Abstract
Vaccination of mice with Mycobacterium vaccae or M. smegmatis induces some protection against M. tuberculosis challenge. The 19-kDa lipoprotein of M. tuberculosis, expressed in M. vaccae or M. smegmatis (M. smeg19kDa), abrogates this protective immunity. To investigate the mechanism of this suppression of immunity, human monocyte-derived macrophages (MDM) were infected with M. smeg19kDa. Infection resulted in reduced production of tumor necrosis factor alpha (TNF-α) (P < 0.01), interleukin-12 (IL-12) (P < 0.05), IL-6 (P < 0.05), and IL-10 (P < 0.05), compared to infection with M. smegmatis vector (M. smegV). Infection with M. smeg19kDa and with M. smegV had no differential effect on expression of costimulatory molecules on MDM, nor did it affect the proliferation of presensitized T cells cocultured with infected MDM. When MDM were infected with M. smegmatis expressing mutated forms of the 19-kDa lipoprotein, including non-O-glycosylated (M. smeg19NOG), nonsecreted (M. smeg19NS), and nonacylated (M. smeg19NA) variants, the reduced production of TNF-α or IL-12 was not observed. When the purified 19-kDa lipoprotein was added directly to cultures of infected monocytes, there was little effect on either induction of cytokine production or its inhibition. Thus, the immunosuppressive effect is dependent on glycosylated and acylated 19-kDa lipoprotein present in the phagosome containing the mycobacterium. These results suggest that the diminished protection against challenge with M. tuberculosis seen in mice vaccinated with M. smegmatis expressing the 19-kDa lipoprotein is the result of reduced TNF-α and IL-12 production, possibly leading to reduced induction of T-cell activation.
To reduce the burden of tuberculosis (TB), particularly in resource-poor countries, an effective vaccine against primary TB and against reactivation of latent infection is urgently required. The development of an effective vaccine against Mycobacterium tuberculosis requires detailed knowledge of the host immune response to mycobacterial antigens and insight into which aspects of this immune response contribute to protection. Currently M. bovis BCG is widely used as a vaccine. BCG immunization of humans is associated with the development of a type 1 T-cell memory response, mediated by antigen-specific gamma interferon (IFN-γ)-producing T cells (29, 35). This memory response results in dermal delayed-type hypersensitivity responses to purified protein derivative of tuberculin (PPD). Despite widespread administration, the current BCG vaccine has failed to provide significant protection in adults against pulmonary TB. Several alternative vaccination strategies are therefore currently being explored (18, 19, 31).
In the mouse model, exposure to M. tuberculosis is followed by recruitment of IFN-γ-producing lymphocytes to the site of infection (12). When mice are vaccinated with BCG and then challenged with M. tuberculosis, an accelerated recruitment of activated IFN-γ-producing lymphocytes to the infected site is observed (37). This is associated with a reduction in the tissue bacillary load but not with elimination of the infection. Many of the new candidate vaccines against tuberculosis are now being tested in the mouse model (23, 25, 33).
In addition to BCG, other Mycobacterium spp. have been tested in mice for their ability to provide protection against M. tuberculosis challenge. When administered to mice as a vaccine, the saprophytic mycobacteria M. vaccae and M. smegmatis induce a short-lived type 1 T-cell response, as demonstrated by the ability of splenocytes to proliferate, produce IFN-γ, and mediate cytotoxic T-lymphocyte (CTL) activity in response to mycobacterial antigens (38). This immune response affords some protection against M. tuberculosis challenge (1, 40). Secreted M. tuberculosis antigens, such as the 19-kDa lipoprotein, are potent inducers of memory T-cell responses in vitro (3). The 19-kDa antigen is capable of stimulating a recall response in T cells and B cells obtained from M. tuberculosis-infected and BCG-vaccinated humans or mice (10, 20, 22, 41). Fast-growing mycobacteria such as M. vaccae and M. smegmatis do not express the 19-kDa antigen (1, 17). The gene encoding the 19-kDa antigen was therefore cloned into a shuttle plasmid to allow the expression of this lipoprotein on the surface of M. vaccae and M. smegmatis, with the expectation that vaccination with such strains would result in enhanced protection against M. tuberculosis challenge. However, even the limited protection afforded by immunization with M. vaccae or M. smegmatis was abrogated when the 19-kDa antigen was expressed by these mycobacteria (1, 40). In addition, immunization of mice with recombinant mycobacterial strains expressing the 19-kDa antigen failed to induce the expected accelerated recruitment of IFN-γ-producing T cells to the infected site following M. tuberculosis challenge. Moreover, the mice immunized with the recombinant mycobacteria expressing the 19-kDa lipoprotein had impaired delayed-type hypersensitivity responses to intradermal PPD (40).
To better understand the impaired protection following vaccination of mice with the M. smegmatis-expressed 19-kDa antigen (M. smeg19kDa), we infected human monocyte-derived macrophages (MDM) with M. smegmatis vector (M. smegV) or M. smeg19kDa. We evaluated the ability of the infected MDM to produce the cytokines tumor necrosis factor alpha (TNF-α), interleukin-12 (IL-12), IL-10, and IL-6. The effect of posttranslational modifications of the 19-kDa protein on cytokine production was also studied by infecting the cells with M. smegmatis recombinants expressing mutated 19-kDa genes. In addition, the effect of exogenously added purified 19-kDa lipoprotein to infected MDM on TNF-α and IL-12 production was examined. Finally, the possibility that the 19-kDa antigen affected the expression of stimulatory and costimulatory molecules (HLA-DR, CD40, CD80, and CD86) on the MDM cell surface was explored.
MATERIALS AND METHODS
Recombinant mycobacteria.
M. smegmatis mc2/1-2c was used for these experiments. Plasmids used for construction of recombinant mycobacteria were based on p16R1, a shuttle vector carrying a hygromycin resistance determinant and origins of replication suitable for maintenance in mycobacteria (16). The gene encoding the glycosylated and acylated 19-kDa antigen was cloned as an XbaI-HindIII fragment in a p16R1 derivative, as described previously, to generate pSMT3-19 (21). These polypeptides were extractable by Triton X-114, suggesting that they are acylated lipoproteins. The gene encoding the 19-kDa antigen was also modified by site-directed mutagenesis to alter posttranslational modification. In pSMT3-19NOG, O-linked glcosylation was inhibited by substitution of two threonine clusters by valine residues (21). A nonsecreted form (pSMT3-19NS) was produced by removing the signal sequence of the protein, and a nonacylated form (pSMT3-19NA) was generated by replacement of the N-terminal cysteine residue of the mature wild-type protein with alanine (32). The mutant proteins were expressed at levels comparable to those of the wild-type protein. Each of the plasmids was introduced into M. smegmatis by electroporation, and its expression was characterized as described previously (32). As a control, M. smegV was used in all infection experiments.
Isolation of monocytes.
Peripheral blood mononuclear cells (PBMC) were isolated from blood obtained from healthy volunteers. Blood was layered on 15 ml of Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) and centrifuged at 400 × g for 25 min. PBMC were harvested from the gradient, washed twice with RPMI 1640 (Gibco BRL, Gaithersburg, Md.), and kept on ice until use. Adherent monolayers were obtained by incubating PBMC in 24-well Falcon tissue culture plates (Becton Dickenson Labware, Lincoln Park, N.J.) for 1 to 2 h at 37°C. Nonadherent cells were washed off, and approximately 3 × 105 monocytes per well were incubated for 6 days at 37°C under 5% CO2 in fresh RPMI medium enriched with 10% human AB serum (R10) (Gemini Bio Products, Calabasas, Calif.).
Infection of monocytes.
Recombinant M. smegmatis was cultured in lipopolysaccharide-free 7H9 broth (Difco) enriched with 2% glucose, glycerol, and hygromycin B (Boehringer Mannheim, Indianapolis, Ind.) at 50 μg/ml. Mycobacterial stocks were frozen at −80°C until use. MDM (6 days old) were infected with the mycobacteria at a multiplicity of infection (MOI) of 3:1 and 3 h later were pulsed with gentamicin (Gibco BRL) (final concentration, 200 μg/ml) to inhibit the growth of extracellular organisms. At 5 h postinfection, the culture medium was removed, stored at −70°C for cytokine analysis, and replaced with fresh R10 medium without antibiotics.
The effect of exogenous recombinant 19-kDa lipoprotein on cytokine production by infected monocytes was explored by adding 100 to 400 ng of mycobacterium-derived 19-kDa antigen per ml to the MDM prior to infection with M. smegmatis. Native antigen purified from M. tuberculosis H37Rv was obtained from John Belisle, Colorado State University, Fort Collins, Colo. (the purified native 19-kDa lipoprotein was obtained as part of the Tuberculosis Research Materials and Vaccine Testing Contract from the National Institute of Allergy and Infectious Diseases, NOI AI-753230). Recombinant 19-kDa antigen expressed in M. vaccae was purified as described previously (1). Two preparations of purified protein were tested. Recombinant M. vaccae 19-kDa culture filtrates (rCF) and recombinant M. vaccae 19-kDa whole-cell lysate (rWCL) purified from the sonicated bacterial pellet. Since only 19-kDa protein produced by mycobacteria shows posttranslational acylation and glycosylation (17, 34), recombinant 19-kDa antigen from mycobacteria and not an Escherichia coli source was used.
CFU assay.
At each time point, 500 μl of supernatant for cytokine analysis was removed from each well of infected cells and stored at −70°C until use. The remaining contents of each well, to which 500 μl of phosphate-buffered saline (PBS) containing 0.25% Tween 80 (Sigma) had been added, was probe sonicated for 20 s and harvested for colony counts. Tenfold serial dilutions of the mycobacterial suspension were plated on 7H11 agar containing glucose (2%) and hygromycin B (50 μg/ml). Culture plates were incubated at 37°C, and the number of colonies was determined after 48 to 72 h.
Cytokine assays.
The supernatant was sampled for the cytokines TNF-α, IL-6, IL-12 (p70 and p40), and IL-10. Cytokine enzyme-linked immunosorbent assay kits (Endogen, Woburn, Mass.) were used as specified by the manufacturer, and samples were run in duplicate or triplicate. Since the cytokines produced during phagocytosis were removed along with the gentamicin-containing medium, initial (5-h) cytokine values were added to those at subsequent time points (24 to 96 h). A large donor-to-donor variability in MDM cytokine production in response to infection in vitro was noted, yielding nonparametric data. Therefore, when the results from multiple donors were reported, cytokine levels were normalized; 100% was defined as the amount produced by infection with M. smegV at 24 h postinfection. In experiments using the 19-kDa mutants, data for TNF-α production were normalized to the levels of cytokine induced at 5 h by infection with M. smegV. When results reported are from a single donor, the data are given as absolute values (picograms per milliliter).
T-cell proliferation.
T lymphocytes (105) from the blood of a PPD+ donor, enriched by E-rosetting and depleted of monocytes by passage through a nylon wool fiber column (Polysciences Inc., Warrington, Pa.), were added to MDM infected for 5 h with M. smegV or M. smeg19kDa. T-cell proliferative responses were assayed by [3H]thymidine (NEN Research Products, Boston, Mass.) incorporation during the last 6 or 18 h of 72- to 120-h cultures. The cells were harvested onto fiber mats with an automatic cell harvester (Skatron Instruments Inc., Sterling, Va.), and [3H]thymidine incorporation was measured with a betaplate liquid scintillation counter (model LKB 1205; Wallac, Gaithersburg, Md.).
FACS analysis.
Adherent cells were harvested 24, 48, and 72 h postinfection after a 30-min incubation with 0.02% EDTA (pH 7.2) in ice-cold PBS followed by a wash in ice-cold PBS containing 3% fetal calf serum and 0.1% sodium azide (fluorescence-activated cell sorter [FACS] buffer). Macrophages were labeled on ice for 30 min with one of the following monoclonal antibodies: phycoerythrin (PE)–anti-CD14, PE–anti-CD80 (B7.1), or PE–anti-HLA-DR (all from Becton Dickinson, San Jose, Calif.) PE-anti-CD86 (B7.2) or fluorescein isothiocyanate–anti-CD40 (Pharmingen, San Diego, Calif.), washed once in FACS buffer, and fixed with 2% glutaraldehyde in PBS before being subjected to analysis by flow cytometry (FACScan, Becton Dickinson).
Statistics.
The paired, one-tailed Student t test was used to compare CFU and cytokine levels for each time point. P < 0.05 was considered statistically significant.
RESULTS
Survival of M. smegmatis strains in cultures of human MDM.
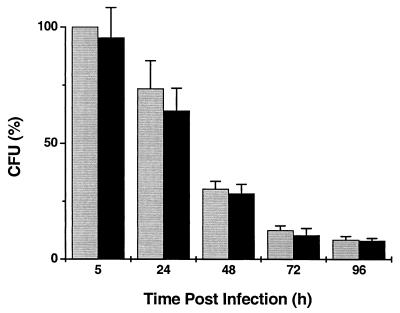
Monocytes, maintained for 6 days in tissue culture (MDM), were infected with M. smegV or M. smeg19kDa at an MOI of 3:1. At 5 h postinfection, 22 to 56% (mean, 36%) of the organisms were phagocytosed. Pulsing of the infected cultures with gentamicin resulted in killing of the extracellular bacilli. Further incubation of the cells led to a progressive decline in the number of viable intracellular organisms. At all time points, bacterial counts were similar for both strains (Fig. 1). MDM viability, as determined by trypan blue exclusion following detachment with EDTA, was similar for uninfected, M. smegV-infected, and M. smeg19kDa-infected cells (range, 79 to 97%).
FIG. 1.
Number of intracellular viable mycobacteria following infection of MDM with M. smegV (grey bars) or M. smeg19kDa (black bars). Cells were infected at an MOI of 3:1 for 3 h and pulsed with gentamicin for 2 h. The results are expressed as the percentage of CFU normalized to the number of CFU of M. smegV at 5 h. Results are means of six experiments done in duplicate or triplicate, carried out on monocytes of unrelated donors. Error bars indicate standard error of the mean.
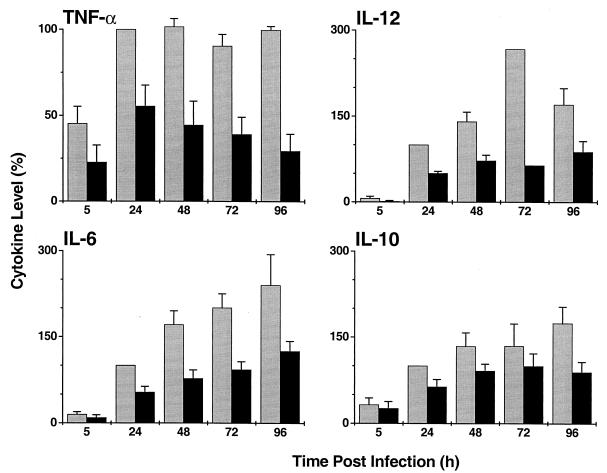
Cytokine production by MDM following infection with M. smegmatis strains.
The levels of cytokines produced by MDM following infection with M. smegV or M. smeg19kDa are shown in Fig. 2. In response to infection with M. smegV, the kinetics of production of the various cytokines differed. By 5 h postinfection, significant levels of TNF-α were noted, and its levels peaked by 24 h. In contrast, at 5 h, there were very low (if any) levels of IL-12, IL-6, and IL-10. Significant amounts of all cytokines were detected at 24 h. Therefore, in these experiments, results for all cytokines were normalized to the 24-h time point (see Materials and Methods). IL-12 production peaked at 72 h and then decreased, while IL-6 and IL-10 levels continued to increase over the course of the experiment. Infection with M. smeg19kDa resulted in up to threefold-lower production of TNF-α (P < 0.01), IL-12 (P < 0.05), and IL-6 (P < 0.05) compared to infection with M. smegV. Since IL-10 production was also lower in the M. smeg19kDa-infected MDM (Fig. 2) (P < 0.05), we concluded that reduced production of TNF-α, IL-6, and IL-12 did not result from increased production of IL-10. When M. smegV and M. smeg19kDa were mixed 1:1 prior to infection and the same total number of bacilli was used to infect MDM at an MOI of 3:1, the 19-kDa antigen-induced inhibition of TNF-α and IL-12 production was not significantly affected (data not shown). In this mixed infection, the 19-kDa antigen-induced inhibition was still evident even though only 50% of the infecting organisms expressed this antigen.
FIG. 2.
Cytokine production by MDM infected with M. smegV (grey bars) or M. smeg19kDa (black bars). Results were normalized to cytokine levels induced by M. smegV at 24 h of infection. Results are means and standard error of the mean of four to six experiments.
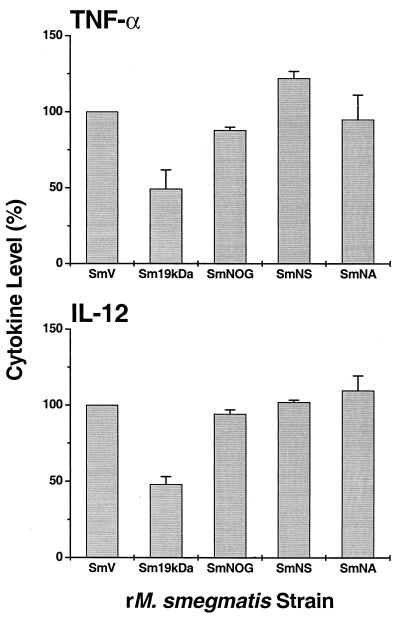
Effect of posttranslational modification of the 19-kDa antigen on cytokine production by MDM infected with M. smegmatis strains.
To study the effect of acylation and glycosylation of the 19-kDa antigen on cytokine production, MDM were infected with M. smegmatis expressing the complete glycosylated and acylated molecule (M. smeg19kDa), the non-O-glycosylated (M. smeg19NOG), nonsecreted (M. smeg19NS), or nonacylated (M. smeg19NA) forms of the 19-kDa antigen, or M. smegV. Reduced production of TNF-α and IL-12 was observed only when the 19-kDa antigen was intact (acylated and O glycosylated) (Fig. 3).
FIG. 3.
Effect of posttranslational modification of the 19-kDa antigen on cytokine production. TNF-α (5 h postinfection) and IL-12 (24 h postinfection) production by MDM infected with M. smegV (SmV) (100%), M. smeg19kDa (Sm19kDa), or M. smegmatis expressing truncated forms of the 19-kDa lipoprotein, non-O-glycosylated (SmNOG), nonsecreted (SmNS) or nonacylated (SmNA) is shown. The results are means and standard error of the mean of three or four experiments and are normalized to M. smegV.
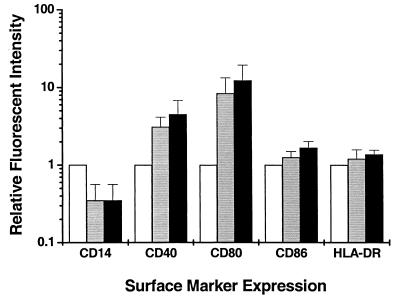
Effect of the 19-kDa antigen on costimulatory molecule expression and T-cell proliferation.
Adherent MDM infected with M. smegV or M. smeg19kDa were detached at 24, 48, and 72 h postinfection and analyzed for cell surface markers by flow cytometry. Comparative analysis of the mean fluorescent intensity for uninfected, M. smegV-infected, and M. smeg19kDa-infected cells revealed an infection-induced down-regulation of CD14 and increased expression of CD40 and CD80, which reached a maximum at 48 h postinfection (Fig. 4). CD14 down-regulation and increased CD40 and CD80 expression were similar following M. smegV or M. smeg19kDa infection. No significant differences in CD86 or HLA-DR expression were observed among uninfected MDM or MDM infected with either M. smegV or M. smeg19kDa (Fig. 4). When T cells from PPD+ individuals were exposed to autologous infected MDM, the recall proliferative responses were slightly higher (22% ± 16% higher) in the presence of M. smeg19kDa than in the presence of M. smegV. These results suggest that the 19-kDa antigen did not directly affect the expression of costimulatory molecules on MDM. The lipoprotein may have been recognized by presensitized T cells, resulting in slight increases in in vitro proliferation of the cells.
FIG. 4.
Expression of cell surface markers on uninfected MDM (open bars) or MDM infected for 48 h with M. smegV (grey bars) or M. smeg19kDa (black bars). The results are normalized to the values for uninfected cells (set at 1) and are means and standard error of the mean of three experiments.
Effect of exogenously added 19-kDa antigen on cytokine production by MDM infected with M. smegmatis strains.
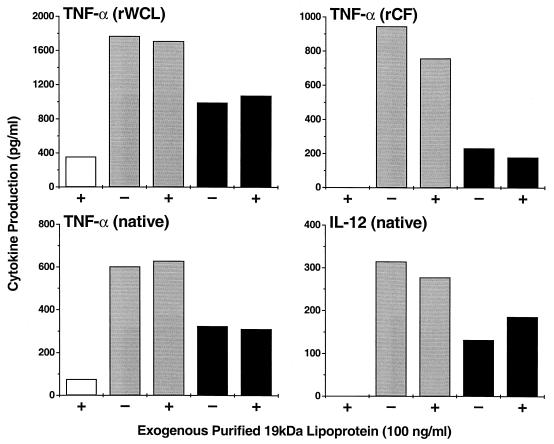
We next investigated whether cytokine production by MDM could be modified by addition of purified recombinant or purified native 19-kDa lipoprotein to the culture medium during infection with M. smegmatis strains. When different concentrations of recombinant 19-kDa lipoprotein purified from whole-cell lysates (rWCL) were added to uninfected MDM, low levels of TNF-α were produced in a dose-dependent manner. When added to MDM infected with either M. smegV or M. smeg19kDa, rWCL at 100 ng/ml had no effect on TNF-α production (Fig. 5). The extent of inhibition (∼50%) of TNF-α production in the M. smeg19kDa-infected cells compared to that in the M. smegV-infected MDM remained the same. Addition of recombinant 19-kDa antigen purified from culture filtrate (rCF) to uninfected MDM had no stimulatory effect on TNF-α production (Fig. 5). When rCF at 100 ng/ml was added to MDM infected with M. smegV or M. smeg19kDa, the inhibitory effect on TNF-α production was minimal. When the purified native 19-kDa antigen (100 ng/ml) was added to uninfected MDM, very low levels of TNF-α and no IL-12 were produced (Fig. 5). Native lipoprotein added to MDM infected with M. smegV or M. smeg19kDa had little or no effect on TNF-α or IL-12 production. Taken together, these results suggest that exogenously added 19-kDa antigen has only limited effects on cytokine production. It appeared that the 19-kDa lipoprotein must be intracellular and probably must be in the same cellular compartment as M. smeg19kDa to exert the maximal inhibitory effect on MDM cytokine production.
FIG. 5.
Effect of exogenously added 19-kDa lipoprotein on TNF-α or IL-12 production by MDM. MDM were uninfected (open bars) or infected with M. smegV (grey bars) or M. smeg19kDa (black bars) in the presence (+) or absence (−) of 100 ng of purified recombinant 19-kDa protein per ml isolated from whole recombinant M. smeg19kDa cell lysate (rWCL) or isolated from M. smeg19kDa culture filtrate (rCF), or 19-kDa protein purified from M. tuberculosis (native).
DISCUSSION
Protective immunity against M. tuberculosis depends on TNF-α and IL-12 production to regulate the activation of T-lymphocytes and to stimulate the antimycobacterial capacity of infected macrophages (24). Mice lacking IL-12 have decreased and delayed expression of mRNA for IFN-γ, TNF-α, and inducible nitric oxide synthase in infected tissues, as well as reduced lymphocyte recruitment to the site of infection (7). These mice also display early mortality with overwhelming tissue bacillary loads following infection with mycobacteria. Mice treated with exogenous IL-12 demonstrate an increased, IFN-γ-dependent resistance to infection with M. tuberculosis (8, 14). IL-12 as a vaccine adjuvant enhances the development of a protective Th1 response (2, 15). Similarly, IL-12 DNA therapy of M. tuberculosis-infected mice results in a phenotypic switch, from Th2-type cytokine production to IFN-γ-secreting Th1-type cells (28). Therefore, IL-12 is a key cytokine involved in establishing protective immunity against M. tuberculosis.
A critical role in protection against murine tuberculosis has also been demonstrated for the proinflammatory cytokine TNF-α. Mice lacking TNF-α fail to control M. tuberculosis infection. This is associated with delayed macrophage activation and impaired T-cell migration into granulomas despite the presence of adequate numbers of both CD4+ and CD8+ T cells in the liver and lungs (5, 13). TNF-α enhances IFN-γ-induced production of reactive nitrogen intermediates and may thus contribute to the killing of M. tuberculosis in vitro (11). Recent studies have also suggested an important role for IL-6 in stimulating early IFN-γ production during M. tuberculosis infection (36) and in priming of T cells after vaccination with M. tuberculosis proteins (27). We have shown here that following infection of human MDM in vitro with M. smegmatis expressing the 19-kDa lipoprotein of M. tuberculosis, there is reduced production of inflammatory cytokines, including IL-12 and TNF-α, as well as of IL-6. This reduced cytokine production may explain why M. smegmatis strains expressing the 19-kDa antigen were observed to be ineffective as vaccines (1, 40).
Experiments in which the 19-kDa lipoprotein was used to elicit an immune response have yielded contrasting results. In some studies, immunization of mice with the purified protein or with DNA encoding the 19-kDa antigen resulted in antigen-specific IFN-γ production by splenocytes, with a slight increase in survival time after M. tuberculosis challenge in the case of the DNA vaccine (1, 40). Protection against M. tuberculosis challenge was observed in mice immunized with a 19-kDa recombinant vaccinia virus construct (42). Although Erb et al. could readily detect antibodies against the 19-kDa lipoprotein following DNA vaccination, no proliferative or CTL activity was induced and protection against M. bovis BCG challenge was not achieved (9). In addition, T cells obtained from mice immunized with 19-kDa-antigen-pulsed dendritic cells showed no antigen-specific IFN-γ production (4). However, others have documented that a peptide fragment of the 19-kDa antigen, presented to T cells by human dendritic cells in vitro, could induce HLA class I-restricted CTL activity (30). A recent report suggested that the 19-kDa antigen may be a potent stimulator of IL-12p40 in THP1 cells and of IL-12p70 in fresh human monocytes in vitro (6). However, this observation is not in keeping with our in vitro observations. Here we used a similar preparation of native 19-kDa antigen and showed that the exogenously added lipoprotein induced only small amounts of IL-12 compared to infection with M. smegV. Brightbill et al. added purified 19-kDa antigen at 10 ng/ml to freshly cultured human monocytes and noted the production of IL-12p40 and IL-12p70 (6). In our experiments, we added 100 ng of 19-kDa antigen per ml to MDM and noted no production of IL-12. However, there was some production of TNF-α (50 to 80 pg/ml). By comparison, infection of the MDM with M. smegV at 1:1 induced the production of about 320 pg of IL-12 per ml and 600 pg of TNF-α per ml. We therefore conclude that although the 19-kDa antigen does induce some macrophage cytokine production, the intact mycobacteria induce much higher levels of cytokines (Fig. 5). When Brightbill et al. used THP1 monocytoid cell lines for their experiments, they found high levels of IL-12p40 production (6). Thus, it appears that macrophages and monocytes may differ in their responses to the 19-kDa lipoprotein.
We observed that intracellular M. smeg19kDa has stronger immunomodulating effects than exogenously added 19-kDa antigen. Also, we showed here that the intact 19-kDa molecule is required for the immunomodulatory activity. Interestingly, a previous study had shown that within 1 h of macrophage phagocytosis of M. bovis BCG, M. tuberculosis, or M. smeg19kDa, the 19-kDa lipoprotein traffics out from the phagosome by insertion into plasma membranes. This trafficking did not occur with mutant 19-kDa lipoproteins that were nonacylated and nonglycosylated (32). It is possible that the inhibition of cytokine production by M. smeg19kDa seen here may require the 19-kDa lipoprotein to be initially part of the same cellular compartment as the mycobacterium. This would allow the 19-kDa lipoprotein to leave the phagosome by inserting into the phagosome membrane, thereby gaining access to the regulatory pathways for cytokine production. The idea that a M. tuberculosis component may affect host cell immune function by insertion into the cell membrane has been suggested in another system. Ting et al. have observed that M. tuberculosis blocks human macrophage responses to IFN-γ by preventing the interaction of STAT1 with the transcriptional machinery in the nucleus (39). Recently they have suggested that this occurs through sequestration of transcriptional coactivators at extranuclear sites adjacent to the phagosome membrane (J. D. Ernst, Abstr. IDSA 38th Annu. Meet. p. 46, 2000). The authors suggest that this sequestration may be mediated by a component of the mycobacterial cell wall. It is possible that the 19-kDa lipoprotein may be such a component.
Any factor that contributes to the success of the pathogen by interfering with the host protective response can be considered a virulence factor for M. tuberculosis. Our observation that the 19-kDa antigen influences host cytokine responses is compatible with a role for this protein in mycobacterial virulence. Additional data supporting this view were reported in a study in which a naturally occurring mutant of M. tuberculosis H37Rv (I 2646), which does not express the 19-kDa antigen, showed reduced growth in B10 mice (26). When this strain was transformed with the 19-kDa antigen, increased bacterial loads were observed compared to those in the vector control. Whether mycobacterial strains lacking the 19-kDa antigen are less virulent and could be used as vaccine strains remains to be explored.
ACKNOWLEDGMENTS
These studies were supported by NIH grants AI-22616 and AI-42056 (to G.K.) and by a Wellcome Trust Programme Grant (to D.B.Y.) and Travelling Research Fellowship (to O.N.). F. A. Post was supported by Glaxo-Wellcome Action TB.
We are grateful to Christiane Abou-Zeid and Howard Cooper for purification of recombinant 19-kDa protein. We thank Victoria Freedman for assistance in preparing the manuscript. We thank Marguerite Nulty for secretarial work and Judy Adams for preparing the figures.
REFERENCES
- 1.Abou-Zeid C, Gares M P, Inwald J, Janssen R, Zhang Y, Young D B, Hetzel C, Lamb J R, Baldwin S L, Orme I M, Yeremeev V, Nikonenko B V, Apt A S. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997;65:1856–1862. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso L C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Askgaard D, Ljungqvist L, Bentzon M W, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991;59:1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird M A, Hart D N, Abernethy N, Watson J D. Dendritic cell presentation of PPD and 19 kDa protein of Mycobacterium tuberculosis and emergent T helper cell phenotype. Immunol Cell Biol. 1995;73:537–543. doi: 10.1038/icb.1995.84. [DOI] [PubMed] [Google Scholar]
- 5.Bean A G, Roach D R, Briscoe H, France M P, Korner H, Sedgwick J D, Britton W J. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 6.Brightbill H D, Libraty D H, Krutzik S R, Yang R B, Belisle J T, Bleharski J R, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 7.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 9.Erb K J, Kirman J, Woodfield L, Wilson T, Collins D M, Watson J D, LeGros G. Identification of potential CD8+ T-cell epitopes of the 19 kDa and AhpC proteins from Mycobacterium tuberculosis. No evidence for CD8+ T-cell priming against the identified peptides after DNA-vaccination of mice. Vaccine. 1998;16:692–697. doi: 10.1016/s0264-410x(97)00253-3. [DOI] [PubMed] [Google Scholar]
- 10.Faith A, Moreno C, Lathigra R, Roman E, Fernandez M, Brett S, Mitchell D M, Ivanyi J, Rees A D. Analysis of human T-cell epitopes in the 19,000 MW antigen of Mycobacterium tuberculosis: influence of HLA-DR. Immunology. 1991;74:1–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Flesch I E, Hess J H, Oswald I P, Kaufmann S H. Growth inhibition of Mycobacterium bovis by IFN-gamma stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int Immunol. 1994;6:693–700. doi: 10.1093/intimm/6.5.693. [DOI] [PubMed] [Google Scholar]
- 12.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunology. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 14.Flynn J L, Goldstein M M, Triebold K J, Sypek J, Wolf S, Bloom B R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 15.Freidag B L, Melton G B, Collins F, Klinman D M, Cheever A, Stobie L, Suen W, Seder R A. CpG oligodeoxynucleotides and interleukin-12 improve the efficacy of Mycobacterium bovis BCG vaccination in mice challenged with M. tuberculosis. Infect Immun. 2000;68:2948–2953. doi: 10.1128/iai.68.5.2948-2953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garbe T R, Barathi J, Barnini S, Zhang Y, Abou-Zeid C, Tang D, Mukherjee R, Young D B. Transformation of mycobacterial species using hygromycin resistance as selectable marker. Microbiology. 1994;140:133–138. doi: 10.1099/13500872-140-1-133. [DOI] [PubMed] [Google Scholar]
- 17.Garbe T, Harris D, Vordermeier M, Lathigra R, Ivanyi J, Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993;61:260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grange J M. Effective vaccination against tuberculosis-a new ray of hope. Clin Exp Immunol. 2000;120:232–234. doi: 10.1046/j.1365-2249.2000.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurunathan S, Klinman D M, Seder R A. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 20.Harris D P, Vordermeier H M, Friscia G, Roman E, Surcel H M, Pasvol G, Moreno C, Ivanyi J. Genetically permissive recognition of adjacent epitopes from the 19-kDa antigen of Mycobacterium tuberculosis by human and murine T cells. J Immunol. 1993;150:5041–5050. [PubMed] [Google Scholar]
- 21.Herrmann J L, O'Gaora P, Gallagher A, Thole J E, Young D B. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 1996;15:3547–3554. [PMC free article] [PubMed] [Google Scholar]
- 22.Jackett P S, Bothamley G H, Batra H V, Mistry A, Young D B, Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988;26:2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamath A T, Groat N L, Bean A G, Britton W J. Protective effect of DNA immunization against mycobacterial infection is associated with the early emergence of interferon-gamma (IFN-gamma)-secreting lymphocytes. Exp Immunol. 2000;120:476–482. doi: 10.1046/j.1365-2249.2000.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan G, Freedman V H. The role of cytokines in the immune response to tuberculosis. Res Immunol. 1996;147:565–572. doi: 10.1016/s0923-2494(97)85223-6. [DOI] [PubMed] [Google Scholar]
- 25.Kumar M, Behera A K, Matsuse H, Lockey R F, Mohapatra S S. A recombinant BCG vaccine generates a Th1-like response and inhibits IgE synthesis in BALB/c mice. Immunology. 1999;97:515–521. doi: 10.1046/j.1365-2567.1999.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lathigra R, Zhang Y, Hill M, Garcia M J, Jackett P S, Ivanyi J. Lack of production of the 19-kDa glycolipoprotein in certain strains of Mycobacterium tuberculosis. Res Microbiol. 1996;147:237–249. doi: 10.1016/0923-2508(96)81384-2. [DOI] [PubMed] [Google Scholar]
- 27.Leal I S, Smedegard B, Andersen P, Appelberg R. Interleukin-6 and interleukin-12 participate in induction of a type 1 protective T-cell response during vaccination with a tuberculosis subunit vaccine. Infect Immun. 1999;67:5747–5754. doi: 10.1128/iai.67.11.5747-5754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowrie D B, Tascon R E, Bonato V L, Lima V M, Faccioli L H, Stavropoulos E, Colston M J, Hewinson R G, Moelling K, Silva C L. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 29.Marchant A, Goetghebuer T, Ota M O, Wolfe I, Ceesay S J, De Groote D, Corrah T, Bennett S, Wheeler J, Huygen K, Aaby P, McAdam K P, Newport M J. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999;163:2249–2255. [PubMed] [Google Scholar]
- 30.Mohagheghpour N, Gammon D, Kawamura L M, van Vollenhoven A, Benike C J, Engleman E G. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J Immunol. 1998;161:2400–2406. [PubMed] [Google Scholar]
- 31.Morris S, Kelley C, Howard A, Li Z, Collins F. The immunogenicity of single and combination DNA vaccines against tuberculosis. Vaccine. 2000;18:2155–2163. doi: 10.1016/s0264-410x(99)00540-x. [DOI] [PubMed] [Google Scholar]
- 32.Neyrolles O, Gould K, Gares M-P, Brett S, Janssen R, O'Gaora P O, Herrmann J-L, Prevost M-C, Perret E, Thole J E R, Young D. Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J Immunol. 2001;166:447–457. doi: 10.4049/jimmunol.166.1.447. [DOI] [PubMed] [Google Scholar]
- 33.Olsen A W, Hansen P R, Holm A, Andersen P. Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. Eur J Immunol. 2000;30:1724–1732. doi: 10.1002/1521-4141(200006)30:6<1724::AID-IMMU1724>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 34.Prestidge R L, Grandison P M, Chuk D W, Booth R J, Watson J D. Production of the 19kDa antigen of Mycobacterium tuberculosis in Escherichia coli and its purification. Gene. 1995;164:129–132. doi: 10.1016/0378-1119(95)00470-q. [DOI] [PubMed] [Google Scholar]
- 35.Ravn P, Boesen H, Pedersen B K, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 36.Saunders B M, Frank A A, Orme I M, Cooper A M. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun. 2000;68:3322–3326. doi: 10.1128/iai.68.6.3322-3326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva C L, Bonato V L, Lima V M, Faccioli L H, Leao S C. Characterization of the memory activated T cells that mediate the long-lived host response against tuberculosis after bacillus Calmette-Guerin or DNA vaccination. Immunology. 1999;97:573–581. doi: 10.1046/j.1365-2567.1999.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skinner M A, Yuan S, Prestidge R, Chuk D, Watson J D, Tan P L. Immunization with heat-killed Mycobacterium vaccae stimulates CD8+ cytotoxic T cells specific for macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1997;65:4525–4530. doi: 10.1128/iai.65.11.4525-4530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ting L M, Kim A C, Cattamanchi A, Ernst J D. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J Immunol. 1999;163:3898–3906. [PubMed] [Google Scholar]
- 40.Yeremeev V V, Lyadova I V, Nikonenko B V, Apt A S, Abou-Zeid C, Inwald J, Young D B. The 19-kD antigen and protective immunity in a murine model of tuberculosis. Clin Exp Immunol. 2000;120:274–279. doi: 10.1046/j.1365-2249.2000.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young D B, Garbe T R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991;142:55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Venkataprasad N, Ivanyi J, Vordermeier M. Vaccination with recombinant vaccinia viruses protects mice against Mycobacterium tuberculosis infection. Immunology. 1997;92:6–9. doi: 10.1046/j.1365-2567.1997.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]