Summary
Recent breakthroughs in human stem cell technologies have enabled the generation of 3D brain organoid platforms for modeling human neurodevelopment and disease. Here, we review advances in brain organoid development, approaches for generating whole-brain or cerebral organoids and region-specific brain organoids, and their applications in disease modeling. We present a comprehensive overview of various brain organoid generation protocols, including culture steps, media, timelines, and technical considerations associated with each protocol, and highlight the advantages and disadvantages of each protocol. We also discuss the current limitations as well as increasing sophistication of brain organoid technology, and future directions for the field. These insights provide a valuable assessment of multiple commonly used brain organoid models and main considerations for investigators who are considering implementing brain organoid technologies in their laboratories.
Subject areas: organoids, stem cells, cell culture
Recent breakthroughs in human stem cell technologies have enabled the generation of 3D brain organoid platforms for modeling human neurodevelopment and disease. Here, we review advances in brain organoid development, approaches for generating whole-brain or cerebral organoids and region-specific brain organoids, and their applications in disease modeling. We present a comprehensive overview of various brain organoid generation protocols, including culture steps, media, timelines, and technical considerations associated with each protocol, and highlight the advantages and disadvantages of each protocol. We also discuss the current limitations as well as increasing sophistication of brain organoid technology, and future directions for the field. These insights provide a valuable assessment of multiple commonly used brain organoid models and main considerations for investigators who are considering implementing brain organoid technologies in their laboratories.
Introduction
Studies of human brain are challenging, largely due to the complexities of the human brain and inaccessibility of primary brain tissue. Animal models have traditionally been used as model organisms for neuroscience research since human brain tissue is limited by availability and ethical considerations. However, rodents can only model the human brain to a limited extent and are not fully representative of human disease pathology, which likely contributes to the overall low success of translating findings from animal studies to the clinic. The development of human pluripotent stem cell (hPSC)-based neural models in the last two decades has provided new systems for studying human brain development and disease.1 These models generate a variety of platforms of varying complexity, beginning with least complex monolayer cultures of neural stem cells that can be differentiated into specialized neural cell types, to more complex neural tube-like structures called rosettes, which exhibit spontaneous radial organization reminiscent of an embryonic neural tube, or the highly complex 3D spheroid and organoid models that have organ-like tissue morphology and composition of certain brain regions or contain multiple regions within a single organoid called cerebral or whole-brain organoids.1,2 Collectively, brain organoids represent physiologically relevant 3D in vitro neural systems for functional modeling of human brain development and disease.3
The balance between complexity and heterogeneity is an important feature to consider when deciding which approach to use for a particular study. 2D cultures are simplistic models to study cell types in isolation; however, they fail to capture complex cellular interactions.As such they have limited utility in modeling many disease-relevant neurobiological phenomenon, but at the same time they offer the advantage of homogeneity needed for robust and reliable assay readouts. 3D culture models are more physiologically relevant as they contain diverse cell types, which undergo complex spatiotemporal structural organization to mimic functional features of the brain with greater complexity than 2D models. However, their inherent heterogeneity results in issues of reproducibility. All these models have accelerated our understanding of neurobiology and led to promising findings. In this review we will focus on protocols to produce brain organoids, three-dimensional brain-like tissues.
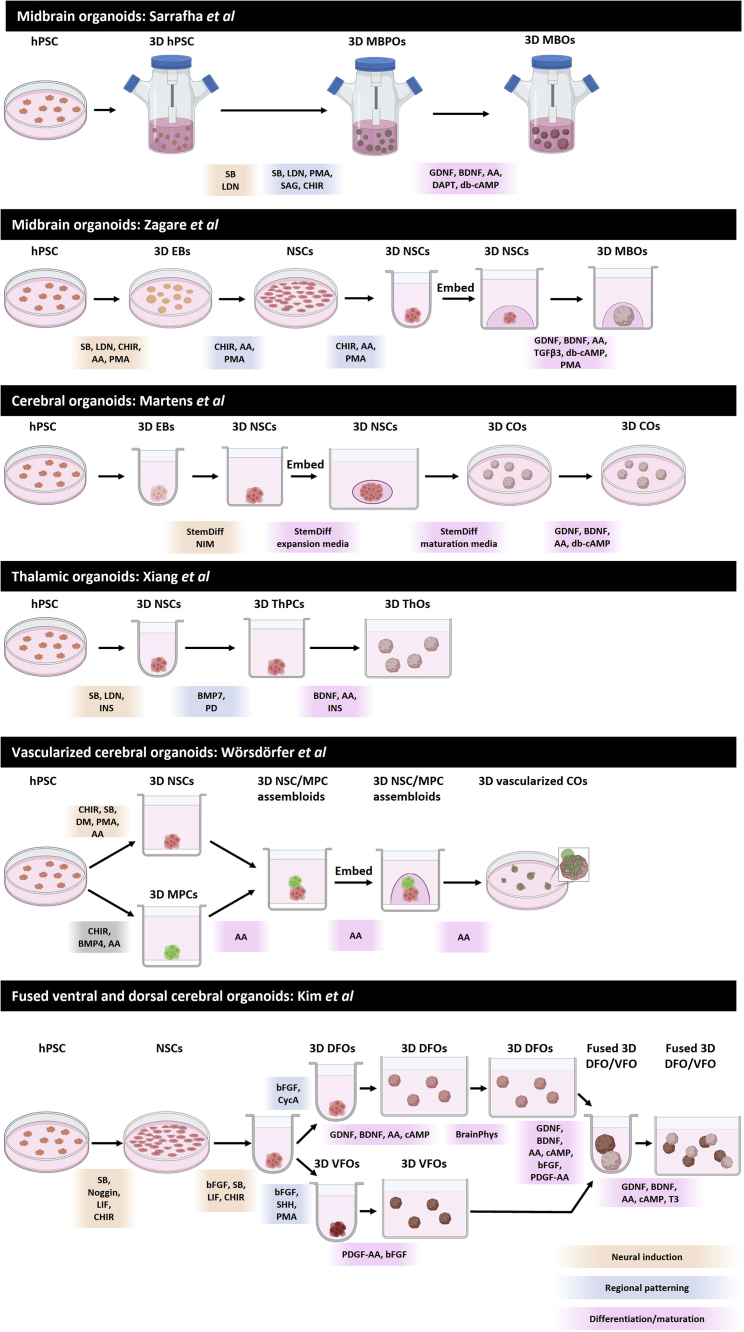
Recent years have seen an explosion in development of brain organoid systems, from whole-brain or cerebral organoids to region specific organoids—cerebellar, pituitary, midbrain, forebrain, cortical, thalamic, and choroid plexus organoids, and more recently fused organoids or assembloids (Figure 1, Table 1). The continually expanding set of brain organoid systems has provided researchers with fantastic tools to unlock the complexities of normal and diseased brain. With the plethora of brain organoid protocols available, each consisting of multiple steps with specialized media systems and equipment, it can be a daunting task for scientists looking to adopt this emerging technology for their research.
Figure 1.
Graphical overview of stages of brain organoid generation protocols presented in Tables 2, 3, and 4, including key stages, culture vessels, and media components
Notes: media compositions are simplified to show an overview of only key factors at each stage. Carefully review detailed media composition in protocols for exact composition, concentrations, and timings used. Depicted culture vessels indicate those described by protocol authors. Where possible, we recommend researchers use these vessels, but in some cases, alternatives may be used. Created with BioRender.com.
Table 1.
Advances in brain organoid development
| Brain organoid technologies | Reference |
|---|---|
| First 3D neural organoid in the form of self-organized optic cup | Eiraku et al.4 |
| First brain organoid in the form of self-patterned cerebral organoids and self-organizing cortical tissue | Kadoshima et al.5; Lancaster et al.6 |
| Enhanced reproducibility, survival, and maturation of brain organoids | Giandomenico et al.7; Lancaster et al.8; Qian et al.9; Quadrato et al.10; Velasco et al.11 |
| Increased cellular diversity in brain organoids | Madhavan et al.12; Marton et al.13; Ormel et al.14; Pasca et al.15; Quadrato et al.10; Sakaguchi et al.16; Velasco et al.11 |
| Development of region-specific brain organoids: cerebellar, pituitary, midbrain, forebrain, cortical, and thalamic organoids | Birey et al.17; Jo et al.18; Muguruma et al.19; Ozone et al.20; Qian et al.21; Schukking et al.22; Trujillo et al.23; Xiang et al.24 |
| Intracerebral transplantation to achieve vascularized brain organoids | Mansour et al.25; Wang et al.26 |
| Functional neural circuit and oscillatory waves in brain organoids | Giandomenico et al.7; Trujillo et al.23 |
| Axially patterned brain organoids | Cederquist et al.27 |
| CNS-barrier forming brain organoids with cerebrospinal fluid production | Pellegrini et al.28 |
| Organoid/microglia co-cultures to recapitulate neural-immune interactions in the brain | Abud et al.29; Brownjohn et al.30; Lin et al.31; Xu et al.32 |
| Organoid/endothelial cell co-cultures to recapitulate neural-vascular interactions in the brain | Cakir et al.33; Pham et al.34; Shi et al.35; Worsdorfer et al.36 |
| Organoid/tumor cell co-cultures to recapitulate brain tumor invasion | Goranci-Buzhala et al.37; Krieger et al.38; Linkous et al.39 |
| Fusion of organoids (assembloids) to study brain regional interconnectivity and neural circuit formation | Andersen et al.40; Bagley et al.41; Birey et al.17; Miura et al.42; Xiang et al.24; Xiang et al.43 |
To provide scientists a summary of the major considerations for implementing brain organoid technologies we provide an overview of the different detailed brain organoid protocols published in STAR Protocols, as well as the groundbreaking whole-brain protocol published by the Knoblich group (a complete list of brain organoid protocols published in STAR Protocols is available at: https://star-protocols.cell.com/search?categories = Organoids&query = brain).44 Given the intensive nature of cell culture needed to generate these complex 3D structures, several metrics such as protocol steps, reagents and timing, special equipment, and technical expertise needed, as well as advantages and disadvantages of each protocol are discussed. Additionally, we examine protocols for fusing different brain-region specific organoids such as dorso-ventral forebrain assembloids to model oligodendroglial development and myelination, and generation of hybrid organoids by fusion of neural and mesenchymal organoids to form vascularized neural organoids. Lastly, we look at how these systems have been applied in disease modeling of glioblastoma tumors, Alzheimer’s disease and traumatic brain injury, the current disadvantages of brain organoids and highlight future directions of this promising technology. Our aim in reviewing this expanding toolkit of brain organoid models is to foster their wider adoption by the scientific community.
Summaries of brain organoid protocols published in STAR protocols
See Box 1 for a list of abbreviations used in Tables 2, 3, 4, and 5.
Box 1. Abbreviations.
AA: ascorbic acid, B27-RA: B-27 supplement without vitamin A (retinyl acetate), BDNF: brain-derived neurotrophic factor, bFGF: basic fibroblast growth factor, BME: β-mercaptoethanol, BMP4: bone morphogenetic protein 4, BMP7: bone morphogenetic protein 7, cAMP: cyclic adenosine monophosphate, CHIR: CHIR99021 (glycogen synthase kinase 3 inhibitor, Wnt pathway agonist), CO: cerebral organoid, CycA: cyclopamine (smoothened receptor inhibitor, hedgehog pathway antagonist), DAPT: γ-secretase inhibitor (NOTCH pathway antagonist), db-cAMP: dibutyryl-cyclic adenosine monophosphate, DFO: dorsal forebrain organoid, DM: dorsomorphin (BMP type I receptor inhibitor, BMP pathway antagonist), DA: dopaminergic, EB: embryoid body, GDNF: glial cell line-derived neurotrophic factor, GLICO: glioma cerebral organoid, GSC: glioma stem cells, hESC: human embryonic stem cell, hPSC: human pluripotent stem cell, INS: insulin, iPSC: induced pluripotent stem cell, LDN: LDN139189 (BMP type I receptor inhibitor, BMP pathway antagonist), L-Glu: L-glutamine, LIF: leukemia inhibitory factor, MBO: midbrain organoid, MBPO: midbrain progenitor organoid, MPC: mesodermal progenitor cell, N2: N-2 supplement, NEAA: Minimum Essential Medium-non-essential amino acids, NSC: neural stem cell, PD: PD325901 (MEK inhibitor, MAPK/ERK pathway antagonist), PDOX: patient derived orthotopic xenografts, PMA: purmorphamine (smoothened receptor activator, hedgehog pathway agonist), P/S: penicillin/streptomycin, SAG: smoothened agonist (smoothened receptor activator, hedgehog pathway agonist), SB: SB431542 (TGF-β type I receptor inhibitor, TGF-β/Activin/NODAL pathway antagonist), T3: Triiodo-L-thyronine (thyroid hormone), TGFβ3: transforming growth factor-β3, ThPC: thalamic progenitor cell, ThO: thalamic organoid, PDO: patient derived organoids, ULA: ultra-low attachment, VFO: ventral forebrain organoid.
Table 2.
Generation of whole-brain or cerebral organoids
| Organoid type: Cerebral organoids (CO) | ||
| Protocol title | Generation and validation of APOE knockout human iPSC-derived cerebral organoids | Generation of cerebral organoids from human pluripotent stem cells |
| STAR Protocols reference/Seminal Reference | Martens et al.45 | Lancaster and Knoblich46 |
| Original publication | Zhao et al.47 | Lancaster et al.6 |
| Protocol length (before long-term culture) | ∼5 weeks | |
| Specialized equipment needed | Orbital shaker or Spinning bioreactor | |
| Note | Martens et al.45 protocol uses the StemDiff Cerebral Organoid Kit available commercially from StemCell Technologies. While this media is based on published recipes,6,46 the precise formulation is proprietary. | |
| Protocol stages overview | 1) 2D hPSC culture, 2) EB formation, 3) Neural induction, 4) Matrigel embedding and expansion of NSCs, 5) Cerebral organoid differentiation, 6) Cerebral organoid maturation, 7) Long-term culture | |
| Protocol stage 1 | 2D hPSC culture | |
| Stage timing | 1–2 h | |
| Culture format | Cell culture dishes | Cell culture dishes |
| Culture system/Culture medium | mTeSR1, hESC-qualified Matrigel, dispase clump passaging | mTeSR1, Matrigel-coated plates |
| Cell number/preparation for differentiation | 70% confluence, Accutase to single cells, seed at 15,000 cells/well of U-bottomed 96-well ULA plate | EDTA/accutase-treatment to obtain single cell suspension, seed at 9,000 cells/well of U-bottomed 96-well ULA plate. One hPSC well will yield approximately an entire 96-well plate of EBs |
| Protocol stage 2 | 3D EB formation | |
| Stage timing | 5 days | 5–7 days |
| Culture format | U-bottomed 96-well ULA plate | |
| Culture medium, growth factors and small molecules | StemDiff Cerebral Organoid Kit: complete EB Formation medium + 10μM Y27632 | DMEM-F12 medium + 20% knockout serum replacement + 3% ESC-quality Fetal Bovine Serum + 1 mM β-Mercaptoethanol (BME) + 1% non-essential amino acids (NEAA) + 1% Glutamax + 4 ng/mL Basic Fibroblast Growth Factor (bFGF) + 50μM Y27632 |
| Protocol stage 3 | Neural induction | |
| Stage timing | 2–5 days | 4–5 days |
| Culture format | 48-well ULA plate | 24-well ULA plate |
| Culture medium, growth factors and small molecules | StemDiff Cerebral Organoid Kit: complete Neural Induction Medium | DMEM-F12 medium + 1% N2 + 1% Glutamax + 1% NEAA + 1 μg/mL heparin |
| Protocol stage 4 | Matrigel embedding and expansion of neuroepithelial buds | |
| Stage timing | 3 days | 4 days |
| Culture format | EBs embedded in Matrigel in 6-well ULA cell culture plate | EBs embedded in Matrigel droplets in 6-cm dish |
| Culture medium, growth factors and small molecules | StemDiff Cerebral Organoid Kit: complete Expansion Medium | Cerebral organoid differentiation medium without vitamin A: DMEM-F12 medium + Neurobasal medium (1:1) + 0.5x N2 + 1x B27-RA + 2.5 μg/mL INS + 50μM BME + 1% Glutamax + 1x P/S |
| Protocol stage 5 | CO maturation | |
| Stage timing | 4 weeks | |
| Culture format | 10 cm dish on orbital shaker @ 40 rpm | Transfer embedded organoids to a 125 mL spinning bioreactor or 6 cm dish on orbital shaker @ 85 rpm |
| Culture medium, growth factors and small molecules | StemDiff Cerebral Organoid Kit: complete Maturation Medium | Cerebral organoid differentiation medium containing vitamin A: DMEM-F12 medium + Neurobasal medium (1:1) + 0.5x N2 + 1x B27 + 2.5 μg/mL INS + 50μM BME + 1% Glutamax + 1x P/S |
| Protocol stage 6 | Long-term culture | |
| Stage timing | Up to 1 year | |
| Culture format | 10 cm dish on orbital shaker @ 40 rpm | 125-mL spinning bioreactor or 6 cm dish on orbital shaker @ 85 rpm |
| Culture medium | DMEM-F12 medium + Neurobasal medium (1:1) + 0.5x B27-RA + 0.5x N2 + 1x Glutamax + 1x Sodium pyruvate + 200 ng/mL AA + 1x NEAA + 500 ng/mL dcAMP + 1x P/S + 10 ng/mL BDNF + 10 ng/mL GDNF | Cerebral organoid differentiation medium containing vitamin A |
| Analysis & summary | ||
| Marker analysis of organoid cellular identity in protocol and original publication | D28 cerebral organoids: neural progenitor cells (SOX2), dorsal region marker (PAX6), ventral region marker (NKX2.1), intermediate progenitors (TBR2), deep cortical layer neurons (CTIP2), early neurons (TUJ1), astrocytes (GFAP); D84 cerebral organoids: superficial cortical layer neurons (SATB2), deep cortical layer neurons (CTIP2), astrocytes (GFAP, S100β) | 1–2-month-old COs: progenitors (SOX2), neurons (Tuj1 or DCX), forebrain (Foxg1), choroid plexus (TTR), hippocampus (Prox1 and Fzd9), mitotic radial glia (P-vim), cortical layer neurons (CTIP2 and SATB2) |
| Functional analysis of organoids | Assessment of the expression of mature neuronal markers (MAP2, CTIP, SATB2) pre- and post-synaptic markers (synaptophysin and PSD95) by immunoblot. No direct assessment of organoid function (e.g., electrophysiology). | Calcium imaging (in original publication) |
| Additional protocols described in STAR Protocols paper | Guide RNA design, cloning oligos into px459 plasmid, human iPSC electroporation, genotyping to identify gene edited clones, neural marker immunostaining, gene knockout efficiency analysis by RT-qPCR | N/A |
| Protocol applications | Modeling aspects of human brain and assessment of gene function in brain development and disease | |
| Protocol advantages | Provides complete workflow for generating iPSC clones containing targeted INDELs for knockout studies, as well as protocols for quality assessment of targeted clones. Commercially sourced differentiation media simplifies organoid production and improves consistency. Simple, optimized workflow with access to technical support from differentiation kit vendor. | Provides in-depth and easy to follow protocol for generation and analysis of 3D cerebral organoids from hPSCs. Highlighted critical steps, provided representative images of optimal and suboptimal organoids at various stages, and compared with alternative methods |
| Protocol limitations | CO are not regionally restricted, containing tissue resembling multiple brain regions. These tissues develop stochastically and are highly heterogeneous, making COs challenging to use in some applications. Commercially sourced media adds cost to the protocol. Neither protocol nor original publication describe functional assessment of generated organoids | |
| Technical expertise neededa | Proficiency with undifferentiated hPSC culture is essential. Some experience with organoid production and manipulation is helpful but not necessary | Proficiency with undifferentiated hPSC culture is essential. Proficiency with organoid production and manipulation is useful |
| Additional protocols described | Guide RNA design, cloning oligos into px459 plasmid, human iPSC electroporation, genotyping to identify gene edited clones, neural marker immunostaining, gene knockout efficiency analysis by RT-qPCR | Cryosectioning and immunostaining of COs |
| Conclusion | These protocols are based on the seminal work of Lancaster et al., 2013 and organoids produced using this method have been widely used and extensively validated. The use of consistent commercially sourced media in Martens et al., 2021 protocol and associated technical support from the vendor is a significant advantage making this protocol an excellent choice for novices where “whole-brain” organoids are appropriate | |
NB: in addition to protocol-specific details outlined below, there are many additional factors to consider when implementing an organoid generation protocol. These are reviewed in “Practical Considerations”.
Proficiency in the culture of high-quality undifferentiated hPSCs is essential for the success of any differentiation protocol and is thus a prerequisite for implementing all brain organoid protocols described. All organoid generation protocols described in this review can be implemented by skilled technical staff. However, differentiation can be highly variable from line-to-line, warranting optimization of the protocol for each line. Furthermore, the steps involved in some protocols are more complex than others (e.g., organoid fusion). Therefore, here we provide our assessment of the ideal level of technical expertise needed to successfully implement this protocol.
Table 3.
Generation of region-specific brain organoids
| Organoid type: midbrain dopaminergic organoids (MBO) | |
| Protocol title | High-throughput generation of midbrain dopaminergic neuron organoids from reporter human pluripotent stem cells. |
| STAR Protocols reference | Sarrafha et al.48 |
| Original publication | Ahfeldt et al.49 |
| Protocol length (before long-term culture) | ∼6 weeks |
| Specialized equipment needed | Magnetic stir plate, spinner flasks |
| Protocol stages overview | 1) 2D hPSC culture, 2) 3D hPSC culture, 3) Generation of 3D midbrain progenitor organoids, 4) Differentiation to 3D dopaminergic-neuron organoids, 5) Long-term culture |
| Protocol stage 1 | 2D hPSC culture |
| Stage timing | Feeder-free maintenance culture |
| Culture format | Cell culture dishes |
| Culture system/Culture medium | StemFlex (ThermoFisher) + Geltrex (ThermoFisher), Accutase clump passaging |
| Cell number/preparation for differentiation | Four 10-cm dishes in monolayer used to seed spinner flasks @ ∼40x10e6 per flask |
| Protocol stage 2 | 3D hPSC culture |
| Stage timing | 6 days |
| Culture format | Spinner flask |
| Culture system/Culture medium | StemFlex (ThermoFisher) |
| Protocol stage 3 | Generation of 3D midbrain progenitor organoids (MBPOs) |
| Stage timing | 12 days |
| Culture format | Spinner flask |
| Culture medium, growth factors and small molecules | D0-1: DMEM-F12 medium + 1x Glutamax + 1x B27 Supplement minus vitamin A (B27-RA) + 1x N-2 supplement (N2) + 100 nM LDN193189 (LDN) + 10μM SB431542 (SB) D2-3: DMEM-F12 medium + 1x Glutamax + 1x B27-RA + 1x N2 + 100nM LDN + 10μM SB + 2μM Purmorphamine (PMA) + 1μM Smoothened Agonist (SAG) D4-7: DMEM-F12 medium + 1x Glutamax + 1x B27-RA + 1x N2 + 100nM LDN + 10μM SB + 2μM PMA + 1μM SAG + 1.5μM CHIR99021 (CHIR) D8-11: DMEM-F12 medium + 1x Glutamax + 1x B27-RA + 1x N2 + 100nM LDN + 1.5μM CHIR |
| Protocol stage 4 | Differentiation to 3D MBOs |
| Stage timing | 24 days |
| Culture format | Spinner flask |
| Culture medium, growth factors and small molecules | D12-35: DMEM-F12 medium + 1x Glutamax + 1x B27-RA + 1x N2 + 20 ng/mL Brain-derived neurotrophic factor (BDNF) + 20 ng/mL Glial cell line-derived neurotrophic factor (GDNF) + 0.2mM Ascorbic Acid (AA) + 10μM DAPT + 0.1mM Dibutyryl-Cyclic Adenosine Monophosphate (db-cAMP) |
| Protocol stage 5 | Long-term culture |
| Stage timing | Up to 200 days |
| Culture format | Spinner flask |
| Culture medium, growth factors and small molecules | D36+: DMEM-F12 medium + 1x Glutamax + 1x B27-RA + 1x N2 + 20 ng/mL BDNF + 20 ng/mL GDNF + 0.2mM AA |
| Analysis & summary | |
| Cell types generated | D15: midbrain progenitor organoids: dopaminergic (DA) neurons (TH), midbrain progenitors (FoxA2, LMX1A); D20 midbrain progenitor organoids: TH midbrain neurons (TH, TUBB3); D30 dopaminergic neuron organoids: DA neurons (TH, TUBB3, NURR1, GIRK2); D80: midbrain DA neuron organoid: astrocytes (GFAP); D200: midbrain DA neuron organoid: (TH, Melanin) |
| Additional information | Midbrain organoids can be plated on PLO-LN plates to generate DA-neuron outgrowths, or dissociated to single cells and plated on human astrocytes |
| Functional analysis of organoids | Whole patch clamp recordings showing voltage-gated sodium and potassium currents, and evoked and spontaneous action potential generation of isolated TH-neurons from midbrain organoids. Live Ca2+ imaging showing spontaneous tetrodotoxin-sensitive activity |
| Protocol applications | Analysis of human midbrain development; identification of pathophysiological mechanisms in disease affecting midbrain (e.g., Parkinson’s disease) |
| Protocol advantages | Protocol facilitates scalability and high throughput midbrain organoid production |
| Protocol limitations | Expensive relative to other midbrain organoid protocols due to large volume of media/growth factors used for differentiation. However, protocol can be scaled down to 6-well ultra-low attachment plates on orbital shaker |
| Technical expertise neededa | Proficiency with undifferentiated hPSC culture is essential. Proficiency with organoid production and manipulation is useful |
| Additional protocols described in STAR Protocols paper | Reporter iPSC line generation by CRISPR/Cas |
| Conclusion | Protocol generates MBOs that express midbrain markers and contain DA neurons exhibiting electrophysiological activity and astrocytes. However, the Zagare protocol described below appears to generate organoids containing more DA neurons (>60% at D30) than this protocol (plateau at ∼30% by D40. MBOs generated with this method contain astrocytes, but no oligodendrocyte formation is described. This protocol facilitates significant scale up using spinner flasks (although increased media consumption will increase cost), however the protocol is also amenable to scale down in 6-well plates. We recommend implementing and optimizing this protocol in 6-well plates before scaling up. Technically, this protocol will present few difficulties to users with experience culturing and differentiating hPSCs, especially those familiar with organoid production. |
| Organoid type: MBO | |
| Protocol title | A robust protocol for the generation of human midbrain organoids |
| STAR protocols reference | Zagare et al.50 |
| Original publication | Monzel et al.51 |
| Protocol length (before long-term culture) | ∼4 weeks (cryopreserved NSCs), ∼10 weeks (new derivation) |
| Specialized equipment needed | Multichannel pipet (optimally), horizontal shaker |
| Protocol stages overview | 1) 2D hPSC culture, 2) 3D embryoid body (EB) formation 3) 2D NSC culture, 4) Generation of 3D NSCs, 5) Embedding 3D NSCs in Geltrex, 6) Differentiation to 3D midbrain organoids, 7) Long-term culture |
| Protocol stage 1 | 2D hPSC culture (Reinhardt et al.52) |
| Stage timing | Feeder-dependent maintenance culture |
| Culture format | Cell culture dishes |
| Culture system/culture medium | DMEM-F12 medium + 20% knockout serum replacement + 1 mM BME + 1% non-essential amino acids (NEAA), 1% P/S + 1% L-Glu + 5 ng/mL Basic Fibroblast Growth Factor (bFGF). Inactivated MEFs substrate. Collagenase IV for routine passaging and EB generation. |
| Protocol stage 2 | 3D EB generation (Reinhardt et al.52) |
| Stage timing | 6 days |
| Culture format | 10 cm petri dishes |
| Culture system/culture medium | D1-2: DMEM-F12 medium+ 20% knockout serum replacement + 1 mM BME + 1% non-essential amino acids (NEAA), 1% P/S + 1% L-Glu + 1μM Dorsomorphin (DM) + 3μM CHIR + 0.5μM PMA D3-4: DMEM/F12 medium + Neurobasal medium (1:1) + 2mM L-Glu + 0.5x N2 + 1x B27-RA + 1x P/S + 1μM Dorsomorphin (DM) + 3μM CHIR + 0.5μM PMA D5-6: DMEM-F12 medium + Neurobasal medium + 2mM L-Glu + 0.5x N2 + 1x B27-RA + 1x P/S + 0.5μM PMA + 150μM AA. EBs triturated to break into smaller pieces. |
| Protocol stage 3 | 2D NSC culture |
| Stage timing | 2 months (new derivation), 2 weeks (cryopreserved NSCs) |
| Culture format | Cell culture dishes |
| Culture medium, growth factors and small molecules | DMEM/HAM’s F12 medium + Neurobasal medium (1:1) + 2mM Glutamax + 1x Penicillin/Streptomycin (P/S) + 0.5x N2 + 1x B27-RA + 3μM CHIR + 0.75μM PMA + 150μM AA. Matrigel or Geltrex-coated plates. Single cells prepared by Accutase passaging |
| Cell number/preparation | 9,000 NSCs required per well of a 96-well ultra-low attachment (ULA) plate |
| Protocol stage 4 | Generation of 3D NSCs |
| Stage timing | 6 days |
| Culture format | 96-well U-bottomed ULA (D0-5), 24-well cell culture plate (D6-7) |
| Culture medium, growth factors and small molecules | N2B27 maintenance medium: DMEM/HAM’s F12 medium + Neurobasal medium (1:1) + 2mM Glutamax + 0.5x N2 + 1x B27-RA + 1x P/S |
| Protocol stage 5 | Embedding 3D NSCs in Geltrex |
| Stage timing | 2 days |
| Culture format | 24-well cell culture plate, embedded in Geltrex |
| Culture medium, growth factors and small molecules | N2B27 maintenance medium |
| Protocol stage 6 | Differentiation to 3D MBOs |
| Stage timing | 6 days |
| Culture format | 24-well cell culture plate, embedded in Geltrex; plate placed on horizontal shaker (80 rpm) for remainder of protocol starting on d14 |
| Culture medium, growth factors and small molecules | N2B27 maintenance medium + 10 ng/mL BDNF + 10 ng/mL GDNF + 200μM AA + 1 ng/mL Transforming Growth Factor-β3 (TGFβ3) + 500μM db-cAMP + 1μM PMA |
| Protocol stage 7 | Long-term culture |
| Stage timing | Up to 1 year |
| Culture format | 24-well cell culture plate, embedded in Geltrex |
| Culture medium, growth factors and small molecules | N2B27 maintenance medium + 10 ng/mL BDNF + 10 ng/mL GDNF + 200μM AA + 1 ng/mL TGFβ3 + 500μM db-cAMP |
| Analysis & summary | |
| Marker analysis of organoid cellular identity in protocol and original publication | D0: NSCs (NESTIN, SOX2, PAX6); D30 midbrain organoids: DA neurons (TUJ1B, TH, NURR1); D44 midbrain organoids: oligodendrocytes (O4); D60 midbrain organoids: DA neurons (FOXA2, LMX1A, TH, NURR1, DAT, DDC), astrocytes (S100b, GFAP); D149 DA neurons: neuromelanin granules (Fontana Masson staining) |
| Functional analysis of organoids | Synaptic connections determined by detection of SYP-positive pre-synapses directly contacting PSD95-positive post-synapses by immunoanalysis; Spontaneous electrophysiological activity detected by Ca2+ imaging and multi-electrode array analysis; dopamine production confirmed by immunoanalysis; Oligodendrocyte function demonstrated by ensheathment of TUJ1-positive neurites by myelin sheets of CNPase+/MBP + oligodendrocytes |
| Protocol applications | Analysis of human midbrain development; identification of pathophysiological mechanisms in disease affecting midbrain (e.g., Parkinson’s disease) |
| Protocol advantages | NSCs can be cryopreserved and thawed for organoid production, enhancing consistency if using quality-controlled banks of cryopreserved NSCs. Oligodendrocytes co-develop with neurons. |
| Protocol limitations | Utilizes undefined hPSC culture media and feeders. Requires production of NSCs from hPSCs (∼2 months) or prior production and cryopreservation of NSCs.52 Requires time-consuming embedding 3D NSC spheres in semi-solid matrix (Geltrex). |
| Technical expertise neededa | Proficiency with undifferentiated hPSC culture is essential. Some experience with organoid production and manipulation is helpful but not necessary |
| Additional protocols described in STAR Protocols paper | Derivation of neural stem cells (NSCs) |
| Conclusion | Protocol describes generation of MBOs that express midbrain markers and contain DA neurons exhibiting electrophysiological activity. This protocol generates organoids containing a higher percentage of DA neurons (>60% at D30) than the Sarrafha protocol (plateau at ∼30% by D40). MBOs contain both astrocytes and oligodendrocytes and are amenable to long-term culture. Although this protocol requires production of NSCs, these can be cryopreserved and thawed for organoid production, facilitating increased reproducibility as well as organoid generation without a need to consistently culture hPSCs. Technically, this protocol will present few difficulties to users with experience culturing and differentiating hPSCs, especially those familiar with organoid production. However, the undefined nature of the hPSC culture system described for generating NSCs may make implementation of this protocol somewhat more challenging |
| Organoid type: thalamic organoids (ThOs) | |
| Protocol title | Generation of Regionally Specified Human Brain Organoids Resembling Thalamus Development |
| STAR Protocols reference | Xiang et al.53 |
| Original publication | Xiang et al.24 |
| Protocol length (before long-term culture) | 16 days |
| Specialized equipment needed | Orbital shaker |
| Protocol stages overview | 1) 2D hPSC culture, 2) Neural induction, 3) Thalamic patterning, 4) Neural maturation and long-term culture |
| Protocol stage 1 | 2D hPSC culture |
| Stage timing | Feeder-free maintenance culture |
| Culture format | Cell culture dishes |
| Culture system/Culture medium | mTeSR1, Matrigel, dispase clump passaging |
| Cell number/preparation for differentiation | 9,000 cells per well of a ULA 96-well U-bottomed plate. Single cells prepared by Accutase |
| Protocol stage 2 | Neural induction |
| Stage timing | 8 days |
| Culture format | EB formation in ULA 96-well U-bottomed plate |
| Culture medium, growth factors and small molecules | DMEM-F12 medium + 15% Knockout serum replacement + 1x NEAA+ 1x Glutamax + 100μM BME + 100nM LDN + 10μM SB+ 4 μg/mL Insulin (INS) |
| Protocol stage 3 | Thalamic patterning |
| Stage timing | 8 days |
| Culture format | Transfer EBs to ULA 24-well plate and incubate on an orbital shaker |
| Culture medium, growth factors and small molecules | DMEM-F12 medium + 0.15% (w/v) Dextrose + 100μM BME + 1% N2 + 2% B27-RA + 30 ng/mL Bone Morphogenetic Protein 7 (BMP7) + 1μM PD325901 (PD) |
| Protocol stage 4 | Neural maturation and long-term culture |
| Stage timing | Up to 1 year |
| Culture format | 6-well plate on orbital shaker |
| Culture medium, growth factors and small molecules | DMEM-F12 medium + Neurobasal medium (1:1) + 0.5% N2 + 1% B27 + NEAA + 1% Glutamax + 1% P/S+ 0.025% INS + 50μM BME + 20 ng/mL BDNF + 200μM AA |
| Analysis & Summary | |
| Marker analysis of organoid cellular identity in protocol and original publication | D60 thalamic organoids: caudal forebrain (OTX2); ventral thalamus (DBX1); thalamus marginal zone (GBX2); thalamus (TCF7L2). D89: Astrocytes (GFAP, EAAT1). scRNA-seq analysis identified cell types human fetal thalamus cell types |
| Functional analysis of organoids | Spontaneous action potential production detected by whole-cell patch clamp analysis and Ca2+ imaging (GCaMP6s); and synapse formation by immunoanalysis demonstrating adjacency of presynaptic marker SYP and postsynaptic marker PSD95. Axonal projections to and from fused COs |
| Protocol applications | Analysis of thalamus development and disease; mechanisms of axon targeting and synapse formation (following fusion with cortical organoids) |
| Protocol advantages | Thalamic organoids can be fused with other region-specific brain organoids to model axonal connections and function of neural circuits between the thalamus and different regions in the brain. |
| Protocol limitations | Challenging to generate specific thalamic nuclei |
| Technical expertise neededa | Proficiency with undifferentiated hPSC culture is essential. Some experience with organoid production and manipulation is helpful but not necessary |
| Conclusion | Protocol generates thalamic organoids containing fetal thalamic cell types and electrophysiologically active thalamic neurons. Technically, the production of thalamic organoids is straightforward, although fusion with other organoid types is more challenging |
Proficiency in the culture of high-quality undifferentiated hPSCs is essential for the success of any differentiation protocol and is thus a prerequisite for implementing all brain organoid protocols described. All organoid generation protocols described in this review can be implemented by skilled technical staff. However, differentiation can be highly variable from line-to-line, warranting optimization of the protocol for each line. Furthermore, the steps involved in some protocols are more complex than others (e.g., organoid fusion). Therefore, here we provide our assessment of the ideal level of technical expertise needed to successfully implement this protocol.
Table 4.
Generation of fused organoids (assembloids)
| Assembloid type: Fusion of neural and mesenchymal organoids to form vascularized neural organoids | ||
| Protocol title | Generation of Vascularized Neural Organoids by Co-culturing with Mesodermal Progenitor Cells | |
| STAR protocols reference | Worsdorfer et al.54 | |
| Original publication | Worsdorfer et al.36 | |
| Protocol length (before long-term culture) | ∼4 weeks | |
| Specialized equipment needed | 2D rocking plate | |
| Protocol stages overview | 1) hPSC culture, 2) Generation of neuro-mesenchymal organoids, 3) Assembly of Neural and Mesenchymal aggregates, 4) Culture neuromesenchymal organoids for vascular network formation, 5) Maturation of vascularized neuromesenchymal organoids, 6) Long-term culture | |
| Protocol stage 1 | 2D hPSC culture | |
| Stage timing | Feeder-free maintenance culture + 3 days | |
| Culture format | Cell culture dishes | |
| Culture system/Culture medium | StemMACS iPSC Brew Medium, Matrigel, Accutase single cell passaging | |
| Cell number/preparation for differentiation | 4,000 cells/well of 6-well plate. hPSCs should be at ∼80% confluency before seeding organoid formation. | |
| Protocol stage 2 | Generation of neuro-mesenchymal organoids | |
| Neural organoids | Mesenchymal organoids | |
| Stage timing | 8 days | 4 days |
| Timing note | The timing of neural and mesenchymal organoid induction should be coordinated to be completed on the same day | |
| Culture format | Agarose-coated F-bottom 96-well plate (agarose to prevent attachment) | |
| Culture medium, growth factors and small molecules | D1-2: DMEM-F12 medium + Neurobasal medium (1:1) + 1x B27-RA + 1x N2 + 2mM L-Glu + 3μM CHIR + 10μM SB + 1μM DM + 0.5μM PMA D3-5: DMEM-F12 medium + Neurobasal medium (1:1) + 1x B27-RA+ 1x N2 + 2mM L-Glu + 3μM CHIR + 62.5 μg/mL AA + 0.5μM PMA D6-7 Neural differentiation medium: DMEM-F12 medium + Neurobasal medium (1:1) + 1x B27-RA+ 1x N2 + 2mM L-Glu + 62.5 μg/mL AA + 1x P/S |
Advanced DMEM-F12 medium + 2mM L-Glu + 62.5 μg/mL AA + 10μM CHIR + 25 ng/mL Bone Morphogenetic Protein 4 (BMP4) |
| Protocol stage 3 | Assembly of Neural and Mesenchymal aggregates | |
| Stage timing | 2 days | |
| Assembly approach | Manually transfer a single d4 mesodermal organoid into each well of an F-bottom 96-well plate already containing a d7 neural organoid. Incubate for 2 days before embedding in Matrigel in stage 4 | |
| Culture format | Agarose-coated F-bottom 96-well plate (agarose to prevent attachment) | |
| Culture medium, growth factors and small molecules | Neural differentiation medium | |
| Protocol stage 4 | Embed neuromesenchymal organoids for vascular network formation | |
| Stage timing | 20 days | |
| Culture format | Assembloids embedded in Matrigel in 96-well plate D9. Transferred to 10cm dish on day 10 | |
| Culture medium, growth factors and small molecules | Neural differentiation medium | |
| Protocol stage 5 | Maturation of vascularized neuromesenchymal organoids | |
| Stage timing | Up to 210 days | |
| Culture format | 10 cm dish on 2D rocking plate | |
| Culture medium, growth factors and small molecules | Neural differentiation medium | |
| Analysis & Summary | ||
| Marker analysis of organoid cellular identity in protocol and original publication | D20 assembloids: neurons (TUJ1, MAP2), endothelial cells (CD31), basement membrane (COL I, COL IV), peri-endothelial cells (SMA); D210 vascularized neural organoids: neuroepithelial cells (SOX1, Nestin), neurons (MAP2), radial glia cells or astrocytes (GFAP), microglia-like cells (IBA1) | |
| Functional analysis of organoids | In vitro differentiation of mesodermal progenitors to smooth muscle and endothelial cells. Formation of vasculature containing endothelial cell-cell junctions, a collagen IV-positive basement membrane and peri-endothelial cells. | |
| Protocol applications | Facilitates analysis of role of vascularization and potentially other stromal cell types in neural development | |
| Protocol advantages | Additional organoid complexity via incorporation of vascularity more closely mimics human brain cellular composition. Mesodermal organoids provide a source of additional stromal cell types including microglia-like cells. Mesodermal organoids can be mixed with tumor cells to generate vascularized tumor models and form connections with existing blood vessels when fused with chick chorion allantois membrane. | |
| Protocol limitations | Regional identity of neural organoids is not described. Variable and only partial vascularization of neural components in assembloids. Vasculature that forms is immature | |
| Technical expertise neededa | Proficiency with undifferentiated hPSC culture is essential. Proficiency with organoid production and manipulation is useful | |
| Additional protocols described in STAR Protocols paper | Paraffin sectioning and histological analysis, H&E staining, immunofluorescence analysis, tissue clearing for microscopic analysis | |
| Conclusion | Protocol generates partially vascularized organoids with undefined neural identity, but the additional cellular complexity achieved by fusing neural and mesodermal organoids may be useful for studying the role of specific mesenchymal cell types in early stages of brain development and development of neurovasculature. Technically, this protocol will present few difficulties to users with experience culturing hPSCs and with organoid production | |
| Assembloid type: Fusion of dorsal and ventral forebrain organoids to model oligodendroglial development and myelination | ||
| Protocol title | Generation of human Pluripotent stem cell-derived fused organoids with oligodendroglia and myelin | |
| STAR Protocols reference | Kim and Jiang55 | |
| Original publication | Kim et al.56 | |
| Brain organoid type(s) | Ventral (VFO) and dorsal (DFO) forebrain organoids | |
| Protocol length (before long-term culture) | 12 weeks | |
| Specialized equipment needed | Orbital shaker | |
| Protocol stages overview | 1) hPSC culture, 2) Generation and culture of NSCs, 3) Organoid formation from NSCs, 4) Generation of ventral and dorsal forebrain organoids, 5) Oligodendroglial and neuronal differentiation, 6) Neuronal maturation of DFOs, 7) Assembly of VFOs and DFOs | |
| Protocol stage 1 | hPSC culture (Chen et al.57) | |
| Stage timing | Feeder-free maintenance culture | |
| Culture format | Cell Culture dishes | |
| Culture system/Culture medium | mTeSR1, Matrigel, dispase clump passaging | |
| Protocol stage 2 | Generation and culture of NSCs (Chen et al.57) | |
| Stage timing | 4 weeks | |
| Culture format | EB formation in ULA plate, Rosette formation in cell culture dishes, ULA dishes, NSC formation in cell culture dishes | |
| Culture medium, growth factors and small molecules | D1-7 (neural induction): DMEM-F12 medium + 1x N2 + 5μM SB + 50 ng/mL Noggin D8-14 (rosette formation): DMEM-F12 medium + 1x N2 + 1 μg/mL Laminin D15-D28 (NSC expansion): DMEM-F12 medium + Neurobasal medium (1:1) + 1x N2 + 1x B27-RA + 20 ng/mL bFGF + 10 ng/mL Leukemia Inhibitory Factor (LIF) + 3μM CHIR + 2μM SB + 10μM Y27632 |
|
| Cell number/preparation for differentiation | 9,000 NSCs, prepared by TrypLE passaging to single cells, plated per well of a 96-well ULA plate | |
| Protocol stage 3 | Cerebral organoid formation from NSCs | |
| Stage timing | 4 days | |
| Culture format | 96-well ULA plate (2 days), 6-well ULA plate on an orbital shaker (2 days) | |
| Culture medium, growth factors and small molecules | DMEM-F12 medium + Neurobasal medium (1:1) + 1x N2 + 1x B27-RA + 20 ng/mL bFGF + 10 ng/mL LIF + 3μM CHIR 99021 + 2μM SB + 1x P/S | |
| Protocol stage 4 | Generation of regionally patterned forebrain organoids | |
| Ventral forebrain organoids (VFOs) | Dorsal forebrain organoids (DFOs) | |
| Stage timing | 2 weeks | 2 weeks. DFOs produced at the end of this stage are fused with VFOs produced in stage 6 |
| Culture format | 6-well ULA plate on an orbital shaker | 6-well ULA plate on an orbital shaker |
| Culture medium, growth factors and small molecules | DMEM-F12 medium + Neurobasal medium (1:1) + 1x N2 + 1x B27-RA + 20 ng/mL bFGF + 50 ng/mL SHH + 1 μM PMA + 10μM Y27632 + 1x P/S | DMEM-F12 medium + Neurobasal medium (1:1) + 1x N2 + 1x B27-RA + 20 ng/mL bFGF + 5μM Cyclopamine A (CycA) 10μM Y27632 + 1x P/S |
| Protocol stage 5 | Oligodendroglial differentiation | Neuronal differentiation |
| Stage timing | 2 weeks | 2 weeks |
| Culture format | 6-well ULA plate on an orbital shaker | 6-well ULA plate on an orbital shaker |
| Culture medium, growth factors and small molecules | OPC medium: DMEM-F12 medium + 1x N2 + 1x B27-RA + 10 ng/mL PDGF-AA + 10 ng/mL bFGF + 1x P/S | ND medium: DMEM-F12 medium + Neurobasal medium (1:1) + 1x N2 + 1x B27-RA + 10 ng/mL GDNF + 10 ng/mL BDNF + 1μM cAMP + 200nM AA+ 1x P/S |
| Protocol stage 6 | Neuronal maturation of DFOs | |
| Stage timing | 2 weeks. DFOs produced at the end of this step are fused with VFOs produced in stage 4 | |
| Culture format | 6-well ULA plate on an orbital shaker | |
| Culture medium, growth factors and small molecules | BrainPhys neuronal medium | |
| Note | BrainPhys medium is available commercially from StemCell Technologies. While this media is based on published recipes,58 the precise formulation is proprietary | |
| Protocol stage 7 | Assembly of VFOs and DFOs | |
| Stage timing | 2 days (week 2 VFOs and week 6 DFOs are used for assembly) | |
| Assembly approach | Manually transfer a single VFO and a single DFO into each well of a 96-well ULA round-bottomed plate. Incubate for 2h without agitation and then 8h with hourly gentle trituration of media without disturbing organoids. Transfer to 6 well ULA plate for remainder of protocol | |
| Culture format | 96-well ULA round-bottom plate for fusion and transferred to 6 well ULA plate on orbital shaker | |
| Culture medium, growth factors and small molecules | 1:1 ratio of OPC and ND medium | |
| Protocol stage 8 | Myelination and maturation | |
| Stage timing | Up to 6 weeks | |
| Culture format | 6 well ULA plate on orbital shaker | |
| Culture medium, growth factors and small molecules | OL medium: DMEM-F12 medium + Neurobasal medium (1:1) + 1x N2 + 1x B27-RA + 1x P/S + 10 ng/mL GDNF + 10 ng/mL BDNF + 1μM cAMP + 200nM AA + 10 ng/mL Triiodo-L-thyronine (T3) Feed fused organoids with a 1:1 ratio of OL and ND medium |
|
| Analysis & Summary | ||
| Marker analysis of organoid cellular identity in protocol and original publication | D0 NSCs (PAX6). Week 2 ventral forebrain (NKX2.1, NKX2.2, DLX1, LHX6) and dorsal forebrain (PAX6, EMX1, TBR2). Week 4 oligodendrocytes (OLIG2), astrocytes (S100β), neurons (βIII-TUBULIN). Week 6 DFOs (c-FOS, SYNAPSIN 1, PSD-95). Week 9 oligodendrocytes (PDGFR, MBP), excitatory post-synaptic components (HOMER1, SHANK3), inhibitory post-synaptic components (ARHGEF9, GPHN) | |
| Functional analysis of organoids | Spontaneous action potential production in functional neurons and glia detected by Ca2+ imaging. Myelination of neurons by electron microscopy | |
| Protocol applications | Study of the mechanisms of human oligodendrogenesis, migration and myelination in ventral and dorsal forebrain. Study of diseases of defective myelination | |
| Protocol advantages | NSCs can be cryopreserved and thawed for organoid production, enhancing consistency if using quality-controlled banks and avoiding the need for continual culture of undifferentiated hPSCs. The use of VFOs and DFOs may permit analysis of the first 2 oligodendrogenic waves identified in mouse brain. Analysis of fused VFOs and DFOs permits analysis of the third wave of oligodendrogenesis. | |
| Protocol limitations | Most oligodendrocytes do not form compact myelin sheaths in organoids. Necrotic core forms in fused organoids | |
| Technical expertise neededa | Proficiency with undifferentiated hPSC culture is essential. Proficiency with organoid production and manipulation is useful | |
| Conclusion | Protocol generates forebrain organoids that recapitulate oligodendrogenesis, promote oligodendrocyte maturation, and demonstrate functional myelination. Technically, this protocol will present few difficulties to users with experience culturing hPSCs and with organoid production | |
Proficiency in the culture of high-quality undifferentiated hPSCs is essential for the success of any differentiation protocol and is thus a prerequisite for implementing all brain organoid protocols described. All organoid generation protocols described in this review can be implemented by skilled technical staff. However, differentiation can be highly variable from line-to-line, warranting optimization of the protocol for each line. Furthermore, the steps involved in some protocols are more complex than others (e.g., organoid fusion). Therefore, here we provide our assessment of the ideal level of technical expertise needed to successfully implement this protocol.
Table 5.
Application of brain organoids in disease modeling
| Glioblastoma (GBM) tumors | ||||||
| Oudin et al.59 | Gamboa et al.60 | Linkous and Fine, 202061 | ||||
| Model type |
|
|
Glioma cerebral organoid (GLICO) |
|||
| Original publication(s) | Golebiewska et al.62 | Chadwick et al.63 | Linkous et al.39 Pine et al.64 | |||
| Methods details | ||||||
|
Mechanical | Mechanical + Enzymatic | Mechanical + Enzymatic | |||
|
Small tissue fragments | Single cell suspension | Single cell suspension to derive GSCs | |||
|
With serum | Serum free + growth factors | Serum free + growth factors | |||
|
PDO – Agar coated flasks PDOX – Intracranial transplantation |
PDO – Matrigel droplet | GLICO – Co-culture GSCs with cerebral organoids | |||
| Phenotypes observed | Recapitulate histological, genetic, epigenetic, and transcriptomic features of patient tumors | Recapitulate key expression profiles and tumor cell phenotypes seen in GBM patients | Recapitulate invasive behavior (tumor microtubes), molecular heterogeneity and transcriptional cellular states found in primary GBM tumors | |||
| Tumor formation timeline | PDO – 7–14 days PDOX – up to16 months |
GSC – 7 days PDO – 2–12 weeks PDOX – 2–12 weeks |
GLICO – 2–14 days | |||
| Additional protocols described in STAR Protocols paper provided | Intracranial implantation, MRI imaging of tumor growth in vivo, PDOX tumor processing and purification of human tumor cells | RNA extraction, RNA sequencing, qPCR, IF, IHC, drug synergy assays | Development of cerebral organoids from hESCs | |||
| Technical expertise neededa | Knowledge of advanced stem cell/organoid culture techniques; training/certification to work with patient specimens; mouse skull anatomy, intracranial implantation procedure and animal xenograft studies (for Oudin and Gamboa protocols) | |||||
| Specialized equipment needed | Surgical instruments and reagents, stereotactic apparatus with drill and automated injector pump, MRI machine or IVIS system | Orbital shaker | ||||
| Advantages | These protocols provide clinically relevant 3D glioma models to interrogate key aspects of glioma biology and preclinical drug testing in a patient-specific manner. GLICO and PDOX models allow for assessing the cross talk of tumor with its microenvironment. GSCs and PDOs offer the advantage of cryopreservation for downstream applications. | |||||
| Limitations | These protocols are limited by the quality and quantity of tumor tissue, and rate of success varies between patients. Necrotic or less proliferative tumor fragments may yield organoids of poor quality, therefore close coordination with neurosurgeons/neuropathologists for providing biopsies with high tumor content (preferably proliferative in nature) is necessary. Additionally, optimal tissue storage upon surgical removal and immediate transfer to the tissue processing facility should be prearranged to keep the tissue viable. Processing fresh tissue increases the likelihood of establishment of successful organoids. | |||||
| Alzheimer’s disease (AD) | ||||||
| Martens et al.45 | ||||||
| Model type | Cerebral organoid | |||||
| Original publication | Zhao et al.47 | |||||
| Method details | Timeline | Steps | ||||
| 1 week | gRNA/Cas9 cloning | |||||
| 1–2 weeks | iPSC electroporation | |||||
| 3–4 weeks | Single cell clone isolation and expansion | |||||
| 3–4 weeks | Positive clone selection and characterization | |||||
| 3–4 months | APOE−/− iPSC-derived cerebral organoid culture (covered in detail in Table 2) | |||||
| 2 weeks | Analyses of APOE−/− COs | |||||
| Phenotypes observed | APOE−/− COs show greater apoptosis and synaptic loss and have increased levels of Aβ and phosphorylated tau. | |||||
| Additional protocols described in STAR Protocols paper provided | Design of gRNAs; PCR analysis of iPSC clones, iPSC characterization - karyotyping analysis, immunostaining of pluripotency markers and differentiation into 3 germ layers; immunostaining for neural markers for CO validation, RT-qPCR and western blotting for validation of APOE−/− COs | |||||
| Technical expertise neededa | Knowledge of CRISPR/Cas9 technology and advanced stem cell/organoid culture techniques | |||||
| Specialized equipment needed | Thermocycler, Invitrogen Neon™ transfection system or similar system for electroporation, gel electrophoresis equipment, Cytovision GSL-120 scanner, orbital shaker, iCycler thermocycler or similar RT-qPCR system, western blotting equipment | |||||
| Advantages | Provides a useful protocol for CRISPR-Cas9-based genetic editing of human iPSCs and generating iPSC-derived cerebral organoids to study gene function and consequent mechanisms in disease pathology | |||||
| Limitations | Using iPSCs for cerebral organoid production can be challenging, especially for beginners as the differentiation can be highly variable between iPSC lines used, thus warranting optimization of the protocol for each line. | |||||
| Traumatic brain injury (TBI) | ||||||
| Ramirez et al.65 | ||||||
| Model type | Cerebral organoid | |||||
| Original publication | Ramirez et al.66 | |||||
| Method details | Timeline | Steps | ||||
|
80 days | Fibroblast culture Reprogramming iPSC characterization |
||||
|
50 days and up | EB formation EB induction EB embedding Maturation |
||||
|
4–7 days | Skull cleaning Skull craniotomy and sealing Gas sterilization |
||||
|
20 min | Gelatin and agarose mixture Skull filling with the phantom brain |
||||
|
2 h | Stereotaxic frame sterilization Positioning COs CCI procedure CO recovery and analysis |
||||
| Phenotypes observed | CCI induced apoptosis in COs and recapitulated pathological features of TBI, including neuronal damage, neuron loss, and astrogliosis. | |||||
| Additional protocols described in STAR Protocols paper provided | Freezing, immunostaining and alkaline phosphatase staining of iPSCs; Formalin fixation, paraffin embedding and immunostaining of COs | |||||
| Technical expertise neededa | Knowledge of advanced stem cell/organoid culture techniques, mouse skull anatomy, stereotaxic frames, vernier scale, CCI procedures | |||||
| Specialized equipment needed | Orbital shaker, CCI device, H2O2 steamer | |||||
| Advantages | CO-based model of TBI is a useful system to understand TBI-induced brain damage when compared to 2D-systems and animal models. Spatial organization and cellular heterogeneity of COs allow the study of functional consequences of disease-associated gene variants and drug screening in a more realistic setting. | |||||
| Limitations | This protocol uses agarose-based polymer in an open skull. However, using closed skull and/or transplanting COs in live mouse brain will be more suitable to model TBI. Moreover, COs in their current form lack cellular and structural features to accurately model TBI pathology. | |||||
Proficiency in the culture of high-quality undifferentiated hPSCs is essential for the success of any differentiation protocol and is thus a prerequisite for implementing all brain organoid protocols described. All organoid generation protocols described in this review can be implemented by skilled technical staff. However, differentiation can be highly variable from line-to-line, warranting optimization of the protocol for each line. Furthermore, the steps involved in some protocols are more complex than others (e.g., organoid fusion). Therefore, here we provide our assessment of the ideal level of technical expertise needed to successfully implement this protocol.
Practical considerations
How to choose the right protocol for your work
The choice of the optimal protocol to generate brain organoids is largely dependent on your research question. You should validate the identity of cells generated in these organoids and document appropriate functional properties. If multiple protocols exist for generating the same regional organoid type, you should confirm the presence and function of the desired cells type(s). Typically, protocols that most closely mirror developmental paradigms produce neurons that exhibit the greatest in vitro maturation and functionality.67 Other factors to consider include the length and complexity of each protocol and the availability of appropriate resources (staffing, equipment, and key reagents).
Key factors for success
It can be expensive, challenging, and time consuming to introduce the culture and directed differentiation of hPSCs to a lab. Key factors that affect the successful implementation of a brain organoid protocol include the availability of trained staff, and the reproducible quality of hPSCs and reagents. The availability of a shared resource/core facility that can facilitate access to high-quality undifferentiated hPSCs or provide training in their culture and differentiation can significantly alleviate this issue. Ideally, hPSCs should be obtained from a well-characterized, cryopreserved bank. The identity of banked cells must be authenticated, and hPSCs should be mycoplasma-free, contain a stable karyotype, and demonstrate tri-lineage differentiation capacity. Furthermore, the activity of growth factors and small molecules can vary from batch-to-batch and vendor-to-vendor. We recommend assessing the quality of batches of these reagents and purchasing validated lots in bulk. Although expensive, the increasing availability of commercial differentiation kits can alleviate some of the challenges associated with protocol implementation.
Regardless of the brain organoid type generated and differentiation method used, robust assessment of the identity of the organoids produced is critical. The identity of cells in brain organoids can be heterogeneous and exhibit significant variability from batch-to-batch and from line-to-line. We recommend establishing robust quality assessment criteria that allow unequivocal, quantitative analysis of the key cell types that should be present in specific organoids. This can be a resource intensive process but is essential for reproducible and robust data interpretation. Optimal quality analysis includes implementation at intermediate steps in addition to at the completion of the differentiation protocol to enable the rapid identification of a suboptimal batch.
Finally, given that generation of brain organoids frequently takes several months, it is crucial to have a well-developed plan to ensure a consistent supply of validated reagents and to account for production disruptions such as microbial contamination of cultures. We recommend generating brain organoids in staged batches to enable faster recovery from unexpected loss of a single batch. Some protocols enable cryopreservation of intermediate cell types.50 This not only facilitates more rapid recovery from production disruptions but permits generation of banks of quality-controlled cells for organoid production that can help reduce batch to batch variation.
Disadvantages and improvements
Cerebral organoids model early brain development with remarkable fidelity; however, their further neuronal maturation and size is limited by interior hypoxia and cell death due to lack of vasculature. Efforts are underway to achieve in vitro vascularization of tissue-engineered constructs; however, until a fully functional vascular system, especially an authentic blood-brain-barrier, can be developed, scientists have achieved vascularization by transplanting brain organoids into the rodent brain, which allowed extensive growth and invasion of host blood vessels into the human organoid and resulted in greater survival of cells owing to effective blood perfusion.25 To circumvent the problem of insufficient surface diffusion of nutrients and oxygen, scientists have applied the classic method of organotypic slice culture to organoids.7,9 By sectioning and culturing mature cerebral organoid slices at the air-liquid interface, not only does the tissue remain healthy over an extended time but also exhibits improved neuronal maturation. Another study suggested adding BDNF to the maturation medium to obtain long-term growth and improved development.10 Additionally, Lancaster et al.6 improved the reproducibility of their original cerebral organoid protocol by using PLGA microfilaments as a floating scaffold to generate elongated embryoid bodies, performing short treatment with WNT activator, and adding dissolved Matrigel to maturation medium to increase reproducibility and improve forebrain tissue architecture and cortical development.8
Current brain organoids also lack an inherent microglia component, the resident immune cells of the brain. Given that microglia are critical for brain development and homeostasis, generation of more complex organoids integrating these cells is an area of significant interest. Scientists have utilized a co-culture approach to incorporate iPSC-derived microglia cells or primary human microglia in brain organoids to investigate the neuro-immune crosstalk that plays a critical role in either protecting or enhancing neuronal pathology.29,31,68
Since the initial establishment of brain organoid protocols, subsequent studies have further modified protocols to generate organoids with more specific brain regional identities using a combination of specific growth factors and signaling molecules or inhibitors resembling the in vivo regional brain developmental programs, as well as models with increased cellular diversity (Table 1). These directed differentiation protocols.11,15 generate organoids with greater reproducibility, addressing the issue of morphological and cellular variability seen with the organoids generated via self-patterning, non-guided differentiation process.
Existence of functional neural networks required for producing complex brainwaves in organoids is a significant advance in brain organoid technology and could have huge implications for studying neuronal processes in normal vs disease state.23 Moreover, the approach of fusing brain region-specific organoids expands the applicability of organoids to study more brain disorders by allowing studies of brain regional interconnectivity and neural circuit formation in a dish.69
Future perspectives
The advent of brain organoid technology has heralded a new era for human brain biology and neurological disease modeling that would not have been possible little more than a decade ago. Brain organoids provide a powerful human-specific platform to study mechanisms of normal brain development, interrogate complex disease phenotypes of various neurological disorders, and facilitate the discovery of novel neurotherapeutics. Organoids are chronologically relevant models to study brain development for their ability to be maintained for long periods of time in culture to achieve maturation milestones necessary for investigating late-stage processes such as gliogenesis, axonal myelination, neuronal migration, and connectivity, which have been previously difficult to study in vitro. Additionally, brain organoids have been used as experimental models to understand the pathological effects of viruses on the central nervous system, such as Zika21 and more recently SARS-CoV-2.70 Brain organoids have also been used for performing comparative studies in other primates to understand the evolution of human brain.71,72
Researchers now have access to patient-specific human brain models, which presents a useful and clinically relevant strategy to study brain disorders. As we look to the future, by combining brain organoid and genome editing technologies, disease-associated mutations can be introduced or corrected in patient-derived iPSCs, and subsequently be differentiated into brain organoids for functional analyses and small molecule screening. Moreover, single cell sequencing and protein analysis tools can be applied to organoids to decipher disease mechanisms of neurological disorders. These advances highlight the exciting prospects for their application towards drug discovery, personalized medicine and possibly cell therapy. The biological questions that can be applied to brain organoids will only increase as we gain deeper insights into experimentally manipulating and scaling these mini-tissues and make rapid progress towards generating high-quality and reproducible models as well as developing more specialized model subtypes. As brain organoid protocols evolve to produce more complex and functional human neural tissues, ethical considerations related to consciousness and the treatment of research animals transplanted with human brain tissues, will become more acute.73
Acknowledgments
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Benito-Kwiecinski S., Lancaster M.A. Brain organoids: human neurodevelopment in a dish. Cold Spring Harb Perspect Biol. 2020;12:a035709. doi: 10.1101/cshperspect.a035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidhaye J., Knoblich J.A. Brain organoids: an ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ. 2020;28:52–67. doi: 10.1038/s41418-020-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian X., Song H., Ming G.L. Brain organoids: advances, applications and challenges. Development. 2019;146:dev166074. doi: 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T., Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 5.Kadoshima T., Sakaguchi H., Nakano T., Soen M., Ando S., Eiraku M., Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giandomenico S.L., Mierau S.B., Gibbons G.M., Wenger L.M.D., Masullo L., Sit T., Sutcliffe M., Boulanger J., Tripodi M., Derivery E., et al. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 2019;22:669–679. doi: 10.1038/s41593-019-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancaster M.A., Corsini N.S., Wolfinger S., Gustafson E.H., Phillips A.W., Burkard T.R., Otani T., Livesey F.J., Knoblich J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017;35:659–666. doi: 10.1038/nbt.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian X., Su Y., Adam C.D., Deutschmann A.U., Pather S.R., Goldberg E.M., Su K., Li S., Lu L., Jacob F., et al. Sliced human cortical organoids for modeling Distinct cortical layer formation. Cell Stem Cell. 2020;26:766–781.e9. doi: 10.1016/j.stem.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quadrato G., Nguyen T., Macosko E.Z., Sherwood J.L., Min Yang S., Berger D.R., Maria N., Scholvin J., Goldman M., Kinney J.P., et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velasco S., Kedaigle A.J., Simmons S.K., Nash A., Rocha M., Quadrato G., Paulsen B., Nguyen L., Adiconis X., Regev A., et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 2019;570:523–527. doi: 10.1038/s41586-019-1289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhavan M., Nevin Z.S., Shick H.E., Garrison E., Clarkson-Paredes C., Karl M., Clayton B.L.L., Factor D.C., Allan K.C., Barbar L., et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods. 2018;15:700–706. doi: 10.1038/s41592-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marton R.M., Miura Y., Sloan S.A., Li Q., Revah O., Levy R.J., Huguenard J.R., Pașca S.P. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 2019;22:484–491. doi: 10.1038/s41593-018-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ormel P.R., Vieira de Sa R., van Bodegraven E.J., Karst H., Harschnitz O., Sneeboer M.A.M., Johansen L.E., van Dijk R.E., Scheefhals N., Berdenis van Berlekom A., et al. Microglia innately develop within cerebral organoids. Nat. Commun. 2018;9:4167. doi: 10.1038/s41467-018-06684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasca A.M., Sloan S.A., Clarke L.E., Tian Y., Makinson C.D., Huber N., Kim C.H., Park J.Y., O'Rourke N.A., Nguyen K.D., et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi H., Kadoshima T., Soen M., Narii N., Ishida Y., Ohgushi M., Takahashi J., Eiraku M., Sasai Y. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 2015;6:8896. doi: 10.1038/ncomms9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birey F., Andersen J., Makinson C.D., Islam S., Wei W., Huber N., Fan H.C., Metzler K.R.C., Panagiotakos G., Thom N., et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jo J., Xiao Y., Sun A., Cukuroglu E., Tran H.D., Goke J., Tan Z., Saw T., Tan C.P., Lokman H., et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and Neuromelanin-producing neurons. Cell Stem Cell. 2016;19:248–257. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muguruma K., Nishiyama A., Kawakami H., Hashimoto K., Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015;10:537–550. doi: 10.1016/j.celrep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 20.Ozone C., Suga H., Eiraku M., Kadoshima T., Yonemura S., Takata N., Oiso Y., Tsuji T., Sasai Y. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat. Commun. 2016;7 doi: 10.1038/ncomms10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian X., Nguyen H., Song M., Hadiono C., Ogden S., Hammack C., Yao B., Hamersky G., Jacob F., Zhong C., et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schukking M., Miranda H.C., Trujillo C.A., Negraes P.D., Muotri A.R. Direct generation of human cortical organoids from primary cells. Stem Cells Dev. 2018;27:1549–1556. doi: 10.1089/scd.2018.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trujillo C.A., Gao R., Negraes P.D., Gu J., Buchanan J., Preissl S., Wang A., Wu W., Haddad G.G., Chaim I.A., et al. Complex Oscillatory Waves emerging from cortical organoids model early human brain network development. Cell Stem Cell. 2019;25:558–569.e7. doi: 10.1016/j.stem.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang Y., Tanaka Y., Cakir B., Patterson B., Kim K.Y., Sun P., Kang Y.J., Zhong M., Liu X., Patra P., et al. hESC-derived thalamic organoids form Reciprocal Projections when fused with cortical organoids. Cell Stem Cell. 2019;24:487–497.e7. doi: 10.1016/j.stem.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansour A.A., Goncalves J.T., Bloyd C.W., Li H., Fernandes S., Quang D., Johnston S., Parylak S.L., Jin X., Gage F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018;36:432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z., Wang S., Xu T., Hong C., Cheng M., Zhu P., Lin J., Su D., Miao C. Cerebral organoids transplantation improves neurological motor function in rat brain injury. CNS Neurosci. Ther. 2020;26:682–697. doi: 10.1111/cns.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cederquist G.Y., Asciolla J.J., Tchieu J., Walsh R.M., Cornacchia D., Resh M.D., Studer L. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 2019;37:436–444. doi: 10.1038/s41587-019-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini L., Bonfio C., Chadwick J., Begum F., Skehel M., Lancaster M.A. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science. 2020;369:eaaz5626. doi: 10.1126/science.aaz5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abud E.M., Ramirez R.N., Martinez E.S., Healy L.M., Nguyen C.H., Newman S.A., Yeromin A.V., Scarfone V.M., Marsh S.E., Fimbres C., et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 2017;94:278–293.e9. doi: 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownjohn P.W., Smith J., Solanki R., Lohmann E., Houlden H., Hardy J., Dietmann S., Livesey F.J. Functional studies of Missense TREM2 mutations in human stem cell-derived microglia. Stem Cell Rep. 2018;10:1294–1307. doi: 10.1016/j.stemcr.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y.T., Seo J., Gao F., Feldman H.M., Wen H.L., Penney J., Cam H.P., Gjoneska E., Raja W.K., Cheng J., et al. APOE4 Causes Widespread molecular and cellular Alterations associated with Alzheimer's disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98:1141–1154.e7. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu R., Boreland A.J., Li X., Erickson C., Jin M., Atkins C., Pang Z.P., Daniels B.P., Jiang P. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Rep. 2021;16:1923–1937. doi: 10.1016/j.stemcr.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cakir B., Xiang Y., Tanaka Y., Kural M.H., Parent M., Kang Y.J., Chapeton K., Patterson B., Yuan Y., He C.S., et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods. 2019;16:1169–1175. doi: 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pham M.T., Pollock K.M., Rose M.D., Cary W.A., Stewart H.R., Zhou P., Nolta J.A., Waldau B. Generation of human vascularized brain organoids. Neuroreport. 2018;29:588–593. doi: 10.1097/wnr.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Y., Sun L., Wang M., Liu J., Zhong S., Li R., Li P., Guo L., Fang A., Chen R., et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worsdorfer P., Dalda N., Kern A., Kruger S., Wagner N., Kwok C.K., Henke E., Ergun S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-52204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goranci-Buzhala G., Mariappan A., Gabriel E., Ramani A., Ricci-Vitiani L., Buccarelli M., D'Alessandris Q.G., Pallini R., Gopalakrishnan J. Rapid and Efficient invasion assay of glioblastoma in human brain organoids. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107738. [DOI] [PubMed] [Google Scholar]
- 38.Krieger T.G., Tirier S.M., Park J., Jechow K., Eisemann T., Peterziel H., Angel P., Eils R., Conrad C. Modeling glioblastoma invasion using human brain organoids and single-cell transcriptomics. Neuro Oncol. 2020;22:1138–1149. doi: 10.1093/neuonc/noaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linkous A., Balamatsias D., Snuderl M., Edwards L., Miyaguchi K., Milner T., Reich B., Cohen-Gould L., Storaska A., Nakayama Y., et al. Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep. 2019;26:3203–3211.e5. doi: 10.1016/j.celrep.2019.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersen J., Revah O., Miura Y., Thom N., Amin N.D., Kelley K.W., Singh M., Chen X., Thete M.V., Walczak E.M., et al. Generation of functional human 3D cortico-motor assembloids. Cell. 2020;183:1913–1929.e26. doi: 10.1016/j.cell.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagley J.A., Reumann D., Bian S., Levi-Strauss J., Knoblich J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods. 2017;14:743–751. doi: 10.1038/nmeth.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miura Y., Li M.Y., Birey F., Ikeda K., Revah O., Thete M.V., Park J.Y., Puno A., Lee S.H., Porteus M.H., Pașca S.P. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat. Biotechnol. 2020;38:1421–1430. doi: 10.1038/s41587-020-00763-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiang Y., Tanaka Y., Patterson B., Kang Y.J., Govindaiah G., Roselaar N., Cakir B., Kim K.Y., Lombroso A.P., Hwang S.M., et al. Fusion of regionally specified hPSC-derived organoids models human brain development and Interneuron migration. Cell Stem Cell. 2017;21:383–398.e7. doi: 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lancaster M.A., Knoblich J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345 doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 45.Martens Y.A., Xu S., Tait R., Li G., Zhao X.C., Lu W., Liu C.C., Kanekiyo T., Bu G., Zhao J. Generation and validation of APOE knockout human iPSC-derived cerebral organoids. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancaster M.A., Knoblich J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014;9:2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao J., Fu Y., Yamazaki Y., Ren Y., Davis M.D., Liu C.C., Lu W., Wang X., Chen K., Cherukuri Y., et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer's disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020;11:5540. doi: 10.1038/s41467-020-19264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarrafha L., Parfitt G.M., Reyes R., Goldman C., Coccia E., Kareva T., Ahfeldt T. High-throughput generation of midbrain dopaminergic neuron organoids from reporter human pluripotent stem cells. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahfeldt T., Ordureau A., Bell C., Sarrafha L., Sun C., Piccinotti S., Grass T., Parfitt G.M., Paulo J.A., Yanagawa F., et al. Pathogenic pathways in early-Onset Autosomal Recessive Parkinson's disease discovered using Isogenic human dopaminergic neurons. Stem Cell Rep. 2020;14:75–90. doi: 10.1016/j.stemcr.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zagare A., Gobin M., Monzel A.S., Schwamborn J.C. A robust protocol for the generation of human midbrain organoids. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monzel A.S., Smits L.M., Hemmer K., Hachi S., Moreno E.L., van Wuellen T., Jarazo J., Walter J., Bruggemann I., Boussaad I., et al. Derivation of human midbrain-specific organoids from Neuroepithelial stem cells. Stem Cell Rep. 2017;8:1144–1154. doi: 10.1016/j.stemcr.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinhardt P., Glatza M., Hemmer K., Tsytsyura Y., Thiel C.S., Hoing S., Moritz S., Parga J.A., Wagner L., Bruder J.M., et al. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang Y., Cakir B., Park I.H. Generation of regionally specified human brain organoids resembling Thalamus development. STAR Protoc. 2020;1 doi: 10.1016/j.xpro.2019.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Worsdorfer P., Rockel A., Alt Y., Kern A., Ergun S. Generation of vascularized neural organoids by Co-culturing with mesodermal progenitor cells. STAR Protoc. 2020;1 doi: 10.1016/j.xpro.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim H., Jiang P. Generation of human pluripotent stem cell-derived fused organoids with oligodendroglia and myelin. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H., Xu R., Padmashri R., Dunaevsky A., Liu Y., Dreyfus C.F., Jiang P. Pluripotent stem cell-derived cerebral organoids Reveal human Oligodendrogenesis with dorsal and ventral Origins. Stem Cell Rep. 2019;12:890–905. doi: 10.1016/j.stemcr.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen C., Kim W.Y., Jiang P. Humanized neuronal chimeric mouse brain generated by neonatally engrafted human iPSC-derived primitive neural progenitor cells. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bardy C., van den Hurk M., Eames T., Marchand C., Hernandez R.V., Kellogg M., Gorris M., Galet B., Palomares V., Brown J., et al. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc. Natl. Acad. Sci. USA. 2015;112:E2725–E2734. doi: 10.1073/pnas.1504393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oudin A., Baus V., Barthelemy V., Fabian C., Klein E., Dieterle M., Wantz M., Hau A.C., Dording C., Bernard A., et al. Protocol for derivation of organoids and patient-derived orthotopic xenografts from glioma patient tumors. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gamboa C.M., Jara K., Pamarthy S., Liu L., Aiken R., Xiong Z., Danish S., Sabaawy H.E. Generation of glioblastoma patient-derived organoids and mouse brain orthotopic xenografts for drug screening. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linkous A., Fine H.A. Generating patient-derived gliomas within cerebral organoids. STAR Protoc. 2020;1 doi: 10.1016/j.xpro.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golebiewska A., Hau A.C., Oudin A., Stieber D., Yabo Y.A., Baus V., Barthelemy V., Klein E., Bougnaud S., Keunen O., et al. Patient-derived organoids and orthotopic xenografts of primary and recurrent gliomas represent relevant patient avatars for precision oncology. Acta Neuropathol. 2020;140:919–949. doi: 10.1007/s00401-020-02226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chadwick M., Yang C., Liu L., Gamboa C.M., Jara K., Lee H., Sabaawy H.E. Rapid processing and drug Evaluation in glioblastoma patient-derived organoid models with 4D bioprinted arrays. iScience. 2020;23 doi: 10.1016/j.isci.2020.101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pine A.R., Cirigliano S.M., Nicholson J.G., Hu Y., Linkous A., Miyaguchi K., Edwards L., Singhania R., Schwartz T.H., Ramakrishna R., et al. Tumor Microenvironment is critical for the Maintenance of cellular states Found in primary glioblastomas. Cancer Discov. 2020;10:964–979. doi: 10.1158/2159-8290.CD-20-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramirez S., Mukherjee A., Sepulveda S.E., Gherardelli C., Becerra-Calixto A., Bravo-Vasquez N., Soto C. Protocol for controlled cortical impact in human cerebral organoids to model traumatic brain injury. STAR Protoc. 2021;2 doi: 10.1016/j.xpro.2021.100987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramirez S., Mukherjee A., Sepulveda S., Becerra-Calixto A., Bravo-Vasquez N., Gherardelli C., Chavez M., Soto C. Modeling Traumatic Brain Injury in Human Cerebral Organoids. Cells. 2021;10 doi: 10.3390/cells10102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A., et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popova G., Soliman S.S., Kim C.N., Keefe M.G., Hennick K.M., Jain S., Li T., Tejera D., Shin D., Chhun B.B., et al. Human microglia states are conserved across experimental models and regulate neural stem cell responses in chimeric organoids. Cell Stem Cell. 2021;28:2153–2166.e6. doi: 10.1016/j.stem.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasca S.P. Assembling human brain organoids. Science. 2019;363:126–127. doi: 10.1126/science.aau5729. [DOI] [PubMed] [Google Scholar]
- 70.Pellegrini L., Albecka A., Mallery D.L., Kellner M.J., Paul D., Carter A.P., James L.C., Lancaster M.A. SARS-CoV-2 Infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27:951–961.e5. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanton S., Boyle M.J., He Z., Santel M., Weigert A., Sanchis-Calleja F., Guijarro P., Sidow L., Fleck J.S., Han D., et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature. 2019;574:418–422. doi: 10.1038/s41586-019-1654-9. [DOI] [PubMed] [Google Scholar]
- 72.Benito-Kwiecinski S., Giandomenico S.L., Sutcliffe M., Riis E.S., Freire-Pritchett P., Kelava I., Wunderlich S., Martin U., Wray G.A., McDole K., Lancaster M.A. An early cell shape transition drives evolutionary expansion of the human forebrain. Cell. 2021;184:2084–2102.e19. doi: 10.1016/j.cell.2021.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sawai T., Sakaguchi H., Thomas E., Takahashi J., Fujita M. The ethics of cerebral organoid research: Being conscious of consciousness. Stem Cell Rep. 2019;13:440–447. doi: 10.1016/j.stemcr.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]