Abstract
The rhizomes of ginger have been in use in many forms of traditional and alternative medicines. Besides being employed as condiment and flavoring agent, it is used in the treatment of nausea, osteoarthritis, muscle pain, menstrual pain, chronic indigestion, Alzheimer’s disease, and cancer. Ginger rhizome contains volatile oils, phenolic compounds and resins, and characterization studies showed that [6]-gingerol, [6]-shogaol, and [6]-paradol are reported to be the pharmacologically active components. Gingerol is a major chemical constituent found as volatile oil in the rhizomes of ginger. It has several medicinal benefits and used for the treatment of rheumatoid arthritis, nausea, cancer, and diabetes. Many studies have been carried out in various parts of the world to isolate and standardize gingerol for their use as a complementary medicine. The present review summarizes wide range of research studies on gingerol and its pharmacological roles in various metabolic diseases.
Graphical Abstract

Keywords: Ginger, Gingerol, Zingiber officinale, Volatile oil, Resins, Cancer, Inflammation
Introduction
Ginger (Zingiber officinale) has always been used as a remedy for a wide range of diseases (Bode and Dong 2004). The plant was first cultivated in China and other Southeast Asian parts, but it is now extensively grown throughout Asia, including the Western regions of the world (Park and Pezzuto 2011). Ginger is popular as a spice and its therapeutic benefits such as nausea, asthma, arthritis, gastrointestinal disorders, headache, and other diseases. It is also helpful in chemotherapy-related nausea, vomiting, and motion sickness (Bode and Dong 2011; Butt and Sultan 2011).
For decades, people had quest for curing different types of diseases by the use of drugs derived from different medicinal plants (Jindal et al. 2021; Regassa et al. 2022; Shukla et al. 2022). The records show that the drug preparations from plants were found in Nagpur, India, about 6000 years old. These records comprise of about 12 recipes for different types of drug preparation from 250 plant species (Kelly 2009). Besides this, the Vedas also mentioned the treatment of various diseases with plants. Scientific work on plants was started as the knowledge on identification and isolation of various phytoconstituents from the beginning of the nineteenth century (Bhardwaj et al. 2018). Over the last few decades, people started the use of plant-based medicines in their diets as supplements (Cohen et al. 2002).
Ginger is most commonly used medicinal plant in Asia and many other countries for combating various digestive disorders like indigestion, constipation, headache, rheumatism, cold, and cough (Bhargava et al. 2012). Besides these applications, it has also been found to possess anticancer, antioxidant, antidiabetic, hepatoprotective, larvicidal, analgesic, anti-inflammation, and immunomodulatory properties (Kumar et al. 2011; Ghasemzadeh et al. 2010; Ho et al. 2013). Various secondary plant metabolites and compounds have been reported from ginger for their pharmaceutical uses among which flavonoids and phenolics are the important groups (Ho et al. 2013). All the three compounds namely gingerol, shogaol, and paradol possess various types of medicinal properties which include antioxidant, antitumor, and COX inhibitor activities (Mohammad 2016).
To understand the mechanism of action of ginger has piqued researchers in to the ginger related research in recent years. There has been remarkable research and reviews on the therapeutic effects of ginger and its principal components (Bode and Dong 2004, 2011, 2008; Butt and Sultan 2011; Ali et al. 2008; Ding et al. 1991; Habib et al. 2008; Jolad et al. 2005; Poltronieri et al. 2014; Semwal et al. 2015; Shukla and Singh 2007; Surh et al 1998). Keeping in view the importance of gingerols in treating various human diseases, this review discusses the use and the mechanism of action of gingerols against potential human diseases.
Mode of action of gingerol
Several researchers have revealed that [6]-, [8]-, [10]-gingerols, and [6]-shogaol exhibited antiemetic effects. These compounds bind to a modulatory region on the 5-HT3 receptor of ion-channel complex (Abdel-Aziz 2006).
According to Radhakrishnan et al., [6]-gingerol is responsible for inhibition of cell augmentation in human colon cancer SW-480 cells and HCT116 cells, as well as inducing cell death in SW-480 cells. The mode of action is related to caspase-8, caspase-9, caspase-3, and caspase-7 activation, and also breakdown of poly-ADP ribose polymerase (PARP) (Radhakrishnan et al. 2014).
One of the mechanisms by which gingerol affects cancerous cells has been discovered to be protein breakdown. These compounds hinder the transformation of healthy tissue into cancerous cells via inhibition of AP-1 proteins. On the occurrence of cancer, paradol promotes apoptosis because of its cytotoxic effect (Bode et al. 2001; Wei et al. 2005).
[6]-Gingerol has also been reported to exhibit cell cycle arrest, apoptosis, and enzyme-coupled cell signaling receptor degeneration in tumor cells. Besides this, gingerol was found to inhibit proliferative action by blocking the translation of cyclin required for duplication during the G1 and G2 stages of cell division (Mao et al. 2019).
Additionally, gingerol has been shown to weaken A549 cells to TNF-related apoptosis instigating ligand (TRAIL)-induced apoptosis via decreasing autophagy flux (Nazim et al. 2015).
Chemical constituents and properties of gingerols
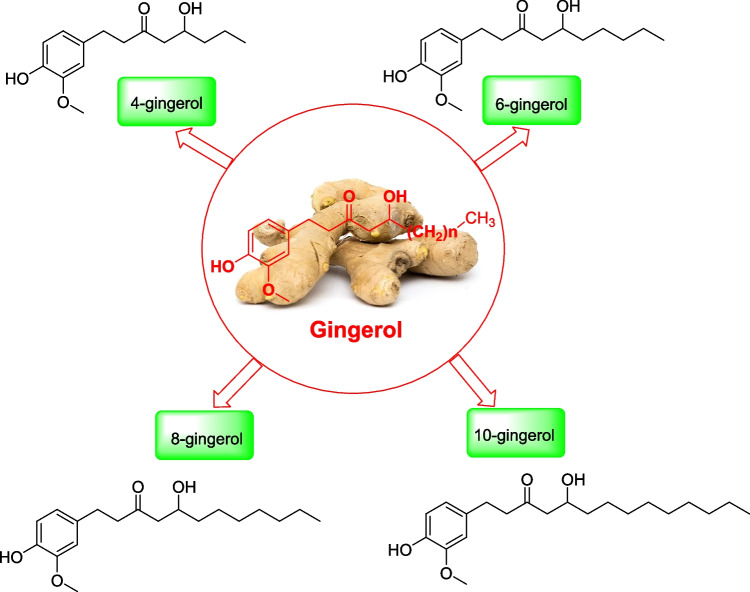
Chemically, gingerol or [6]-gingerol (Fig. 1) is a relative of piperine and capsaicin, which occurs in black pepper and chili pepper for their respective spiciness (Girhepunje et al. 2017). It is low-melting crystalline solid with pungent smell. On heating, ginger form gingerol, a less pungent and spicy-sweet aromatic compound, namely, zingerone. Also, on drying or heating gingerol turns to shogaols by dehydration reaction which is more pungent than gingerol. This type of conversion is more at higher temperature (Jung et al. 2018). [8]-Gingerol, [10]-gingerol [31], and [12]-gingerol [32] are also found in ginger and these together are called as the deemed gingerols. Various investigations have revealed that gingerols and shogoals are found to be mutagenic in nature (Fig. 2 Mao et al., 2019).
Fig. 1.
Signaling pathways involved in anticancer mechanism of [6]-gingerol (Mao et al. 2019)
Fig. 2.
Different kinds of gingerols with their structures (Girhepunje et al. 2017)
Isolation of gingerols
Countercurrent chromatography was used by Farthing et al. for separating [6]-, [8]-, and [10]-gingerols from the powdered root of ginger. Further, [4]-gingerol was also isolated with some minor modifications of this process. In these techniques, diol-bonded columns were used which separated the gingerols present in crude methanol extracts from the interfering constituents [34]. [6]-, [8]-, and [10]-Gingerols were isolated from fresh ginger rhizomes via fractionation method normal phase HPLC. These compounds were also characterized by mass spectral studies (Hiserodt et al. 1998).
In a study, dichloromethane was used to extract gingerol and related compounds from ginger rhizomes and then substituted with alkyl groups. Then, these compounds using chromatographic techniques were evaluated for the antioxidant activities and were further evaluated (Yuki et al. 2004). In another study, 6-gingerol was isolated from ginger rhizomes and was chemically modified. This modified compound was tested against diabetic mice which improved the insulin signaling (Chakraborty et al. 2012).
Synthesis of gingerols
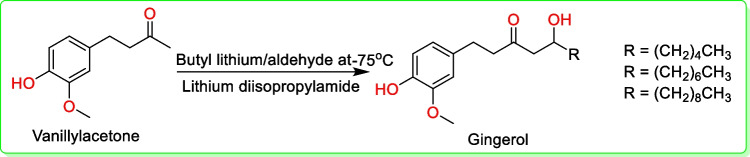
In 1999, Flemming and co-workers synthesized [6]-, [8]-, and [10]-gingerols in a single-step synthesis (Fig. 3). Vanillylacetone was treated with butyl lithium (C4H9Li) and then lithium diisopropylamide (C6H14LiN) along with an appropriate aldehyde to give gingerol (Fig. 3) (Fleming et al. 1999).
Fig. 3.
Synthesis scheme of [6]-, [8]-, and [10]-gingerols (Fleming et al. 1999)
In 1980, Denniff et al. synthesized [6]-gingerol via phenylalanine. This precursor formed p-coumaric acid and later dihydroferulic acid, followed by [6]-gingerdione leading to the resultant compound (Denniff et al. 1980).
Ramirez-Ahumada et al., in 2006 used the same amino acid, phenylalanine, while making use of enzymes such as phenylalanine ammonia lyase, p-coumaroyl quinate transferase, p-coumaroyl shikimate transferase, caffeic acid O-methyltransferase, caffeoyl-CoA-O-methyltransferase, and reductase (Ramirez-Ahumada et al. 2006).
Kumar et al., in 2012 achieved [6]-, [7]-, and [9]-gingerols with the help of eugenol that was converted to the nitro compound and then reacted with terminal alkenes to produce isoxazolines. After catalytic hydrogenation along with Raney nickel, respective gingerols were yielded (Kumar et al. 2012).
In 2009, Ma et al. opted a different approach to synthesize ( +)-(S)-[6]-, [8]-, and [10]-gingerols via enantioselective method for desirable production (Ma et al. 2009).
Therapeutic applications of gingerols
Gingerols are one of the important key ingredients that are found in ginger. [6]-Gingerol has various pharmacological properties that are effective in cancer, diabetes, emesis, inflammation, and many more (Fig. 4) (Bode and Dong 2011; Butt and Sultan 2011).
Fig. 4.
Therapeutic properties of [6]-gingerol in different types of diseased conditions
Anticancer
An anticancer activity of ginger was performed on an animal model having carcinoma cells (Katiyar et al. 1996). The study was concluded that the introduction of [6]-gingerol to SENCAR mice with DMBA-induced skin cancer gave them considerable protection and reduced tumor. Nigam et al. revealed that [6]-gingerol reduced the development of epidermoid carcinoma (A431) by producing reactive oxygen species (ROS) (Nigam et al. 2009). Increased ROS caused a change in mitochondrial membrane potential that resulted in the discharge of cytochrome-C and activation of apoptotic protease activating factor-1 (Apaf-1) that further resulted in caspase expression, implying apoptosis. The findings suggested that [6]-gingerol could be successful in the skin cancer treatment. They also concluded in another study that providing [6]-gingerol before and after benzo[a]pyrene therapy for 32 weeks lowered the skin tumors, elevated repressed p53 and Bax concentrations, and reduced the Bcl-2 levels and survivin in benzo[a]pyrene-induced skin cancer (Nigam et al. 2010).
The most fatal, malignant, and dangerous astrocytoma in adults is glioblastoma multiforme (GBM). Lee et al. discovered that [6]-gingerol induces TRAIL-mediated glioblastoma cell death. The bioactive component in ginger increased death receptor 5 (DR5) levels in a p53-dependent pathway, decreased antiapoptotic proteins (survivin, c-FLIP, Bcl-2, and XIAP), and increased pro-apoptotic proteins, Bax, and reduced Bid (ROS). The findings implied that TRAIL and [6]-gingerol could be used together to treat TRAIL-resistant glioblastoma tumors (Lee et al. 2008).
In an in vitro research, [6]-gingerol increased caspase-3 and caspase-7 activation and triggered apoptosis in gastric cancer cells. The stimulation of apoptosis was mediated by [6]-gingerol via blocking TRAIL-induced NF-kB activation and downregulating cellular inhibitor of apoptosis 1 (cIAP)-1 (Ishiguro et al. 2007; Prasad and Tyagi 2015). Mahady et al. studied the impact of [6]-, [8]-, and [10]-gingerols on 19 strains of Helicobacter pylori including the most vulnerable type cagA + . Infection with cagA + Helicobacter pylori strain significantly increased the probability of gastritis (Mahady et al. 2003).
According to Yagihashi et al., [6]-gingerol suppressed the growth and infiltration of rat ascites hepatoma cells. Cell cycle disruption and apoptosis are responsible for the therapeutic action of [6]-gingerol in these malignant cells (Yagihashi et al. 2008). Yusof et al. showed that ginger oleoresin had a chemoprotective effect against hepatocellular carcinoma in rats (Yusof et al. 2009), whereas Habib et al. discovered that ginger oleoresin reduced inflammation by lowering elevated levels of NF-kB and TNF-α in hepatocellular carcinoma cells (Habib et al. 2008). [6]-Gingerol triggered cell death in human HepG2 hepatoma cells by releasing cathepsin D prior to the production of ROS and the release of cytochrome-C from mitochondria (Yang et al. 2012). In HepG2 cells, [6]-gingerol suppressed the invasive and metastatic capabilities of phorbol 12-myristate 13-acetate (PMA) via blockage of MMP-9 and urokinase-type plasminogen activator (uPA), and also enhanced the production of tissue inhibitor metalloproteinase protein-1 (TIMP-1). Suppression of the MAPK and PI3k/Akt pathways, along with the functions of NF-kB and STAT3, revealed the process of invasion and metastasis (Weng et al. 2010).
Kim and Kim discovered that [6]-gingerol modulates tight junction-related proteins and decreases infiltration and metastasized pancreatic cancer cells via ERK/NF-kB/snail signaling pathway (Kim and Kim 2013). According to Akimoto et al., ginger extract slows down the cell cycle development and, as a result, accelerated cell death in human pancreatic cancer cell lines, notably PANC-1 cells (Akimoto et al. 2015).
Colorectal cancer (CRC) is one of the most prevalent cancers in men followed by lung and prostate cancer and in women after breast cancer (Jemal et al. 2011). A multistage genetic model of cancer development encompassing genetic variations of the APC gene, Kras, PI3k, and Wnt/-catenin, as well as bridge between these mechanisms, had a significant role in cell cycle advancement deregulation, cell death prevention, initiation of genomic instability, and improved intrusiveness and metastasis (Moran et al. 2010; Wu et al. 2013). Reduced apoptosis in the colon epithelium is linked to a higher probability of colorectal cancer. [6]-Gingerol treatment of human colon cancer cell elicited significant G2/M phase arrest having enhanced negative cell cycle regulators p27Kip1 and p21Cip1 while decreasing the amount of cyclin A, cyclin B1, and CDK1 (Lin et al. 2012).
Anti-inflammatory effect
The detailed study of S-[6]-gingerol revealed its anti-inflammatory activities. Tumor necrosis factor (TNF-α) and interleukin 1 (IL-1) are regarded as alarmins that trigger inflammatory cell recruitment by promoting the production of pro-inflammatory genes, whereas anti-inflammatory activities have been reported to be mediated by mitogen-activated protein kinase phosphatase-5 (MKP5) (Apte and Voronov 2002). TNF-α and IL-1β have been reported to enhance p38-dependent nuclear factor κB (NFκB) activation. These have also been reported to increase the expression of pro-inflammatory genes cyclooxygenase-2 (COX-2), IL-6, and IL-8 in normal prostatic epithelial cells. 6-Gingerol is also responsible to increase the regulation of MKP5, while decreasing the cytokine-induced p38-dependent pro-inflammatory changes (Nonn et al. 2007). Li et al. reported that nuclear factor κB (NFκB) and cyclooxygenase-2 (COX-2) are important inflammatory mediators of interleukins (Li et al. 2013).
Antioxidant effect
6-Gingerol possesses antioxidant properties by donating electrons and also behaves as free radical scavenger (Croft 1999; Ma et al. 2004). Many studies have revealed that 6-gingerol possessed significant genotoxic activity (Nonn et al. 2007; Yang et al. 2011; Wang et al. 2014). Moreover, it decreases elevated glucose amount and oxidative stress by raising superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and decreased glutathione concentration (GSH). Gingerols, shogaols, and paradols have also been reported to prevent angiogenesis and metastasis, apoptosis activation and suppression of cell cycle development (Chakraborty et al. 2012).
Cardiovascular effect
At low doses, gingerols and shogaols have been reported to induce depressant activity while at higher doses possessed cardiotonic properties (Wang et al. 2003). Liu et al. investigated that [6]-gingerol blocked the angiotensin-II type 1 receptor activation indicating thereby that ginger regulates the blood pressure and strengthens the cardiovascular system (Liu et al. 2013). The phenolic constituents 6-, 8-, and 10-gingerol were also reported for atropine-resistant and l-NAME-sensitive vasodilator activity (Ghayur et al. 2005). 6-Gingerol was reported to have some beneficial effects on cardiovascular disease due to decrease in intracellular Ca2+ (Han et al. 2019).
Antimicrobial activity
Gingerol and its related compounds possessed antimicrobial activities. Studies on [10]-gingerol have been found to suppress the development of bacteria Mycobacterium avium and Mycobacterium tuberculosis in an in vitro culture, while [6]-gingerol and [12]-gingerol reported to inhibit periodontal bacteria (Miri et al. 2008). In another study, [10]-gingerol and [12]-gingerol are found to inhibit three bacterial growth; Porphyromonas gingivalis, Porphyromonas endodontalis, and Prevotella intermedia (Park et al. 2008).
Anticoagulant effect
Gingerols and other related analogues are also essential for the inhibition of arachidonic acid-induced human platelet serotonin generation and coagulation. They provided a strong base for some more active synthesized gingerol derivatives with aspirin like potencies (Koo et al. 2001). Nurtjahja-Tjendraputra also reported the antiplatelet activities of gingerol, 8-shogaol, and 8-paradol (Nurtjahja-Tjendraputra et al. 2003).
Antiobesity activity
In in vitro studies, gingerols have been found to improve the expression of specific genes and insulin-dependent glucose uptake in mouse 3T3-L1 pre-adipocytes(Sekiya et al. 2004). Also, in a mice model, [6]-gingerol was found to have a potential role in lowering the lipid in treating type 2 diabetes (Singh et al. 2009). It also possesses anti-obesity effect which helps to reduce the accumulation of lipid in mice when fed on high-fat diet (Okamoto et al. 2011). Investigations on 6-gingerol were revealed to depict antiadipogenic effects as compared to 6-shogaol (Tzeng and Liu 2013; Tzeng et al. 2014). Activities of gingerol also reported in rat models which revealed the low levels of glucose, body weight, leptin, insulin, amylase, lipase plasma, and tissue lipids (Saravanan et al. 2014). [6]-Gingerol was also found to inhibit adipogenic differentiation and lipid deposition by activating the Wnt/β-catenin signaling mechanism. The β-catenin is important in adipogenic differentiation (Li and Zhou 2015). In another study, gingerenone A, [6]-shogaol, and [6]-gingerol had been found to show antiobesity property by reducing adipogenesis and enhanced the fatty acid catabolism (Mao et al. 2019).
Antiemetic effect
In the ileum of guinea pig, [6]-gingerol, [6]-shogaol, and galanolactone displayed anti 5-hydroxytryptamine (5-HT) effect (Yamahara et al. 1989; Huang et al. 1991) and the antiemetic effect is related to [6]-, [8]-, and [10]-gingerols and shogaols (Kawai et al. 1994). In another study, gingerols, shogaols, galanolactone, and di-terpenoid have been found to exhibit an important role in nausea and vomiting (Huang et al. 1991; Bhattarai et al. 2001), whereas in minks, gingerol possessed antiemetic activity. Although most of the studies in human beings have been supportive of the pre-clinical observations, yet some of these studies are found to be contradictory. It has also been found that [6]-gingerol, [8]-gingerol, [10]-gingerol, and 6-shogaol played important role as a 5-hydroxytryptamine 3 (5-HT3) antagonist, neurokinin-1 (NK-1) antagonist, antihistaminic, and possessed prokinetic activities (Haniadka et al. 2012). Many researchers have indicated that gingerols exhibited antiemetic activity but there are contradictory findings to prevent chemotherapy-induced nausea and vomiting (CINV) (Palatty et al. 2013). Extracts of gingerols and shogoals were found to increase gastric emptying effect and stimulated gastric antral contractions by their activity on cholinergic M receptors and serotonergic 5-HT and 5-HT receptors (Giacosa et al. 2015). The antiemetic efficacy of gingerol was demonstrated by using two vomiting models which revealed the reduction of cisplatin-induced consumption of kaolin in rats and emesis in minks. The pathway of gingerol has been associated to the modulation of 5-HT, SP, and DA mechanism (Li et al. 2020).
Antidiabetic activity
Antidiabetic properties of gingerols have been studied in a clinical trial with patients having diabetes and hypercholesterolemia. In this trial, a dose of 3 g/day (for a period of 30 days) had been given which reduced the blood glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), and very low-density lipoprotein-cholesterol (VLDL-C) levels (Andallu et al. 2003). In another study, gingerols are reported for the delayed of diabetic complications (Saraswat et al. 2010). The major pungent components of ginger (gingerols) were found to improve diabetes. It also inhibited the enzymes relevant to type 2 diabetes which revealed a high ability to inhibit α-glucosidase and α-amylase in the management of type 2 diabetes (Rani et al. 2011). The ginger extract in ethyl acetate extract (5 μg/mL) had been found to improve glucose absorption in cells and expressed glucose transporter type 4 (GLUT4) on the outer membrane of the cells. These findings revealed that the antidiabetic activity of ginger (Rani et al. 2012). In type 2 diabetic patients, the ginger supplement significantly decreased the glucose concentrations and raised the insulin-sensitivity check index (Mahluji et al. 2013). Gingerols exhibited six types of mechanisms which revealed the antidiabetic effects such as the following: (1) elevated glucose absorption, (2) induction of 5′ adenosine monophosphate-activated protein kinase phosphorylation, (3) advancement of glucose transporter 4 (GLUT-4) translocation to cytoplasm, (4) suppression of glycation end product induced upsurge of ROS levels in pancreatic β-cells, (5) decline in fasting blood glucose amount and enhanced glucose intolerance, (6) optimization of hepato genetic expression of enzymes associated to glucose metabolism towards the reduction of gluconeogenesis and glycogenolysis whilst enhancing glycogenesis, resulting in lower blood glucose levels (Samad et al. 2017). The studies showed that by activation of GLP-1, 6-gingerol enhanced glucose-stimulated insulin production and increased glucose uptake in skeletal muscle (Son et al. 2015). In another study, [10]-gingerol was reported to exhibit potent antidiabetic effects due to decrease in serum glucose concentration (Yadav and Garg 2019).
Antiangiogenesis
Angiogenesis is a critical phase in cancer in which new blood vessels develop, allowing metastasized cancers to survive and expand. Matrix metalloproteinases (MMPs) are proteolytic enzymes which are abundant in a variety of malignant tumors. They are vital in tumor growth and metastasis. MMPs suppression may be a useful technique for preventing the invasion and metastasis of carcinoma cells (Rundhaug 2005).
The antiangiogenic effect of [6]-gingerol has been displayed in numerous investigations. [6]-Gingerol suppressed VEGF-induced multiplication of human endothelial cells by triggering G1 cell cycle arrest, according to Kim and coworkers. It also prevented endothelial cells from forming capillary-like tubes in response to VEGF, as well as endothelial cell sprouting in the rat aorta and the development of a VEGF-induced new blood channel in the mouse cornea (Kim et al. 2005). Weng et al. found that [6]-gingerol inhibited the release of VEGF and IL-8 in Hep3B hepatoma cells. They also revealed that [6]-gingerol can inhibit capillary tube development and shorten its length using HUVEC cells in a tube formation experiment, implying that it has antiangiogenic and anti-invasive properties (Weng et al. 2010, 2012). They also reported that [6]-gingerol might have anti-invasive effect against hepatoma cells (HepG2 and Hep3B) by regulating MMP-9 and TIMP-1 (tissue inhibitor metalloproteinase 1 (Weng et al. 2012). Furthermore, Kim et al. reported that [6]-gingerol had anti-angiogenic characteristics, inhibiting VEGF and fibroblast growth factor-induced multiplication and capillary-like tube development in endothelial and ovarian cancer cells (Kim et al. 2005). In a separate research, it was discovered that [6]-gingerol suppressed MMP-2 and MMP-9 in MDA-MB-231 (human breast cancer cells) and PANC-1 (pancreatic duct-like carcinoma) cells, respectively (Lee et al. 2008; Kim and Kim 2013).
Neuroprotective activity
In neuroblastoma cells, the neuroprotective potential of [6]-gingerol had been investigated in Ab(25–35)-induced oxidative stress. [6]-Gingerol inhibited intracellular ROS accumulation as well as stabilized Ab(25–35)-depleted endogenous antioxidant glutathione quantity, and also stimulated the mRNA and protein expression levels of antioxidant enzymes like c-glutamylcysteine ligase (GCL) and heme oxygenase-1 (HO-1) via induction of NF factor 2 (NFf2) (Lee et al. 2011).
Clinical studies
Recently, the clinical studies of ginger are gaining importance due to its beneficial effects in various types of diseases like cancer, nausea and vomiting, gynecological problems, etc. Various types of studies on ginger have been carried out in different parts of the world. The literature on clinical trials has been revealed with encouraging evidence regarding its efficacy on human health and safety (Marx et al. 2017; Danwilai et al. 2017).
In cancer patients, the multiple randomized clinical trials (RCTs) have revealed the potency of ginger in reducing CINV and also in dysmenorrheal, while in another study, ginger has been shown to improve lipid levels and improved glucose regulation, insulin susceptibility, and glycosylated hemoglobin of type-2 diabetes mellitus. The ginger treatment was reported to enhance the health and quality of life in patients of the CINV and also helped in reducing and delaying the CINV in children and adults (Pillai et al. 2011; Konmun et al. 2017).
Besides this, gingerols have been reported to prevent vomiting induced by an antiretroviral regimen (Dabaghzadeh et al. 2014). On the other hand, its treatment in postoperative nausea and vomiting (PONV) had no significant effect (Anh et al. 2020). The cinical trials on giner and its chemical constituents are given in (Table 1).
Table 1.
Clinical trials performed on ginger and its chemical constituents
| S. no | Ginger | Clinical trials | Phase | Ref |
|---|---|---|---|---|
| 1 | Ginger oil | Reduction in knee pain rating | Double-blind, placebo-controlled experimental study | (Yip and Tam 2008) |
| 2 | [4]-, [6]-, [8]-, [10]-gingerol | No hypo-analgesic impact on quadriceps pain level in contrast to placebo | Double-blind, crossover design | (Black and O'Connor 2008) |
| 3 | Ginger | Equal to ibuprofen and mefenamic acid | Double-blind comparative study | (Ozgoli et al. 2009) |
| 4 | Ginger versus salicylate | This plant mixture is therapeutically useful for individuals with knee AO in reducing pain, muscle tightness, and restricted movement; its impact is equivalent to salicylate ointment | Block randomization method | (Zahmatkash and Vafaeenasab 2011) |
| 5 | Sublingual formulation of fever few/ginger | Reliable and efficacious as first-line therapy for abortion with patients suffering from migraine | Randomized 3:1 | (Cady et al. 2011) |
| 6 | Plygersic gel (Zingiber officinale; Zingiber cassumunar) | Improves knee joint pain following 6 weeks of treatment | Double-blind, randomized, controlled trial | (Niempoog et al. 2012) |
| 7 | Ginger | If provided at the start and 3 days prior, it is an efficient and acceptable medication to alleviate pain in females with primary dysmenorrhea | Randomized, controlled trial | (Rahnama et al. 2012; Jenabi 2013; Kashefi et al. 2014) |
| 8 | Ginger combination (340 mg EV.EXT 35 Zingiber officinalis extract) + glucosamine | Upper SODA pain severity was reduced little but dramatically | Randomized controlled study | (Drozdov et al. 2012) |
| 9 | Ginger supplement in combination with other botanicals | Effective to lessen pain in the knee | Randomized, double-blind, parallel-efficacy, four-arm multicentre equivalence drug trial | (Drozdov et al. 2012; Chopra et al. 2013; Nieman et al. 2013) |
| 10 | Ginger supplement | Accelerates muscular strength recovery after rigorous training | Randomized groups | (Matsumura et al. 2015) |
| 11 | Ginger powder supplementation | Decrease inflammation markers in knee AO population | Double-blind randomized placebo-controlled clinical trial | (Naderi et al. 2016) |
Also, nanoformulation of gingerols has been reported for treating the various types of activities (Table 2).
Table 2.
Reported ginger extract based nanoformulations for the treatment of various types of diseased conditions
| S. no | Nanoformulations | Properties | Experimental results | Ref |
|---|---|---|---|---|
| 1 | Ginger rhizome water extract | Antimicrobial activity | Bacterial growth suppression; Escherichia coli, Pseudomonas aeruginosa, Salmonella typhimurium, Streptococcus pyogenes, Staphylococcus aureus, Bacillus subtilis, Rhizopus sp., Aspergillus niger, Candida albicans thus may be employed as anti-bacterial medication industries | (Norhidayah et al. 2015) |
| 2 | Ginger extract | Protection against alcohol induced liver damage | Oral therapy of GNPs helped in protection of the liver against harmful effects of alcohol mainly due to presence of Shogaols in ginger | (Zhuang et al. 2015) |
| 3 | Ginger extract | Drug bioavailability | GNPs may help in improving in delivery of the nanoparticles to various parts of the body without inducing adverse effects and further improving the bioavailability in the target cells | (Khalil et al. 2016) |
| 4 | Ginger-derived nanoparticles | Molecular targeting and gene regulation | GNPs serve a part in molecular targeting and the regulation of genomic expression | (Zhang et al. 2016a, b) |
| 5 | Ginger-derived lipid nano-vectors | Prevention against IBD (Inflammatory bowel disease) and CAC (Coronary artery calcification) | The GDNP system is simple to develop and might even offer a viable treatment approach for the prevention and treatment of IBD and CAC | (Zhang et al. 2016a, b) |
| 6 | Ginger-derived nanoparticles | Anti-colitis | Useful in treating colitis without any side effect | (Zhang et al. 2016a) |
| 7 | Ginger-derived nanoparticles | Prevention of intestinal inflammation | Could prevent intestinal inflammation in mice suffering from acute colitis and chronic colitis | (Zhang et al. 2016a, b) |
| 8 | Ginger nanoparticles | Apoptosis | Responsible for inducing apoptotic activity against chemical-induced cancer in male rats. GNPs enhanced the amountof enzymatic antioxidants and reduced necrotic/apoptotic rate | (Abdu et al. 2017) |
| 9 | Ginger-derived nanoparticles | Drug delivery | GNPs may be beneficial in drug delivery to colon | (Zhang et al. 2016a, b) |
| 10 | Hydrogel system encapsulating shogaol | Anti-colitis | Reported to weaken symptoms and improve colitis wound repair in mice | (Zhang et al. 2018) |
| 11 | mRNAs of ginger exosome like nanoparticles (GELN) | Anti-colitis | GELN ameliorate mouse colitis | (Teng et al. 2018) |
| 12 | Ginger-derived nanoparticles | Body weight, hematological parameters and histological analysis | GNPs showed no symptoms of cytotoxicity and offer a safe route of medication administration | (Zhang et al. 2018) |
| 13 | Ginger-derived nanoparticles | Prevention against IBD | It has the ability to improve the therapy and management of IBD | (Mao et al. 2019) |
| 14 | Ginger extract | Antihepatotoxicity and nephrotoxicity | Helpfulin improving liver and kidney biochemical markers, oxidative stress and histopathological structure in rats | (Bakr et al. 2020) |
| 15 | Ginger-derived exosome-like nanoparticles | NLRP3 inflammasome assembling, IL-1β, and IL-18 production, pyroptosis | Impede the organization of the NLRP3 inflammasome, production of IL-1 and IL-18, along with pyroptosis in mice macrophages | (Chen et al. 2019) |
| 16 | Ginger-derived nanoparticles | Drug delivery | GNPs were combined with chitosan to provide a drug delivery method for the controlled release of 5-ASA favours at the gastric pH, that is advantageous towards IBD | (Markam and Bajpai 2020) |
| 17 | Silver nanoparticles of ginger | Protection against SARS-CoV-2 | Ginger silver nanoparticles exhibit an inhibitory potential against SARS-CoV-2 | (Mohammad et al. 2021) |
Discussion
Ginger has been reported for various types of diseases like gastrointestinal disorders, inflammatory bowel disease, peptic ulcer, motion sickness, etc. Lately, different gingerols have been found to show strong anticoagulant effects, anti-inflammatory effects, antiemetic effects, antinociceptive effects, antioxidant effects, cardiovascular effects, antitussive effects, immunomodulatory effects, lipid effects, weight loss effects, antimicrobial activities, and chemopreventive activities. Various studies also revealed that gingerol and its derivativesare responsible to inhibit tumor promotion in mouse skin. However, not much is understoodabout the processesthrough which ginger and its derivatives accomplish these properties. Recently, many studies have demonstrated the different types of mechanisms with animalmodels. Moreover, more research is needed to determine the properties and mechanisms of gingerol and its compounds in human intervention trials and gene expression.
Conclusion and future prospects
Ginger and its phytoconstituents are considered as a safe option to treat different diseases due to no significant adverse effects. Among several chemical constituents like gingerols, shogaols, paradols, dihydroparadols, diarylheptanoids, zingiberene, phellandrene, etc., gingerols are found to be accountable for different therapeutic and pharmacological actions. Many studies have revealed its therapeutic applications in the therapy of cancer, its effect as antioxidant, antibiotic, antiemetic, antidiabetic, antiangiogenesis etc., [6]-, [8]-, [10]-gingerols and [6]-shogaol act on the 5-HT3 receptor ion-channel complex, by binding to a modulatory site (Abdel-Aziz 2006). Various studies have thus confirmed that these can be exploited for future phyto-medicine. However, more studies are required for the control of the disease development via modulation of antioxidant, metabolic and genetic activities. Therefore, more systematic research with detailed methodologies and numerous clinical trials are required to address the functional characteristics of ginger.
Current treatments for various types of human diseases are based upon the use of synthetic drugs which give good results. However, these also show adverse side effects. Various constituents of gingerol have been found to be quite effective against many human diseases such as cardiovascular diseases, cancer, diabetes, obesity and many more. Besides, these constituents are safe and competitively inexpensive. Therefore, gingerol and its constituents have created optimism towards the unique and novel therapeutic strategies against these diseases. More research is needed to investigate the molecular pathway for the mechanism of action of gingerol which will open a newvista for its use in large scale.
Author contribution
Samridhi Sharma and Krishan Chander Sharma: conceptualize and written the whole manuscript. Santosh Kumar Upadyay, Monu Kumar Shukla, Tirath: participated in the design of study and improves grammar. Lokender Kumar, Jasha Momo H. Anal, Sanjib Bhattacharyya: helps in validate, review and editing. Deepak Kumar: design, validate and supervised the whole manuscript.
Data Availability
No datasets were generated or analyzed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Since no slaughtering of animal was done so there is no need for ethical approval for publishing this manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Samridhi Sharma, Email: samridhi.sharma1634@gmail.com.
Monu Kumar Shukla, Email: monushukla02@gmail.com.
Krishan Chander Sharma, Email: krishan14chander@gmail.com.
Tirath, Email: tktirathkumar007@gmail.com.
Lokender Kumar, Email: lokenderkumar@shooliniuniversity.com.
Jasha Momo H. Anal, Email: hmunshel.jasha@iiim.res.in
Santosh Kumar Upadhyay, Email: skupadhyay@pu.ac.in.
Sanjib Bhattacharyya, Email: bhattacharyya.Sanjib2k12@gmail.com.
Deepak Kumar, Email: guptadeepak002@gmail.com.
References
- Abdel-Aziz H, Windeck T, Ploch M, Verspohl EJ. Mode of action of gingerols and shogaols on 5-HT3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur J Pharmacol. 2006;530:136–143. doi: 10.1016/j.ejphar.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Abdu SB, Abdu F, Khalil WK. Ginger nanoparticles modulate the apoptotic activity in male rats exposed to dioxin-induced cancer initiation. Int J Pharmacol. 2017;13:946–957. doi: 10.3923/ijp.2017.946.957. [DOI] [Google Scholar]
- Akimoto M, Iizuka M, Kanematsu R, Yoshida M, Takenaga K. Anticancer effect of ginger extract against pancreatic cancer cells mainly through reactive oxygen species-mediated autotic cell death. PLoS ONE. 2015;10:e0126605. doi: 10.1371/journal.pone.0126605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Al-Sanea MM, Abelyan N, Abdelgawad MA, Musa A, et al. Strawberry and ginger silver nanoparticles as potential inhibitors for SARS-CoV-2 assisted by in silico modeling and metabolic profiling. Antibiotics. 2021;10:824. doi: 10.3390/antibiotics10070824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar BS, Nilendra S, Rakesh M, Arvind KS. Anti-hyperglycaemic, lipid lowering and anti-oxidant properties of [6]-gingerol in db/db mice. Int J Med Med Sci. 2009;1:536–544. [Google Scholar]
- Andallu B, Radhika B, Suryakantham V. Effect of aswagandha, ginger and mulberry on hyperglycemia and hyperlipidemia. Plant Foods Hum Nutr. 2003;58:1–7. doi: 10.1023/B:QUAL.0000040352.23559.04. [DOI] [Google Scholar]
- Anh NH, Kim SJ, Long NP, et al. Ginger on human health: a comprehensive systematic review of 109 randomized controlled trials. Nutrients. 2020;12:157. doi: 10.3390/nu12010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte RN, Voronov E. Interleukin-1-a major pleiotropic cytokine in tumor-host interactions. Semin Cancer Biol. 2002;12:277–290. doi: 10.1016/s1044-579x(02)00014-7. [DOI] [PubMed] [Google Scholar]
- Bakr AF, Abdelgayed SS, El-Tawil OS, Bakeer AM. Ginger extract and ginger nanoparticles; characterization and applications. Int J Vet Sci. 2020;9:203–209. doi: 10.37422/IJVS/20.021. [DOI] [Google Scholar]
- Bhardwaj S, Verma R, Gupta J. Challenges and future prospects of herbal medicine. Int Res Med Health Sci. 2018;1:12–15. doi: 10.36437/irmhs.2018.1.1.D. [DOI] [Google Scholar]
- Bhargava S, Dhabhai K, Batra A, Sharma A, Malhotra B. Zingiber officinale: chemical and phytochemical screening and evaluation of its antimicrobial activities. J Chem Res. 2012;4:360–364. [Google Scholar]
- Bhattarai S, Tran VH, Duke CC. The stability of gingerol and shogaol in aqueous solutions. J Pharm Sci. 2001;90:1658–1664. doi: 10.1002/jps.1116. [DOI] [PubMed] [Google Scholar]
- Black CD, O'Connor PJ. Acute effects of dietary ginger on quadriceps muscle pain during moderate-intensity cycling exercise. Int J Sport Nutr Exerc Metab. 2008;18:653–664. doi: 10.1123/ijsnem.18.6.653. [DOI] [PubMed] [Google Scholar]
- Bode AM, Dong Z. Herbal and traditional medicine: biomolecular and clinical aspects. In: Packer L, Ong CN, Halliwell B, editors. The amazing and mighty ginger. New York: CRC Press; 2004. pp. 131–156. [Google Scholar]
- Bode AM, Dong Z. Modulation of cell signal transduction by tea and ginger. In: Dong Z, Surh YJ, editors. Dietary modulation of cell signaling pathways. 1. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. pp. 45–74. [Google Scholar]
- Bode AM, Dong Z. The amazing and mighty ginger. In: Benzie IFF, Wachtel-Galor S, editors. Herbal medicine: biomolecular and clinical aspects. 2. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. pp. 129–154. [Google Scholar]
- Bode AM, Ma WY, Surh YJ, Dong Z. Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Res. 2001;61:850–853. [PubMed] [Google Scholar]
- Butt MS, Sultan MT. Ginger and its health claims: molecular aspects. Crit Rev Food Sci Nutr. 2011;51:383–393. doi: 10.1080/10408391003624848. [DOI] [PubMed] [Google Scholar]
- Cady RK, Goldstein J, Nett R, Mitchell R, Beach ME, Browning R. A double-blind placebo-controlled pilot study of sublingual feverfew and ginger (LipiGesic™M) in the treatment of migraine. Headache. 2011;51:1078–1086. doi: 10.1111/j.1526-4610.2011.01910.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty D, Mukherjee A, Sikdar A, Paul A, GhoshA K-B (2012) [6]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxi Letters 210:34–43 ((PMID: 22285432)) [DOI] [PubMed]
- Chen XY, Zhou Y, Yu JJ (2019) Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol Pharm 16:2690–2699 ((PMID: 31038962)) [DOI] [PubMed]
- Chopra A, Saluja M, Tillu G, Sarmukkaddam S et al (2013) Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: A randomized, double-blind, controlled equivalence drug trial. Rheumatology 52:1408–1417 ((PMID: 23365148)) [DOI] [PubMed]
- Cohen RJ, Ek K, Pan CX (2002) Complementary and alternative medicine (CAM) use by older adults: A comparison of self-report and physician chart documentation. J Gerontol A Biol Sci Med Sci 57:223–227 ((PMID: 11909887)) [DOI] [PubMed]
- Cosmas S, Joshua PE, Durojaye OA. In-silico structure-activity relationship and molecular docking studies of Monosubstitted 6-Gingerol against Streptococcus pneumoniae Phosphomevalonate kinase. Internat J Sci Eng Res. 2018;9:1733–1742. [Google Scholar]
- Croft KD. Antioxidant effects of plant phenolic compounds. In: Basu TK, Temple NJ, Garg ML, editors. Antioxidants in Human Health and Disease. New York: CABI Publishing; 1999. pp. 109–121. [Google Scholar]
- Dabaghzadeh F, Khalili H, Dashti-Khavidaki S, Abbasian L, Moeinifard A (2014) Ginger for prevention of antiretroviral-induced nausea and vomiting: a randomized clinical trial. Expert Opin Drug Saf 13:859–866 ((PMID: 24820858)) [DOI] [PubMed]
- Danwilai K, Konmun J, Sripanidkulchai BO, Subongkot S (2017) Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: a pilot study. Cancer Manag Res 9:11–18 ((PMID: 28203106)) [DOI] [PMC free article] [PubMed]
- Denniff P, Macleod I, Whiting DA. Studies in the biosynthesis of [6]-gingerol, pungent principle of ginger (Zingiber officinale) J Chem Soc Perkin Trans. 1980;1:2637–2644. doi: 10.1039/p19800002637. [DOI] [Google Scholar]
- Ding GH, Naora K, Hayashibara M, Katagiri Y, Kano Y, Iwamoto K (1991) Pharmacokinetics of [6]-gingerol after intravenous administration in rats. Chem Pharm Bull (tokyo) 39:1612–1614 ((PMID: 1934184)) [DOI] [PubMed]
- Drozdov VN, Kim VA, Tkachenko EV, Varvanina GG (2012) Influence of a specific ginger combination on gastropathy conditions in patients with osteoarthritis of the knee or hip. J Altern Complement Med 18:583–588 ((PMID: 22784345)) [DOI] [PubMed]
- Farthing JE, O'neill MJ, Isolation of gingerols from powdered root ginger by countercurrent chromatography. J Liq Chromatogr. 1990;13:941–950. doi: 10.1080/01483919008049223. [DOI] [Google Scholar]
- Fleming SA, Dyer CW, Eggington J. A convenient one-step gingerol synthesis. Synth Commun. 1999;29:1933–1939. doi: 10.1080/00397919908086182. [DOI] [Google Scholar]
- Ghasemzadeh A, Jaafar HZ, Rahmat A, Wahab PE, Halim MR (2010) Effect of different light intensities on total phenolics and flavonoids synthesis and anti-oxidant activities in young ginger varieties (Zingiber officinale Roscoe). Int J Mol Sci 11:3885–3897 ((PMID: 21152306)) [DOI] [PMC free article] [PubMed]
- Ghayur MN, Gilani AH, Afridi MB, Houghton P (2005) Cardiovascular effects of ginger aqueous extract and its phenolic constituents are mediated through multiple pathways. Vascul Pharmacol 43:234–241 ((PMID: 16157513)) [DOI] [PubMed]
- Giacosa A, Morazzoni P, Bombardelli E, Riva A, Porro GB, Rondanelli M (2015) Can nausea and vomiting be treated with ginger extract? Eur Rev Med Pharmacol Sci 19:1291–1296 ((PMID: 25912592)) [PubMed]
- Girhepunje NS, Dumore NG, Swati U, Deepali TV, Shende SN. Indian J of Med Res & Pharmace Sci. 2017;4:24–28. [Google Scholar]
- Habib SH, Makpol S, Abdul Hamid NA, et al. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics (sao Paulo) 2008;63:807–813. doi: 10.1590/s1807-59322008000600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniadka R1, Rajeev AG, Palatty PL, Arora R, Baliga MS. Zingiber officinale ginger as an anti-emetic in cancer chemotherapy: a review. J Altern Complement Med. 2012;18(440):444. doi: 10.1089/acm.2010.0737. [DOI] [PubMed] [Google Scholar]
- HanX ZY, Liang Y, Zhang J, Li M, Zhao Z, Zhang X, Xue Y, Zhang Y, Xiao J, Chu L (2019) 6-Gingerol, an active pungent component of ginger, inhibits L-type Ca2+ current, contractility, and Ca2+ transients in isolated rat ventricular myocytes. Food SciNutr 7:1344–1352 ((PMID: 31024707)) [DOI] [PMC free article] [PubMed]
- Hiserodt RD, Franzblau SG, Rosen RG. Isolation of 6-, 8-, and 10-gingerol from ginger rhizome by HPLC and preliminary evaluation of inhibition of Mycobacterium avium and Mycobacterium tuberculosis. J Agric Food Chem. 1998;46:2504–2508. doi: 10.1021/jf970948l. [DOI] [Google Scholar]
- Ho SC, Chang KS, Lin CC (2013) Anti-neuroinflammatory capacity of fresh ginger is attributed mainly to 10-gingerol. Food Chem 141:3183–3191 ((PMID: 23871076)) [DOI] [PubMed]
- Huang QR, Iwamoto M, Aoki S, Tanaka N, Tajima K, Yamahara J, Takaishi Y, Yoshida M, Tomimatsu T, Tamai Y (1991) Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem Pharm Bull (tokyo) 39:397–399 ((PMID: 2054863)) [DOI] [PubMed]
- Ishiguro K, Ando T, Maeda O, Ohmiya N, Niwa Y, Kadomatsu K, Goto H. Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms. Biochem Biophys Res Commun. 2007;362:218–223. doi: 10.1016/j.bbrc.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jenabi E (2013) The effect of ginger for relieving of primary dysmenorrhoea. J Pak Med Assoc 63:8–10 ((PMID: 23865123)) [PubMed]
- Jindal DK, Sah P, Bisht D, Lalhlenmawia H, Kumar D, Kumar D (2021). Role of Medicinal Plants in Pulmonary Hypertension. In: Dua K, Nammi S, Chang D, Chellappan DK, Gupta G, Collet T. (eds) Medicinal Plants for Lung Diseases. Springer, Singapore. 10.1007/978-981-33-6850-7_13
- Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN. Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE2 production. Phytochemistry. 2005;66:1614–1635. doi: 10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Jung MY, Lee MK, Park HJ, Oh EB, Shin JY, Park JS, Jung SY, Oh JH, Choi DS (2018) Heat-induced conversion of gingerols to shogaols in ginger as affected by heat type (dry or moist heat), sample type (fresh or dried), temperature and time. Food Sci Biotechnol 27:687–693. 10.1007/s10068-017-0301-1 [DOI] [PMC free article] [PubMed]
- Kashefi F, Khajehei M, Tabatabaeichehr M, Alavinia M, Asili J. Comparison of the effect of ginger and zinc sulfate on primary dysmenorrhea: a placebo-controlled randomized trial. Pain Manag Nurs. 2014;15:826–833. doi: 10.1016/j.pmn.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Agarwal R, Mukhtar H (1996) Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale rhizome. Cancer Res 56:1023–1030 ((PMID: 8640756)) [PubMed]
- Kawai T, Kinoshita K, Koyama K, Takahashi K. Anti-emetic principles of Magnolia obovata bark and Zingiber officinale rhizome. Planta Med. 1994;60:17–20. doi: 10.1055/s-2006-959399. [DOI] [PubMed] [Google Scholar]
- Kelly K. History of medicine. New York: Facts on file; 2009. pp. 29–50. [Google Scholar]
- Khalil WK, El-Bassyouni GT, Booles HF. Nano-encapsulated form of citrus medica for osteoporosis treatment in animal model. Int J Pharm Clin Res. 2016;8:49–59. [Google Scholar]
- Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, Kim YM, Kwon YG. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335:300–308. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- Kim SO, Kim MR. [6]-Gingerol prevents disassembly of cell junctions and activities of MMPs in invasive human pancreas cancer cells through ERK/NF-κ B/Snail signal transduction pathway. Evid Based Complement Alternat Med. 2013;2013:761852. doi: 10.1155/2013/761852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konmun J, Danwilai K, Ngamphaiboon N, Sripanidkulchai B, Sookprasert A, Subongkot S. A phase II randomized double-blind placebo-controlled study of 6-gingerol as an anti-emetic in solid tumor patients receiving moderately to highly emetogenic chemotherapy. Med Oncol. 2017;34:69. doi: 10.1007/s12032-017-0931-4. [DOI] [PubMed] [Google Scholar]
- Koo KL, Ammit AJ, Tran VH, Duke CC, Roufogalis BD. Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thromb Res. 2001;103:387–397. doi: 10.1016/s0049-3848(01)00338-3. [DOI] [PubMed] [Google Scholar]
- Kumar G, Karthik K, Rao B. Review on pharmacological and phytochemical properties of Zingiber officinale Roscoe (Zingiberaceae) J Phar Res. 2011;4:2963–2969. [Google Scholar]
- Kumar NV, Srinivas P, Bettadaiah BK. New scalable and eco-friendly synthesis of gingerols. Tetrahedron Lett. 2012;53:2993–2995. doi: 10.1016/j.tetlet.2012.03.092. [DOI] [Google Scholar]
- Lee C, Park GH, Kim CY, Jang JH. [6]-Gingerol attenuates β-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem Toxicol. 2011;49:1261–1269. doi: 10.1016/j.fct.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Lee HS, Seo EY, Kang NE, Kim WK. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J Nutr Biochem. 2008;19:313–319. doi: 10.1016/j.jnutbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Li C, Zhou L. Inhibitory effect 6-gingerol on adipogenesis through activation of the Wnt/β-catenin signaling pathway in 3T3-L1 adipocytes. Toxicol in Vitro. 2015;30:394–401. doi: 10.1016/j.tiv.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Li X, Kristine CYM, Van HT, Li Y, Duke CC, Roufogalis BD, Heather AK (2013) Attenuation of proinflammatory responses by S-[6]-gingerol via inhibition of ROS/NF-kappa B/COX2 activation in HuH7 cells. Evid Based Complement Alternat Med pp 1–8. 10.1155/2013/146142 [DOI] [PMC free article] [PubMed]
- Lin CB, Lin CC, Tsay GJ. 6-Gingerol inhibits growth of colon cancer cell LoVo via induction of G2/M arrest. Evid Based Complement Alternat Med. 2012 doi: 10.1155/2012/326096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu J, Guo H, Sun S, Wang S, Zhang Y, Li S, Qiao Y. [6]-Gingerol: a novel AT1 antagonist for the treatment of cardiovascular disease. Planta Med. 2013;79:322–326. doi: 10.1055/s-0032-1328262. [DOI] [PubMed] [Google Scholar]
- Ma J, Jin X, Yang L, Liu ZL. Diarylheptanoids from the rhizomes of Zingiber officinale. Phytochem. 2004;65:1137–1143. doi: 10.1016/j.phytochem.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Ma S, Zhang S, Duan W, Wang W. An enantioselective synthesis of (+)-(S)-[n]-gingerols via the l-proline-catalyzed aldol reaction. Bioorg Med Chem Lett. 2009;19:3909–3911. doi: 10.1016/j.bmcl.2009.03.081. [DOI] [PubMed] [Google Scholar]
- Mahady GB, Pendland SL, Yun GS, Lu ZZ, Stoia A (2003) Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of Cag A+ strains of Helicobacter pylori. Anticancer Res 23:3699 ((PMID: 14666666)) [PMC free article] [PubMed]
- Mahluji S, Attari VE, Mobasseri M, Payahoo L, Ostadrahimi A, Golzari SE. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int J Food SciNutr. 2013;64:682–686. doi: 10.3109/09637486.2013.775223. [DOI] [PubMed] [Google Scholar]
- Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T, Bin LH. Bioactive compounds and bioactivities of ginger (zingiber officinale roscoe) Foods. 2019;8:185. doi: 10.3390/foods8060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markam R, Bajpai AK. Functionalization of ginger derived nanoparticles with chitosan to design drug delivery system for controlled release of 5-amino salicylic acid (5-ASA) in treatment of inflammatory bowel diseases: An in vitro study. React Funct Polym. 2020;149:104520. doi: 10.1016/j.reactfunctpolym.2020.104520. [DOI] [Google Scholar]
- Marx W, Ried K, McCarthy AL, Vitetta L, Sali A, McKavanagh D, Isenring L. Ginger—Mechanism of action in chemotherapy-induced nausea and vomiting: a review. Crit Rev Food Sci Nutr. 2017;57:141–146. doi: 10.1080/10408398.2013.865590. [DOI] [PubMed] [Google Scholar]
- Yuki M, Kikuzaki H, Hisamoto M, Nakatani N (2004) Antioxidant properties of gingerol related compounds from ginger. BioFactors 21:293–296. 10.1002/biof.552210157 [DOI] [PubMed]
- Matsumura MD, Zavorsky GS, Smoliga JM. The effects of pre-exercise ginger supplementation on muscle damage and delayed onset muscle soreness. Phytother Res. 2015;29:887–893. doi: 10.1002/ptr.5328. [DOI] [PubMed] [Google Scholar]
- Miri P, Bae J, Lee DS. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytothery Res. 2008;22:1446–1449. doi: 10.1002/ptr.2473. [DOI] [PubMed] [Google Scholar]
- Mohammad YA (2016) Gingerol and its role in chronic diseases. Adv Exp Med & Bio 929:177–207. 10.1007/978-3-319-41342-6_8 [DOI] [PubMed]
- Mohammad M. Al-Sanea, Narek Abelyan, Mohamed A. Abdelgawad, Arafa Musa et al (2021) Strawberry and Ginger Silver Nanoparticles as Potential Inhibitors for SARS-CoV 2 Assisted by In Silico Modeling and Metabolic Profiling. Antibiotics 10:824. 10.3390/antibiotics10070824 [DOI] [PMC free article] [PubMed]
- Morán A, Ortega P, de Juan C, Fernández-Marcelo T, Frías C, Sánchez-Pernaute A, Torres AJ, Díaz-Rubio E, Iniesta P, Benito M. Differential colorectal carcinogenesis: molecular basis and clinical relevance. World J Gastrointest Oncol. 2010;2:151. doi: 10.4251/wjgo.v2.i3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Ortega P, de Juan C, et al. Differential colorectal carcinogenesis: molecular basis and clinical relevance. World J Gastrointest Oncol. 2010;2:151–158. doi: 10.4251/wjgo.v2.i3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi Z, Mozaffari-Khosravi H, Dehghan A, Nadjarzadeh A, Huseini HF. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: a 12-week double-blind randomized placebo-controlled clinical trial. J Tradit Complement Med. 2016;6:199–203. doi: 10.1016/j.jtcme.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazim UM, Jeong JK, Seol JW, Hur J, Eo SK, Lee JH, Park SY. Inhibition of the autophagy flux by gingerol enhances TRAIL-induced tumor cell death. Oncol Rep. 2015;33:2331–2336. doi: 10.3892/or.2015.3869. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Shanely RA, Luo B, Dew D, Meaney MP, Sha W. A commercialized dietary supplement alleviates joint pain in community adults: a double-blind, placebo-controlled community trial. Nutr J. 2013;12:1–9. doi: 10.1186/1475-2891-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niempoog S, Siriarchavatana P, Kajsongkram T (2012) The efficacy of Plygersic gel for use in the treatment of osteoarthritis of the knee. J Med Assoc Thai 1:113–119 ((PMID: 23451449)) [PubMed]
- Nigam N, Bhui K, Prasad S, George J, Shukla Y. [6]-Gingerol induces reactive oxygen species regulated mitochondrial cell death pathway in human epidermoid carcinoma A431 cells. Chem Biol Interact. 2009;181:77–84. doi: 10.1016/j.cbi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Nigam N, George J, Srivastava S, Roy P, Bhui K, Singh M, Shukla Y. Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B [a] P-induced mouse skin tumorigenesis. Cancer Chemother Pharmacol. 2010;65:687–696. doi: 10.1007/s00280-009-1074-x. [DOI] [PubMed] [Google Scholar]
- Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogen. 2007;28:1188–1196. doi: 10.1093/carcin/bgl241. [DOI] [PubMed] [Google Scholar]
- Norhidayah A, Noriham A, Rusop MD. Assessment of antimicrobial activity of nanoparticle ginger rhizome water extract. IJSRP. 2015;11:665–669. [Google Scholar]
- Nurtjahja-Tjendraputra E, Ammit AJ, Roufogalis BD, Tran VH, Duke CC. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res. 2003;111:259–265. doi: 10.1016/j.thromres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Irii H, Tahara Y, Ishii H, Hirao A, Udagawa H, Hiramoto M, Yasuda K, Takanishi A, Shibata S. Synthesis of a new [6]-gingerol analogue and its protective effect with respect to the development of metabolic syndrome in mice fed a high-fat diet. J Med Chem. 2011;54:6295–6304. doi: 10.1021/jm200662c. [DOI] [PubMed] [Google Scholar]
- Ozgoli G, Goli M, Moattar F. Comparison of effects of ginger, Mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J Altern Complement Med. 2009;15:129–132. doi: 10.1089/acm.2008.0311. [DOI] [PubMed] [Google Scholar]
- Palatty PL, Haniadka R, Valder B, Arora R, Baliga MS. Ginger in the prevention of nausea and vomiting: a review. Crit Rev Food Sci Nutr. 2013;53:659–669. doi: 10.1080/10408398.2011.553751. [DOI] [PubMed] [Google Scholar]
- Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2011;21:231–255. doi: 10.1023/A:1021254725842. [DOI] [PubMed] [Google Scholar]
- Park M, Bae J, Lee DS. Antibacterial activity of 10-gingerol and 12-gingerol isolated from ginger rhizome against periodontal bacteria. Phytotherapy Res. 2008;22:1446–1449. doi: 10.1002/ptr.2473. [DOI] [PubMed] [Google Scholar]
- Pillai AK, Sharma KK, Gupta YK, Bakhshi S. Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatr Blood Cancer. 2011;56:234–238. doi: 10.1002/pbc.22778. [DOI] [PubMed] [Google Scholar]
- Poltronieri J, Becceneri B, A, M Fuzer A, Cesar C Filho J, Martin CB, Cezar Vieira P, Pouliot N, R Cominetti M, [6]-gingerol as a cancer chemopreventive agent: a review of its activity on different steps of the metastatic process. Mini Rev Med Chem. 2014;14:313–321. doi: 10.2174/1389557514666140219095510. [DOI] [PubMed] [Google Scholar]
- Prasad S, Tyagi AK. Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Practice. 2015 doi: 10.1155/2015/142979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot–a review of the association between cannabis and psychosis. Front Psychiatry. 2014;22:54. doi: 10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnama P, Montazeri A, Huseini HF, Kianbakht S, Naseri M. Effect of Zingiber officinale R. rhizomes (ginger) on pain relief in primary dysmenorrhea: a placebo randomized trial. BMC Complement Altern Med. 2012;12:1–7. doi: 10.1186/1472-6882-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ahumada MC, Timmermann BN, Gang DR. Biosynthesis of curcuminoids and gingerols in turmeric (Curcuma longa) and ginger (Zingiber officinale): identification of curcuminoid synthase and hydroxycinnamoyl-CoA thioesterases. Phytochem. 2006;67:2017–2029. doi: 10.1016/j.phytochem.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Rani MP, Krishna MS, Padmakumari KP, Raghu KG, Sundaresan A. Zingiber officinale extract exhibits antidiabetic potential via modulating glucose uptake, protein glycation and inhibiting adipocyte differentiation: an in vitro study. J Sci Food Agric. 2012;92:1948–1955. doi: 10.1002/jsfa.5567. [DOI] [PubMed] [Google Scholar]
- Rani PM, Padmakumari KP, Sankarikutty B, Lijo Cherian O, Nisha VM, Raghu KG. Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. Int J Food Sci Nutr. 2011;62:106–110. doi: 10.3109/09637486.2010.515565. [DOI] [PubMed] [Google Scholar]
- Regassa H, Sourirajan A, Kumar V, Pandey S, Kumar D, Dev K (2022) A Review of Medicinal Plants of the Himalayas with Anti-Proliferative Activity for the Treatment of Various Cancers. Cancers 14:3898. 10.3390/cancers14163898 [DOI] [PMC free article] [PubMed]
- Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad MB, Mohsin MN, Razu BA, Hossain MT, Mahzabeen S. Glucose-stimulated insulin secretion pathway in pancreatic β-glucose-stimulated insulin secretion pathway in pancreatic-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Leprdb/db type 2 diabetic mice. BMC Complement Altern Med. 2017;17:395–407. doi: 10.1186/s12906-017-1903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswat M, Suryanarayana P, Reddy PY, Patil MA, Balakrishna N, Reddy GB (2010) Antiglycating potential of Zingiber officinalis and delay of diabetic cataract in rats. Mol vis 16:1525–1537 ((PMID: 20806076)) [PMC free article] [PubMed]
- Saravanan G, Ponmurugan P, Deepa MA, Senthilkumar B. Anti-obesity action of gingerol: effect on lipid profile, insulin, leptin, amylase and lipase in male obese rats induced by a high-fat diet. J Sci Food Agric. 2014;94:2972–2977. doi: 10.1002/jsfa.6642. [DOI] [PubMed] [Google Scholar]
- Sekiya K, Ohtani A, Kusano S. Enhancement of insulin sensitivity in adipocytes by ginger. BioFactors. 2004;22:153–156. doi: 10.1002/biof.5520220130. [DOI] [PubMed] [Google Scholar]
- Semwal RB, Semwal DK, Combrinck S, Viljoen AM. Gingerols and shogaols: important nutraceutical principles from ginger. Phytochem Lett. 2015;117:554–568. doi: 10.1016/j.phytochem.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Singh AB, Akanksha, Singh N, Maurya R, Srivastava AK (2009) Anti-hyperglycaemic, lipid lowering and anti-oxidant properties of [6]-gingerol in db/db mice. Int J Med Med Sci 1:536–544
- Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Shukla MK, Singh SK, Pandey S, Gupta PK, Choudhary A, Jindal DK, Dua K, Kumar D (2022) Potential Immunomodulatory Activities of Plant Products. S Afr J Bot 149:937–943. 10.1016/j.sajb.2022.04.055
- Son MJ, Miura Y, Yagasaki K. Mechanisms for antidiabetic effect of gingerol in cultured cells and obese diabetic model mice. Cytotech. 2015;67:641–652. doi: 10.1007/s10616-014-9730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanoski N (1999) Development of health culture in Veles and its region from the past to the end of the 20th century. Veles Soc of Sci and Art pp13–34
- Surh YJ, Lee E, Lee JM. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutat Res. 1998;402:259–267. doi: 10.1016/S0027-5107(97)00305-9. [DOI] [PubMed] [Google Scholar]
- Teng Y, Ren YI, Sayed M, Hu X, Lei C, Kumar A, Hutchins E, Mu J, Deng Z, Luo C, Sundaram K. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–652. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Qian W, Qian Q, Zhang W, Cai X (2020) Gingerol inhibits cisplatin-induced acute and delayed emesis in rats and minks by regulating the central and peripheral 5-HT, SP, and DA systems. J Nat Med 74:353–370. 10.1007/s11418-019-01372-x [DOI] [PMC free article] [PubMed]
- Tzeng TF, Chang CJ, Liu IM. 6-gingerolinhibits rosiglitazone-induced adipogenesis in 3T3-L1adipocytes. Phytother Res. 2014;28:187–192. doi: 10.1002/ptr.4976. [DOI] [PubMed] [Google Scholar]
- Tzeng TF, Liu IM. 6-Gingerol prevents adipogenesis and the accumulation of cytoplasmic lipid droplets in 3T3-L1 cells. Phytomedicine. 2013;20:481–487. doi: 10.1016/j.phymed.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Wang CC, Chen LG, Lee LT, Yang LL (2003) Effects of 6-gingerol, an antioxidant from ginger, on inducing apoptosis in human leukemic HL-60 cells. In Vivo 17:641–645 ((PMID: 14758732)) [PubMed]
- Wang S, Zhang C, Yang G, Yang Y. Biological properties of 6-gingerol: a brief review. Nat Prod Commun. 2014;9:1934578X1400900736. doi: 10.1177/1934578X1400900736. [DOI] [PubMed] [Google Scholar]
- Wei QY, Ma JP, Cai YJ, Yang L, Liu ZL. Cytotoxic and apoptotic activities of diarylheptanoids and gingerol-related compounds from the rhizome of Chinese ginger. J Ethnopharmacol. 2005;102:177–184. doi: 10.1016/j.jep.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Weng CJ, Chou CP, Ho CT, Yen GC. Molecular mechanism inhibiting human hepatocarcinoma cell invasion by 6-shogaol and 6-gingerol. Mol Nutr Food Res. 2012;56:1304–1314. doi: 10.1002/mnfr.201200173. [DOI] [PubMed] [Google Scholar]
- Weng CJ, Wu CF, Huang HW, Ho CT, Yen GC. Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol Nutr Food Res. 2010;54:1618–1627. doi: 10.1002/mnfr.201000108. [DOI] [PubMed] [Google Scholar]
- Wu WK, Wang XJ, Cheng AS, Luo MX, Ng SS, To KF, Chan FK, Cho CH, Sung JJ, Yu J. Dysregulation and crosstalk of cellular signaling pathways in colon carcinogenesis. Crit Rev Oncol Hematol. 2013;86:251–277. doi: 10.1016/j.critrevonc.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Garg A. Antidiabetic effects of [10]-gingerol in streptozotocin- and high-fat diet-induced diabetic rats. Asian J Pharmaceu & Clinical Res. 2019;12:77–80. doi: 10.22159/ajpcr.2019.v12i11.35421. [DOI] [Google Scholar]
- Yagihashi S, Miura Y, Yagasaki K. Inhibitory effect of gingerol on the proliferation and invasion of hepatoma cells in culture. Cytotech. 2008;57:129–136. doi: 10.1007/s10616-008-9121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahara J, Rong HQ, Iwamoto M, Kobayashi G, Matsuda H, Fujimura H. Active components of ginger exhibiting anti-serotonergic action. Phytother Res. 1989;3:70–71. doi: 10.1002/ptr.2650030208. [DOI] [Google Scholar]
- Yang G, Wang S, Zhong L, et al. [6]-gingerol induces apoptosis through lysosomal-mitochondrial axis in human hepatoma G2 cells. Phytother Res. 2012;26:1667–1673. doi: 10.1002/ptr.4632. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhong L, Jiang L, Geng C, Cao J, Sun X, Liu X, Chen M, Ma Y. 6-Gingerol prevents patulin-induced genotoxicity in HepG2 cells. Phytother Res. 2011;25:1480–1485. doi: 10.1002/ptr.3446. [DOI] [PubMed] [Google Scholar]
- Yip YB, Tam AC. An experimental study on the effectiveness of massage with aromatic ginger and orange essential oil for moderate-to-severe knee pain among the elderly in Hong Kong. Complement Ther Med. 2008;16:131–138. doi: 10.1016/j.ctim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Yusof M, Anum Y (2016) Gingerol and its role in chronic diseases. Drug discovery from mother nature 177–207. 10.1007/978-3-319-41342-6_8
- Yusof Y, Ahmad N, Das S, Sulaiman S, Murad N. Chemopreventive efficacy of ginger (Zingiber officinale) in ethionine induced rat hepatocarcinogenesis. Afr J TraditComplem Altern Med. 2009;6:87–93. doi: 10.4314/ajtcam.v6i1.57078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahmatkash M, Vafaeenasab MR. Comparing analgesic effects of a topical herbal mixed medicine with salicylate in patients with knee osteoarthritis. Pak J Biol Sci. 2011;14:715–719. doi: 10.3923/pjbs.2011.715.719. [DOI] [PubMed] [Google Scholar]
- Zhang M, Collins JF, Merlin D. Do ginger-derived nanoparticles represent an attractive treatment strategy for inflammatory bowel diseases? Nanomedicine. 2016;12:3035–3037. doi: 10.2217/nnm-2016-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Viennois E, Xu C, Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4:e1134415. doi: 10.1080/21688370.2015.1134415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Xu C, Liu D, Han MK, Wang L, Merlin D. Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J Crohns Colitis. 2018;12:217–229. doi: 10.1093/ecco-jcc/jjx115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D, Feng W, McClain CJ, Zhang HG. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi: 10.3402/jev.v4.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick SM, Djuric Z, Ruffin MT, Litzinger AJ, Normolle DP, Alrawi S, Feng MR, Brenner DE. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1930–1936. doi: 10.1158/1055-9965.EPI-07-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.