicol. 2009;5:501–521. doi: 10.1517/17425250902918302. [DOI] [PubMed] [Google Scholar]
- 54.Amara I.E., Anwar-Mohamed A., El-Kadi A.O. Mercury modulates the CYP1A1 at transcriptional and posttranslational levels in human hepatoma HepG2 cells. Toxicol. Lett. 2010;199:225–233. doi: 10.1016/j.toxlet.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Guyot E., Solovyova Y., Tomkiewicz C., Leblanc A., Pierre S., El Balkhi S., Le Frere-Belda M.-A., Lecuru F., Poupon J., Barouki R. Determination of heavy metal concentrations in normal and pathological human endometrial biopsies and in vitro regulation of gene expression by metals in the Ishikawa and Hec-1b endometrial cell line. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korashy H.M., El-Kadi A.O. Regulatory mechanisms modulating the expression of cytochrome P450 1A1 gene by heavy metals. Toxicol. Sci. 2005;88:39–51. doi: 10.1093/toxsci/kfi282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.