Abstract
We found that when group A streptococci are cocultured with human pharyngeal cells, they upregulate and secrete a 25-kDa toxin, determined to be the bacteriophage-encoded streptococcal pyrogenic exotoxin C (SpeC). This prompted us to determine if the bacteriophage themselves are induced during coculture conditions. We found that bacteriophage induction does occur, resulting in the release of ∼105 phage particles during the 3-h coculture. Furthermore, we show that the bacteriophage induction event is mediated by a pharyngeal cell soluble factor for which we provide an initial characterization.
Streptococcus pyogenes (group A streptococcus) is responsible for a large number of serious diseases worldwide, the most common of which is pharyngitis. If gone untreated, this infection could result in rheumatic heart disease in about 3% of cases. While a great deal of data have been accumulated regarding this organism, including its structures and genetic composition, our knowledge of its interaction with host cells during the early stages of infection remains at a rudimentary level. In deciphering the interactions of the streptococcus with its host, both surface and extracellular proteins are likely involved. While the majority of surface proteins act as adhesins, soluble proteins tend to exhibit a variety of activities, some of which are enzymatic.
In an effort to conserve energy, bacterially secreted proteins involved in pathogenesis may not necessarily be constitutively expressed, but instead are induced when required for infection. Upon culture of bacteria together with their host cells, upregulation of specific bacterial genes (some of which are virulence factors) has been observed with both intracellular (1, 2, 9) and nonintracellular organisms (12, 14). The streptococcal class of toxins includes some of the most potent molecules secreted by S. pyogenes, which often results in the destruction of host cells. Thus, analysis of the secretion of toxins by S. pyogenes in response to its host cell environment may help illuminate the regulation of these molecules in one of the earliest stages of streptococcal pathogenesis.
MATERIALS AND METHODS
Growth conditions for pharyngeal cells and S. pyogenes.
The human pharyngeal cell line Detroit 562 (ATCC CCL 138) was grown in minimal essential medium (MEM; Gibco-BRL, Gaithersburg, Md.) containing 10% fetal bovine serum. Cells were grown in Falcon six-well plates (35 mm in diameter) at 37°C under 5% CO2. The strains of S. pyogenes used included D471 (from the Rockefeller University collection), lysogenized strain CS112, and its indicator strain, CS24 (kindly supplied by Patrick Schlievert). All bacteria were cultured at 37°C in Todd-Hewitt broth containing 1% yeast extract (THY; Difco Laboratories, Detroit, Mich.).
S. pyogenes-human pharyngeal cell coculture system.
Pathogenic S. pyogenes (strain D471) cells were grown overnight and suspended in phosphate-buffered saline (PBS). After adjustment of the optical density at 650 nm (OD650) to 1.0 (∼5 × 108 CFU/ml), the bacteria were centrifuged and resuspended in serum-free MEM. Detroit 562 pharyngeal cells grown to confluence were washed with serum-free MEM and then inoculated with the prepared S. pyogenes to a final concentration of ∼108 CFU/ml in a total volume of 1.25 ml of serum-free MEM. The coculture was allowed to incubate for 3 h. at 37°C under 5% CO2. This medium was then centrifuged (9,300 × g for 15 min) and sterile filtered through a 0.45-μm-pore-diameter membrane.
As controls, bacteria and pharyngeal cells were incubated alone. Control medium was processed in parallel to the coculture sample.
Toxin detection in coculture medium.
Both bacterial and pharyngeal cell medium supernatants, along with the coculture medium supernatant, were concentrated 100-fold by trichloroacetic acid precipitation, separated by gel electrophoresis, and blotted onto a polyvinylidene difluoride (PVDF) membrane. The membrane was then blocked at room temperature for 4 h in Tris-Tween buffer (50 mM Tris, 150 mM NaCl, 0.5% Tween [pH 8.0]). After blocking, the membrane was probed with the anti-toxin-specific antibody 442 (kindly supplied by John Zabriskie) (4) at a 1:500 dilution in Tris-Tween buffer. Alkaline phosphatase-conjugated antirabbit immunoglobulin G (Sigma Chemical Co., St. Louis, Mo.) was used for development according to standard procedures.
SpeC identification.
For SpeC identification, the concentrated coculture medium was prepared and blotted as described above. The PVDF membrane was stained with Coomassie brilliant blue-R, and the portion of the membrane containing the 25-kDa toxin band was cut and subjected to internal sequencing via automated Edman degradation in the Rockefeller University Biotechnology Center. The determined sequence was then compared to those in the GenBank database.
Identification of bacteriophage during coculture.
The CS112-Detroit coculture medium was prepared as described for the D471-Detroit coculture; however, coculture medium samples were taken at 1-h intervals for 3 h. Dilutions of the coculture medium were mixed 1:1 (vol/vol) with an overnight culture of S. pyogenes indicator strain CS24 (diluted 1:1 in THY broth). Ten-microliter drops of this mixture were then spotted onto THY agar (1.5%) plates. After overnight incubation at 37°C, the plaques were enumerated. Media from the controls made up of pharyngeal cells only and bacteria only were also tested by this method. Additionally, dilutions of the coculture medium sample and the bacterial control medium sample were spread onto proteose peptone agar (1.5%) plates (Difco Laboratories, Detroit, Mich.) containing 3% defibrinated sheep blood. The plates were incubated overnight at 37°C, and colonies were then enumerated.
The coculture medium supernatant was prepared for electron microscopy by direct negative staining. Samples were processed at the Rockefeller University Electron Microscopy Facility.
speC detection in bacteriophage CS112.
The phage detected by plaque assay were blotted and fixed onto a nylon Hybond-N+ membrane (Amersham) according to the standard blotting protocol for the Amersham ECL (enhanced chemiluminescence) direct nucleic acid detection system. By using the ECL system for Southern blot analysis, the blot was hybridized with a speC-specific gene probe and developed according to the recommended protocol.
Identification of SPIF.
To identify soluble phage inducing factor (SPIF), Detroit 562 pharyngeal cells were grown to confluence, washed with serum-free MEM, and then incubated in 1.25 ml of serum-free medium per well for 3 h. This SPIF-containing medium was then centrifuged (9,300 × g for 15 min) and sterile filtered through a 0.45-μm-pore-diameter membrane filter (Scheicher & Schuell, Keene, N.H.). S. pyogenes CS112 was added to a concentration of ∼108 CFU/ml and allowed to incubate for 3 h at 37°C under 5%CO2. Concurrently, as a positive control for bacteriophage induction, CS112 bacteria were incubated in coculture with Detroit 562 pharyngeal cells according to the standard coculture procedure. Additionally, CS112 bacteria were suspended in serum-free MEM to a concentration of ∼108 CFU/ml and incubated in parallel to the other samples. The resulting culture media were then centrifuged (10,000 rpm for 15 min), and the supernatants were analyzed for both bacteriophage by plaque assay and for SpeC toxin by Western blot analysis with the antitoxin-specific antibody 442.
SPIF characterization.
To initially characterize the size of SPIF, Detroit 562 culture medium was filtered through a 1- or 10-kDa membrane (Amicon, Beverly, Mass.). The filtrates were then assayed for SPIF activity (i.e., phage induction capacity). Further SPIF characterization involved boiling the SPIF-containing Detroit 562 medium for 10 min and then assaying for SPIF activity. Additionally, SPIF was treated with pronase (200 μg/ml) and allowed to digest for 1 h at 37°C. The digest was filtered through a Centricon 10-kDa molecular mass cutoff filter to remove the pronase before assaying for SPIF activity.
When testing for the presence of SPIF in the culture medium of the MCF-7 breast cancer cell line, the same procedure used for the Detroit 562 pharyngeal cell line was followed.
Bacterial growth and phage induction.
S. pyogenes CS112 cells were added to serum-free MEM to a concentration of ∼108 CFU/ml. The bacteria were incubated at 37°C under 5% CO2 for 3 h. At hourly time points, 500 μl of culture was centrifuged (10 min at 14,000 rpm), the supernatant was collected, and pellets were suspended in an equivalent volume of PBS. The OD650 of these hourly time points was measured. Additionally, the supernatant was assayed for bacteriophage by the standard plaque assay. After 3 h of incubation, 250 μl of additional serum-free MEM, MEM containing 30% fetal calf serum (FCS), or spent Detroit medium containing SPIF was added to each 500-μl sample of the incubating culture. After addition of these agents, we continued to assay the OD650 and phage titer in the sample.
RESULTS AND DISCUSSION
Induction of phage-encoded toxin after coculture with human pharyngeal cells.
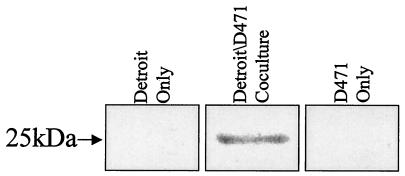
In our in vitro model of streptococcal infection, S. pyogenes cells were added to human Detroit 562 pharyngeal cells and incubated for 3 h, and the supernatant was analyzed for the presence of toxin. Using the antitoxin antibody 442 (4) (which reacts against conserved epitopes over a broad range of staphylococcal and streptococcal toxins) to probe Western blots, we found that a 25-kDa toxin was produced and secreted by S. pyogenes D471 under coculture conditions. This protein is undetectable when the bacteria are incubated alone and is likewise undetectable in the pharyngeal cell supernatants (Fig. 1). Isolation and sequence determination of the 25-kDa protein revealed 100% identity within an open reading frame from the S. pyogenes M1 genome (http: //www.genome.ou.edu/strep). Comparison with the GenBank database determined that the 25-kDa protein was the streptococcal pyrogenic exotoxin C (SpeC), a well-documented bacteriophage gene product.
FIG. 1.
Detection of secreted toxin during coculture. Streptococcal strains were grown alone or in coculture with Detroit human pharyngeal cells for 3 h. Proteins in the culture medium supernatants were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotted, and probed with anti-toxin-specific antibody 442.
Induction of bacteriophage after coculture with human pharyngeal cells.
The literature is replete with examples of bacteriophage-encoded toxin upregulation resulting from bacteriophage induction (8, 11, 13); however, such phage induction is usually the result of wholly artificial and nonspecific conditions (e.g., UV irradiation or mitomycin C). Although bacteriophage induction resulting from the bacterial interaction with the host cell environment has not been previously reported, we speculated that in the presence of pharyngeal cells, the observed upregulation of SpeC may occur as a result of bacteriophage induction. When we examined the D471-pharyngeal cell coculture supernatant by electron microscopy, we observed particles that appeared consistent in size and shape with bacteriophage; however, we were unable to generate plaques by using this supernatant and a variety of indicator strains. This suggests that either the indicators used were not sensitive to this phage, or the phage are in fact induced, but defective. Indeed, studies by Goshorn and Schlievert (6) show that most SpeC-producing bacteriophage are unable to form plaques on a wide range of indicators.
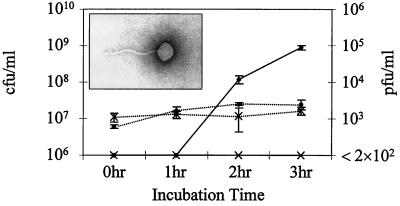
When we used a different S. pyogenes strain (CS112), which harbors a plaque-forming SpeC phage, in our pharyngeal cell coculture assay, we found that within 3 h, supernatants of this coculture medium contained bacteriophage (∼105 PFU/ml) when assayed on indicator lawns of S. pyogenes CS24 (Fig. 2). During the 3 h of coculture, PFU counts increased significantly, while the CS112 CFU demonstrated little change under these minimal medium conditions. Electron microscopy of the 3-h supernatant provided additional evidence of a tailed bacteriophage with a head diameter of ∼40 nm, consistent with other streptococcal bacteriophage (10) (Fig. 2 inset). Our inability to detect plaques in the supernatant of CS112 bacteria, which were incubated in either serum-containing or serum-free MEM, could mean either that the bacteria do not spontaneously induce phage during the incubation period, or the phage are produced at a level lower than the assay detection limit (2 × 102 PFU/ml).
FIG. 2.
Identification of bacteriophage during coculture. Streptococcal strains were grown alone or in coculture with Detroit human pharyngeal cells for 3 h. At timed intervals, the culture medium was examined for bacterial CFU of the CS112 control (--×--) and Detroit-CS112 coculture (--●--) versus PFU of the CS112 control (—∗—) and Detroit-CS112 coculture (—●—). The assay detection limit is 2 × 102 PFU/ml. The values plotted are mean concentrations of duplicate wells ± standard deviations. At the 3-h time point, the PFU concentrations found in the coculture media were significantly higher than those found in the control, even when assuming the highest undetectable concentration of 2 × 102 PFU/ml (P = 0.01). (Inset) Electron micrograph (with direct negative stain) of the bacteriophage found in the Detroit-CS112 coculture medium.
As is the case with many bacteria, group A streptococci often harbor more than one lysogenic bacteriophage. Using Southern blot analysis with a speC-specific probe, we verified that the induced CS112 phage that we detected during pharyngeal cell coculture actually is the bacteriophage harboring speC (not shown). This finding provided conclusive evidence that both the toxin and the phage from which it is encoded are induced during the same event. Whether phage induction is required for SpeC upregulation warrants further investigation.
Pharyngeal cell soluble factor mediates toxin and bacteriophage induction.
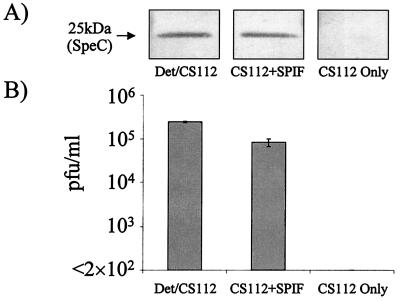
Since these results clearly indicate that the observed toxin and bacteriophage inductions are pharyngeal cell dependent, we questioned whether such induction was mediated by pharyngeal cell contact or by a pharyngeal cell soluble factor. When CS112 bacteria were incubated in cell-free medium in which pharyngeal cells had grown alone for 3 h, both SpeC and CS112 phage (Fig. 3) were induced to levels similar to those detected in coculture. When the same experiment was performed with S. pyogenes D471, only SpeC toxin induction could be detected (not shown). Our continued inability to detect bacteriophage from strain D471 supports the possibility of an incomplete or defective phage. These results provide strong evidence for a SPIF, which induces both phage and the phage-associated exotoxin.
FIG. 3.
SpeC and bacteriophage induction during coculture or SPIF exposure. (A) Streptococcal strain CS112 was incubated in coculture with Detroit 562 pharyngeal cells (Det/CS112), in the presence of SPIF (CS112+SPIF), or alone (CS112 Only). Proteins in the culture medium supernatants were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotted, and probed with anti-toxin-specific antibody 442. (B) The culture medium supernatants were also analyzed by plaque assay for CS112 bacteriophage induction.
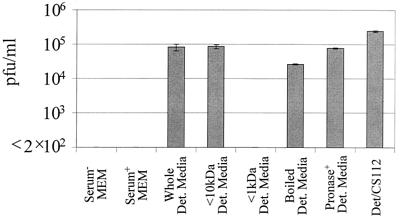
Initial characterization of SPIF demonstrated the molecule to be constitutively produced independent of cycloheximide treatment (where de novo protein synthesis was undetectable). Further characterization of SPIF revealed that its size was relatively small (lying between 1 and 10 kDa) and that it is resistant to heat, remaining active even after boiling (Fig. 4). The latter result and its stability at 4°C for at least 3 months rule out the possibility that SPIF is a highly reactive, short-lived radical. While still a crude characterization, SPIF was found to be resistant to pronase treatment (Fig. 4). These initial studies lead us to believe that SPIF is likely to be a low-molecular-mass compound that is protease resistant.
FIG. 4.
Characteristics of SPIF. Detroit pharyngeal cell culture medium supernatants were analyzed by plaque assay in order to detect CS112 bacteriophage induction. To characterize SPIF, spent medium from Detroit 562 pharyngeal cells was filtered through a 10-kDa membrane, filtered through a 1-kDa membrane, boiled, or pronase digested prior to CS112 bacterial incubation in the medium. CS112 bacteria were also incubated in serum-free MEM and serum-containing MEM as controls. CS112 bacteriophage induction during Detroit-CS112 coculture was examined in parallel and is included as a reference mark. The assay detection limit is 2 × 102 PFU/ml. The values plotted are mean concentrations from duplicate trials ± standard deviations. Phage induction was found to be statistically significant with either spent medium from Detroit 562 pharyngeal cells (Whole Det. Media; P = 0.02), <10-kDa Detroit cell medium (<10kDa Det. Media; P = 0.007), boiled Detroit cell medium (Boiled Det. Media; P = 0.0009), pronase-digested Detroit cell medium (Pronase+ Det. Media; P = 0.001) or Detroit-CS112 coculture (Det/CS112; P = 0.001).
In ascertaining the identity of SPIF, we examined whether this phage-inducing molecule is produced by cells other than pharyngeal cells. Indeed, we determined that SPIF is produced, with comparable activity, by an unrelated, MCF-7 breast cancer cell line (not shown). These findings lead us to believe that the molecule is likely to be a common extracellular component produced over a broad tropism of cells.
Bacterial growth insufficient for phage induction.
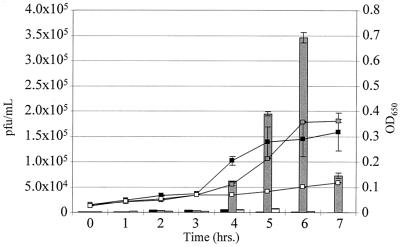
The low bacterial growth detected during the S. pyogenes coculture with human pharyngeal cells (Fig. 2) prompted us to investigate whether SPIF-mediated phage induction was growth dependent. In these studies, we attempted to replicate the mucosal environment in which low bacterial growth would be anticipated. When streptococci were incubated alone for 3 h in MEM, no appreciable phage were detected (Fig. 5); however, by adding fetal calf serum to a concentration of 10% at 3 h, we were able to demonstrate linear bacterial growth, but no appreciable bacteriophage induction. Upon addition of SPIF to S. pyogenes at the 3-h time point, both bacterial growth and phage induction increased (Fig. 5). Thus, while growth appears to coincide with SPIF-mediated phage induction, in itself, it is not sufficient for phage induction.
FIG. 5.
Bacterial growth is insufficient for phage induction. S. pyogenes CS112 was incubated in MEM (without serum) for 3 h. At 3 h, either MEM containing 30% fetal calf serum (▪), spent Detroit medium containing SPIF (░⃞), or additional MEM containing 0% serum (□) was added to the incubating culture. The phage titer is represented by the bar graph, and the OD is represented by the line curve. The values plotted are averages from duplicate trials ± standard deviations. Addition of either SPIF or serum to the CS112 culture resulted in statistically insignificant differences in bacterial growth (P = 0.56); however, SPIF addition resulted in a marked bacteriophage induction (P = 0.002).
The relationship between bacteria and their phage.
Many pathogenic bacteria are lysogenized, often with more than one lysogen, as observed in streptococci and staphylococci; however, except for the production of toxins in some systems (8, 11, 13), the role that these lysogens play in pathogenesis is unknown. Our findings may, for the first time, offer insight into the fundamental relationship between bacteria and their prophage. Group A streptococci are uniquely human pathogens that are asymptomatically carried in the pharynx of up to 20% of adults and children (the streptococcal reservoir). Our data support the idea that upon entry of lysogenized group A streptococci into the pharynx, a constitutively produced trans-acting pharyngeal cell factor (i.e., SPIF) mediates bacteriophage induction, enabling the newly released phage to lysogenize the preexisting colonizing streptococci, which may be sensitive to these phage, thus, allowing the bacteriophage to successfully disseminate its genetic material. While not wholly supported (5), the findings of Lazar and Waldor lend credence to this idea. They showed that CTXφ+ strains of Vibrio cholerae can lysogenize phage-naive strains within the intestines of mice (7). Similar findings were elucidated with the Shiga toxin 1-encoding phage of Escherichia coli (3). Our results suggest a symbiotic relationship between phage and bacteria, in which the bacteria assist the lysogen by providing transport to the proper bacterial environment and the signal for replication, while the phage reciprocates with the release of virulence determinants or other molecules carried on the phage genome. However, the role that these molecules (such as SpeC) play in the infection process has not been completely resolved.
While the induction and secretion of a bacteriophage-encoded toxin after coculture with mammalian cells are novel findings for streptococci, the induction, assembly, and release of complete bacteriophage particles as a result of such an interaction have not been previously described for any lysogenic phage system. Lysogenic phage induction is usually a consequence of DNA damage (induced by UV light, mitomycin C, or similar mutagens); however, in all cases, it results from the destruction or inactivation of the phage repressor. Further characterization of the pharyngeal cell SPIF will be required to determine whether it functions as a DNA mutagen or is utilized to specifically induce the lysogen. Ultimately, phage induction results in the lysis of the induced cocci releasing cytoplasmic contents, some of which may be virulence determinants. Perhaps the remaining cocci in the streptococcal chain exploit these determinants, reaping some pathogenic benefit from the lysis and sacrifice of a few cocci in the chain. Such a phenomenon may help explain why streptococci grow in chains.
ACKNOWLEDGMENTS
We thank Joshua Lederberg and Emil Gotschlich for insightful review of the manuscript. We give many thanks to David Thaler for discussion of the subject matter. Additionally, we thank Patricia Ryan, Daniel Nelson, and Corrie Broudy for critical comments throughout the execution of this work and preparation of the manuscript. We are also indebted to Eleana Sphicas for her electron microscopy expertise.
This work was supported by Public Health Service grant AI11822 to V.A.F.
REFERENCES
- 1.Abshire K Z, Neidhardt F C. Analysis of proteins synthesized by Salmonella typhimuriumduring growth within a host macrophage. J Bacteriol. 1993;175:3734–3743. doi: 10.1128/jb.175.12.3734-3743.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y. Induced expression of the Legionella pneumophilagene encoding a 20-kilodalton protein during intracellular infection. Infect Immun. 1998;66:203–212. doi: 10.1128/iai.66.1.203-212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acheson D W, Reidl J K, Zhang X, Keusch G T, Mekalanos J J, Waldor M K. In vivo transduction with Shiga toxin 1-encoding phage. Infect Immun. 1998;66:4496–4498. doi: 10.1128/iai.66.9.4496-4498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannan J, Visvanathan K, Zabriskie J B. Structure and function of streptococcal and staphylococcal superantigens in septic shock. Infect Dis Clin N Am. 1999;13:387–396. doi: 10.1016/s0891-5520(05)70081-7. [DOI] [PubMed] [Google Scholar]
- 5.Faruque S, Asadulghani M, Abdul Alim A R M, Albert M J, Nasirul Islam K M, Mekalanos J J. Induction of the lysogenic phage encoding cholera toxin in naturally occurring strains of toxigenic Vibrio choleraeO1 and O139. Infect Immun. 1998;66:3752–3757. doi: 10.1128/iai.66.8.3752-3757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goshorn S C, Schlievert P M. Bacteriophage association of streptococcal pyrogenic exotoxin type C. J Bacteriol. 1989;171:3068–3073. doi: 10.1128/jb.171.6.3068-3073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazar S, Waldor M K. ToxR-independent expression of cholera toxin from the replicative form of CTXφ. Infect Immun. 1998;66:394–397. doi: 10.1128/iai.66.1.394-397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda M, Barksdale L. System for the investigation of the bacteriophage-directed synthesis of diphtherial toxin. J Bacteriol. 1967;93:722–730. doi: 10.1128/jb.93.2.722-730.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moors M A, Levitt B, Youngman P, Portnoy D A. Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect Immun. 1999;67:131–139. doi: 10.1128/iai.67.1.131-139.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moynet D J, Colon-Whitt A E, Calandra G B, Cole R M. Structure of eight streptococcal bacteriophages. Virology. 1985;142:263–269. doi: 10.1016/0042-6822(85)90334-4. [DOI] [PubMed] [Google Scholar]
- 11.Mühldorfer I, Hacker J, Keusch G T, Acheson D W, Tschäpe H, Kane A V, Ritter A, Ölschläger T, Donohue-Rolfe A. Regulation of Shiga-like toxin II operon in Escherichia coli. Infect Immun. 1996;64:495–502. doi: 10.1128/iai.64.2.495-502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 13.Zabriskie J B. The role of temperate bacteriophage in the production of erythrogenic toxin by group A streptococci. J Exp Med. 1964;119:761–780. doi: 10.1084/jem.119.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J P, Normark S. Induction of gene expression in Escherichia coliafter pilus-mediated adherence. Science. 1996;273:1234–1236. doi: 10.1126/science.273.5279.1234. [DOI] [PubMed] [Google Scholar]