Abstract
Vector-borne diseases threaten human and agricultural health and are a critical component of the ecology of plants and animals. While previous studies have shown that pathogen spread can be affected by vector preferences for host infection status, less attention has been paid to vector preference for host sex, despite abundant evidence of sex-specific variation in disease burden. We investigated vector preference for host infection status and sex in the sterilizing ‘anther-smut’ pathogen (Microbotryum) of the alpine carnation, Dianthus pavonius. The pathogen is transferred among hosts by pollinators that visit infected flowers and become contaminated with spores produced by infected anthers. The host plant has a mixed breeding system with hermaphrodites and females. In experimental floral arrays, pollinators strongly preferred healthy hermaphrodites over both females and diseased plants, consistently across different guilds of pollinators and over multiple years. Using an agent-based model we showed that pollinator preferences for sex can affect pathogen spread in populations with variable sex-ratios, even if there is no preference for infection status. Our results demonstrate that vector preferences for host traits other than infection status can play a critical role in pathogen transmission dynamics when there is heterogeneity for those traits in the host population.
Keywords: Vector behavior, transmission, sex-ratio, plant-pathogen, pollinators, floral-disease, anther-smut, Microbotryum, Dianthus pavonius
INTRODUCTION
Vector-borne diseases pose serious threats to human health (Gubler 1998; Jones et al. 2008), agriculture (Reisen 2010), and wildlife (Harvell et al. 2009). Understanding the factors that influence vector movement to hosts with particular traits, and the consequences of these preferences for pathogen spread is therefore critically important. Results from a wide range of empirical studies have shown that, in many cases, infected hosts are more attractive to vectors. For example, infection with malaria parasites in both humans (Lacroix et al. 2005; Moraes et al. 2014) and birds (Cornet et al. 2013) increases attractiveness to mosquito vectors. In plants, Ingwell et al. (2012) showed that uninfected aphids preferred wheat plants infected with barley yellow dwarf virus, but that this preference switched when aphids became infected. However, in other cases, infection results in hosts becoming less attractive to vectors. For example, Daugherty et al. (2011) found that grapevines infected with the bacterial pathogen Xyella fastidiosa are discriminated against by its leafhopper vector.
While many vector behavior studies have focused on host infection status, vectors have also been shown to discriminate between other important host traits such as physiological state (Gervasi et al. 2016) and sex (Christe et al. 2007; Burkett-Cadena et al. 2014). For example, a study of blood meals from three avian-feeding mosquito species showed a significant mosquito preference for male birds (Burkett-Cadena et al. 2014). In mouse-eared bats, females have a higher prevalence of ectoparasitic mites, and laboratory choice tests have shown that mites prefer adult females over adult males (Christe et al. 2007). Strong vector preferences with respect to host sex could help explain sex-biases prevalence of vector-borne diseases that have been observed in several systems (Pickering and Christie 1980; Schall et al. 2000; Bruns et al. 2019) and could play a role in the evolution of sexual dimorphism in pathogen defense (Hamilton and Zuk 1982; Zuk and McKean 1996). Additionally, in hosts with mating systems that lack strict determination of 1:1 sex ratios or that that are not fully dioecious (e.g. true males and females), sex-specific pathogen transmission has the potential to drive the evolution of sex-ratio, and ultimately breeding systems (Steets et al. 2007; Miller and Bruns 2016; Bruns et al. 2019).
Theory shows that vector preferences for diseased vs. healthy hosts can significantly impact pathogen transmission rate (McElhany et al. 1995; Sisterson 2008; Shaw 2017; Shoemaker et al. 2019). The effect of preferences on transmission is strongly dependent on disease prevalence; McElhany et al. (1995) showed that early in an epidemic, vector preference for diseased hosts leads to an increase in transmission, because there are more visits to rare diseased hosts than expected by chance. However, late in an epidemic when infected individuals are common, preference for diseased hosts can reduce transmission because vectors are more likely to move between preferred diseased hosts than to rare healthy hosts. Vectors that switch host preferences from diseased to healthy after becoming infected with a pathogen have a larger impact on pathogen spread (Roosien et al. 2013, Shaw 2017).
In contrast, the impact of vector preferences for host sex among healthy hosts on disease transmission has received less attention. These interactions are likely to be particularly important when there is significant variation in host sex-ratio. For example, in a strongly male-biased population, where vectors strongly prefer healthy males to healthy females, vector movement may be primarily between healthy males, with fewer contacts to diseased individuals. But, if the sex ratio of the healthy population is strongly female-biased, vector avoidance of females could increase vector movement to diseased individuals potentially increasing the overall pathogen transmission rate. However, the magnitude of sex-preferences and the sex-ratio that are necessary to impact pathogen transmission are unknown.
Among flowering plants, pollinators can be important vectors of a diverse range of diseases caused by fungi, bacteria, and pollen-infecting viruses (McArt et al. 2014). We investigated pollinator preferences for disease status and sex in Dianthus pavonius, a wild, alpine carnation that is infected throughout its range with a fungal pathogen that causes ‘anther-smut disease’. The fungus causes the anthers to produce fungal spores instead of pollen, resulting in host sterilization. Pollinators play a critical role in transmission; insects visiting diseased flowers get dusted with spores and transmit these to healthy hosts mechanically (there are no fungal lifecycle stages in the host). The host plant has a mixed breeding system composed of hermaphrodites and females. Pollinator preferences for disease status and sex could potentially affect both rates of pathogen spread as well as the evolution of sex-ratio (Miller and Bruns 2016, Bruns et al. 2019). We conducted field experiments over two field seasons to investigate preferences of the pollinator community for diseased, healthy female, and healthy hermaphrodite D. pavonius flowers. We also quantified nectar production of these three flower types in the greenhouse to see if pollinator preferences could be explained by nectar quantity. We then used an agent-based model to theoretically examine the potential effects of vector preferences for infection status and sex on transmission.
We hypothesized that pollinators would prefer healthy flowers over diseased flowers, since this pattern has been observed in the related host species Silene latifolia (Shykoff and Bucheli 1995; Altizer et al. 1998). We also hypothesized that pollinators would prefer hermaphrodite flowers over females, since recent field studies have shown that female D. pavonius plants are smaller and have lower levels of spore deposition on their flowers than hermaphrodites (Bruns et al. 2019).
METHODS
Study system
Dianthus pavonius is a perennial carnation native to western alpine regions of France and Italy. We studied a population near Rifugio Garelli at ca. 2000m in the Parco Naturale del Marguareis, Cuneo Province, Piemonte Region, Italy. The flowers are bright pink and attract a wide range of pollinators including butterflies, bees, syrphid flies, and smaller anthophilid flies. The plant has two different sexual morphs: hermaphrodites and females. Hermaphrodites are protandrous and produce pollen for 3–5 days before the stigma emerges. Females have small aborted anthers and do not produce any pollen. Previous work has shown that the corolla dimeter of female flowers is 22% smaller on average than that of hermaphrodites, and that in this area, the local percentage of females ranges from 20–70% (Bruns et al. 2019).
Dianthus pavonius is infected by the anther-smut fungus Microbotryum dianthorum, (sensu lato), Microbotryales, Basidiomycota. Since infection by the fungus induces anther development in female plants, the sex of infected plants (female or hermaphrodite) is not distinguishable (Uchida et al. 2003). The average prevalence of Microbotryum in the study population is 40% and has remained at this level for the past eight years (Bruns et al. 2017). We have previously shown that female flowers have lower levels of spore deposition in the field (Bruns et al. 2019).
Pollinator abundance and preference:
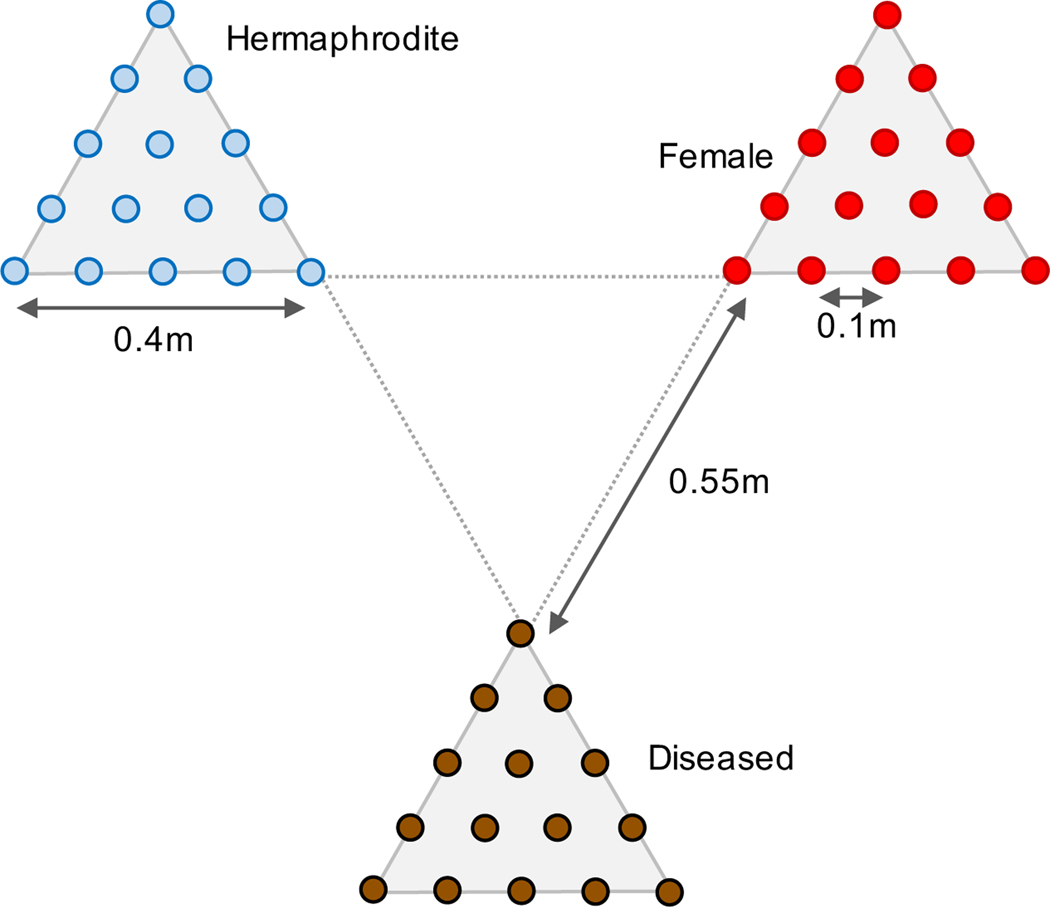
We set up pollinator choice arrays in the field in 2017 and 2019 using ‘virgin’ D. pavonius flowers in small water filled tubes used by florists. Each array had three equally spaced patches of 15 floral tubes, with each patch consisting of a single type of flower (healthy hermaphrodite, healthy female, or diseased) and with two flowers per tube (Fig. 1). In 2017 we set 3 replicate arrays near Rifugio Garelli, randomizing the orientation and order of the block assignment within the plots. In 2019, we set up 4 replicate blocks in the same general area. Each replicate array was observed by a single observer in 15-minute time blocks during sunny periods of the late-morning and mid-afternoon when pollinators were most active. We recorded the total number of pollinators landing on each flower type, separately from the total number of visits made by each pollinator to other flowers within a given patch. Visits were counted as such only if the insect landed on the petals of a flower. We did not track the number of visits between flower types. We categorized insects into the following broad groups: butterflies, bees, syrphids, small anthophilid flies (small flies that were observed crawling into the nectaries), and other (this was mainly composed of small beetles and house flies which landed on petals but were not observed to probe the flowers for nectar or pollen). The experiments were repeated over three separate years from 2017 to 2019 to assess consistency in pollinator abundances and preferences. However, we only report results from 2017 and 2019 because the available observation period in 2018 was colder and wetter and we observed too few pollinators to carry out analyses. In 2017, each plot was observed in 15-minute blocks, rotating observations between the 3 different floral patches every 1 minute. In 2019 we also observed plots in 15-minute increments but observed all 3 patches within an array simultaneously. In total, we carried out 150 observation minutes per floral type in 2017 (7 observation periods on 3 replicate plots) and 660 minutes in 2019 (13 observation periods on 4 replicate plots).
Fig. 1.
Design of field choice test. Each circle represents a floral tube, filled with two virgin flowers of the same type.
Nectar production:
To determine if nectar production varied with disease status and sex, we measured nectar quantity of greenhouse reared D. pavonius plants. We used 1mm diameter microcapillary tubes to collect nectar from 34 hermaphrodite, 32 female, and 32 diseased plants. To avoid clogging the microcapillary tubes with pollen or spores, we cut the flower off just above the ovary immediately prior to collection. Microcapillary tubes were placed into the nectaries at the base of the flower at four different locations around the ovary, and the volume of the accumulated nectar was recorded. We marked individual flowers with the date they opened so that we could account for floral age. The plants were from 24 different full-sib families, with parental seed originating from the Rifugio Garelli population. All individuals had been inoculated with Microbotryum 6 months prior to the experiment. Seventeen of the families used had replicate individuals with at least two flower types, while six families had individuals from all three flower categories (hermaphrodite, female, diseased).
Data analysis:
There was no significant difference in the number of pollinators that visited the three replicate plots in 2017 (X2=2.7732, df = 2, p-value = 0.250) nor the four replicate plots in 2019 (X2=0.10361, df = 3, p-value = 0.991). We therefore pooled results from all plots within a year prior to analysis. We used chi-squared contingency tests on the pooled data within each year to test whether there were differences in the total number of pollinators visiting patches of diseased, female and hermaphrodite flowers (“3-way” preference test). We also carried out planned “2-way” contingency tests to determine whether there were significant preferences for 1) diseased vs. healthy flowers, and 2) female vs. hermaphrodite flowers. To assess disease versus healthy preference, we compared the observed number of pollinators visiting diseased and healthy flowers to the number expected given the 1:2 ratio of these flower types in the arrays. To assess sex preferences, we excluded the visits to diseased plants, and compared the proportion of pollinators visiting females and hermaphrodites to the number expected given the 1:1 sex-ratio of healthy plants in the arrays.
We used the fitdistrplus package (Version 1.0–14) in R to describe the distribution of movements per pollinator within a patch for each pollinator type. We used only the 2019 data because the short individual observation periods in 2017 could have resulted in a truncated number of visits per pollinator. To determine whether pollinators changed behavior after visiting a flower within a patch, we calculated the proportion of pollinators that visited more than one flower within a patch of the same flower type. We used Fisher’s exact test to determine whether the proportion of pollinators making multiple visits varied among: 1) all three flower types, 2) between diseased and healthy plants, and 3) between females and hermaphrodites.
We used ANOVA to compare nectar production among the three flower types in the greenhouse experiments, with flower age as a covariate. We ran a second model using a subset of the data that included only plant families with replicate plants of all three types and included family as a random effect. All analyses were carried out using R (Version 3.6.1).
Simulations of spore dispersal:
We used an agent-based model to estimate how pollinator preferences for disease status and sex might impact pathogen spread. For simplicity, we assumed that pollinator movement was random (i.e. not dependent on physical proximity of the plants in the array). The number of movements each pollinator made was drawn from a fitted lognormal distribution based on the observed visit number for a given pollinator type. This movement pattern resulted in most pollinators visiting just one plant and leaving, with a few individuals visiting more than one flower, representative of the behavior we observed in the field.
The probability of an individual pollinator settling on host type Y for a given visit, v was:
where py is the preference for host type Y, Ny is the abundance of host type Y, and Z is the total number of hosts types (following Shoemaker et al. 2019). When there is no preference between diseased, female, and hermaphrodite plants, py=1/3. We assumed that all direct movements between a diseased and healthy plant (of either sex) resulted in spore deposition, that there was no significant carryover (e.g. a healthy plant receiving spores did not then ‘transmit’ spores), and that there was no difference in the amount of spores transferred by different pollinator types. The model was implemented in R (Version 3.6.1), see DataS1 in the supplemental material.
We first ran simulations to test the following four idealized preference scenarios: 1) null model with no preference, 2) A 2-fold preference for hermaphrodites over females, but no disease preference 3) A 2-fold preference for healthy flowers over diseased, but no sex preference, or 4) A combined 2-fold preference for both healthy and hermaphrodite flowers over female flowers. These magnitudes of sex and disease preferences are similar to the observed preferences in syrphid flies (See results, Table 1). We tested the effect of these preference combinations across a range of sex ratios and at disease prevalence levels of 10 and 40, and 80%, although we note that 40% is at the higher end of prevalence that has been observed in natural populations (Bruns et al. 2018).
Table 1.
Number of pollinators arriving to diseased, healthy female, and healthy hermaphrodite patches by different types of pollinators and results of 3-way, and planned 2-way chi-squared contingency tests.
| Number of visits | 3-way choice | Disease preference | Herm. preference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pollinator type | Diseased | Female | Herm | N | X 2 | p | Preference 1 | X 2 | p | Preference 2 | X 2 | p |
| 2017 | ||||||||||||
| Butterflies | 6 | 11 | 13 | 30 | 2.6 | 0.273 | −0.40 | 1.828 | 0.176 | 0.08 | 0.167 | 0.683 |
| Bees | 4 | 9 | 9 | 22 | 2.273 | 0.321 | −0.45 | 1.634 | 2.011 | 0.00 | 0 | 1 |
| Syrphid flies | 36 | 19 | 44 | 99 | 9.88 | 0.007 | 0.09 | 0.292 | 0.589 | 0.40 | 9.921 | 0.002 |
| Small antho. flies | 25 | 9 | 91 | 125 | 90.697 | <0.0001 | −0.40 | 9.365 | 0.002 | 0.82 | 67.24 | <0.0001 |
| Others | 17 | 20 | 25 | 62 | 1.581 | 0.454 | −0.18 | 0.719 | 0.397 | 0.11 | 0.556 | 0.456 |
|
| ||||||||||||
| Total | 88 | 68 | 182 | 338 | 65.775 | <0.0001 | −0.22 | 8.101 | 0.004 | 0.46 | 51.98 | |
|
| ||||||||||||
| 2019 | 0 | |||||||||||
| Butterflies | 2 | 3 | 4 | 9 | 0.667 | 0.717 | −0.33 | 0.124 | 0.725 | 0.14 | 0.143 | 0.706 |
| Bees | 13 | 15 | 21 | 49 | 2.123 | 0.346 | −0.20 | 0.729 | 0.393 | 0.17 | 1 | 0.173 |
| Syrphid flies | 42 | 76 | 106 | 224 | 27.467 | <0.0001 | −0.44 | 27.709 | <0.0001 | 0.16 | 4.945 | 0.026 |
| Small antho. flies | 25 | 22 | 38 | 85 | 5.106 | 0.078 | −0.12 | 0.417 | 0.591 | 0.27 | 4.267 | 0.039 |
| Others | 21 | 26 | 22 | 69 | 0.609 | 0.738 | −0.09 | 0.142 | 0.706 | −0.08 | 0.333 | 0.564 |
|
| ||||||||||||
| Total | 103 | 142 | 191 | 436 | 26.757 | <0.0001 | −0.29 | 18.5 | <0.0001 | 0.15 | 7.21 | 0.008 |
(Observed -Expected)/Expected. Negative numbers indicate a preference for healthy patches over diseased patches.
Observed -Expected/Expected. Positive numbers indicate a preference for hermaphrodite patches over female patches
We introduced pollinators into a population of 500 plants and tracked their movement between diseased and healthy plants. We chose 500 as a compromise between run length and adequate representation of disease and floral type proportions. Each simulation was run with 500 pollinators and replicated 100 times, to approximate theoretical outcomes. We used the visit distribution estimated for syrphid files, since these were estimated to have the largest impact on pathogen transmission (see results). We ran additional simulations to test the effect of varying pollinator numbers and different visit distributions.
We then used the model to evaluate the likely impact of our observed preference data on transmission, in pollinator communities. We first estimated the relative contribution of each pollinator group to transmission, we ran simulations that included all five pollinator types, adjusting by the abundance (Table 1) and visit distribution (Table 3), of each pollinator type. Then, we compared the total transmission in simulations with and without observed pollinator preferences. We only accounted for preferences within pollinator groups that were found to be statistically significant. Simulations were run 100 times, assuming a disease prevalence of 40% (similar to that observed in the natural population; Bruns et al. 2017), and a range of sex ratios.
Table 3.
Proportion of pollinators that made more than one visit within a patch of either diseased, female, or hermaphrodite flowers. N is the number of pollinators arriving at a patch of given floral type. P-values are shown for Fisher exact tests comparing persistence on all three flower types, and comparison of disease vs. healthy, and healthy female vs. healthy hermaphrodite.
| Diseased | Female | Herm. | P-values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pollinator type | prop. | N | prop. | N | prop. | N | 3 way choice | Dis. Pref | Sex. Pref |
| 2017 | |||||||||
| Butterflies | 0 | 6 | 0.545 | 11 | 0.385 | 13 | 0.105 | 0.061 | 0.682 |
| Bees | 0.5 | 4 | 0.333 | 9 | 0.556 | 9 | 0.853 | 1 | 0.637 |
| Syrphid flies | 0.25 | 36 | 0.632 | 19 | 0.545 | 44 | 0.007 | 0.003 | 0.721 |
| Small antho. flies | 0.32 | 25 | 0.333 | 9 | 0.231 | 91 | 0.53 | 0.447 | 0.445 |
| Others | 0 | 17 | 0.05 | 20 | 0.08 | 25 | 0.775 | 0.555 | 1 |
|
| |||||||||
| Total | 0.22 | 88 | 0.37 | 68 | 0.31 | 182 | 0.108 | 0.058 | 0.367 |
|
| |||||||||
| 2019 | |||||||||
| Butterflies | 0 | 2 | 0.667 | 3 | 0.5 | 4 | 0.524 | 0.444 | 1 |
| Bees | 0.615 | 13 | 0.533 | 15 | 0.667 | 21 | 0.706 | 1 | 0.499 |
| Syrphid flies | 0.429 | 42 | 0.461 | 76 | 0.594 | 106 | 0.088 | 0.232 | 0.097 |
| Small antho. flies | 0.08 | 25 | 0.091 | 22 | 0.079 | 38 | 1 | 1 | 1 |
| Others | 0.333 | 21 | 0.238 | 26 | 0.091 | 22 | 0.143 | 0.105 | 0.429 |
|
| |||||||||
| Total | 0.34 | 103 | 0.37 | 142 | 0.44 | 191 | 0.205 | 0.206 | 0.260 |
RESULTS
Pollinator abundance and preference:
Number of pollinators:
The most common pollinators on Dianthus in both 2017 and 2019 were syrphid flies and smaller anthophilid flies (Table 1) with moderate visitation from butterflies, bees, and others (primarily small beetles, house flies, and gnats). There were significant differences among the three flower types in the total number of pollinators visiting in both 2017 (Table 1). When the pollinator counts were analyzed by pollinator-type, only syrphid flies and small anthophilid flies showed statistically significant preference patterns among the three flower types (Table 1). In both cases, healthy hermaphrodites attracted more pollinators than either diseased or healthy female plants.
Planned two-way comparisons showed significant preferences for disease status among small anthophilid flies in 2017 and syrphid flies in 2019. In both cases, pollinators visited diseased plants less often than expected by chance (Table 1). Significant preferences for sex were detected in both small anthophilid and syrphid flies and were consistent across years. In both cases, pollinators preferred hermaphrodites over females.
Visits per pollinator:
The number of movements per pollinator within a patch (including single visits) fit a log normal distribution (Table 2, Appendix S1: Fig. S1). Bees had the greatest average number of sequential movements, followed by syrphid flies, while small Anthophilid flies had the least (Table 2). However, flower type only had a significant effect on probability of visiting more than one flower within a patch among syrphid flies (Table 3). In 2017, syrphids were more likely to make multiple visits within healthy patches than diseased patches. This pattern was not significant in 2019.
Table 2.
Abundance and movement rate of different pollinator groups. The average number of pollinators per hour in 2017 and 2019, and the mean and standard deviation from a fitted log-normal distribution of number of movements per pollinator in 2019.
| Ave num of poll. per hour | movements per pollinator2 | |||
|---|---|---|---|---|
| 2017 | 20191 | Log-normal mean | Log-normal Sd | |
| Syrphid flies | 13.5 | 20.22* | 0.5672 | 0.6385 |
| Anthophilid flies | 8.16 | 4.905 | 0.0685 | 0.2580 |
| Bees | 2.90 | 4.44 | 0.6415 | 0.6268 |
| Butterflies | 4.44 | 0.18 | 0.3531 | 0.4118 |
| Others | 8.16 | 4.92 | 0.2119 | 0.4609 |
Asterisks indicate a significant difference between years (p<0.05).
Across all flower types
Nectar production:
Nectar quantity increased from young (1–3 days) to old (4–7 days) in all flowers (F1,94=5.029, p=0.027). There was a significant difference in nectar quantity among diseased, female, and hermaphrodite flowers for both young and old flowers (F2,94=11.599, p <0.0001), with diseased plants producing less nectar than healthy flowers of either sex (Fig. 2). Young female flowers had more nectar than young hermaphrodite flowers, but these differences disappeared as flowers aged. Diseased flowers still had significantly lower nectar quantity when we controlled for family (X2=27.463, df=7, p=0.0003; Appendix S1: Fig. S2).
Fig. 2.
Nectar quantity in greenhouse reared plants. Error bars represent 1 SEM. Numbers indicate sample size.
Effect of vector preference on pathogen transmission:
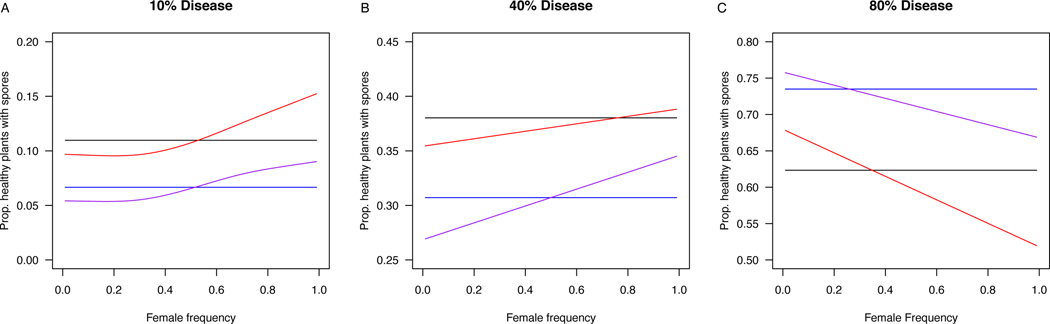
Simulations with the agent-based model showed that vector preferences for healthy hosts in a population with low prevalence led to 39% reduction in pathogen transmission at all female frequencies, compared to a null model with no preferences (Fig. 3A). Among healthy hosts, vector preferences for sex also affected transmission relative to the no-preference model, but the outcome was strongly dependent on host sex ratio. When females were rare (10%), vector preference for hermaphrodites led to a 10% decrease in pathogen transmission, relative to the no-preference model, but when females were common (90%), transmission increased by 32% relative to the no-preference model (Fig. 3A). Preferences for hermaphrodites also led to 42–45% reduction of risk of disease exposure for females relative to hermaphrodites, that was consistent across sex ratios (Appendix S1: Fig. S3). Preferences for both disease status and sex (Fig 3A, purple lines) led to a 44% reduction in transmission relative to the no preference model, and a 21% reduction in transmission relative to the healthy-preference model, when females were rare (10%). However, when females were common (90%), preferences for both disease status and sex only reduced transmission relative to the no preference model by 20% and resulted in a 30% increase in transmission relative to the healthy-preference model.
Fig. 3.
Results of simulations with the agent based model showing the individual and combined effects of vector preferences for disease-status (2-fold preference for healthy over diseased hosts) and host sex (2-fold preference for hermaphrodites over female among healthy hosts) on spore transmission when there is variation in population sex-ratio. A) 10% prevalence, B) 40% prevalence, C) 80% prevalence. Color indicates preference model: Black-No preference (py=0.333), Blue- preference for healthy hosts (pd =0.2, pf =0.4, ph =0.4), Red- Preference for hermaphrodite over female hosts (pd =0.333, pf =0.222, ph=0.445), Purple – Combined preference for disease status and sex (pd =0.2, pf =0.267, ph=0.533). All simulations were run by introducing 500 pollinators into a population of 500 plants. The lines show the spline fit from a generalized additive model.
The effects of vector preferences on transmission were mediated by disease prevalence. At 40% prevalence, (Fig. 3B) pollinator preferences for disease status and sex had smaller proportional effects on transmission than at 10% prevalence. Preference for healthy individuals led to a 19% decrease in spore transmission relative to the no-preference model, while preference for hermaphrodites over females led to a just a 6% decrease in spore transmission when females were common, and a 2% increase in transmission when females were rare. At very high prevalence (80%) the effects of vector preferences are reversed (Fig. 3C): preference for healthy plants led to an increase in transmission relative to the no-preference model, and at high female frequencies, preference for hermaphrodites led to an increase in transmission.
Simulations were run with 500 pollinators, which was an approximate estimate of the average number of syrphid pollinators visiting our arrays over the span of one week. Under these finite conditions, there was considerable variation among simulation runs (Appendix S1: Fig. S4). However, pollinator preferences for healthy individuals led to clear differences in transmission (non-overlapping standard deviations) at all prevalence levels. Preferences for hermaphrodites led to discernable changes in transmission, at extreme values of sex ratio (Appendix S1; Fig. S4). Increasing the total number of pollinators (Appendix S1: Fig. S5) or the number of visits per pollinator (Appendix S1: Fig. S6) increased the overall transmission rate, and the effect of preferences relative to the null model.
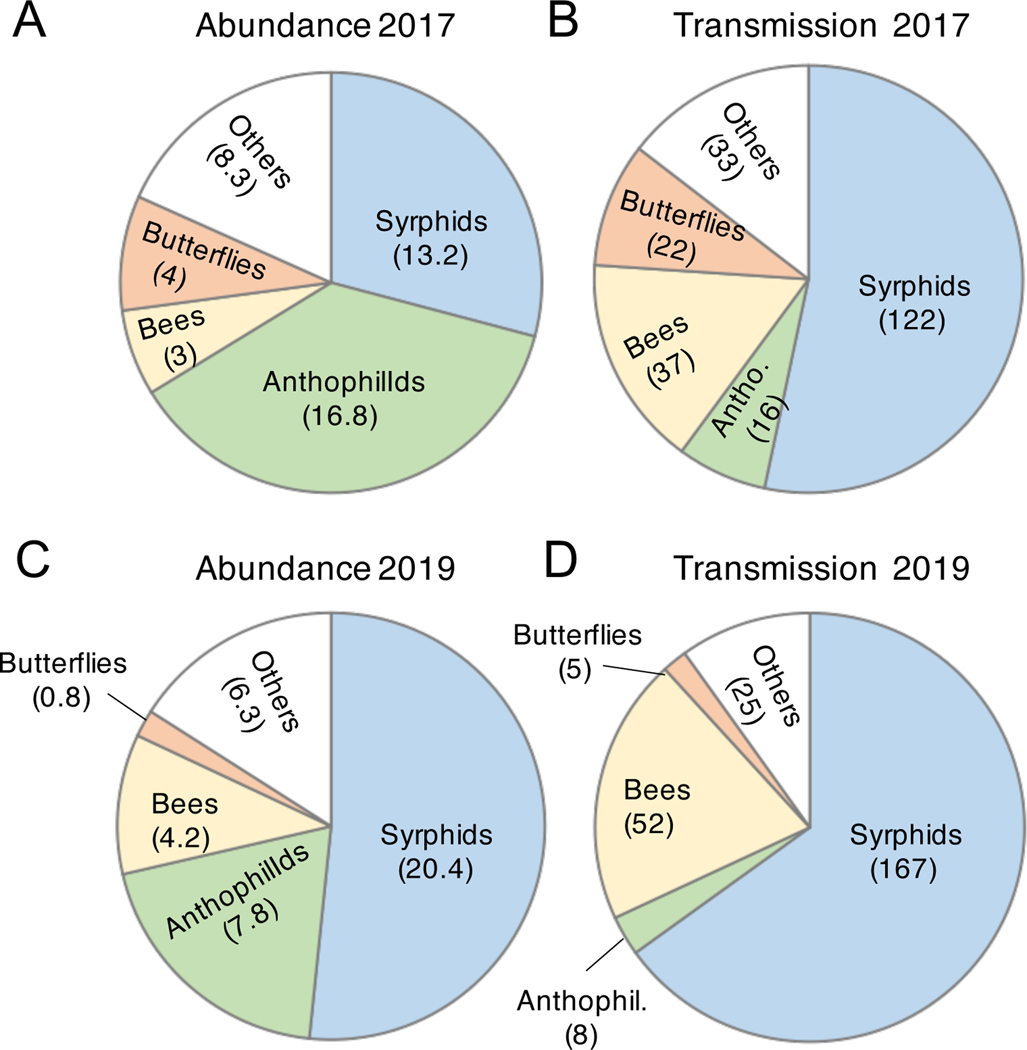
Contribution of pollinator guilds to transmission
In simulations with the full pollinator community, syrphid flies contributed more to transmission than any other guild (Fig. 4). Bees were the second highest contributor to transmission, despite relatively low abundance. Indeed, both syrphids and bees contributed more to transmission than predicted by their proportional abundance alone (Fig. 4A, C), an effect that was driven by the high number of visits per pollinator in these two groups (Table 2, Appendix S1: Fig. S1). In contrast, small anthophilid flies contributed very little to transmission, despite their high abundance.
Fig. 4.
Contribution of different pollinator guilds to: A,C) the total abundance of pollinators observed in D. pavonius arrays (numbers indicate visits per hour), and B,D) the predicted number of transmission events in population of 500 plants assuming 40% disease prevalence, a 50:50 sex ratio, and no pollinator preferences.
Accounting for the significant syrphid preferences observed in 2019 led to a 10% decrease in total transmission in simulations, relative to a null model with no preferences. The 95% confidence interval for proportion of healthy plants becoming infected was 0.62 to 0.73 in the absence of syrphid preferences and dropped to 0.54 to 0.66 in the presence of preferences. Pathogen transmission was decreased further when females were rare (Appendix S1: Table S1). In contrast, accounting for the significant pollinator preferences observed among syrphid and small anthophilid flies in 2017 had little discernable effect on transmission at any sex ratio (Appendix S1: Table S1).
DISCUSSION
Pollinators displayed preferences for host infection status and sex, but the strength of these preferences varied across years and were only present for some groups of pollinators. Where preferences were detected, healthy hermaphrodite flowers were preferred over both healthy female flowers and flowers infected with anther-smut. We also found evidence that some groups of pollinators preferred healthy flowers (regardless of sex) over diseased flowers. However, the bias against disease was less consistent across pollinator guilds and years than the preference for hermaphrodites over females. Results from our agent-based model show that while preferences for healthy hosts can reduce pathogen spread, preferences for sex can also impact rates of pathogen spread, depending upon the sex ratio of the population. Taken together, our results show that vector preferences are ecologically important when there is variation in disease prevalence and host sex ratio.
Vector preferences for healthy hermaphrodites
Our finding that pollinators tended to prefer healthy flowers is broadly consistent with pollinator behavior in the related, dioecious species Silene latifolia. Altizer et al. (1998) found that when the number of inflorescences on a plant was accounted for, healthy S. latifolia plants in natural populations had more visits from nocturnal moths per flower than diseased plants. Shykoff and Bucheli (1995) found that nocturnal pollinators strongly discriminated against diseased plants, but diurnal pollinators did not. However, both these previous studies used whole plants, and a significant portion of the variation in visitation rates were explained by differences in inflorescence number. We controlled for flower age and inflorescence display size by using cut flowers of the same age. Thus, the differences in visitation rate that we observed can be attributed to properties of the flowers themselves (e.g. size, smell, nectar quantity, and the presence or absence of pollen), rather than differences in phenology or plant size.
We showed that healthy D. pavonius plants consistently produced more nectar than diseased plants, which could help explain the preference for healthy plants that was observed in syrphid and small anthophilid flies. However, the strong preference for hermaphrodite flowers over female flowers, exhibited by these same pollinators cannot be explained by nectar rewards, as we did not find any significant difference in nectar production between the two sexes. The patterns of nectar production we found in D. pavonius differ from those of other closely related species. Shykoff et al. (1997) found that nectar production of the gynomonecious plant Dianthus sylvestris was significantly higher for hermaphrodites compared to either diseased or female plants. In S. latifolia, Shykoff and Kaltz (1998) found that nectar sugar was highest in male flowers, and lowest in female flowers, with diseased flowers having intermediate levels of sugar. The impact of infection on nectar production, especially as a potential example of pathogen manipulation of host phenotype, is a subject deserving of further study. Previous work has shown that Microbotryum can manipulate several floral traits that are likely important to pollinator choice, such as increased inflorescence number (Alexander 1987; Shykoff and Kaltz 1998; Bruns et al. 2017) and earlier flowering (Shykoff et al. 1997; Tang et al. 2019).
Sex-biased visitation rates in D. pavonius could also result from differences in flower size and pollen availability. In D. pavonius, we have previously shown that hermaphrodite flowers are significantly larger than female flowers (Bruns et al. 2019), which could account for some of the bias in favor of hermaphrodites. (Diseased flowers, which are a mix of the two sexes, have an intermediate size, but higher variance.) Sex-specific difference in flower size has been shown to positively correlate with pollinator visitation rate in several species (Eckhart 1991; Kaczorowski et al. 2012; McCall and Barr 2012). In D. sylvestris, Shykoff (1997) showed that corolla size was a strong, positive predictor of anther-smut spore deposition. The presence of pollen is also likely to be important, since pollinator preference for hermaphrodites was strongest in syrphid flies which we commonly observed collecting pollen in the field.
The preferences we observed for pollinators are unlikely to be a result of pathogen manipulation of vector behavior, a phenomenon that has been shown in many other vector-borne pathogens (Ingwell et al. 2012; Fang et al. 2013; Gandon 2018), because pollinators are passive vectors of anther-smut disease. Microbotryum spores are mechanically transmitted to new hosts on the bodies of pollinators, and the fungus does carry out any stages of its lifecycle inside the vector. Passive pollinator transmission occurs for several other plant pathogens (McArt et al. 2014) including economically important diseases such as fire blight of apples and pears caused Erwinia bacteria (Alexandrova et al. 2002, Sasu et al. 2010). This mode of transmission is also similar to some ‘non-persistent’ aphid transmitted plant viruses that bind to the mouth parts of the aphid (Ng and Falk 2006; Mauck et al. 2012), or non-biting ‘filth flies’ that can mechanically transmit the bacteria causing anthrax and yaws to primates (Gogarten et al. 2019).
Consequences for pathogen spread and evolution
Our simulations show that the observed pollinator preferences have the potential to influence pathogen spread. When pollinators discriminated against diseased plants, the average number of spore transmission events declined 27% relative to a model without preferences. The reduction in spore transmission was greater when prevalence was lower (10%), compared to the higher (40%) level observed in nature, consistent with prior models (McElhany et al. 1995; Sisterson 2008). Importantly, we also showed that if the sex ratio deviated significantly from 50:50, pollinator preferences for sex can affect spore transmission even if there was no bias against disease per se. This occurred because the difference in vector preference between the two healthy sex morphs, caused preference for the diseased host to become intermediate between that of females and hermaphrodites. For example, when the preferred sex (hermaphrodites in this case) was rare, pathogen spread was enhanced because vectors preferred diseased plants over the common sex (females) and therefore had more contacts to diseased individuals than when the preferred sex was common.
To our knowledge, this is the first study to show that vector preferences for host traits not related to infection status can affect pathogen transmission. The epidemiological impact of vector sex-preferences are more likely to be important where there is substantial variation in sex-ratio among populations. Among Dianthus pavonius populations, female frequency can range from 0 to 70% (Bruns et al. 2019). Previously, we reported that populations of D. pavonius with anther-smut disease had a higher frequency of females than healthy populations, a pattern we interpreted at the time as evidence of the evolution of female-bias sex ratios following selection from disease (Bruns et al. 2019). In light of the current study, an alternative explanation may be that pollinator preferences make disease more likely to spread when the population is female-biased, because aversion to females leads to more movement between diseased and hermaphrodite plants.
The strong pollinator preferences for hermaphrodites over females can also help explain sex-specific patterns of spore dispersal observed in the field (Bruns et al. 2019). We have previously shown that hermaphrodite flowers have higher average levels of spore deposition, a pattern that is partially explained by longer floral lifespans, which increases the greater total number of visits (Bruns et al. 2019). Our current results show that inherent pollinator preferences may also be critical to understanding this pattern. In 2017, syrphids flies showed a strong bias against female plants relative to both hermaphrodites and diseased plants. In simulations, this preference led to a 26% reduction in spore transmission events in females relative to hermaphrodites.
Plants with mixed breeding systems are not the only hosts with sex-ratios that deviate from 50:50. In addition to species with environmental sex determination and sequential hermaphroditism, sex-ratio distorting elements in organisms with chromosomal sex-determination can drive uneven sex ratios in plants and animals (Wood and Newton 1991; Taylor and Ingvarsson 2003). ‘Functional’ sex-ratio biases are also common among social animals with cooperative breeding (Komdeur 1996; Allaine 2000), and those that form female-biased ‘harems’ or roving ‘bachelor herds’ (Bonenfant et al. 2004; Campbell 2008). Vector-preferences for sex could therefore have significant effect on the spread of vector-borne diseases in these types of social animals.
CONCLUSIONS
We found evidence of pollinator preferences for healthy hermaphrodites over both diseased and female flowers, and that these preferences have the potential to impact pathogen spread. Critically, our modeling results show that vector preferences for sex among healthy hosts can impact transmission when host sex-ratio varies, a process which could both affect patterns of pathogen spread and, generate sex-specific risks of disease with potential consequences for selection on sex ratios
Supplementary Material
ACKNOWLEDGEMENTS:
We thank Indigo Ballister, Rachel Cohen, Jae-Hoon Cho, Valentina Carasso, Ivan Pace, and Zach Stern for help with flower collections and pollinator observations and Liz Troy and Kortney Bugg for help with nectar experiments. We are also grateful to Guido and Adriana Colombo and the staff at Rifugio Garelli for their hospitality. The research was made possible with support from NIH R01GM122061 to J.A. E.B., and M.H., as part of the joint NSF-NIH-USDA Ecology and Evolution of Infectious Diseases program.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Alexander HM 1987. Pollination limitation in a population of Silene alba infected by the anther-smut fungus, Ustilago violaceum. The Journal of Ecology 75:771. [Google Scholar]
- Alexandrova M, Cimini B, Carpana E, Massi S, Sabatini AG & Bazzi C. 2002. The role of honeybees in spreading Erwinia amylovora. Acta Horticulturae, 590: 55–60 [Google Scholar]
- Allaine D. 2000. Male-biased sex ratio in litters of Alpine marmots supports the helper repayment hypothesis. Behavioral Ecology 11:507–514. [Google Scholar]
- Altizer SM, Thrall PH, and Antonovics J. 1998. Vector behavior and the transmission of anther-smut infection in Silene alba. American Midland Naturalist 139:147–163. [Google Scholar]
- Bonenfant C, Gaillard J-M, Klein F, and Maillard D. 2004. Variation in harem size of red deer (Cervus elaphus L.): the effects of adult sex ratio and age-structure. Journal of Zoology 264:77–85. [Google Scholar]
- Bradshaw CJA, Harcourt RG, and Davis LS 2003. Male-biased sex ratios in New Zealand fur seal pups relative to environmental variation. Behavioral Ecology Sociobiology 53:297–307. [Google Scholar]
- Bruns EL, Antonovics J, Carasso V, and Hood M. 2017. Transmission and temporal dynamics of anther-smut disease (Microbotryum) on alpine carnation (Dianthus pavonius). Journal of Ecology 105:1413–1424. [Google Scholar]
- Bruns EL, Miller I, Hood ME, Carasso V, and Antonovics J. 2019. The role of infectious disease in the evolution of females: Evidence from anther-smut disease on a gynodioecious alpine carnation. Evolution 73:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett-Cadena ND, Bingham AM, and Unnasch TR 2014. Sex-biased avian host use by arbovirus vectors. Royal Society Open Science 1:140262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. 2008. The Relationship between roosting ecology and degree of polygyny in harem-forming bats: perspectives from Cynopterus. J Mammal 89:1351–1360. [Google Scholar]
- Charlesworth B, and Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. The American Naturalist 112:975–997. [Google Scholar]
- Christe P, Glaizot O, Evanno G, Bruyndonckx N, Devevey G, Yannic G, Patthey P, Maeder A, Vogel P, and Arlettaz R. 2007. Host sex and ectoparasites choice: preference for, and higher survival on female hosts. Journal of Animal Ecology 76:703–710. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH 1986. Sex ratio variation in birds. Ibis 128:317–329. [Google Scholar]
- Cornet S, Nicot A, Rivero A, and Gandon S. 2013. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecology Letters 16:323–329. [DOI] [PubMed] [Google Scholar]
- Daugherty MP, Rashed A, Almeida RPP, and Perring TM 2011. Vector preference for hosts differing in infection status: sharpshooter movement and Xylella fastidiosa transmission. Ecological Entomology 36:654–662. [Google Scholar]
- Eckhart VM 1991. The effects of floral display on pollinator visitation vary among populations of Phacelia linearis (Hydrophyllaceae). Evol Ecol 5:370–384. [Google Scholar]
- Fang Y, Jiao X, Xie W, Wang S, Wu Q, Shi X, Chen G, Su Q, Yang X, Pan H, and Zhang Y. 2013. Tomato yellow leaf curl virus alters the host preferences of its vector Bemisia tabaci. Scientific Reports 3:2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest J, and Thomson JD 2009. Pollinator experience, neophobia and the evolution of flowering time. Proceedings of the Royal Society B: Biological Sciences 276:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandon S. 2018. Evolution and Manipulation of Vector Host Choice. The American Naturalist 192:23–34. [DOI] [PubMed] [Google Scholar]
- Gervasi Stephanie S, Burkett-Cadena Nathan, Burgan Sarah C, Schrey Aaron W, Hassan Hassan K, Unnasch Thomas R, and Martin Lynn B. 2016. Host stress hormones alter vector feeding preferences, success, and productivity. Proceedings of the Royal Society B: Biological Sciences 283:20161278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogarten JF, Düx A, Mubemba B, Pléh K, Hoffmann C, Mielke A, Müller-Tiburtius J, Sachse A, Wittig RM, Calvignac-Spencer S, and Leendertz FH 2019. Tropical rainforest flies carrying pathogens form stable associations with social nonhuman primates. Molecular Ecology 28:4242–4258. [DOI] [PubMed] [Google Scholar]
- Gubler DJ 1998. Resurgent vector-borne diseases as a global health problem. Emerging Infectious Disease 4:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD, and Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites? Science 218:384–387. [DOI] [PubMed] [Google Scholar]
- Harvell D, Altizer S, Cattadori IM, Harrington L, and Weil E. 2009. Climate change and wildlife diseases: When does the host matter the most? Ecology 90:912–920. [DOI] [PubMed] [Google Scholar]
- Ingwell LL, Eigenbrode SD, and Bosque-Pérez NA 2012. Plant viruses alter insect behavior to enhance their spread. Scientific Reports 2:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steets Janette A., Wolf Diana E., Auld Josh R., and Ashman Tia-Lynn. 2007. The role of natural enemies in the expression and evolution of mixed mating in hermaphroditic plants and animals. Evolution 61:2043–2055. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, and Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451:990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski RL, Seliger AR, Gaskett AC, Wigsten SK, and Raguso RA 2012. Corolla shape vs. size in flower choice by a nocturnal hawkmoth pollinator. Functional Ecology 26:577–587. [Google Scholar]
- Kingsolver JG 1987. Mosquito host choice and the epidemiology of malaria. The American Naturalist 130:811–827. [Google Scholar]
- Komdeur J. 1996. Facultative sex ratio bias in the offspring of Seychelles warblers. Proceedings of the Royal Society B: Biological Sciences 263:661–666. [Google Scholar]
- Lacroix R, Mukabana WR, Gouagna LC, and Koella JC 2005. Malaria infection increases attractiveness of humans to mosquitoes. PLOS Biology 3:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck K, Bosque-Pérez NA, Eigenbrode SD, Moraes CMD, and Mescher MC 2012. Transmission mechanisms shape pathogen effects on host–vector interactions: evidence from plant viruses. Functional Ecology 26:1162–1175. [Google Scholar]
- McArt SH, Koch H, Irwin RE, and Adler LS 2014. Arranging the bouquet of disease: floral traits and the transmission of plant and animal pathogens. Ecology Letters 17:624–636 [DOI] [PubMed] [Google Scholar]
- McCall AC and Barr CM, 2012. Why do florivores prefer hermaphrodites over females in Nemophila menziesii (Boraginaceae)?. Oecologia, 170: 147–157. [DOI] [PubMed] [Google Scholar]
- McElhany P, Real LA, and Power AG 1995. Vector preference and disease dynamics: a study of barley yellow dwarf virus. Ecology 76:444–457. [Google Scholar]
- Miller I, and Bruns E. 2016. The effect of disease on the evolution of females and the genetic basis of sex in populations with cytoplasmic male sterility. Proc. R. Soc. B 283:20153035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes CMD, Stanczyk NM, Betz HS, Pulido H, Sim DG, Read AF, and Mescher MC 2014. Malaria-induced changes in host odors enhance mosquito attraction. PNAS 111:11079–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JCK, and Falk BW 2006. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annual Review of Phytopathology 44:183–212. [DOI] [PubMed] [Google Scholar]
- Pickering AD, and Christie P. 1980. Sexual differences in the incidence and severity of ectoparasitic infestation of the brown trout, Salmo trutta L. Journal of Fish Biology 16:669–683. [Google Scholar]
- Reisen WK 2010. Landscape epidemiology of vector-borne diseases. Annual Review of Entomology 55:461–483. [DOI] [PubMed] [Google Scholar]
- Roosien BK, Gomulkiewicz R, Ingwell LL, Bosque-Pérez NA, Rajabaskar D, and Eigenbrode SD 2013. Conditional vector preference aids the spread of plant pathogens: Results from a model. Environmental Entomology 42: 1299–1308. [DOI] [PubMed] [Google Scholar]
- Schall JJ, Pearson AR, and Perkins SL 2000. Prevalence of malaria parasites (Plasmodium floridense and Plasmodium azurophilum) infecting a Puerto Rican lizard (Anolis gundlachi): a nine-year study. The Journal of Parasitology 86:511–515. [DOI] [PubMed] [Google Scholar]
- Wood RJ and Newton ME 1991. Sex-ratio distortion caused by meiotic drive in mosquitoes. The American Naturalist. 137: 270–391. [Google Scholar]
- Sasu MA, Seidl-Adams I, Wall K, Winsor JA & Stephenson AG 2010. Floral transmission of Erwinia tracheiphila by cucumber beetles in a wild Cucurbita pepo. Environmental. Entomology, 39, 140–148. [DOI] [PubMed] [Google Scholar]
- Shaw AK, Peace a. Power AG & Bosque-Perez NA 2017. Vector population growth and condition-dependent movement drive the spread of plant pathogens. Ecology. 98: 2145–2157. [DOI] [PubMed] [Google Scholar]
- Shoemaker LG, Hayhurst E, Weiss-Lehman CP, Strauss AT, Porath-Krause A, Borer ET, Seabloom EW, and Shaw AK 2019. Pathogens manipulate the preference of vectors, slowing disease spread in a multi-host system. Ecology Letters 22:1115–1125. [DOI] [PubMed] [Google Scholar]
- Shykoff JA, and Bucheli E. 1995. Pollinator visitation patterns, floral rewards and the probability of transmission of Microbotryum violaceum, a venereal disease of plants. Journal of Ecology 83:189–1. [Google Scholar]
- Shykoff JA, Bucheli E, and Kaltz O. 1997. Anther smut disease in Dianthus silvester (caryophyllaceae): natural selection on floral traits. Evolution 51:383. [DOI] [PubMed] [Google Scholar]
- Shykoff JA, and Kaltz O. 1998. Phenotypic changes in host plants diseased by Microbotryum violaceum: parasite manipulation, side effects, and trade-offs. International Journal of Plant Sciences 159:236–243. [Google Scholar]
- Sisterson MS 2008. Effects of insect-vector preference for healthy or infected plants on pathogen spread: insights from a model. Journal of Economic Entomology 101:8. [DOI] [PubMed] [Google Scholar]
- Smithson A, and Macnair MR 1996. Frequency-dependent selection by pollinators: mechanisms and consequences with regard to behaviour of bumblebees Bombus terrestris (L.) (Hymenoptera: Apidae). Journal of Evolutionary Biology 9:571–588. [Google Scholar]
- Taylor DR and Ingvarsson PK 2003. Common features of segregation distortion in plants and animals. Genetica 117: 27–35. [DOI] [PubMed] [Google Scholar]
- Tang H, Hood ME, Ren Z-X, Li H-D, Zhao Y-H, Wolfe LM, Li D-Z, and Wang H. 2019. Specificity and seasonal prevalence of anther smut disease Microbotryum on sympatric Himalayan Silene species. Journal of Evolutionary Biology 32. [DOI] [PubMed] [Google Scholar]
- Uchida W, Matsunaga S, Sugiyama R, Kazama Y, and Kawano S. 2003. Morphological development of anthers induced by the dimorphic smut fungus Microbotryum violaceum in female flowers of the dioecious plant Silene latifolia. Planta 218:240–248. [DOI] [PubMed] [Google Scholar]
- Wood RJ and Newton ME 1991. Sex-ratio distortion caused by meiotic drive in mosquitoes. The American Naturalist. 137: 270–391. [Google Scholar]
- Zuk M, and McKean KA 1996. Sex differences in parasite infections: patterns and processes. International Journal for Parasitology 26:1. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.